Abstract
Interleukin-18 (IL-18) produced by activated antigen-presenting cells stimulates natural killer (NK) cells, natural killer T (NKT) cells, and T cells to secrete gamma interferon (IFN-γ). In this study, injection of a single 10-μg dose of recombinant murine IL-18 rapidly, reversibly, and noncytopathically inhibited hepatitis B virus (HBV) replication in the livers of HBV transgenic mice. Furthermore, HBV replication was inhibited by as little as 1 μg of IL-18 injected repetitively, and also by a single 0.1-μg dose of IL-18 injected together with 1 ng of IL-12, neither of which inhibited HBV replication individually, demonstrating synergy between these cytokines in this system. The antiviral effect of IL-18 was mediated by its ability to activate resident intrahepatic NK cells and NKT cells to produce IFN-γ and by its ability to induce IFN-α/β production in the liver. These results suggest that IL-18 has the potential to contribute to the control of HBV replication during self-limited infection and that it may have therapeutic value for the treatment of patients with chronic hepatitis.
We have previously shown that the intrahepatic induction of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IFN-α/β inhibits hepatitis B virus (HBV) replication noncytopathically in the livers of transgenic mice (7). This antiviral effect can be induced by the adoptive transfer of HBV-specific CD8 and CD4 T cells (4, 8), in response to inflammatory cytokines that are produced in the liver during lymphocytic choriomeningitis virus (6, 13), murine cytomegalovirus (1), and adenovirus (1) infections; by the IFN-γ and IFN-α/β inducers poly(I/C), alpha-galactosylceramide, and IL-12; and by an agonistic anti-CD40 antibody that induces IL-12 and IL-18 production in the liver (2, 11, 13, 26).
IL-18 is a new member of the IL-1 family (3, 15, 17, 18). IL-18 is produced as a biologically inactive precursor, and active IL-18 is secreted after cleavage with caspase-1 or other caspases (15). Originally, IL-18 was identified as an IFN-γ-inducing factor that can act on Th1 cells, nonpolarized T cells, natural killer (NK) cells, B cells, and dendritic cells to produce IFN-γ in the presence of IL-12 (15-18). In addition, to its potent induction of IFN-γ, IL-18 activates CD8+ T cells, which play a central role in viral clearance. Thus, the published record suggests that IL-18 might play a role in viral infection. Indeed, a protective effect of IL-18 has been shown in mouse models of herpes simplex virus infection (5) and vaccinia virus infection (22). However, the mechanism of this antiviral effect and its relationship to viral replication have not been defined.
In this study we show that recombinant murine IL-18 (mIL-18) inhibits HBV replication noncytopathically in the livers of HBV transgenic mice in an IFN-γ- and IFN-α/β-dependent manner and that its antiviral effect is synergistic with IL-12.
MATERIALS AND METHODS
Mice.
HBV transgenic mouse lineages 1.3.32 and 1.3.46 have been previously described (9). Both lineages of mice replicate HBV at high levels in the liver and kidney without any evidence of cytopathology. Lineage 1.3.32 was expanded by repetitive backcrossing (more than 20 generations) against C57BL/6 parental strain and then bred one generation against B10.D2. mice to produce the F1 hybrids used in this study. Transgenic mice from lineage 1.3.46 were backcrossed against mice genetically deficient for the IFN-α/β receptor (IFN-α/βR−/−) (14) kindly provided by Michel Aguet (Genentech), exactly as described previously (13). In all experiments, the mice were matched for age (8 weeks), sex (male), and hepatitis B e antigen (HBeAg) levels in serum before experimental manipulations. All animals were housed in pathogen-free rooms under strict barrier conditions.
Reagents.
Recombinant mIL-18 was kindly provided by Klaus M. Esser (Glaxo-SmithKline, Collegeville, Pa.). In all experiments, groups of three to four transgenic mice were injected subcutaneously with IL-18 (doses ranging from 100 ng to 100 μg in a 300-μl volume). IL-18 was diluted in nonpyrogenic phosphate-buffered saline (PBS) (GIBCO Invitrogen Corporation, Carlsbad, Calif.). Recombinant mIL-12, kindly provided Maurice Gately (Hoffmann-La Roche, Nutley, N.J.), was administered intraperitoneally. Anticytokine monoclonal antibodies (MAb) were administered intraperitoneally 24 h before IL-18 injection. Hamster MAb H22 specific for mIFN-γ and hamster MAb TN3 19.12 specific for mTNF-α (generously provided by Robert Schreiber, Washington University St. Louis) were used in this study. In those studies, purified hamster immunoglobulin G (IgG) (Jackson ImmunoResearch, West Grove, Pa.) was used as a control. The hamster antibodies were diluted to 250 μg/200 μl/mouse with PBS (GIBCO Invitrogen Corporation) immediately prior to administration. Goat polyclonal antibody specific for mIL-12, kindly provided by Maurice Gately (Hoffmann-La Roche), was administered intraperitoneally 2 h before IL-18 injection (23). In those studies, purified polyclonal goat IgG (Sigma-Aldrich, St. Louis, Mo.) was used as a control. Rat MAb specific for mIL-18 (clone 74, generously provided by Klaus M. Esser, Glaxo-SmithKline) and rat IgG2a MAb (BD PharMingen, San Diego, Calif.) was used as a control.
DNA and RNA analysis.
Frozen liver tissue was mechanically pulverized under liquid nitrogen and total genomic DNA and RNA were isolated for Southern blotting and RNase protection assay (RPA). Quantification of various cytokine RNAs was performed by RPA exactly as previously described (8, 10). Total DNA was extracted as described previously (9). Twenty micrograms of total DNA was analyzed by Southern blotting with a 32P-labeled full-length HBV DNA probe after HindIII digestion (9, 20, 21).
Biochemical and histological analysis.
The extent of hepatocellular injury was monitored histologically and biochemically by measuring serum alanine aminotransferase (sALT) activity at multiple time points after infection. sALT activity was measured in a Paramax chemical analyzer (Baxter Diagnostics Inc., McGaw Park, Ill.) exactly as previously described (8). For histological analysis, liver tissue was fixed in 10% zinc-buffered formalin (Anatech, Battle Creek, Mich.), embedded in paraffin, sectioned (3 μm), and stained with hematoxylin and eosin.
Intrahepatic lymphomononuclear cell preparation.
Single-cell suspensions were prepared from liver that was perfused with PBS via the inferior vena cava and pressed through a 70-μm-pore-size cell strainer (Becton Dickinson). Total liver cells were digested with 10 ml of RPMI 1640 (Life Technologies) containing 0.02% (wt/vol) collagenase IV (Sigma-Aldrich) and 0.002% (wt/vol) DNase I (Sigma-Aldrich) for 40 min at 37°C. Cells were washed with RPMI 1640 and then underlaid with 24% (wt/vol) metrizamide (Sigma-Aldrich) in PBS. After centrifugation for 20 min at 400 × g, intrahepatic leukocytes (IHLs) were isolated at the interface. Red blood cells were lysed by lysing buffer (0.15 M NH4Cl, 10.0 mM KHCO3, and 0.1 mM Na2EDTA at pH 7.2). The cells were washed once with RPMI 1640 and used for further analysis.
Detection of intracellular cytokines.
IHLs were harvested from HBV transgenic mice at various indicated times after IL-18 injection and cultured ex vivo for 4 h in brefeldin A (BD PharMingen) to allow target protein to be sequestered in the Golgi apparatus. Cells were then surface stained with anti-CD3-FITC, anti-NK1.1-PE MAb (BD PharMingen) washed in fluorescence-activated cell sorter (FACS) buffer (PBS with 1% fetal calf serum), and fixed in 2% paraformaldehyde for 30 min at room temperature. After fixation, cells were permeabilized for 30 min in 25 μl PBS plus 0.5% saponin. Antimouse IFN-γ allophycocyanin (APC) or isotype control-APC MAb (BD PharMingen) was added at a final dilution of 1/100, and cells were incubated for 30 min at room temperature. Cells were washed and resuspended in 1 ml of FACS buffer for analysis on FACScalibur flow cytometer as described previously (11).
RESULTS
IL-18 inhibits HBV replication: dose response.
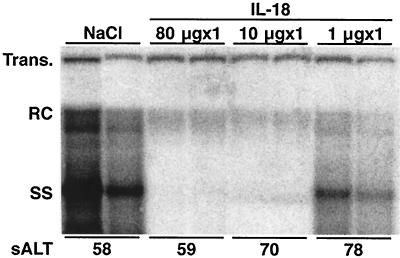
Three age-matched (8- to 10-week), sex-matched (male), and serum HBeAg-matched transgenic mice from lineage 1.3.32 were injected subcutaneously with 1, 10, and 80 μg of recombinant mIL-18 and sacrificed 24 h later. Total hepatic DNA was analyzed for HBV DNA by Southern blot analysis. As shown in Fig. 1 for two representative mice per group, a dose dependent antiviral effect was observed. HBV replication was almost completely abolished by the administration of 10 and 80 μg of IL-18, and it was slightly inhibited by 1 μg of IL-18 compared with control. As shown at the bottom of Fig. 1, the administration of IL-18 did not cause a significant elevation of sALT activity, indicating that the antiviral effect was essentially noncytopathic even at the 80 μg dose.
FIG. 1.
IL-18 inhibits HBV replication: dose response. Age-, sex-, and serum HBeAg-matched lineage 1.3.32 HBV transgenic mice were injected subcutaneously with 80, 10, and 1 μg IL-18 and sacrificed at the indicated time points. Total hepatic DNA was analyzed for HBV DNA by Southern blot analysis. All DNA samples were RNase treated before quantitation and gel electrophoresis. Bands corresponding to the integrated transgene (Trans.), relaxed circular double stranded (RC) and single stranded (SS) HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. Results were compared with those observed in livers from two age-, sex-, and serum HBeAg-matched transgenic littermates injected with saline (NaCl). The mean sALT activity, measured at the time of autopsy, is indicated for each group and is expressed in units/liter.
IL-18 inhibits HBV replication: time course.
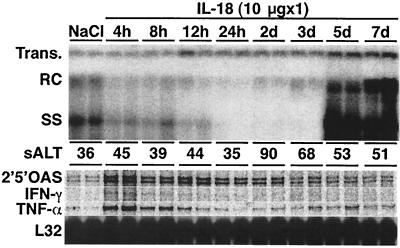
To examine the kinetics and duration of the antiviral effect of IL-18, groups of three transgenic mice from lineage 1.3.32 were injected subcutaneously with 10 μg of IL-18 and killed at various time points thereafter and their livers were analyzed by Southern blot for hepatic HBV DNA. As shown in Fig. 2 for two representative mice per group, hepatic HBV DNA content decreased as early as 4 h after injection, and it was nearly completely abolished within 24 h. The antiviral effect continued for 3 days and the HBV DNA content of the liver returned to baseline by day 5. These changes were accompanied by the early induction IFN-γ, TNF-α, and 2′5′-OAS (an IFN-α/β-inducible gene) mRNA expression 4 h after IL-18 injection which subsequently subsided and returned to baseline levels by day 3. These results suggested that the administration of IL-18 rapidly induced inflammatory cytokines in the liver that have been previously shown to inhibit HBV replication (8, 11, 13, 26). Serum ALT activity was unchanged in these animals until it increased slightly and very transiently on day 2 after IL-18 injection, again indicating that IL-18 inhibits HBV replication in the liver noncytopathically.
FIG. 2.
IL-18 inhibits HBV replication: time course. Age-, sex-, and serum HBeAg-matched lineage 1.3.32 HBV transgenic mice were injected subcutaneously with a single dose (10 μg) of IL-18 and sacrificed at the indicated time points. Total hepatic DNA was analyzed for HBV DNA by Southern blot analysis. The mean sALT activity, measured at the time of autopsy, is indicated for each group and is expressed in units/liter. Total hepatic RNA from the same mice was also analyzed by RPA for the expression of various cytokines as indicated. The RNA encoding the ribosomal protein L32 was used to normalize the amount of RNA loaded in each lane. Results were compared with those observed in livers from two age-, sex-, and serum HBeAg-matched transgenic littermates injected with saline (NaCl).
Cellular source of intrahepatic IFN-γ.
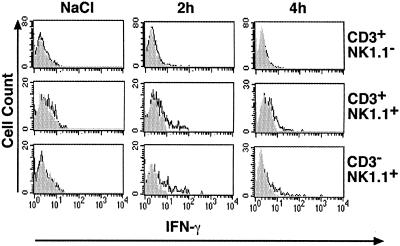
To demonstrate which intrahepatic cell populations were producing IFN-γ in response to IL-18, intracellular cytokine expression was examined by flow cytometry at very early time points (2 to 4 h) after IL-18 injection. Groups of 3 transgenic mice from lineage 1.3.32 were injected subcutaneously with 10 μg of IL-18 and IHLs were harvested 2 to 4 h later, incubated for 4 h with Brefeldin A, and analyzed for IFN-γ production by surface staining with anti-CD3-FITC, anti-NK1.1-PE and anti-IFN-γ-APC. As shown in Fig. 3, we found that NK cells (CD3−/NK1.1+) and NKT cells (CD3+/NK1.1+) but not T cells (CD3+/NK1.1−) produced IFN-γ as early as 2 and 4 h after injection, suggesting that they probably secreted this antiviral cytokine.
FIG. 3.
Cellular source of intrahepatic IFN-γ. IHLs harvested from HBV transgenic mice at the indicated time after IL-18 were surface stained with anti-CD3-FITC and anti-NK1.1-PE MAb and fixed in 2% paraformaldehyde. After fixation, cells were permeabilized, stained with anti-mouse IFN-γ-APC or isotype control-APC and analyzed by flow cytometry.
Repetitive IL-18 injection.
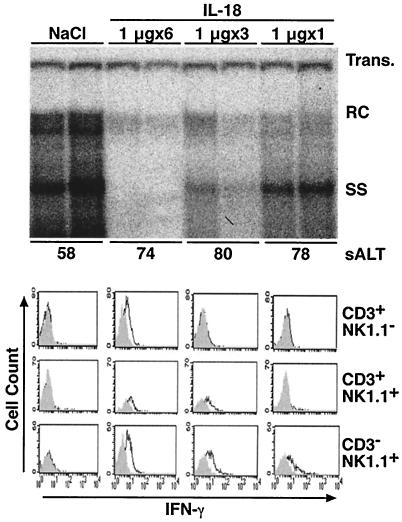
Groups of mice were injected daily for up to 6 days with 1 μg of IL-18 to determine if it was possible to inhibit HBV replication efficiently with this low dose. Mice were sacrificed 24 h after the last injection. As shown in Fig. 4 for two representative mice per group, hepatic HBV DNA content was almost completely abolished by six consecutive daily injections of 1 μg of IL-18 without a significant increase in sALT activity. Thus, even this low dose of IL-18 can inhibit HBV replication without destroying hepatocytes. To determine which IHL populations produce IFN-γ in response to repetitive injection of IL-18, we analyzed this function by flow cytometry as described above. As shown at the bottom of Fig. 4, CD3−/NK1.1+ NK cells were the first cell type to produce IFN-γ in response to a single 1 μg injection of IL-18. This was followed, after 3 daily injections, by CD3+/NK1.1+ NKT cells, and lastly, after 6 daily injections, by CD3+/NK1.1− T cells. Thus, IL-18 can stimulate intrahepatic NK cells, NKT cells and conventional T cells to produce IFN-γ depending on the duration of the treatment, implying that all 3 of these cell types can contribute to the antiviral effect at various time points.
FIG. 4.
Repetitive IL-18 injection. (Top panel) Age-, sex-, and serum HBeAg-matched lineage 1.3.32 HBV transgenic mice were injected s.c. daily for 6, 3, and 1 days with 1 μg of IL-18 and sacrificed 24 h after the last injection. Total hepatic DNA was analyzed for HBV DNA by Southern blot analysis, as indicated. The mean sALT activity, measured at the time of autopsy, is indicated for each group and is expressed in units/liter. (Bottom panel) IHLs from these animals were isolated and analyzed by flow cytometry for IFN-γ expression as described in the legend to Fig. 4. Results were compared with those observed in livers from two age-, sex-, and serum HBeAg-matched transgenic littermates injected six times with saline (NaCl).
The antiviral effect of IL-18 is mediated by IFN-γ and IFN-α/β.
To determine which of the inflammatory cytokines induced by IL-18 is/are responsible for the antiviral effect, IL-18 was injected into groups of transgenic mice from lineage 1.3.32 that were also injected with anti-mouse IFN-γ or TNF-α MAb and the mice were sacrificed 24 h later. Control mice were injected with IL-18 plus irrelevant hamster IgG. Southern blot analysis was performed on total hepatic DNA extracted either from these animals or NaCl-injected controls. As shown in Fig. 5 (top panel), simultaneous administration of anti-IFN-γ MAb efficiently blocked the antiviral effect of IL-18 on HBV replication, whereas simultaneous administration of anti-TNF-α MAb was much less effective. These results suggest that IFN-γ plays a much larger role in the antiviral effect of IL-18 than TNF-α. Since we demonstrated that IL-18 induces the expression of 2′5′ OAS, a well-known IFN-α/β-induced gene, we attempted to determine whether the IFN-α/β pathway also plays a role in the antiviral effect of IL-18. Accordingly, groups of HBV transgenic mice that were either heterozygous (IFN-α/βR+/−) or homozygous (IFN-α/βR−/−) for the IFN-α/βR-null mutation (13) were injected with IL-18 or NaCl, and the animals were sacrificed 24 h later. As shown in Fig. 5 (lower panel), IL-18 did not inhibit HBV replication in the absence of the IFN-α/β receptor, indicating that the IFN-α/β pathway also contributes importantly to the antiviral effect of IL-18.
FIG. 5.
The antiviral effect of IL-18 is mediated by IFN-γ and IFN-α/β. (Top Panel) Age- and serum HBeAg-matched male transgenic mice (lineage 1.3.32) were intraperitoneally injected with 250 μg of hamster MAbs to IFN-γ or TNF-α and sacrificed 24 h after administration of a single dose (10 μg) of IL-18. Control mice were simultaneously injected with 250 μg of irrelevant hamster IgG and sacrificed at the same time after IL-18 administration. Results were compared with those observed in livers from 2 age-, sex-, and serum HBeAg-matched transgenic littermates injected with saline (NaCl). Total hepatic DNA was analyzed for HBV DNA by Southern blot analysis as indicated. The sALT activity values at the time of autopsy are indicated for each mouse and expressed in units/liter. (Bottom panel) Age- and serum HBeAg-matched male transgenic mice (lineage 1.3.46) that were either heterozygous (+/−) or homozygous (−/−) for the IFN-α/β receptor null mutation were injected with 10 μg of IL-18, sacrificed 24 h later and their livers were processed as described above.
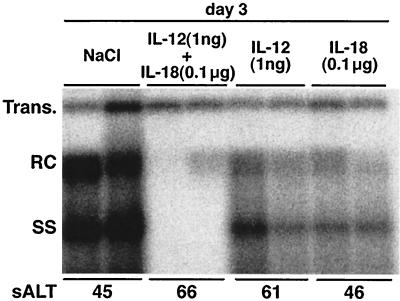
IL-18 synergizes with IL-12 to inhibit HBV replication.
IL-18 is known to act synergistically with IL-12 to regulate NK cell and T cell function (15, 25). To determine whether they also act synergistically to inhibit HBV replication, groups of transgenic mice from lineage 1.3.32 were injected simultaneously with doses of IL-18 (0.1 μg) and IL-12 (1 ng), which were previously determined to not inhibit HBV replication individually (not shown). The mice were sacrificed 3 days after injection and their livers were analyzed by Southern blot for hepatic HBV DNA content. As shown in Fig. 6 and quantitated by phosphorimager analysis relative to the integrated transgene signal, HBV DNA replication was inhibited 33-fold in the livers of animals that received IL-12 and IL-18 simultaneously whereas it was inhibited only 2-fold by either IL-12 or IL-18 alone, indicating that IL-18 and IL-12 act synergistically to inhibit HBV replication. Consistent with this result, administration of IL-12 and IL-18 simultaneously upregulated 2′5′OAS, IFN-γ and TNF-α mRNA expression in the liver compared with each injection (not shown), and there was no evidence of liver disease in these animals, suggesting that the synergistic antiviral effect of IL-18 and IL-12 is noncytopathic. Importantly, however, the antiviral effect of each cytokine is not mediated by the other because we showed in separate studies that the ability of each cytokine to inhibit HBV replication was not blocked by the coadministration of neutralizing antibodies to the reciprocal cytokine (not shown).
FIG. 6.
IL-18 synergizes with IL-12 to inhibit HBV replication. Age-, sex-, and serum HBeAg-matched lineage 1.3.32 HBV transgenic mice were injected either with 0.1 μg of IL-18 subcutaneously or with 1 ng of IL-12 intraperitoneally or both. Mice were sacrificed 3 days after injection. Total hepatic DNA was analyzed for HBV DNA by Southern blot analysis, as indicated. The mean sALT activity, measured at the time of autopsy, is indicated for each group and is expressed in units/liter.
DISCUSSION
In this study, we demonstrated that IL-18 inhibits HBV replication in the livers of transgenic mice, that this effect is noncytopathic, and that it is mediated by IFN-γ and IFN-α/β which are produced by hepatic NK cells and NKT cells within a few hours of IL-18 administration. IFN-γ and IFN-α/β have been shown previously to inhibit HBV replication in vivo and in vitro (13, 20, 21, 26). We also demonstrated that IL-18 can synergize with IL-12 to inhibit HBV replication at extremely low doses of each cytokine, but that it doesn't require IL-12 to express its antiviral potential. Similarly, we showed that IL-12, which is also known to inhibit HBV replication in an IFN-γ-dependent manner (2), doesn't require IL-18 to mediate its antiviral effect.
IL-18 is known to induce NK and NKT cells to produce IFN-γ (12, 16), but it requires IL-12 in order to induce IFN-γ in Th1 cells (15, 17). In keeping with these findings, we showed that IL-18 could rapidly induce intrahepatic NK and NKT cells to produce IFN-γ within 2 h of injection and also to virtually abolish HBV replication less than 24 h later. Importantly, this effect was so rapid it did not require the recruitment of inflammatory cells into the liver (data not shown). This enabled IL-18 to exert its antiviral effects very rapidly and with minimal inflammatory infiltrate or liver cell necrosis.
Nonetheless, repetitive IL-18 administration amplified the antiviral effect of the cytokine in the liver, apparently by recruiting activating intrahepatic T cells to produce IFN-γ as well as NK cells and NKT cells. We suspect that the T cells were induced to express the IL-18 receptor in response to the earlier induction of IFN-γ thereby enabling them to respond to IL-18 by producing additional IFN-γ as already demonstrated (27).
Our results indicate that the IL-18-induced antiviral effect is mediated by IFN-γ since it was blocked by the administration of neutralizing antibody to IFN-γ. We also found that IL-18 did not inhibit HBV replication in IFN-α/βR-deficient mice, indicating that IFN-α/β also contributes to the antiviral effect. The results do not indicate which cells in the liver were triggered to produce IFN-α/β, nor do they reveal whether IL-18 directly induced IFN-α/β production in the liver or whether the IFN-α/β was induced indirectly by IFN-γ. Additional experiments will be required to answer these interesting questions.
Others have shown that IL-18 is not cytotoxic for the hepatocyte (24). We confirmed these observations and extended them to demonstrate that extremely low nontoxic doses of IL-18 could be administered either alone or together with IL-12 to inhibit HBV replication. In addition, microscopic analysis of liver tissue after IL-18 injection revealed virtually normal liver histology with, at most, the infrequent accumulation of small nests of lymphomononuclear cells in the liver and the absence of hepatocellular necrosis or apoptosis. Collectively, these results suggest that IL-18 might have therapeutic potential because it's antiviral effect is rapid and profound and because its cytopathic potential is quite restrained.
The synergistic antiviral effect of IL-12 and IL-18 was quite impressive in this study, since very low doses of each cytokine could be combined to achieve profound inhibition of HBV replication in the absence of cytopathology. This is especially important because IL-12 is known to be rather cytotoxic for the liver at therapeutic doses (2), yet we were able to achieve complete inhibition of HBV replication with noncytotoxic doses of IL-12 when they were administered together with IL-18. We suspect that synergy is due to the ability of IL-12 to induce the expression of the IL-18 receptor as others have shown in T cells (15, 16). Synergy was evident not only in terms of the antiviral effect on HBV replication, it was also reflected by enhanced IFN-γ and IFN-α/β expression, and by a 20-, 6-, and 17-fold increase in the number of intrahepatic NK cells, T cells, and macrophages observable after coadministration of these cytokines compared with each cytokine independently (data not shown). Collectively, these results suggest that coadministration of IL-18 and IL-12 have potential for therapeutic treatment for infectious disease.
In conclusion, our observation that IL-18 can inhibit HBV replication in the livers of these transgenic mice, especially in concert with IL-12, raises the possibility that both of these cytokines may contribute to the control of HBV replication during natural HBV infection. The fact that both IL-18 and IL-12 are produced by professional antigen presenting cells raises the interesting possibility that these cells (which are very abundant in the liver) may participate in the outcome of HBV infection, similar to the resident and recruited T cells, NK cells and NKT cells that become activated in the infected liver. This notion is supported by the observation that HBV replication is inhibited by cytokines produced by hepatic macrophages after the injection of P. yoelii infected mouse erythrocytes into the HBV transgenic mice (19). It is also supported by our recent observations that HBV replication is rapidly abolished in these animals by injection of an agonistic anti-CD40 MAb that selectively activates professional antigen-presenting cells in the liver to produce IL-18 and IL-12 and to, thereby, induce IFN-γ. Thus, we suggest that IL-18, alone or together with IL-12, might have therapeutic potential for the treatment of patients with chronic HBV infection.
Acknowledgments
We thank Klaus M. Esser (Glaxo-SmithKline) for providing recombinant mIL-18 and anti-mouse IL-18 antibody; Maurice Gately (Hoffmann-La Roche) for providing recombinant mIL-12 and anti-mouse IL-12 antibody; Monte Hobbs for providing probe sets used in the RNase protection assays; Alana Altage, Rick Koch, Heike Mendez, Amber Morris, and Margie Chadwell for excellent technical assistance; and Catalina Padilla for assistance with manuscript preparation.
This work was supported by grants CA40489 (F.V.C.) and AI40696 (L.G.G.) from the National Institutes of Health. K. Kimura was supported by a fellowship from the Skaggs Institute.
Footnotes
This is manuscript number 14979-MEM from The Scripps Research Institute.
REFERENCES
- 1.Cavanaugh, V. J., L. G. Guidotti, and F. V. Chisari. 1998. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J. Virol. 72:2630-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavanaugh, V. J., L. G. Guidotti, and F. V. Chisari. 1997. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J. Virol. 71:3236-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello, C. A., D. Novick, A. J. Puren, G. Fantuzzi, L. Shapiro, H. Muhl, D. Y. Yoon, L. L. Reznikov, S. H. Kim, and M. Rubinstein. 1998. Overview of interleukin-18: more than an interferon-gamma inducing factor. J. Leukoc. Biol. 63:658-664. [PubMed] [Google Scholar]
- 4.Franco, A., L. G. Guidotti, M. V. Hobbs, V. Pasquetto, and F. V. Chisari. 1997. Pathogenetic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice. J. Immunol. 159:2001-2008. [PubMed] [Google Scholar]
- 5.Fujioka, N., R. Akazawa, K. Ohashi, M. Fujii, M. Ikeda, and M. Kurimoto. 1999. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J. Virol. 73:2401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidotti, L. G., P. Borrow, A. Brown, H. McClary, R. Koch, and F. V. Chisari. 1999. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J. Exp. Med. 189:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 8.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti, L. G., B. Matzke, H. Schaller, and F. V. Chisari. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbs, M. V., W. O. Weigle, D. J. Noonan, B. E. Torbett, R. J. McEvilly, R. J. Koch, G. J. Cardenas, and D. N. Ernst. 1993. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J. Immunol. 150:3602-3614. [PubMed] [Google Scholar]
- 11.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leite-De-Moraes, M. C., A. Hameg, A. Arnould, F. Machavoine, Y. Koezuka, E. Schneider, A. Herbelin, and M. Dy. 1999. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J. Immunol. 163:5871-5876. [PubMed] [Google Scholar]
- 13.McClary, H., R. Koch, F. V. Chisari, and L. G. Guidotti. 2000. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74:2255-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 16.Okamura, H., S. Kashiwamura, H. Tsutsui, T. Yoshimoto, and K. Nakanishi. 1998. Regulation of interferon-gamma production by IL-12 and IL-18. Curr. Opin. Immunol. 10:259-264. [DOI] [PubMed] [Google Scholar]
- 17.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 18.Okamura, H., H. Tsutsui, S. Kashiwamura, T. Yoshimoto, and K. Nakanishi. 1998. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 70:281-312. [DOI] [PubMed] [Google Scholar]
- 19.Pasquetto, V., L. G. Guidotti, K. Kakimi, M. Tsuji, and F. V. Chisari. 2000. Host-virus interactions during malaria infection in hepatitis B virus transgenic mice. J. Exp. Med. 192:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquetto, V., S. F. Wieland, S. L. Uprichard, M. Tripodi, and F. V. Chisari. 2002. Cytokine-sensitive replication of hepatitis B virus in immortalized mouse hepatocyte cultures. J. Virol. 76:5646-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robek, M. D., S. F. Wieland, and F. V. Chisari. 2002. Inhibition of hepatitis B virus replication by interferon requires proteasome activity. J. Virol. 76:3570-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka-Kataoka, M., T. Kunikata, S. Takayama, K. Iwaki, K. Ohashi, M. Ikeda, and M. Kurimoto. 1999. In vivo antiviral effect of interleukin 18 in a mouse model of vaccinia virus infection. Cytokine 11:593-599. [DOI] [PubMed] [Google Scholar]
- 23.Tripp, C. S., M. K. Gately, J. Hakimi, P. Ling, and E. R. Unanue. 1994. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J. Immunol. 152:1883-1887. [PubMed] [Google Scholar]
- 24.Tsutsui, H., N. Kayagaki, K. Kuida, H. Nakano, N. Hayashi, K. Takeda, K. Matsui, S. Kashiwamura, T. Hada, S. Akira, H. Yagita, H. Okamura, and K. Nakanishi. 1999. Caspase-1-independent, Fas/Fas ligand-mediated IL-18 secretion from macrophages causes acute liver injury in mice. Immunity 11:359-367. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsui, H., K. Matsui, H. Okamura, and K. Nakanishi. 2000. Pathophysiological roles of interleukin-18 in inflammatory liver diseases. Immunol. Rev. 174:192-209. [DOI] [PubMed] [Google Scholar]
- 26.Wieland, S. F., L. G. Guidotti, and F. V. Chisari. 2000. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 74:4165-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimoto, T., K. Takeda, T. Tanaka, K. Ohkusu, S. Kashiwamura, H. Okamura, S. Akira, and K. Nakanishi. 1998. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 161:3400-3407. [PubMed] [Google Scholar]