Abstract
Background: There has been increasing interest in the role of immunologic processes, notably cytokines, in the development of behavioral alterations, especially in medically ill patients. Interferon (IFN)-α is notorious for causing behavioral symptoms, including depression, fatigue, and cognitive dysfunction, and has been used to investigate the effects of cytokines on the brain.
Methods: In the present study we assessed the effects of low-dose IFN-α on brain activity, using functional magnetic resonance imaging during a task of visuospatial attention in patients infected with hepatitis C virus (HCV).
Results: Despite endorsing symptoms of impaired concentration and fatigue, IFN-α-treated patients (n = 10) exhibited task performance and activation of parietal and occipital brain regions similar to that seen in HCV-infected control subjects (n = 11). Interestingly, however, in contrast to control subjects, IFN-α-treated patients exhibited significant activation in the dorsal part of the anterior cingulate cortex (ACC), which highly correlated with the number of task-related errors. No such correlation was found in control subjects.
Conclusions: Consistent with the role of the ACC in conflict monitoring, ACC activation during IFN-α administration suggests that cytokines might increase processing conflict or reduce the threshold for conflict detection, thereby signaling the need to exert greater mental effort to maintain performance. Such alterations in ACC activity might in turn contribute to cytokine-induced behavioral changes.
Keywords: Cytokines, interferon-alpha, depression, cognition, fMRI, anterior cingulate
Patients with a wide variety of medical illnesses exhibit behavioral alterations, including depression, at rates 5–10 times higher than in the general population (Evans et al 1999). Recent theories have proposed that inflammatory mediators, notably cytokines, are involved in the etiology of these behavioral changes (Dantzer et al 1999; Evans et al 1999; Schneider et al 2002; Yirmiya et al 2000). In support of this notion, a rich database has been developed that substantiates the capacity of proinflammatory cytokines, including tumor necrosis factor α, interleukin (IL)-1, and IL-6, to induce behavioral symptoms referred to as "sickness behavior" (Dantzer et al 1999; Yirmiya et al 2000). Sickness behavior is typically associated with the behavioral changes seen in humans and laboratory animals suffering from microbial infections and includes symptoms of cognitive dysfunction, fatigue, psychomotor slowing, anorexia, anhedonia, sleep alterations, and increased sensitivity to pain (Kent et al 1992). Relevant to its mediation by proinflammatory cytokines, sickness behavior can be reliably reproduced by administration of each of the proinflammatory cytokines in isolation or by administering agents (e.g., endotoxin or lipopolysaccharide) that induce the proinflammatory cytokine cascade. Although proinflammatory cytokines are too large to freely pass through the blood–brain barrier, several relevant pathways by which cytokine signals can access the brain have been elucidated, including passage of cytokines through leaky regions in the blood–brain barrier, active transport and transmission of cytokine signals by afferent nerve fibers (e.g., vagus) (Plotkin et al 1996; Rivest et al 2000; Watkins et al 1995). Within the brain, a cytokine network has been described that consists of cell types that can both produce cytokines (glia/neurons) and receive their signals through relevant receptors (Benveniste 1998; Rothwell et al 1996). In terms of behavior, cytokines have also been shown to alter the metabolism of key monoamines, including serotonin, norepinephrine, and dopamine, all of which are believed to play a role in psychopathology (Dunn et al 1999).
To further investigate the effects of cytokines on the brain, recent work has focused on patients undergoing treatment with the cytokine interferon (IFN)-α for infectious diseases and cancer. Interferon-alpha is a potent activator of the inflammatory cytokine network and is well known to cause neuropsychiatric symptoms, including alterations in mood (depression, anxiety, tension/irritability), cognition, and neurovegetative function (Capuron et al 2002; Trask et al 2000). These symptoms not only negatively impact quality of life but also compromise treatment efficacy.
Mood and cognitive symptoms generally arise together during IFN-α treatment (Capuron et al 2002). Cognitive alterations primarily manifest as disturbances in attention and memory and depend on the dose, duration, and route of administration of the cytokine. For example, high doses of IFN-α in patients with leukemia have been associated with marked neuropsychological impairments suggestive of fronto–subcortical brain dysfunction (Pavol et al 1995). More recently, we showed that high-dose IFN-α for malignant melanoma resulted in significant and persistent psychomotor slowing, as manifested by slower reaction time (RT) on the Cambridge Neuropsychological Test Automated Battery (CANTAB) (Capuron et al 2001; Fray and Robbins, 1996). This psychomotor slowing was most pronounced when the task involved increased attentional demands (i.e., when the stimulus appeared in an unpredictable vs. a predictable location on the screen) (Capuron et al 2001). Normal volunteers acutely injected with low-dose IFN-α exhibit more subtle cognitive changes, which include slower RT to stimuli appearing at unpredictable times or locations, but normal performance on tasks of pursuit tracking and syntactic reasoning (Smith et al 1988).
In the present study, we assessed the effect of low-dose IFN-α therapy on performance and brain activation in a task of visuospatial attention, modeled on the RT task of CANTAB, using functional magnetic resonance imaging (fMRI). To probe neurocognitive responses to increasing attentional demand, a simple RT ("detection") task (low demand) and a choice RT ("location") task (higher demand) requiring discrimination and response selection based on the spatial localization of stimuli, were used. To our knowledge, there has been no previous fMRI study of the neurocognitive consequences of IFN-α treatment.
Methods and Materials
Study Population
Twenty-one patients with hepatitis C virus (HCV) were enrolled in the study. Ten patients (8 male, 2 female, mean [SD] age 44 [7] years) had received pegylated (PEG) IFN-α (1.5 μg/kg once weekly s.c.) plus oral ribavirin (800–1400 mg/day) for a mean (SD) duration of 12 (1.7) weeks. The remaining 11 patients (8 male, 3 female, aged 42 [9] years) were awaiting PEG IFN-α therapy and were enrolled as control subjects. No significant differences were found between groups in terms of age, gender, history of depression, or history of substance abuse.
All 21 subjects were right-handed. Patients with the following were excluded: history of brain damage/trauma and/or neurologic disease; metallic implants of any kind; antidepressant or antipsychotic drug treatment within 2 weeks (8 weeks for fluoxetine); ingestion of benzodiazepine or non-benzodiazepine sedative/hypnotics within 72 hours; diagnosis of DSM-IV Axis I psychiatric disorder before starting IFN-α therapy; alcohol/psychoactive substance abuse or dependence within the past 2 years; uncontrolled renal, hematologic, metabolic, cardiac/pulmonary disorders (determined by routine laboratory testing); and prior treatment with IFN or ribavirin. All participants were adults and provided written informed consent. The study was approved by the institutional review board of the Emory University School of Medicine.
Cognitive Task: Visuospatial Attention
To minimize the movement of participants during the fMRI acquisition, we developed a task of visuospatial attention that involves both attentional processes and motor responses. This task was derived from the RT task of the CANTAB battery (Fray and Robbins 1996) but without the requirement of reaching and pointing to a target on the computer screen. All stimuli presentations and recordings of RT and response errors were performed with Presentation software (Neurobehavioral Systems, San Francisco, California).
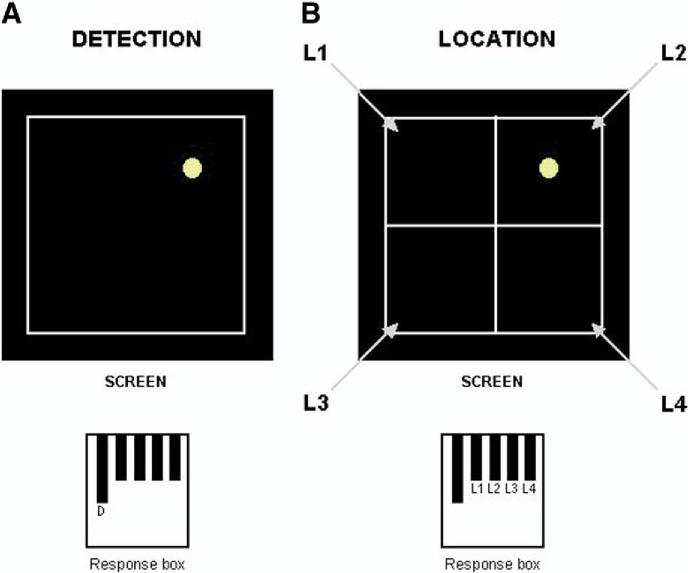
A practice trial was completed outside the scanner room under the supervision of the experimenter. Participants were then instructed to perform the task in the scanner, using a 5-key response box. The task was presented on a screen placed in front of the scanner bed and was monitored through a computer located outside the scanner room. Participants were able to see the screen through a mirror attached to the head coil. The task included a simple "detection" condition and a more complex "location" condition, similar to the "simple" and "choice" variants of the CANTAB RT task, which involves touching a target that appears either in a fixed (simple) or variable (choice) location, using a touch screen computer (Figure 1). In our fMRI version of the task, the "detection" condition consisted of a yellow dot appearing at a random location on the screen at a fixed interval of 4 sec. The subject was instructed to press the response key ("detection" key) each time he/she detected the yellow dot on the screen, independently of its location. The dot was displayed eight times during each "detection" block. In the "location" condition, the screen was divided into four equal quadrants, and the yellow dot was displayed eight times randomly in one of the quadrants, with an interstimulus interval of 4 sec. The subject was instructed to press one of the four possible response keys (L1, L2, L3, or L4), corresponding to the location of the quadrant in which the yellow dot appeared. It should be noted that the display and the keyboard layouts were not isomorphic (the display had a square layout and the keyboard a linear one), making the location condition significantly more challenging than the detection condition. The experimental session included two runs, both of them consisting of alternating "detection" (D), "location" (L), and "rest" (R) blocks with respective durations of 32 sec, 32 sec, and 24 sec. The order of the condition blocks was D-L-R-L-D-R-D-L-R for run 1 and L-D-R-D-L-R-L-D-R for run 2. Each run lasted 5 min, and the order of the two runs was randomized across participants.
Figure 1.
Visuospatial attention task. In the detection condition (A), subjects were instructed to press the detection key ("D") on the response box each time they detected the yellow dot on the screen. In the location condition (B), subjects were instructed to press the key corresponding to the location of the quadrant in which the yellow dot appeared (on the given example, the key to be pressed is L2).
fMRI Data Acquisition and Analysis
Scanning was performed on a 1.5-T Philips NT scanner (Philips Medical Systems, Eindhoven, The Netherlands). The acquisition of a high-resolution T1-weighted anatomic scan was followed by two whole-brain functional runs of 150 scans each (echo-planar imaging, repetition time = 2000 msec, echo time = 40 msec, flip angle = 90°, 64 × 64 matrix, 24 5-mm axial slices) for measurement of the blood oxygen level–dependent effect.
Data were analyzed with the software SPM2 (Wellcome Department of Imaging Neuroscience, London, United Kingdom), running in Matlab 6.1 (Mathworks, Sherborn, Massachusetts). Motion correction to the first functional scan was performed within subject with a six-parameter rigid-body transformation. The mean of the realigned images was spatially normalized to the Montreal Neurological Institute echo-planar template, and the computed transformation was applied to all the realigned images, to bring the functional data into standard space. Finally, a spatial gaussian filter (full width at half maximum = 10 mm, isotropic) was applied to the images to accommodate intersubject variability. A random-effects statistical analysis was performed. For each subject, a general linear model was specified and estimated. "Location" and "detection" effects were modeled as box-cars convolved with a synthetic hemodynamic response function. The motion parameters estimated during the realignment phase were also entered in the general linear model as potential confounds. The images representing the voxel-wise subject-level estimates of the contrast of interest (i.e., "location – detection") were then entered into a two-sample t test for the assessment of the differences in the effect between the two experimental groups. To screen out differential patterns of activity due to the presence of deactivations in one group (the interpretation of which is highly problematic), regions identified in this between-group (interaction) analysis were reported only if they were significantly activated in at least one of the two experimental groups. The statistical threshold was set at p < .001, uncorrected, limited to clusters of volume ≥270 mm3 (≥10 voxels).
Neuropsychiatric Evaluation
Before the scan, neuropsychiatric symptoms were assessed with the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979). The MADRS is a clinician-administered instrument that measures the intensity of depressive symptoms in 10 specific domains (apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, and suicidal thoughts). Symptoms were rated on a scale from 0 (absent) to 6 (severe).
Results
As shown in Table 1, consistent with the increasing demands of the task, the mean RT was slower in the "location" condition compared with the "detection" condition in both IFN-α-treated and control groups (p < .001 for each group). No significant between-group differences were found for either the "detection" or "location" condition. In addition, performance accuracy was similar between groups. Of note, the number of errors within location blocks, as well as the number of shift errors (errors on the first trial of each "location" block, reflecting performance on task-switching processes) were low and were comparable between IFN-α-treated patients and HCV-infected control subjects. Even though no significant differences were measured in the performance of the two groups in the task of visuospatial attention, patients treated with IFN-α exhibited higher symptom severity scores on the MADRS scale compared with control subjects, especially on items assessing fatigue and loss of concentration (Table 2).
Table 1.
Reaction Time and Performance Accuracy on the Task of Visuospatial Attention in Control Subjects and Patients Undergoing IFN-Alpha Therapy
| Control Subjects (n = 11) | IFN-Alpha (n = 10) | Between-Group Difference (p) | |
|---|---|---|---|
| Reaction time (msec) | |||
| Detection time | 466 (133) | 475 (149) | .89 |
| Location time | 839a (168) | 796a (97) | .49 |
| Location errors | |||
| Within errorsb | 1.91 (2.02) | 1.30 (1.49) | .45 |
| Shift errorsc | .64 (.92) | .40 (.70) | .52 |
Data are shown as mean (SD). IFN, interferon.
p < .001 compared with respective detection time (intragroup comparison).
Location within errors: errors within detection blocks.
Location shift errors: errors on the first trial of each location block (either from detection to location or from rest to location).
Table 2.
Neurobehavioral Symptoms (as Measured by the MADRS) in HCV Patients Treated with IFN-Alpha Versus HCV Patients Awaiting IFN-Alpha Therapy (Control Subjects)
| Control Subjects | IFN-Alpha | Between-Group Difference (p) | |
|---|---|---|---|
| Total MADRS score | 2.91 (3.01) | 10.80 (6.37) | <.01 |
| MADRS Domains | |||
| Apparent Sadness | .18 (.40) | .80 (.91) | .06 |
| Reported Sadness | .18 (.40) | .50 (.52) | .14 |
| Inner Tension | .46 (1.03) | 1.30 (1.41) | .13 |
| Reduced Sleep | .82 (1.40) | 1.80 (1.93) | .20 |
| Reduced Appetite | .18 (.60) | 1.40 (1.35) | <.05 |
| Concentration Difficulties | .36 (.80) | 1.60 (1.57) | <.05 |
| Fatigue | .45 (1.03) | 1.70 (1.49) | <.05 |
| Inability to Feel | .09 (.30) | .90 (1.28) | .06 |
| Pessimistic Thoughts | .18 (.40) | .70 (.82) | .08 |
| Suicidal Thoughts | .00 (.00) | .10 (.31) | .31 |
Data are given as mean (SD). MADRS, Montgomery-Asberg Depression Rating Scale; HCV, hepatitis C virus; IFN, interferon.
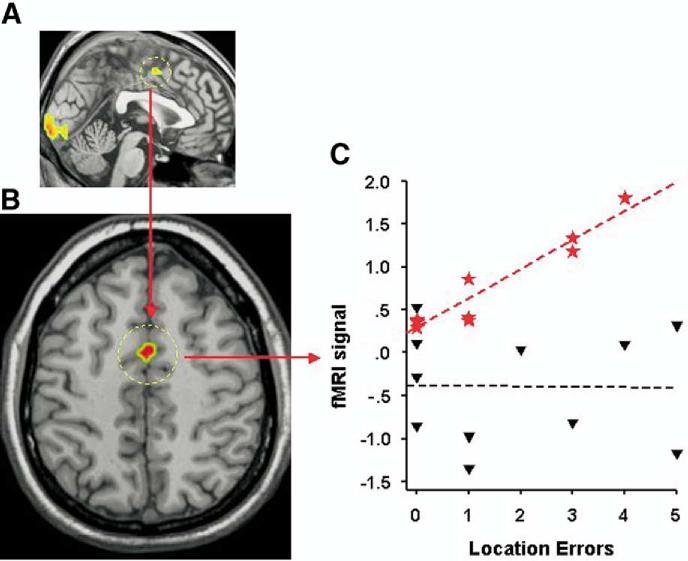
For the effect of interest ("location – detection"), both IFN-α-treated patients and control subjects exhibited significant bilateral activation in occipital (lingual gyrus) and parietal (post-central gyrus and superior parietal gyrus) brain regions (Table 3). In contrast to control patients, however, IFN-α-treated patients showed additional activation in the frontal lobe, including the left superior frontal sulcus, the left inferior frontal gyrus, and the dorsal part (Brodmann’s area 24) of the left anterior cingulate cortex (ACC) (Table 3, Figure 2A). Between-group comparisons indicated that ACC activation was significantly greater in IFN-α-treated patients compared with control subjects (Table 3, Figure 2B).
Table 3.
Brain Regions Displaying Significant Changes in Measured Activity for the Contrast "Location — Detection"
| Peak MNI |
|||||
|---|---|---|---|---|---|
| Brain Region | Z score | Volume (mm3) | X | Y | Z |
| Control Subjects | |||||
| Parietal lobe | |||||
| Postcentral gyrus (R) | 4.44 | 972 | 51 | -27 | 48 |
| Postcentral gyrus (L) | 4.02 | 1566 | -48 | -33 | 51 |
| Superior parietal gyrus (L) | 3.46 | 648 | -15 | -75 | 57 |
| Occipital Lobe | |||||
| Lingual gyrus (bilateral) | 4.13 | 9693 | -6 | -96 | -3 |
| IFN-α | |||||
| Frontal lobe | |||||
| Superior frontal sulcus (L) | 4.43 | 1863 | -27 | 0 | 60 |
| Inferior frontal gyrus (L) | 4.15 | 405 | -51 | 21 | -12 |
| Anterior cingulate (BA24) (L) | 3.47 | 270 | -3 | -3 | 51 |
| Parietal lobe | |||||
| Superior parietal gyrus (R) | 4.04 | 4023 | 15 | -72 | 54 |
| Superior parietal gyrus (L) | 3.80 | 1728 | -9 | -63 | 63 |
| Postcentral gyrus (L) | 3.90 | 405 | -30 | -39 | 69 |
| Postcentral gyrus (R) | 3.72 | 1107 | 45 | -33 | 48 |
| Occipital lobe | |||||
| Lingual gyrus (bilateral) | 5.22 | 9963 | -9 | -99 | -3 |
| Control Subjects > IFN-α | |||||
| None | |||||
| IFN-α > Control Subjects | |||||
| Frontal lobe | |||||
| Anterior cingulate (BA24) (L) | 4.37 | 324 | 0 | 0 | 48 |
Statistical threshold was set at p <.001, uncorrected, limited to clusters of volume ≥270 mm3 (≥10 voxels). MNI, Montreal Neurological Institute; R, right; L, left; IFN, interferon; BA, Brodmann's area.
Figure 2.
(A) Activation of the anterior cingulate cortex (ACC) in patients treated with interferon (IFN)-α (contrast "location – detection"). (B) Activation of the ACC in patients treated with IFN-α compared with control subjects (CTRL) (contrast "location – detection: IFN > CTRL"). (C) Relationship between ACC activation and the rate of errors within the location blocks in patients treated with IFN-α (red stars) (R = .954, p < .0001) and in control subjects (black triangles) (R =–.018, p = .96).
Correlation analyses indicated that the degree of activation measured in the ACC in IFN-α-treated patients was highly correlated with the number of errors made during the location blocks (R = .954, p < .0001). Thus, more errors within location blocks were associated with higher activation of the ACC in patients receiving IFN-α (Figure 2C). In contrast, no significant correlation between activity in the ACC and number of errors was found in control patients (R = –.018, p = .96). Finally, no correlation was found between activity in the ACC, RT, and complaints of loss of concentration and fatigue (measured by the MADRS independently of the fMRI task) in both IFN-α-treated patients and control subjects.
Discussion
Despite complaints of concentration difficulties and fatigue, HCV patients treated with IFN-α for 12 weeks exhibited normal performance on a task of visuospatial attention, as indicated by RT and performance accuracy (in both detection and location conditions) similar to control subjects. In addition, both IFN-α-treated patients and control subjects exhibited significant bilateral activation in the occipital and parietal lobes, consistent with involvement of the parieto–occipital network in visual attention processes (Corbetta et al 1998, 2000; Wager et al 2004). In contrast to control subjects, however, IFN-α-treated patients exhibited significant activation in the dorsal part of the ACC (Brodmann’s area 24), which highly correlated with the number of task-related errors. No such correlation was found in control subjects. Given the role of the ACC in conflict monitoring (especially during attention-demanding tasks), ACC changes during IFN-α administration suggest that cytokines might increase processing conflict and/or reduce the threshold for conflict detection, thereby signaling the need to exert greater executive control and mental effort to maintain performance. Such changes in ACC information processing might in turn contribute to the development of cytokine-induced behavioral alterations, including depression, fatigue, and cognitive dysfunction.
Many studies have implicated a role for the ACC (notably, the dorsal part) in cognitively demanding tasks that involve stimulus discrimination and motor response selection (Bush et al 2000; Paus et al 1993). Accordingly, it has been proposed that activation of the ACC reflects the degree of intentional effort or willed control (volition) needed to perform a task, with increased activity in this region correlating with greater deployment of effort (Duncan and Owen 2000; Paus 2001; Paus et al 1998; Winterer et al 2002). Our finding that ACC activation was only apparent in IFN-α-treated patients suggests that the task might have been processed by IFN-α-treated patients as being more demanding in terms of attentional resources and cognitive effort. Consistent with this notion, patients treated with IFN-α exhibited greater symptom severity scores compared with control subjects on items assessing loss of concentration and fatigue. In turn, fatigue has been shown to significantly impact cognitive performance, including RT, in tasks involving higher attentional or cognitive demand (Van der Linden et al 2003). Thus, activation of the ACC in IFN-α-treated patients, in the absence of performance impairment, might reflect the need for greater deployment of executive control and therefore cognitive effort to overcome the potential negative effect of IFN-α-induced fatigue on performance, especially at higher levels of task difficulty. Nevertheless, in the absence of objective measures of perceived difficulty/effort related to the task itself, the relationship between mental effort and ACC activation cannot be addressed in this study.
Recently, and consistent with the involvement of the ACC in cognitively demanding tasks involving stimulus discrimination/response selection, much interest has been devoted to theories considering the role of the ACC in error detection and conflict monitoring. These theories were derived from event-related-brain-potential studies during speeded response tasks showing a significant negative scalp-potential, time-locked (error-related negativity, ERN) to the time onset of an erroneous response, likely generated by the ACC (Falkenstein et al 1991; Gehring et al 1993). Further neuroimaging studies have indicated, however, that activity in the ACC was also apparent during correct responses under conditions of increased response competition. On the basis of these findings, which emphasize the "evaluative" function of the ACC, it has been suggested that the ACC provides an on-line signal of conflicts rather than errors per se, which in turn indicates the need to engage other brain regions to implement problem-solving strategies (Botvinick et al 2004; Carter et al 1998; Kerns et al 2004). In the present study, activation of the ACC was found to be highly correlated with the number of location errors in IFN-α-treated patients only, a result that is consistent with the hypothesized role of the ACC in error/conflict monitoring (Botvinick et al 2004; Carter et al 1998; Kerns et al 2004). Nevertheless, as noted above, the rate of errors during the task was low and was no different in IFN-α-treated patients compared with control subjects. Therefore, it seems that in patients treated with IFN-α, the task was processed as a condition of higher conflict, despite an error rate that was insufficient to induce a central response in control subjects. It is unclear whether ACC activation in IFN-α-treated patients is an indirect manifestation of an actual increase in processing conflict (e.g., secondary to fatigue or other competing sensory inputs/response outputs) or whether IFN-α alters the sensitivity of the ACC to conflict detection. Future studies of the relationship between ACC activation and a range of error rates (as a function of increasing task difficulty) in IFN-α-treated patients will shed more light on this question. For example, such a strategy has been used to investigate cognitive processing alterations in patients with schizophrenia (Carter et al 1997; Nordahl et al 2001).
Interestingly, increased ACC activation in response to low error rate has been found in individuals vulnerable to psychiatric conditions, including mood and anxiety disorders. For example, Paulus et al (2004) have recently shown increased activation of the ACC during a low-error-rate, decision-making task in individuals with high-trait anxiety, a personality characteristic pre-disposing to psychopathologic (notably anxious) reactions in the context of situations perceived as potentially threatening. This finding was interpreted as suggesting that increased ACC activation during low-error-rate tasks in high-trait anxiety subjects could reflect anticipation of adverse outcomes and errors and contribute to fearfulness of future conflict processing. Other psychological/psychiatric conditions, including neuroticism, distress/negative affect, bipolar disorder, and obsessive-compulsive disorder, have also been associated with either larger ERN amplitude or greater ACC activation during cognitive tasks (Chang et al 2004; Luu et al 2000; Ursu et al 2003). Taken together, the data suggest that cytokine-induced increases in ACC activity might underlie an increased sensitivity and responsiveness to negative events or tasks perceived as potentially challenging and thereby contribute to cognitive distortions that influence emotion regulation. Thus, aside from signaling the need for greater deployment of cognitive effort, increased activation of the ACC in patients treated with IFN-α might also impart an increased vulnerability to negative affects and conflicting situations, and more generally to mood disorders. Because IFN-α-induced behavioral alterations serve to model cytokine-induced behavioral changes, the data also suggest that alterations in cognitive processing secondary to cytokine effects on the brain might represent a risk factor for the development of depression, fatigue, and cognitive dysfunction in patients with increased endogenous cytokines due to various medical illnesses and/or their treatment.
Relevant to potential neurobiological mechanisms of alterations in ACC activation during cytokine administration, it should be noted that the ACC exhibits a high concentration of corticotrophin-releasing hormone (CRH) (Lewis et al 1989). Corticotrophin-releasing hormone is well known to be elevated in patients with mood and anxiety disorders, and its release is activated by inflammatory cytokines, such as IFN-α (Raber et al 1997). Of note, evidence of CRH hypersensitivity (as manifested by increased adrenocorticotropic hormone and cortisol release to the first injection of IFN-α) has been found to predict the development of IFN-α-induced depression in cancer patients (Capuron et al 2003). In addition, it has been suggested that dopaminergic pathways might influence neuronal activity in the ACC (Gabriel and Taylor 1998; Morgan et al 2002). Interestingly, IFN-α has also been associated with evidence of altered dopaminergic function, which in turn has been posited to mediate some core symptoms of cytokine-induced depression (Capuron and Miller 2004; Dunn et al 1999; Shuto et al 1997). These data indicate that both CRH and dopaminergic pathways, known to be activated by cytokines, might participate in cytokine effects on ACC function, thereby providing a potential cognitive pathway to cytokine-induced neuropsychiatric symptoms.
It should be noted that the lack of performance deficits in IFN-α-treated patients might reflect the lower dosage and once-weekly administration of IFN-α used in the present study compared with previous reports. Indeed, most of the previous studies showing performance deficit during IFN-α therapy were conducted in patients either receiving high doses of IFN-α or treated with multiple injections of IFN-α per week (Capuron et al 2001; Pavol et al 1995; Smith et al 1988). In the present study, however, HCV patients were treated with PEG IFN-α, administered once weekly at a relatively low dosage. Alternatively, the task used in this study might not have been cognitively demanding enough to significantly disturb psychomotor speed and efficiency in IFN-α-treated patients, or it might have remained within the boundaries of compensatory mechanisms. Finally, it is possible that increased ACC activation after 12 weeks of IFN-α administration might reflect an increased vulnerability to cognitive dysfunction (and/or psychopathology), which might occur after more prolonged (or intense) cytokine exposure. Nevertheless, the presence of normal task performance in the context of IFN-α treatment provides a unique opportunity to examine cytokine-induced alterations in regional brain activation that are not confounded by cytokine-induced performance deficits.
Acknowledgments
We thank Valerie Watson and Natalia Revzina for their assistance with the study protocol.
Footnotes
LC and GP contributed equally to this work.
This work was supported by the Centers for Disease Control and Prevention, the National Institute of Biomedical Imaging and Bioengineering (EB002635), the National Institute of Mental Health (NIMH) (MH067990 and MH069124), and the National Institute of Drug Abuse (DA00367).
AHM receives grants/research support from GlaxoSmithKline, Schering Plough, Janssen Pharmaceutica, and NIMH.
CBN receives grants/research support from Abbott Laboratories, American Foundation for Suicide Prevention, AstraZeneca, Bristol-Myers-Squibb, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, the National Alliance for Research on Schizophrenia and Depression, NIMH, Pfizer Pharmaceuticals, Stanley Foundation/National Alliance for the Mentally Ill, and Wyeth-Ayerst; acts as a consultant for Abbott Laboratories, Acadia Pharmaceuticals, AstraZeneca, Bristol-Myers-Squibb, Corcept, Cypress Biosciences, Cyberonics, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Ono Pharma, Otsuka, Pfizer Pharmaceuticals, Quintiles, Sanofi, Somerset, and Wyeth-Ayerst; serves on the speakers bureau for Abbott Laboratories, AstraZeneca, GlaxoSmithKline, Janssen Pharmaceutica, and Pfizer Pharmaceuticals; is a stockholder in Corcept, Cypress Biosciences, Neurocrine Biosciences, Acadia Pharmaceuticals, and Revaax; is on the Board of Directors for the American Foundation for Suicide Prevention, the American Psychiatric Institute for Research and Education, the George West Mental Health Foundation, and Novadel Pharma; and holds patents for a method and devices for transdermal delivery of lithium (US 6,375,990 B1) and a method to estimate serotonin and norepinephrine transporter occupancy after drug treatment using patient or animal serum (provisional filing April 2001).
References
- Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: Lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O] H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Wollman EE, Vitkovic L, Yirmiya R. Cytokines, stress, and depression. Conclusions and perspectives. Adv Exp Med Biol. 1999;461:317–329. doi: 10.1007/978-0-585-37970-8_17. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- Evans DL, Staab JP, Petitto JM, Morrison MF, Szuba MP, Ward HE, et al. Depression in the medical setting: Biopsychological interactions and treatment considerations. J Clin Psychiatry. 1999;60:40–55. [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of cross-modal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW. CANTAB battery: Proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18:499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Taylor C. Prenatal exposure to cocaine impairs neuronal coding of attention and discriminative learning. Ann N Y Acad Sci. 1998;846:194–212. [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Foote SL, Cha CI. Corticotropin-releasing factor immunoreactivity in monkey neocortex: An immunohistochemical analysis. J Comp Neurol. 1989;290:599–613. doi: 10.1002/cne.902900412. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J Exp Psychol Gen. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morgan RE, Garavan HP, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Enduring effects of prenatal cocaine exposure on attention and reaction to errors. Behav Neurosci. 2002;116:624–633. doi: 10.1037//0735-7044.116.4.624. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Carter CS, Salo RE, Kraft L, Baldo J, Salamat S, et al. Anterior cingulate metabolism correlates with stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology. 2001;25:139–148. doi: 10.1016/S0893-133X(00)00239-6. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biol Psychiatry. 2004;55:1179–1187. doi: 10.1016/j.biopsych.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: A review of 107 PET activation studies. Neuroreport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. J Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Pavol MA, Meyers CA, Rexer JL, Valentine AD, Mattis PJ, Talpaz M. Pattern of neurobehavioral deficits associated with interferon alpha therapy for leukemia. Neurology. 1995;45:947–950. doi: 10.1212/wnl.45.5.947. [DOI] [PubMed] [Google Scholar]
- Plotkin SR, Banks WA, Kastin AJ. Comparison of saturable transport and extracellular pathways in the passage of IL-1 alpha across the blood-brain barrier. J Neuroimmunol. 1996;67:41–47. doi: 10.1016/0165-5728(96)00036-7. [DOI] [PubMed] [Google Scholar]
- Raber J, Koob GF, Bloom FE. Interferon-alpha and transforming growth factor-beta 1 regulate corticotropin-releasing factor release from the amygdala: Comparison with the hypothalamic response. Neurochem Int. 1997;30:455–463. doi: 10.1016/s0197-0186(96)00082-4. [DOI] [PubMed] [Google Scholar]
- Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi G, Toulmond S. Cytokines and their receptors in the central nervous system: Physiology, pharmacology, and pathology. Pharmacol Ther. 1996;69:85–95. doi: 10.1016/0163-7258(95)02033-0. [DOI] [PubMed] [Google Scholar]
- Schneider RK, Robinson MJ, Levenson JL. Psychiatric presentations of non-HIV infectious diseases. Neurocysticercosis, Lyme disease, and pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection. Psychiatr Clin North Am. 2002;25:1–16. doi: 10.1016/s0193-953x(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747:348–351. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- Smith A, Tyrrell D, Coyle K, Higgins P. Effects of interferon alpha on performance in man: A preliminary report. Psychopharmacology. 1988;96:414–416. doi: 10.1007/BF00216072. [DOI] [PubMed] [Google Scholar]
- Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: Prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316–2326. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: Evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Van der Linden D, Frese M, Meijman TF. Mental fatigue and the control of cognitive processes: Effects on perseveration and planning. Acta Psychol (Amst) 2003;113:45–65. doi: 10.1016/s0001-6918(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: A meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: Evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- Winterer G, Adams CM, Jones DW, Knutson B. Volition to action—an event-related fMRI study. Neuroimage. 2002;17:851–858. [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, et al. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]