Abstract
Transgenic sickle mice expressing βS hemoglobin have activated vascular endothelium that exhibits enhanced expression of NF-κB and adhesion molecules that promote vascular stasis in sickle, but not in normal, mice in response to hypoxia/reoxygenation. Sickle mice hemolyze rbcs in vivo as demonstrated by increased reticulocyte counts, plasma hemoglobin and bilirubin, and reduced plasma haptoglobin. The heme content is elevated in sickle organs, which promotes vascular inflammation and heme oxygenase-1 expression. Treatment of sickle mice with hemin further increases heme oxygenase-1 expression and inhibits hypoxia/reoxygenation–induced stasis, leukocyte-endothelium interactions, and NF-κB, VCAM-1, and ICAM-1 expression. Heme oxygenase inhibition by tin protoporphyrin exacerbates stasis in sickle mice. Furthermore, treatment of sickle mice with the heme oxygenase enzymatic product carbon monoxide or biliverdin inhibits stasis and NF-κB, VCAM-1, and ICAM-1 expression. Local administration of heme oxygenase-1 adenovirus to subcutaneous skin increases heme oxygenase-1 and inhibits hypoxia/reoxygenation–induced stasis in the skin of sickle mice. Heme oxygenase-1 plays a vital role in the inhibition of vaso-occlusion in transgenic sickle mice.
Introduction
Sickle cell disease (SCD) is a devastating hemolytic disease characterized by recurring episodes of painful vaso-occlusion, leading to ischemia/reperfusion injury and organ damage. Despite significant advances in our knowledge of the molecular defects in sickle hemoglobin (Hb) and rbcs, we still lack a clear understanding of the pathophysiology and treatment of vaso-occlusion. Recently, the critical roles of oxidative stress, EC activation, and inflammation in vaso-occlusion have been recognized, in part because of the development of transgenic murine models of SCD (1). Intravital microscopy studies in murine models of SCD have demonstrated the critical role of adhesion molecules in the interaction of sickle rbcs and leukocytes with the vessel wall (2–8). P-selectin, VCAM-1, and ICAM-1 on the endothelium are required; blockade or knockout of any one of these adhesion molecules prevents either cytokine- or hypoxia-induced stasis (2, 4, 6, 7). Antiinflammatory agents such as dexamethasone or antioxidants also can ameliorate hypoxia/reoxygenation–induced abnormalities in blood flow of transgenic sickle mice (2, 3, 5).
Excessive production of ROS in transgenic sickle mice plays a central role in promoting vascular inflammation, primarily through the activation of redox-sensitive transcription factors in the endothelium such as NF-κB and activator protein-1 (9). ROS are signaling molecules for a wide variety of inflammatory stimuli, such as LPS (10, 11) and TNF (12). Thus, it is not surprising that the excess oxidative stress seen in transgenic sickle mice is accompanied by a proinflammatory state.
A major source of oxidative stress in SCD is heme iron. Because of enhanced rates of rbc hemolysis, the endothelium of SCD patients is exposed to higher levels of ROS catalyzed by plasma Hb, heme, and free iron (13). Heme, a ubiquitous, hydrophobic, iron-containing compound, greatly enhances cellular susceptibility to oxidant-mediated injury (14, 15). Heme readily escapes from met-hemoglobin (met-Hb) (16, 17) and rapidly intercalates into the membranes of cells.
The vasculature defends itself against reactive heme released during hemolysis of rbcs by inducing 2 cytoprotective genes, heme oxygenase (HO) and ferritin (18). Three isoforms of HO have been described: 2 constitutively expressed isoforms, HO-2 and HO-3 (19, 20), and a 32-kDa inducible isoform, HO-1. HO-1 is transcriptionally inducible by a variety of agents, such as heme, oxidants, hypoxia, endotoxin, and cytokines (21).
HO-1 is the rate-limiting enzyme in the catabolism of the heme. It breaks down the porphyrin ring to yield equal molar amounts of biliverdin, free iron, and carbon monoxide (CO). HO-1 plays a vital role in the cytoprotection of tissues (22). The induction of HO-1 is accompanied by the induction of apoferritin (18). Apoferritin, by its capacity to bind 4,500 iron molecules and through its heavy chain ferroxidase activity (23), can store nonreactive Fe3+ in the core of the ferritin complex. Induction of HO-1 has been shown to protect tissues and cells against ischemia/reperfusion injury, oxidative stress, inflammation, transplant rejection, apoptosis, and cell proliferation (22, 24). Conversely, humans and mice deficient in HO-1 are especially prone to oxidant-mediated injury (25–27). HO-1 upregulation ameliorates inflammation, in part, through its ability to inhibit EC adhesion molecule expression and leukocyte adhesion in vivo and in vitro (26, 28, 29). In contrast, inhibition of HO-1 increases adhesion molecule expression (30–32). Thus, it is reasonable to surmise that HO-1 plays an important antiinflammatory role, particularly in SCD.
We now demonstrate that HO-1 is upregulated in transgenic sickle mice in response to an increased heme burden. Furthermore, we report that promoting additional increases in HO-1 expression in transgenic sickle mice inhibits NF-κB/EC activation and downregulates EC adhesion molecule expression, which inhibits the binding of leukocytes to the vessel wall and prevents hypoxia/reoxygenation–induced vascular stasis. These findings suggest that HO-1 is upregulated in SCD in response to the hemolysis of sickle rbcs and that additional increases in HO-1 activity and/or its downstream products, in excess of the adaptive upregulation seen in transgenic sickle mice, inhibit vascular inflammation and vaso-occlusion. Agents that increase HO-1 activity or its products may provide new therapies to prevent or treat vaso-occlusion in SCD patients.
Results
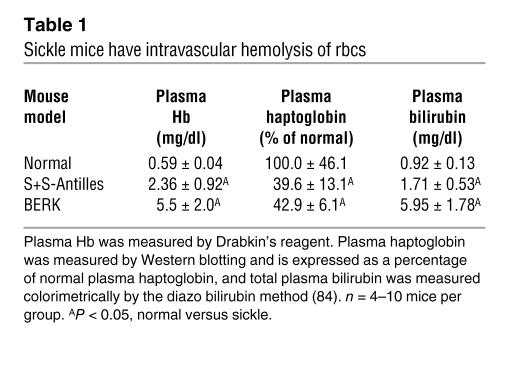
S+S-Antilles and BERK sickle mice, based on their comparison with normal C57BL/6 control mice, have intravascular hemolysis as demonstrated by increased plasma Hb, decreased plasma haptoglobin, and increased plasma bilirubin (Table 1). In data not shown, S+S-Antilles mice were minimally anemic (hematocrit 40.1% ± 3.0%, P < 0.05) and BERK mice were severely anemic (21.6% ± 3.3%, P < 0.05) compared with normal control mice (42.9% ± 1.5%). Reticulocyte counts, expressed as percentage rbcs, were elevated in S+S-Antilles (11.2% ± 2.4%, P < 0.05) and BERK mice (31.6% ± 2.9%, P < 0.05) compared with normal controls (3.1% ± 0.9%). S+S-Antilles and BERK mice also had elevated levels of ferric (Fe3+) met-Hb in their blood (1.0% ± 0.2% and 2.3% ± 0.8%, respectively) compared with normal mice (<0.1%). One way in which met-Hb can form in plasma is by reaction of ferrous (Fe2+) Hb with NO, resulting in potential effects on NO and heme bioavailability (33, 34). Ferric (Fe3+) heme can rapidly dissociate from met-Hb (16, 17), thereby potentially providing a ready source of heme to the vasculature.
Table 1.
Sickle mice have intravascular hemolysis of rbcs
In data not shown, the organ histopathology of S+S-Antilles and BERK mice was examined; rbc congestion was seen in the lungs and livers of sickle mice but not in normal mice. The livers of sickle mice had areas of infarction and inflammation. Red blood cell congestion also was seen in other organs of sickle mice, including the kidneys and brain. Thus, both the mildly anemic S+S-Antilles and the severely anemic BERK models mimic the rbc congestion and infarction pathology seen in humans with SCD.
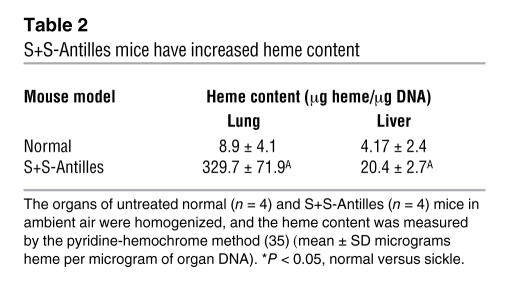
The rbc congestion was estimated by measurement of organ heme content by the pyridine-hemochrome method (35). The lungs and livers of S+S-Antilles mice had significantly more heme than those of normal mice (Table 2).
Table 2.
S+S-Antilles mice have increased heme content
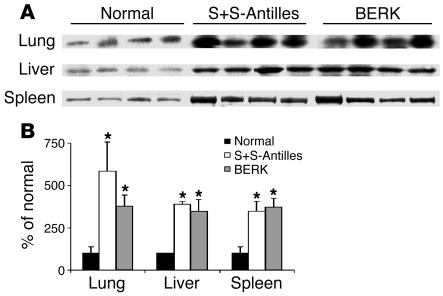
Do the excessive heme content and inflammation found in sickle organs promote increased HO-1 expression? To answer this question, HO-1 expression was measured by Western blotting in organ homogenates from normal and sickle mice. HO-1 expression was significantly increased in the lungs, livers, and spleens of S+S-Antilles and BERK mice compared with normal control mice (Figure 1). HO-1 expression was similar in S+S-Antilles and BERK mice despite higher plasma Hb concentrations in the BERK model, shown in Table 1.
Figure 1.
HO-1 expression is elevated in the organs of sickle mice. Western blots for HO-1 were performed on organ homogenates (1 μg of organ DNA per lane) from lungs, livers, and spleens of untreated normal, S+S-Antilles, and BERK mice. (A) The 32-kDa HO-1 bands are shown for each organ and each mouse. (B) The mean HO-1 band intensities (n = 4) ± SD are expressed as a percentage of those in normal control mice. *P < 0.05, normal versus sickle.
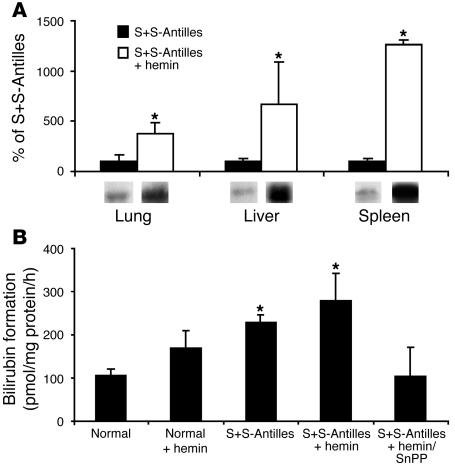
Since HO-1 and its products have documented antiinflammatory properties, a key question is whether additional upregulation of HO-1 expression can inhibit vaso-occlusion and vascular inflammation in sickle mice. Since hemin is the most powerful inducer of HO-1, S+S-Antilles mice were injected i.p. with hemin at 40 μmol/kg/d for 3 days. HO-1 levels in the lungs, liver, and spleen increased markedly in response to hemin injections (Figure 2A), demonstrating that HO-1 can be even further upregulated in sickle mice that already have significant elevations in HO-1 expression compared with normal mice. Figure 2B demonstrates HO-1 activity in a 105,000-g microsomal fraction isolated from normal and sickle livers (36). The data in Figure 2 suggest that (a) hemin treatment of S+S-Antilles mice increases HO-1 even further; (b) HO-1 activity is elevated in the livers of S+S-Antilles mice compared with normal mice; (c) hemin treatment of normal mice increases HO-1 activity in the liver, but is still lower than that of untreated S+S-Antilles mice without hemin treatment; and (d) tin protoporphyrin (SnPP), a powerful inhibitor of HO-1, blocks hemin-induced increases in HO-1 activity. Immunohistochemistry of HO-1 in frozen sections from the lungs, liver, and spleen of normal control mice and untreated and hemin-treated sickle mice demonstrated increased endothelial staining of HO-1 in vessels of all organs from sickle mice compared with normal mice as well as increased parenchymal cell staining in the livers of hemin-treated sickle mice (data not shown).
Figure 2.
Hemin increases HO-1 expression. (A) HO-1 expression can be further upregulated in the organs of sickle mice with hemin treatment. S+S-Antilles mice were either untreated or injected with hemin (40 μmol/kg/d, i.p.) for 3 days. Twenty-four hours after the third injection, the organs were harvested, and Western blots for HO-1 were performed on lung, liver, and spleen homogenates (1 μg of organ homogenate DNA per lane). The mean HO-1 band intensities (n = 3) ± SD are expressed as a percentage of those in untreated S+S-Antilles mice (100%), which represent the same untreated S+S-Antilles organs shown in Figure 1. Below each bar is a representative HO-1 band from the Western blot. *P < 0.05, untreated versus hemin. (B) HO-1 activity in normal and S+S-Antilles livers. HO-1 activity was measured in microsomes isolated from another group of normal and S+S-Antilles sickle mice as previously described (36). Mice were untreated, injected with hemin (40 μmol/kg/d, i.p.) for 3 days, or injected with hemin plus SnPP (40 μmol/kg/d of each porphyrin, i.p.) for 3 days. Twenty-four hours after the third injection, the livers were harvested, microsomes were isolated at 105,000 g, and HO-1 enzymatic activity was measured. The results in triplicate are expressed as mean ± SEM picomoles of bilirubin generated per milligram microsomal protein per hour. *P < 0.05, normal versus sickle.
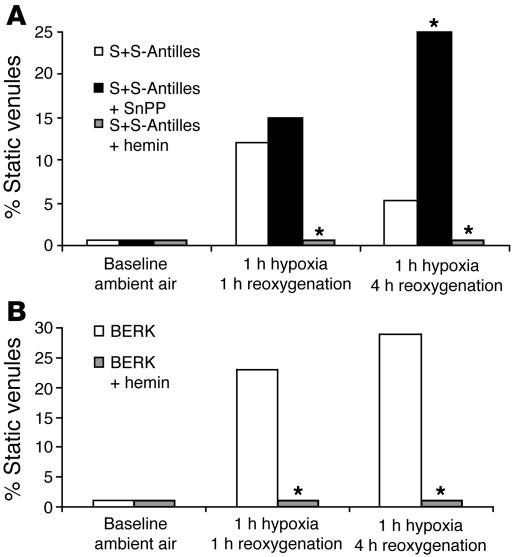
To test whether further upregulation of HO-1 could prevent stasis in subcutaneous venules, S+S-Antilles and BERK mice with an implanted dorsal skin fold chamber (DSFC) were treated with injections of hemin (40 μmol/kg/d, i.p.) or SnPP, a powerful inhibitor of HO-1 activity (40 μmol/kg/d, i.p.) for 3 days before exposure to hypoxia/reoxygenation. Remarkably, hemin-treated sickle mice whose HO-1 was further upregulated showed no evidence of stasis (Figure 3, A and B) after 1 hour of hypoxia and 1 hour of reoxygenation, while untreated S+S-Antilles mice had 12% of venules occluded and untreated BERK mice had 23% of vessels occluded. The HO-1 inhibitor SnPP did not prevent stasis in the venules of S+S-Antilles mice at 1 hour of reoxygenation (15% static) and, in fact, made stasis worse after 4 hours of reoxygenation (25% static) (Figure 3A). Thus, further upregulation of HO-1 by injection of hemin in both these mouse models of SCD prevented hypoxia/reoxygenation–induced stasis.
Figure 3.
Further upregulation of HO-1 by hemin inhibits stasis and HO-1 inhibition by SnPP exacerbates stasis in sickle mice. S+S-Antilles (A) and BERK (B) mice with an implanted DSFC were untreated, injected with hemin (40 μmol/kg/d, i.p.) for 3 days, or injected with SnPP (40 μmol/kg/d, i.p.) for 3 days. Twenty-four hours after the third injection, stasis was measured after 1 hour of hypoxia (7% O2/93% N2) and 1 hour and 4 hours of reoxygenation in room air. n = 3–10 mice and a minimum of 20 venules per mouse. *P < 0.05, untreated versus hemin or SnPP. The proportions of venules exhibiting stasis at each time point were compared using a z test.
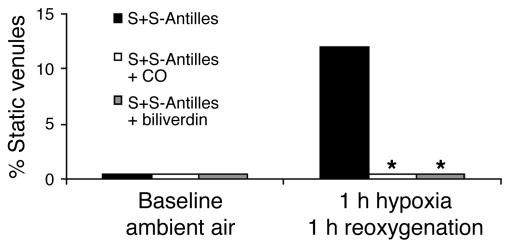
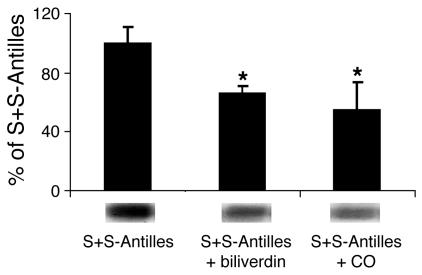
But how does HO-1 inhibit stasis? To address this question, we examined the products of HO-1 enzymatic activity, CO and biliverdin, in the hypoxia/reoxygenation stasis model. S+S-Antilles mice breathing CO at 250 parts per million (ppm) CO in air for 1 hour each day for 3 days or injected with biliverdin (50 μmol/kg i.p.) at 16 hours and 2 hours before exposure to hypoxia (7% O2/93% N2) for 1 hour and then reoxygenated in room air for 1 hour were protected against the development of stasis (Figure 4). Treatment with CO increased carboxy-Hb levels to 11.2% 1 hour after treatment with CO, and the levels decreased to less than 1% 24 hours after each CO exposure (data not shown). Thus, CO inhalation prevented stasis even after carboxy-Hb levels had returned to base line. We see this same timing effect after balloon angioplasty in rats, in which 1 hour of CO inhalation given 1 week before angioplasty still protects against lesion development (data not shown).
Figure 4.
CO and biliverdin inhibit stasis in sickle mice. S+S-Antilles mice with an implanted DSFC were either untreated or treated with inhaled CO (250 ppm CO in air for 1 hour per day) for 3 days or biliverdin injections (50 μmol/kg i.p. twice, at 16 hours and 2 hours, before hypoxia). Twenty-four hours after the third CO treatment or 2 hours after the second biliverdin injection, stasis was measured after 1 hour of hypoxia (7% O2/93% N2) and 1 hour of reoxygenation in room air. n = 3–10 mice and a minimum of 20 venules per mouse. *P < 0.05, untreated versus CO or biliverdin. The proportions of venules exhibiting stasis at each time point were compared using a z test.
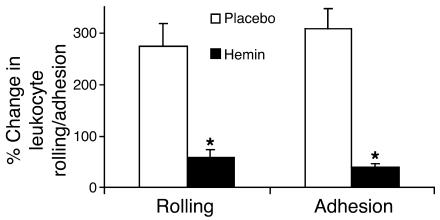
We have previously shown that activation of vascular endothelium plays an essential role in vaso-occlusion in transgenic sickle mice (2, 3). Antioxidants (3), antiinflammatory agents (2), and adhesion molecule–blocking antibodies (2) inhibit EC activation and hypoxia-induced vaso-occlusion. This inhibition is accompanied by decreased interactions of leukocytes with the vessel wall. We wondered whether hemin treatment would also decrease leukocyte rolling and adhesion along the endothelium of sickle mice. This was indeed the case. Hemin injections in S+S-Antilles mice blunted the increases in leukocyte rolling and adhesion induced by hypoxia/reoxygenation (Figure 5).
Figure 5.
Further upregulation of HO-1 by hemin inhibits hypoxia/reoxygenation–induced increases in leukocyte-endothelium interactions. S+S-Antilles mice with an implanted DSFC were treated with either placebo (saline) or hemin injections (40 μmol/kg/d, i.p.) for 3 days. Twenty-four hours after the third injection, leukocyte rolling and adhesion were measured in the subcutaneous venules at base line in ambient air and again in the same venules after exposure of the mice to 1 hour of hypoxia (7% O2/93% N2) and 1 hour of reoxygenation in room air. Results are mean ± SEM percentage change in leukocyte rolling and adhesion after hypoxia/reoxygenation. n = 2 mice and a minimum of 20 venules per group. *P < 0.05, placebo versus hemin.
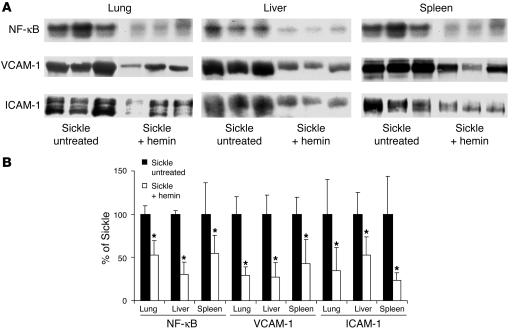
The decrease in leukocyte rolling and adhesion in sickle mice treated with hemin suggested that hemin treatments and HO-1 upregulation were inhibiting adhesion molecule expression on the vessel wall. We have previously shown that sickle mouse organs overexpress NF-κB, VCAM-1, and ICAM-1 compared with organs of normal mice (2, 3, 37). So next we examined the effect of hemin on the expression of adhesion molecules and the inflammatory transcription factor NF-κB. Treatment of sickle mice with i.p. injections of hemin for 3 days markedly decreased NF-κB activation and VCAM-1 and ICAM-1 overexpression in sickle lungs, liver, and spleen (Figure 6). These decrements in vascular inflammation correlate with decreased hypoxia/reoxygenation–induced leukocyte-endothelium interactions and venular stasis in hemin-treated sickle mice, consistent with HO-1 inhibition of inflammation and vaso-occlusion.
Figure 6.
Further upregulation of HO-1 by hemin inhibits NF-κB activation and VCAM-1 and ICAM-1 overexpression in the organs of sickle mice. S+S-Antilles mice were either untreated or injected with hemin (40 μmol/kg/d, i.p.) for 3 days. Twenty-four hours after the third injection, the organs were harvested from mice in ambient air. NF-κB activation was measured by EMSA, and VCAM-1 and ICAM-1 expression was measured by Western blotting in organ homogenates of the lungs, liver, and spleen of sickle mice. (A) The NF-κB, VCAM-1, and ICAM-1 bands are shown for each organ and each sickle mouse. (B) The bar graph shows the mean band intensity (n = 3 mice per group) ± SD for each organ treatment group. *P < 0.05, untreated versus hemin.
Could the products of HO-1, biliverdin and CO, also be working their antistasis effects through inhibition of these inflammatory pathways? CO inhalation or i.p. injections of biliverdin markedly decreased NF-κB activation in the lungs of sickle animals (Figure 7). Thus, it appears that both CO and biliverdin, products of HO-1 degradation of heme, are antiinflammatory and can inhibit stasis in sickle mice.
Figure 7.
Biliverdin or CO treatment inhibits NF-κB activation in the lungs of sickle mice. S+S-Antilles mice were untreated, treated with biliverdin injections (50 μmol/kg i.p. twice, at 16 hours and 2 hours), or treated with inhaled CO (250 ppm in air for 1 hour per day for 3 days). Two hours after the second biliverdin injection or 24 hours after the third CO treatment, mice were exposed to 3 hours of hypoxia (7% O2/93% N2) and 2 hours of reoxygenation in room air. After 2 hours of reoxygenation, the lungs were harvested, and NF-κB activation was measured in organ homogenates by EMSA. n = 3 mice per group. Below each bar is a representative NF-κB band from the EMSA. *P < 0.05, untreated versus biliverdin or CO.
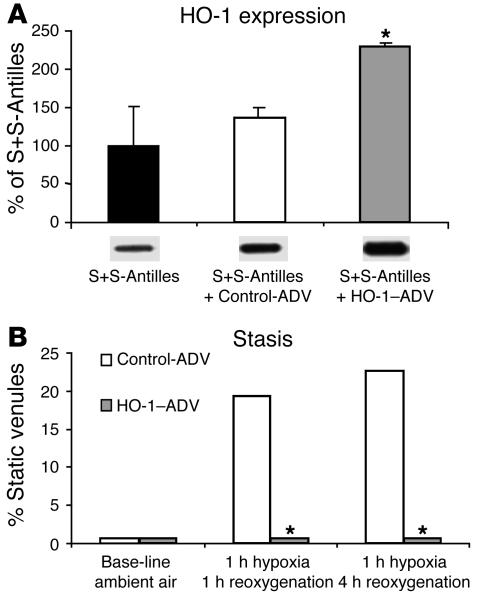
Since hemin can have nonspecific effects in vivo, we also used a rat HO-1 adenovirus construct (HO-1-ADV) to further increase HO-1 expression in S+S-Antilles sickle mice. This construct has been used successfully to overexpress HO-1 in mouse organs in vivo (38). We added HO-1-ADV (2 × 107 MOI) for 48 hours directly to the subcutaneous skin of S+S-Antilles mice with an implanted DSFC and saw approximately 225% induction of HO-1 expression in the skin (Figure 8A) compared with that in untreated S+S-Antilles mice. Control adenovirus (Control-ADV) alone (empty vector without HO-1, 2 × 107 MOI) did not significantly increase HO-1 further. Most importantly, hypoxia/reoxygenation–induced stasis was inhibited after both 1 hour and 4 hours of reoxygenation in the subcutaneous skin of mice treated with HO-1-ADV (Figure 8B). Mice treated with the Control-ADV vector exhibited worsened stasis. These data support our hypothesis that additional upregulation of HO-1 will be beneficial in preventing vaso-occlusion in SCD.
Figure 8.
HO-1-ADV increases HO-1 expression and inhibits stasis. Local administration of HO-1-ADV increases HO-1 expression (A) and inhibits hypoxia/reoxygenation–induced stasis (B) in the skin. S+S-Antilles sickle mice with an implanted DSFC were treated with either a rat HO-1-ADV construct (n = 3 mice and 84 venules) or an empty Control-ADV construct (n = 4 mice and 64 venules). The adenovirus constructs (2 × 107 MOI) in sterile saline were dripped onto the subcutaneous skin inside the DSFC. Forty-eight hours after adenovirus treatment, hypoxia/reoxygenation–induced stasis was measured (B). After measurement of stasis, the skin inside the DSFC window was harvested, and HO-1 expression was measured in the skin homogenates by Western blotting (A). Below each bar is a representative HO-1 band from the Western blot. *P < 0.05, Control-ADV versus HO-1-ADV.
Discussion
These data highlight the critical importance of HO-1 in ameliorating vascular inflammation and vaso-occlusion in murine models of SCD. Nath and colleagues demonstrated increased HO-1 in the kidney vasculature of a patient with SCD as well as increased HO-1 in circulating ECs (39). Jison et al. (40) showed that sickle blood mononuclear cells have elevated HO-1 and biliverdin reductase mRNA even during steady state. Thus, chronic hemolysis and oxidative stress in SCD result in upregulation of HO-1 in patients as well as in transgenic sickle mice. It is likely that during acute hemolytic crises the patient with SCD increases HO-1 even further to deal with the increased heme burden and oxidative stress. It is this upregulation that may facilitate the resolution of vaso-occlusion. This adaptation allows the degradation of toxic heme and the production of CO, an antiinflammatory vasodilator; biliverdin and bilirubin, both antioxidants; and ferritin, an iron chelator.
Since we do not completely understand the pathobiology of human SCD, it is impossible to know how well the sickle mouse models mimic human disease. Probably none of the models displays all of the attributes of human SCD. They can best be described as tools to understand human SCD (41). For our studies, the sickle mouse models mimic human SCD in several important ways: they have rbc sickling in response to hypoxia; hemolysis; organ pathology with rbc congestion and infarction as in the human disease; excessive ROS production; a vigorous and chronic inflammatory response with activated endothelium; and impaired blood flow, especially in response to hypoxia/reoxygenation (2, 4, 7, 37, 42–45). Most of the studies described in this paper focused on the less severe murine model of SCD, the S+S-Antilles mouse (46), and fewer on the more severe murine model, BERK mice (47). However, BERK mice, which are more anemic (hematocrit ∼22%) than S+S-Antilles mice, have similar levels of HO-1 expression, EC activation (2, 37), and organ pathology with rbc congestion and infarction but respond to hypoxia/reoxygenation with even more vaso-occlusion. Manci and colleagues (48) reported that BERK mice were similar to humans with SCD in having erythrocytic sickling, vascular ectasia, intravascular hemolysis, exuberant hematopoiesis, cardiomegaly, glomerulosclerosis, visceral congestion, hemorrhages, multiorgan infarcts, pyknotic neurons, and progressive siderosis. However, the BERK mice differed from humans with SCD in having splenomegaly; splenic hematopoiesis; more severe hepatic infarcts; less severe pulmonary manifestations; no significant vascular intimal hyperplasia; and only a trend toward vascular medial hypertrophy. Thus, these notable differences warrant careful consideration when parallels to human SCD are drawn (48). Our studies suggest that HO-1 upregulation may be important in inhibiting vascular inflammation in these murine transgenic models of human SCD. It is uncertain whether HO-1 plays a similar role in human SCD patients. Linkage of additional HO-1 expression in these SCD mouse models to other endpoints such as improvements in hemolysis and anemia or organ pathology and lifespan, conditions more directly relevant to the human disease, would strengthen these findings as being potentially relevant to human SCD.
Based on our current understanding of the interrelationships of hemolysis, oxidative stress, EC activation, blood cell adhesion, and vaso-occlusion, we have proposed a model that delineates how oxidative stress and inflammation contribute to the pathophysiology of vaso-occlusion in SCD (2, 3). We hypothesize that Hb, heme, and iron derived from hemolysis of sickle rbcs promote excessive ROS production, leading to EC activation and adhesion molecule expression on the vessel wall, which in turn promote the adhesion of sickle rbcs and leukocytes to endothelium, leading to vaso-occlusion. This paper demonstrates that the same pathophysiology induces adaptive cytoprotective proteins such as HO-1 in the endothelium that can ameliorate and prevent vaso-occlusion and the accompanying vascular inflammation commonly seen in SCD patients. We propose that it is the balance between these pro-oxidative and antioxidative forces that determines whether or not a vessel becomes occluded. In steady state these forces are likely balanced. However, a pro-oxidative insult such as hypoxia or an infection can easily tip the delicate balance in favor of hemolysis, oxidative stress, inflammation, and vaso-occlusion, causing the vessel wall to adapt by producing more cytoprotective proteins such as HO-1, which put the vasculature back in a balanced steady state. Induction of HO-1 arrives too late in the pathology of crises, and perhaps those patients that do not have significant clinical manifestations of the disease are those that have a greater homeostatic HO-1 response.
HO-1 degrades heme, which not only removes a major catalytic source of ROS and oxidative stress but also at the same time produces CO and biliverdin, which have their own antiinflammatory effects. CO gas is produced by HO-1–mediated opening of the heme ring. CO is a colorless, odorless gas that has traditionally been considered a dangerous poison. This toxicity is in part due to its high affinity for Hb (245 times greater than that of O2), which alters O2 transport and delivery. CO also can interact with other heme proteins as discussed below. At low concentrations CO can be therapeutic. CO mimics many of the protective effects of HO-1, as well as some of the functions of NO (22, 24). Like NO, CO activates the heme protein guanylate cyclase and inhibits platelet activation and aggregation (22, 24). CO participates in the regulation of vascular tone in hepatic sinusoidal cells, suggesting that NO and CO share control of these relaxation processes (49). Exogenous inhaled CO, at approximately 250 ppm, and in some studies as low as 10 ppm (50), reduces inflammatory responses in several models of oxidant injury in ways similar to those of HO-1 overexpression (22). Also, CO liberated by CO-releasing molecules significantly suppresses the inflammatory response elicited by LPS in cultured macrophages (51). Like NO (52), CO interacts with signal transduction pathways, inhibits proinflammatory genes, and augments antiinflammatory cytokines (22, 24, 53, 54). Specifically, it selectively activates p38 MAPK signaling pathways in a guanylate cyclase–independent manner (22, 53). CO also inhibits proliferation of VSMCs and has antiapoptotic effects on cells (22, 24).
As noted above, CO seems to play a role similar to that of NO. NO has taken an important role in the pathogenesis and therapy of SCD (34, 55, 56). SCD patients have a reduction in basal and stimulated NO production and bioavailability. The deficiency of NO is in part due to elevated plasma Hb and excessive ROS production in both SCD patients and transgenic sickle mice (34). Therapeutic CO may be more effective, because the efficacy of NO is hampered by its ability to form reactive nitrogen species. Under oxidative conditions, NO reacts with ROS, resulting in the formation of highly reactive ONOO– (peroxynitrite) (57). Peroxynitrite does not prevent or ameliorate disease like NO does but, in contrast, exacerbates oxidative inflammatory stress (58, 59). Unlike NO, CO does not contain any unpaired free electrons and is, therefore, relatively inert.
Studies over 30 years ago by Beutler (60) suggested that CO binding to hemoglobin S shifts the oxygen dissociation curve to the left and can actually inhibit hemoglobin S deoxygenation, hemoglobin S polymerization, and rbc hemolysis. Less hemolysis means less plasma Hb, less oxidative stress, less vascular inflammation, enhanced bioavailability of NO, and ultimately less vaso-occlusion. NO and peroxynitrite can induce HO-1 activity and thus form a feedback loop, where CO takes over NO functions under conditions of oxidative stress (21). In our studies, inhaled CO was able to inhibit vaso-occlusion in the skin and NF-κB activation in the lungs, suggesting a potential therapeutic benefit at low doses in SCD patients.
Another product of heme metabolism by HO-1, as noted above, is biliverdin. In mammals, biliverdin is rapidly converted by biliverdin reductase to bilirubin. Both biliverdin and bilirubin are antioxidants with probable physiological relevance in plasma and the extravascular space (61). Exogenous bilirubin has been demonstrated to be cytoprotective in models of ischemia/reperfusion injury (22, 24). At micromolar concentrations, both biliverdin and bilirubin efficiently scavenge peroxyradicals and thereby inhibit lipid peroxidation (62, 63). Biliverdin and bilirubin also may counteract intracellular nitrosative stress reactions (64, 65). In our studies, biliverdin injected i.p. was able to inhibit vascular stasis in the skin and NF-κB activation in the lungs similarly to inhaled CO, suggesting that biliverdin also may have therapeutic benefits in SCD. Biliverdin reductase might also be involved in transcriptional regulation of HO-1 (66).
Some of HO-1’s protective effects in sickle mice were likely due to ferritin induction. In our initial studies, the induction of HO-1 was accompanied by the induction of apoferritin (18). Apoferritin, made up of heavy and light chains, protects cells by its capacity to bind 4,500 iron molecules and through its heavy chain ferroxidase activity (23, 67, 68). Ferritin can store nonreactive Fe3+ in the core of the ferritin complex. This handling of catalytic Fe2+ is essential for ferritin’s ability to protect cells against oxidative stress (13, 69, 70) by interrupting Fenton chemistry, exemplified in the Haber-Weiss reaction for hydroxyl radical generation. Animal models that overexpress the H-ferritin chain withstand ischemia/reperfusion injury and oxidative stress without the concomitant increase in HO-1 (71). In vivo models that increased HO-1 by hemin or Hb injections all increased ferritin levels (36, 72, 73). Dissecting the intimate relationship between HO-1, Fe release from heme, and ferritin has depended on the use of HO-1 inhibitors and iron chelators. Additional studies in sickle mice are needed to dissect the relative contributions of HO-1 and ferritin to inhibition of oxidative stress, inflammation, and vaso-occlusion in SCD. We conjecture that ferritin may be an important cytoprotectant in iron-burdened SCD animals and patients.
Other defenses against Hb and heme released into the vasculature include plasma haptoglobin and hemopexin. However, these defenses are likely overwhelmed in SCD, as evidenced by the low haptoglobin concentrations seen in our sickle mice and low plasma hemopexin levels in SCD patients (74–77). Moreover, the heme content and the overexpression of HO-1 in organs of sickle mice suggest that this is indeed the case.
Markers of hemolysis are associated with a clinical subphenotype of pulmonary hypertension, leg ulceration, priapism, and risk of death in SCD patients (78, 79). Hemolysis may drive vasculopathy, but, by inducing HO-1 expression, hemolysis may ultimately limit vaso-occlusion. Perhaps HO-1 induction by hemolysis may explain the protection against atherosclerosis in SCD patients. Ironically, we used hemin, a “bad actor” in SCD, to therapeutically increase HO-1 and inhibit vascular inflammation and vaso-occlusion. Hematin therapy could be an approach in SCD patients. However, before HO-1 induction, hematin could potentially be pro-oxidative and also contribute to iron overload. Future therapeutic studies in our laboratory will focus on pharmacological therapies such as statins to increase HO-1 expression in SCD. Two recent papers demonstrated that some of the vasoprotective effects of statins, drugs used to decrease cholesterol and vascular inflammation in atherosclerosis, may be due to their ability to induce HO-1 (80, 81). Alternatively, direct administration of CO or biliverdin might be a therapeutic modality by which to treat or prevent sickle crises. In addition, functional polymorphisms in the HO-1 gene promoter region that control the ability to upregulate HO-1 (82, 83) could potentially explain some of the phenotypic differences in severity seen in SCD patients. These findings suggest that HO-1 and its products are promising new avenues of therapy in SCD.
Methods
Mice.
All animal experiments were approved by the University of Minnesota’s Institutional Animal Care and Use Committee. We used male and female S+S-Antilles (46) and BERK (47) transgenic sickle mice as our models for human SCD. S+S-Antilles mice are homozygous for deletion of the mouse β-major globin locus and express human α, βS, and βS-Antilles globin transgenes. βS-Antilles globins contain, in addition to the βS mutation at β6, a second mutation at β23 (Val→Ile). βS-Antilles has low oxygen affinity and decreased solubility under deoxygenated conditions, resulting in a more severe form of SCD. In S+S-Antilles mice, approximately 42% of the β globins expressed are βS and 36% are βS-Antilles.
BERK mice have full knockout of murine α and β globins and replacement with a human α and βS globin transgene (47). BERK mice have more severe organ pathology than S+S-Antilles mice, exhibit multiorgan pathology, including areas of fibrosis and/or infarction in liver, kidney, and spleen, and have loss of urine-concentrating ability.
Normal male and female mice (C57BL/6) were used as genetic controls for the sickle mice. The mice used in these studies were 8–12 weeks of age. The mice weighed 20–30 g and were housed in specific pathogen–free housing to prevent common murine infections that could cause an inflammatory response. All the mice were maintained on a standard chow diet.
Treatments of mice.
In experiments to either upregulate HO-1 expression or inhibit HO-1 activity, mice were injected i.p. with hemin (Sigma-Aldrich) or tin protoporphyrin (SnPP; Frontier Scientific Inc.), respectively, at a dose of 40 μmol/kg/d for 3 days. HO-1, NF-κB, VCAM-1, and ICAM-1 were measured in organ homogenates, and hypoxia/reoxygenation–induced stasis and leukocyte rolling and adhesion were measured in the subcutaneous skin.
In experiments to evaluate the effects of CO and biliverdin, sickle mice were placed in a special chamber and exposed to an atmosphere of CO at a dose of 250 ppm CO in air (Air Products and Chemicals Inc.) for 1 hour per day for 3 days or were injected with biliverdin (Frontier Scientific Inc.) at a dose of 50 μmol/kg i.p. twice, at 16 hours and 2 hours, before harvesting of lungs or exposure to hypoxia (7% O2/93% N2). Twenty-four hours after the third inhaled CO treatment or 2 hours after the second biliverdin injection, NF-κB was measured in lung homogenates, and hypoxia/reoxygenation–induced stasis was measured in the subcutaneous skin.
In experiments to further evaluate the effect of HO-1 upregulation on stasis, S+S-Antilles sickle mice with an implanted DSFC were treated with either a rat HO-1 adenovirus construct (HO-1-ADV) or an empty control adenovirus (Control-ADV). The recombinant adenovirus containing rat HO-1 cDNA was generated as previously described (38). The adenovirus constructs (2 × 107 MOI) in sterile saline were dripped onto the subcutaneous skin inside the DSFC. Forty-eight hours after adenovirus treatment, hypoxia/reoxygenation–induced stasis was measured.
After the treatments described above, sickle mice with an implanted DSFC were exposed to hypoxia/reoxygenation to measure vascular stasis or leukocyte rolling and adhesion. After base-line measurements in room air, sickle mice were placed in a special chamber and exposed to 1 hour of hypoxia (7% O2/93% N2) followed by reoxygenation in room air for 1 hour. The measurements were repeated in the same venules after exposure to hypoxia/reoxygenation. The sickle mice could tolerate the brief (1-hour) exposure to hypoxia without dying.
Markers of hemolysis.
Hematocrit levels and reticulocytes were measured in EDTA blood collected from the tail veins of mice as previously described (43). Plasma Hb was measured by Drabkin’s reagent. Plasma haptoglobin was measured by Western blotting, and total plasma bilirubin was measured colorimetrically by the diazo bilirubin method (84).
Mouse tissue collection.
The mice in ambient air were sacrificed and tissues harvested as previously described (2). Mice were asphyxiated in a CO2 chamber for approximately 60 seconds. Samples of lungs, livers, and spleens were taken for homogenate preparation. Organ samples for homogenate preparation were immediately frozen in liquid nitrogen and stored at –80°C. Organ samples also were collected for histopathology.
Organ heme content.
The heme content of lungs and livers was measured in organ homogenates by the pyridine-hemochrome method (35).
Western blots of lung, liver, and spleen HO-1, VCAM-1, and ICAM-1.
Lung, liver, and spleen tissue samples were collected and frozen as described above. Thawed tissues were homogenized and homogenate DNA concentrations were measured as previously described (2). Tissue homogenates containing an equal amount of homogenate DNA per well were subjected to SDS-PAGE (7.5%). After SDS-PAGE, the samples were transferred electrophoretically to PVDF membranes, and immunoblotting of the organ homogenates was performed with rabbit anti–rat HO-1 antiserum (Stressgen Biotechnologies Inc.), or goat anti–mouse VCAM-1 or ICAM-1 IgG directed against the C-terminus of the target protein (Santa Cruz Biotechnology Inc.). Sites of primary antibody binding were visualized with HRP-conjugated donkey anti-rabbit or anti-goat IgG (Jackson ImmunoResearch Laboratories Inc.). The final detection of immunoreactive bands was performed using a chemiluminescent detection substrate (Pierce).
EMSA for NF-κB activation.
NF-κB activation was measured in organ extracts by EMSA as previously described (2). To confirm the identity of NF-κB bands, some reactions were run with an excess of unlabeled consensus or mutant double-stranded DNA (dsDNA) for competition experiments or with antibodies to the p50 or p65 subunit of NF-κB for supershift experiments. The mutant dsDNA for NF-κB differed by 1 bp from the consensus binding sequence. A 10-fold excess of mutant dsDNA added to the binding reaction was unable to inhibit binding of the radiolabeled consensus dsDNA sequence to NF-κB, while a 10-fold excess of unlabeled consensus dsDNA completely abolished binding of the radiolabeled consensus dsDNA. Mouse NF-κB EMSA bands contained the p50 and p65 subunits (data not shown).
Quantitation of Western blots and EMSAs.
To quantitate expression of HO-1, VCAM-1, ICAM-1, and NF-κB, exposed films of chemiluminescent Western blots and radioactive gel shifts were scanned on a GS-700 Imaging Densitometer (Bio-Rad Laboratories Inc.). Bands on each image were quantified with Molecular Analyst software (version 2.1; Bio-Rad Laboratories Inc.) using local background subtraction. The intensity of HO-1, VCAM-1, and ICAM-1 bands on film was linear between 25 and 1,000 ng of tissue homogenate DNA with correlation coefficients (r2) of 1.00, 1.00, and 0.98, respectively. The intensity of NF-κB bands on film was linear between 12 and 500 ng of tissue homogenate DNA with a correlation coefficient (r2) of 0.98. This permitted accurate quantitation of the relative intensities of HO-1, VCAM-1, ICAM-1, and NF-κB bands on film and their corresponding levels of tissue expression.
Measurement of vascular stasis.
Hypoxia/reoxygenation–induced stasis of venular blood flow in the subcutaneous skin was measured in mice with an implanted DSFC using intravital microscopy as previously described (2, 43). All measurements of blood flow parameters in the DSFC were made 4–7 days after DSFC implantation. At base line, with the mice in ambient air, flowing venules were selected at random, and their relative locations were noted on a map of the microscopic field. After base-line selection of flowing venules in ambient air, the mice were subjected to 1 hour of hypoxia (7% O2/93% N2), followed by reoxygenation in room air. After 1 and 4 hours of reoxygenation, the same venules were reexamined for blood flow. Venules with no observable blood flow were counted as static. The percentage of static vessels was calculated by division of the number of static venules at any given time point by the total number of flowing venules selected at base line. A minimum of 20 subcutaneous venules were examined in each mouse. We equate vascular stasis with vaso-occlusion for experimental purposes. Certainly, prolonged vaso-occlusion seen clinically is associated with activation of coagulation and thrombosis leading to organ infarction.
Leukocyte rolling and adhesion.
Leukocyte-endothelium interactions were measured in venules of the subcutaneous skin after staining of leukocytes in vivo with the intravital fluorescent dye rhodamine 6G as previously described (2, 43, 85). S+S-Antilles mice with an implanted DSFC were treated with either placebo (saline) or hemin injections (40 μmol/kg/d, i.p.) for 3 days. Twenty-four hours after the third injection, leukocyte rolling and adhesion were measured in the subcutaneous venules at base line in room air and again after exposure of the mice to 1 hour of hypoxia (7% O2/93% N2) and 1 hour of reoxygenation in room air. The rolling flux was determined as the total number of leukocytes rolling through a given section of vessel per minute. To assess leukocyte adhesion, 100-μm venular segments were examined, and a leukocyte was considered adherent if it remained stationary for at least 30 seconds (4). Results are expressed as the percentage change in leukocyte rolling and adhesion after hypoxia/reoxygenation. A minimum of 20 vessels were examined in each mouse.
CO-Hb and met-Hb.
Oximeters (682 [Instrumentation Laboratory] and ABL 700 [Radiometer America Inc.], respectively) were used to measure CO-Hb and met-Hb levels in blood samples collected by cardiac puncture (86).
Statistics.
All statistical analyses were performed with SigmaStat 2.0 for Windows (SPSS). Comparisons of data groups were made using a 2-tailed Student’s t test or a Mann-Whitney rank sum test. The proportions of venules exhibiting stasis at each time point were compared using a z test. P values less than or equal to 0.05 were considered statistically significant.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute grants HL67367 and HL55552. We would like to thank Stephana Choong for breeding and characterizing the transgenic sickle mice used for these studies.
Footnotes
Nonstandard abbreviations used: CO, carbon monoxide; Control-ADV, control adenovirus; dsDNA, double-stranded DNA; DSFC, dorsal skin fold chamber; Hb, hemoglobin; HO, heme oxygenase; HO-1-ADV, heme oxygenase-1 adenovirus; met-Hb, met-hemoglobin; ppm, parts per million; SCD, sickle cell disease; SnPP, tin protoporphyrin.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Nagel RL, Fabry ME. The panoply of animal models for sickle cell anaemia. Br. J. Haematol. 2001;112:19–25. doi: 10.1046/j.1365-2141.2001.02286.x. [DOI] [PubMed] [Google Scholar]
- 2.Belcher JD, et al. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2715–H2725. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 3.Mahaseth H, et al. Polynitroxyl albumin inhibits inflammation and vasoocclusion in transgenic sickle mice. J. Lab. Clin. Med. 2005;145:204–211. doi: 10.1016/j.lab.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J. Clin. Invest. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaul DK, et al. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H293–H301. doi: 10.1152/ajpheart.01150.2003. [DOI] [PubMed] [Google Scholar]
- 6.Embury SH, et al. The contribution of endothelial cell P-selectin to the microvascular flow of mouse sickle erythrocytes in vivo. Blood. 2004;104:3378–3385. doi: 10.1182/blood-2004-02-0713. [DOI] [PubMed] [Google Scholar]
- 7.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setty BN, Stuart MJ. Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood. 1996;88:2311–2320. [PubMed] [Google Scholar]
- 9.Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000;35:521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 10.Park HS, et al. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 11.Victor VM, Rocha M, De la Fuente M. N-acetylcysteine protects mice from lethal endotoxemia by regulating the redox state of immune cells. Free Radic. Res. 2003;37:919–929. doi: 10.1080/1071576031000148727. [DOI] [PubMed] [Google Scholar]
- 12.Chen XL, Zhang Q, Zhao R, Medford RM. Superoxide, H2O2, and iron are required for TNF-alpha-induced MCP-1 gene expression in endothelial cells: role of Rac1 and NADPH oxidase. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1001–H1007. doi: 10.1152/ajpheart.00716.2003. [DOI] [PubMed] [Google Scholar]
- 13.Balla J, et al. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol. Dial. Transplant. 2003;18:v8–v12. doi: 10.1093/ndt/gfg1034. [DOI] [PubMed] [Google Scholar]
- 14.Balla G, Vercellotti G, Eaton JW, Jacob HS. Heme uptake by endothelium synergizes polymorphonuclear granulocyte-mediated damage. Trans. Assoc. Am. Physicians. 1990;103:174–179. [PubMed] [Google Scholar]
- 15.Balla G, Vercellotti GM, Eaton JW, Jacob HS. Iron loading of endothelial cells augments oxidant damage. J. Lab. Clin. Med. 1990;116:546–554. [PubMed] [Google Scholar]
- 16.Bunn HF, Jandl JH. Exchange of heme among hemoglobins and between hemoglobin and albumin. J. Biol. Chem. 1968;243:465–475. [PubMed] [Google Scholar]
- 17.Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc. Natl. Acad. Sci. U. S. A. 1988;85:237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balla G, et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 19.Rublevskaya I, Maines MD. Interaction of Fe-protoporphyrin IX and heme analogues with purified recombinant heme oxygenase-2, the constitutive isozyme of the brain and testes. J. Biol. Chem. 1994;269:26390–26395. [PubMed] [Google Scholar]
- 20.McCoubrey WK, Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 21.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 22.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 23.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. . Biochim. Biophys. Acta. 1996; 1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 24.Wagener FA, et al. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 2003;55:551–571. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- 25.Yachie A, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagener FA, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–1811. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 27.Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum. Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 28.Soares MP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 29.Wagener FA, et al. Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J. Pharmacol. Exp. Ther. 1999;291:416–423. [PubMed] [Google Scholar]
- 30.Hayashi S, et al. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ. Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 31.Vachharajani TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1613–H1617. doi: 10.1152/ajpheart.2000.278.5.H1613. [DOI] [PubMed] [Google Scholar]
- 32.Rucker M, et al. Reduction of inflammatory response in composite flap transfer by local stress conditioning-induced heat-shock protein 32. Surgery. 2001;129:292–301. doi: 10.1067/msy.2001.111079. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, et al. Biological activity of nitric oxide in the plasmatic compartment. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiter CD, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 35.Berry EA, Trumpower BL. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 1987;161:1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- 36.Balla J, et al. Endothelial cell heme oxygenase and ferritin induction in rat lung by hemoglobin in vivo. Am. J. Physiol. 1995;268:L321–L327. doi: 10.1152/ajplung.1995.268.2.L321. [DOI] [PubMed] [Google Scholar]
- 37.Belcher JD, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 38.Otterbein LE, et al. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J. Clin. Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nath KA, et al. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am. J. Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jison ML, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagel RL. Lessons from transgenic mouse lines expressing sickle hemoglobin. Proc. Soc. Exp. Biol. Med. 1994;205:274–281. doi: 10.3181/00379727-205-43708. [DOI] [PubMed] [Google Scholar]
- 42.Osarogiagbon UR, et al. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- 43.Kalambur VS, et al. Microvascular blood flow and stasis in transgenic sickle mice: utility of a dorsal skin fold chamber for intravital microscopy. Am. J. Hematol. 2004;77:117–125. doi: 10.1002/ajh.20143. [DOI] [PubMed] [Google Scholar]
- 44.Embury SH, Mohandas N, Paszty C, Cooper P, Cheung AT. In vivo blood flow abnormalities in the transgenic knockout sickle cell mouse. J. Clin. Invest. 1999;103:915–920. doi: 10.1172/JCI5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaul DK, Fabry ME, Costantini F, Rubin EM, Nagel RL. In vivo demonstration of red cell-endothelial interaction, sickling and altered microvascular response to oxygen in the sickle transgenic mouse. J. Clin. Invest. 1995;96:2845–2853. doi: 10.1172/JCI118355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabry ME, et al. A second generation transgenic mouse model expressing both hemoglobin S (HbS) and HbS-Antilles results in increased phenotypic severity. Blood. 1995;86:2419–2428. [PubMed] [Google Scholar]
- 47.Paszty C, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 48.Manci, E.A., et al. 2005. Pathology of “Berkeley” sickle cell mice: similarities and differences with human sickle cell disease. Blood. doi:10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed]
- 49.Hata K, et al. Induction of heme oxygenase-1 and dilation of hepatic sinusoids by an administration of pyrrolidine dithiocarbamate in rat livers. J. Surg. Res. 2003;115:310–317. doi: 10.1016/j.jss.2003.08.240. [DOI] [PubMed] [Google Scholar]
- 50.Sarady JK, et al. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J. 2004;18:854–856. doi: 10.1096/fj.03-0643fje. [DOI] [PubMed] [Google Scholar]
- 51.Sawle P, et al. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br. J. Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marshall HE, Hess DT, Stamler JS. S-nitrosylation: physiological regulation of NF-kappaB. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8841–8842. doi: 10.1073/pnas.0403034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol. Cell. Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohta K, Yachie A. Development of vascular biology over the past 10 years: heme oxygenase-1 in cardiovascular homeostasis. J. Endovasc. Ther. 2004;11(Suppl. 2):II140–II150. doi: 10.1177/15266028040110S607. [DOI] [PubMed] [Google Scholar]
- 55.Gladwin MT, Schechter AN. Nitric oxide therapy in sickle cell disease. Semin. Hematol. 2001;38:333–342. doi: 10.1016/s0037-1963(01)90027-7. [DOI] [PubMed] [Google Scholar]
- 56.Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Curr. Opin. Hematol. 2003;10:99–107. doi: 10.1097/00062752-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Wolin MS, Davidson CA, Kaminski PM, Fayngersh RP, Mohazzab HK. Oxidant–nitric oxide signalling mechanisms in vascular tissue. Biochemistry Mosc. 1998;63:810–816. [PubMed] [Google Scholar]
- 58.Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem. Soc. Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 59.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 60.Beutler E. The effect of carbon monoxide on red cell life span in sickle cell disease. Blood. 1975;46:253–259. [PubMed] [Google Scholar]
- 61.Stocker R, McDonagh AF, Glazer AN, Ames BN. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol. 1990;186:301–309. doi: 10.1016/0076-6879(90)86123-d. [DOI] [PubMed] [Google Scholar]
- 62.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 63.Dailly E, Urien S, Barre J, Reinert P, Tillement JP. Role of bilirubin in the regulation of the total peroxyl radical trapping antioxidant activity of plasma in sickle cell disease. Biochem. Biophys. Res. Commun. 1998;248:303–306. doi: 10.1006/bbrc.1998.8960. [DOI] [PubMed] [Google Scholar]
- 64.Kaur H, et al. Interaction of bilirubin and biliverdin with reactive nitrogen species. FEBS Lett. 2003;543:113–119. doi: 10.1016/s0014-5793(03)00420-4. [DOI] [PubMed] [Google Scholar]
- 65.Mancuso C, Bonsignore A, Di Stasio E, Mordente A, Motterlini R. Bilirubin and S-nitrosothiols interaction: evidence for a possible role of bilirubin as a scavenger of nitric oxide. Biochem. Pharmacol. 2003;66:2355–2363. doi: 10.1016/j.bcp.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 66.Miralem T, Hu Z, Torno MD, Lelli KM, Maines MD. Small interference RNA-mediated gene silencing of human biliverdin reductase, but not that of heme oxygenase-1, attenuates arsenite-mediated induction of the oxygenase and increases apoptosis in 293A kidney cells. J. Biol. Chem. 2005;280:17084–17092. doi: 10.1074/jbc.M413121200. [DOI] [PubMed] [Google Scholar]
- 67.Theil EC. Ferritin: at the crossroads of iron and oxygen metabolism. J. Nutr. 2003;133:1549S–1553S. doi: 10.1093/jn/133.5.1549S. [DOI] [PubMed] [Google Scholar]
- 68.Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 2002;33:457–463. doi: 10.1016/s0891-5849(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 69.Cozzi A, et al. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J. Biol. Chem. 2000;275:25122–25129. doi: 10.1074/jbc.M003797200. [DOI] [PubMed] [Google Scholar]
- 70.Epsztejn S, et al. H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood. 1999;94:3593–3603. [PubMed] [Google Scholar]
- 71.Berberat PO, et al. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003;17:1724–1726. doi: 10.1096/fj.03-0229fje. [DOI] [PubMed] [Google Scholar]
- 72.Erario MA, Gonzales S, Noriega GO, Tomaro ML. Bilirubin and ferritin as protectors against hemin-induced oxidative stress in rat liver. Cell. Mol. Biol. (Noisy-le-grand). 2002;48:877–884. [PubMed] [Google Scholar]
- 73.Gonzales S, Erario MA, Tomaro ML. Heme oxygenase-1 induction and dependent increase in ferritin. A protective antioxidant stratagem in hemin-treated rat brain. Dev. Neurosci. 2002;24:161–168. doi: 10.1159/000065686. [DOI] [PubMed] [Google Scholar]
- 74.Foidart M, Liem HH, Adornato BT, Engel WK, Muller-Eberhard U. Hemopexin metabolism in patients with altered serum levels. J. Lab. Clin. Med. 1983;102:838–846. [PubMed] [Google Scholar]
- 75.Wochner RD, Spilberg I, Iio A, Liem HH, Muller-Eberhard U. Hemopexin metabolism in sickle-cell disease, porphyrias and control subjects: effects of heme injection. N. Engl. J. Med. 1974;290:822–826. doi: 10.1056/NEJM197404112901503. [DOI] [PubMed] [Google Scholar]
- 76.Fertakis A, Panitsas G, Angelopoulos B. Serum haemopexin concentration in patients with various haemoglobinopathies. Effect of splenectomy. Acta Haematol. 1973;50:149–153. doi: 10.1159/000208342. [DOI] [PubMed] [Google Scholar]
- 77.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32:811–815. [PubMed] [Google Scholar]
- 78.Kato, G.J., et al. 2005. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood. doi:10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed]
- 79.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106:3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 81.Grosser N, et al. The antioxidant defense protein heme oxygenase 1 is a novel target for statins in endothelial cells. Free Radic. Biol. Med. 2004;37:2064–2071. doi: 10.1016/j.freeradbiomed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 82.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic. Biol. Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Exner M, et al. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J. Endovasc. Ther. 2001;8:433–440. doi: 10.1177/152660280100800501. [DOI] [PubMed] [Google Scholar]
- 84.Martinek RG. Improved micro-method for determination of serum bilirubin. Clin. Chim. Acta. 1966;13:161–170. doi: 10.1016/0009-8981(66)90290-7. [DOI] [PubMed] [Google Scholar]
- 85.Baatz H, Steinbauer M, Harris AG, Krombach F. Kinetics of white blood cell staining by intravascular administration of rhodamine 6G. Int. J. Microcirc. Clin. Exp. 1995;15:85–91. doi: 10.1159/000178955. [DOI] [PubMed] [Google Scholar]
- 86.Mahoney JJ, Vreman HJ, Stevenson DK, Van Kessel AL. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrophotometers (CO-oximeters) in comparison with reference methods. Clin. Chem. 1993;39:1693–1700. [PubMed] [Google Scholar]