Abstract
PACT, a protein activator of PKR, can cause inhibition of cellular protein synthesis and apoptosis. Here, we report that the Us11 protein of herpes simplex virus type 1 can block PKR activation by PACT both in vitro and in vivo. Although Us11 can bind to both PKR and PACT, mutational analyses revealed that the binding of Us11 to PKR, and not to PACT, was essential for its inhibitory action. Similar analyses also revealed that the inhibitory effect was mediated by an interaction between the C-terminal half of Us11 and the N-terminal domain of PKR. The binding of Us11 to PKR did not block the binding of PKR to PACT but prevented its activation. Us11 is the first example of a viral protein that can inhibit the action of PACT on PKR.
PKR, a serine/threonine kinase whose level is increased by interferon treatment of cells, is a latent enzyme that needs to be activated by autophosphorylation (12, 42, 43). Once activated, PKR can phosphorylate a limited set of cellular proteins, including the translation initiation factor eIF-2α whose phosphorylation causes inhibition of translation. Among the many physiological roles of PKR, its antiviral activities are the most well known. In addition, PKR has been shown to be an important element in the transcriptional signal transduction pathways activated by specific cytokines, growth factors, double-stranded (ds) RNA, and extracellular stresses. In these contexts, PKR has been shown to be required for activation of other protein kinases such as p38, JNK, and IKK (10, 21, 46) and transcription factors such as NF-κB, IRF-1, p53, STAT1, ATF, STAT3, and AP-1 (10, 14, 24, 25, 43, 44). PKR has also been shown to be important in cellular differentiation, apoptosis, cell growth, and oncogenic transformation (12, 43).
The classical activator of PKR is dsRNA, which is known to be produced during viral infection of cells. dsRNA binds to two dsRNA-binding motifs present at the amino terminus of PKR (17, 32) and changes its conformation to an active state in which it can bind ATP (2, 3) and autophosphorylate (41). We have shown that the same domain of PKR can also mediate dsRNA-independent protein-protein interactions with proteins containing similar domains (34). One such protein is PACT, whose binding to PKR leads to the activation of PKR in the absence of dsRNA (15, 33). Thus, PACT is a true protein activator of PKR. PACT contains three identifiable domains, of which domains 1 and 2 bind to the amino-terminal dimerization domain (DD) of PKR whereas domain 3 binds to a different region further downstream (36). Domain 3 is the effector domain; it can activate PKR by itself in vitro. In vivo, however, it requires either of the other two domains to anchor it strongly to PKR so that the activation process can occur efficiently. In mammalian cells, PACT-mediated PKR activation and consequent apoptosis happen only upon the application of cellular stresses such as withdrawal of growth factors or treatment with a low dose of actinomycin D or arsenite (22, 31, 36).
To circumvent PKR-mediated blockage of viral protein synthesis, many viruses have evolved mechanisms to block PKR activation and action (42). Both DNA and RNA viruses use a number of different strategies to achieve this goal. Some encode proteins that can bind and remove viral dsRNA, a common activator of PKR (6, 20). Others encode decoy RNAs to compete with dsRNA for PKR binding (11, 18). Several viral proteins or cellular proteins induced in virus-infected cells bind to PKR and block its activation by dsRNA (1, 13, 26). Others can serve as pseudosubstrates or cause dephosphorylation of eIF-2α (16, 23). Among these myriad anti-PKR viral proteins are two herpes simplex virus type 1 (HSV-1)-encoded proteins, γ34.5 and Us11 (28, 39). The former protein functions by recruiting a cellular phosphatase to eIF-2, which restores eIF-2α to its unphosphorylated state (19).
Us11 is an abundant 21-kDa protein produced late in the HSV-1 lytic growth cycle that is packaged into the viral tegument (39). Genetic analysis has revealed that the premature cessation of protein synthesis observed in cells infected with γ34.5 mutants can be prevented by expressing Us11 as a viral immediate-early protein (28, 29). The ability of Us11 to enhance the growth of γ34.5 mutant viruses and allow for sustained translation requires the 68-residue C-terminal domain (38). This segment of Us11 localizes to the nucleolus, associates with polysomes, and contains a region important for interaction with PKR in an RNA-dependent manner (5, 27, 39, 40). Furthermore, this domain binds dsRNA through a novel RNA recognition motif and prevents PKR activation in response to dsRNA (38; D. Khoo, C. Perez, and I. Mohr, submitted for publication).
In this study, we investigated whether Us11 can block PACT-mediated PKR activation. Because Us11 is an RNA-binding protein, it is conceivable that its action against dsRNA-mediated PKR activation is achieved solely by dsRNA binding. In that case, PKR activation by PACT might be unaffected by Us11. Contrary to this expectation, the results presented here clearly demonstrate that Us11 can block PACT-mediated PKR activation in vitro and in vivo. Although Us11 binds to both PACT and PKR, the C-terminal domain of Us11 must bind to the N-terminal domain of PKR in order to inhibit PKR activation by PACT.
MATERIALS AND METHODS
Construction of PACT and PKR mutants.
The generation of PACT and PACT mutant expression constructs has been described previously (36). PCR fragments corresponding to residues 1 to 170, 171 to 551, and 360 to 551 were ligated into restriction enzyme-digested pcDNA3. A FLAG epitope tag was added at the C-terminal coding end of all PKR constructs.
Expression and purification of GST-Us11 and mutants.
Us11C (GST Δ1-87) contains residues 88 to 155 from the HSV-1 (Patton strain) Us11 protein fused to the carboxyl terminus of glutathione S-transferase (GST). Us11N (GST Δ88-155) consists of residues 1 to 87 from Us11 fused to the carboxyl terminus of GST. Proteins were expressed in Escherichia coli BL21(DE3) (lysS) and purified by affinity chromatography on glutathione agarose (Pharmacia). The full-length Us11 protein was expressed in bacteria with a histidine tag and purified on Ni-NTA (nickel-nitrilotriacetic acid) agarose (Qiagen). Eluted fractions were pooled, dialyzed against 20 mM HEPES-KOH (pH 7.4), 100 mM KCl, and 5% glycerol, snap-frozen in a dry ice-ethyl alcohol bath, and stored at −80°C.
PKR activation assay in vitro.
The kinase activation assay of PKR was performed on PKR purified by monoclonal antibody immobilized on protein G-Sepharose (33). HT1080 cells were treated with 1,000 U of beta interferon/ml for 24 h and lysed in high-salt buffer (20 mM Tris-HCl [pH 7.5], 50 mM KCl, 400 mM NaCl, 1% Triton X-100, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], 100 U of aprotinin/ml, 20% glycerol). HT1080 lysate was mixed with 1 μl of PKR monoclonal antibody 71/10 (Ribogene) in high-salt buffer and placed on a spinning wheel for 30 min at 4°C. Twenty-five microliters of protein G-Sepharose was added and spun for an additional 30 min at 4°C. The protein G-Sepharose beads were washed four times in 500 μl of high-salt buffer and two times in 500 μl of activity buffer (10 mM Tris-HCl [pH 7.5], 50 mM KCl, 2 mM magnesium acetate, 7 mM β-mercaptoethanol, 20% glycerol). The activation assay was performed on immobilized PKR in activity buffer containing 1 to 100 nmol of purified protein activator or inhibitor, 2.5 mM MnCl2, 0.1 mM ATP, and 10 μCi [γ-32P]ATP for 20 min at 30°C. Purified Us11C or Us11N protein was added to the reaction before purified PACT or PACT mutant protein. Labeled protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 11% resolving gel. Autoradiography was performed at room temperature.
GST pulldown assay.
Proteins to be tested for interaction with Us11C were independently mixed with 1 μg of GST or GST-Us11C in binding buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1% Triton X-100, 20% glycerol, 100 U of aprotinin/ml, 0.2 mM PMSF) or high-salt buffer containing 25 μl of glutathione-Sepharose 4B (Amersham Pharmacia) and placed on a rotating wheel for 2 h at 4oC. After binding, the beads were washed six times with 500 μl of fresh buffer. The proteins interacting with the GST-containing protein were analyzed by Western blotting for FLAG, histidine, or PACT domain 3. GST-Us11C was detected by Western blotting for GST.
In vitro interaction assay.
In vitro translated, 35S-labeled PACT or PACT mutant proteins were synthesized using the TNT T7-coupled reticulocyte system (Promega) (33). After translation, 3 μl of reticulocyte extract containing the protein to be tested for interaction was mixed with 25 μl of glutathione-Sepharose 4B and 1 μg of purified GST-Us11C in binding buffer and placed on a rotating wheel for 2 h at 4°C. After binding, the beads were washed six times with 500 μl of binding buffer. The washed beads were then boiled in 2× Laemmli buffer (150 mM Tris-HCl [pH 6.8], 5% SDS, 5% β-mercaptoethanol, 20% glycerol) for 2 min and analyzed by SDS-PAGE on a 12% resolving gel.
Expression in mammalian cells and PKR purification.
HT1080 cells were transfected in 100-mm-diameter culture dishes with 5 μg of cytomegalovirus (CMV) PKR (K296R)-FLAG or CMV PKR mutant-FLAG DNA using Lipofectamine 2000 reagent (Invitrogen). At 24 h after transfection, cells were lysed in high-salt buffer (20 mM Tris-HCl [pH 7.5], 50 mM KCl, 400 mM NaCl, 1% Triton X-100, 0.2 mM PMSF, 100 U of aprotinin/ml, 20% glycerol) on ice. In some experiments, the cell extract was used to immunoprecipitate FLAG-PKR constructs with anti-FLAG (M2)-agarose. The agarose beads were washed four times with high-salt buffer and twice with low-salt buffer (10 mM Tris-HCl [pH 7.5], 50 mM KCl, 2 mM magnesium acetate, 100 U of aprotinin/ml, 0.2 mM PMSF, 1% Triton X-100, 20% glycerol). PKR-FLAG was eluted with 0.2 mg of FLAG peptide (Sigma)/ml in elution buffer (10 mM Tris-HCl [pH 7.5], 10% glycerol). Purified PKR-FLAG constructs were analyzed by Western blotting with anti-FLAG monoclonal antibody (Sigma) (33).
Assay for binding of PACT domain 3 to PKR.
Purified maltose binding protein (MBP) or MBP tethered to PACT domain 3 (MBP-3) were independently bound (0.5 μg each) to amylose resin (New England Biolabs) in amylose binding buffer (20 mM Tris-HCl [pH 7.5], 10 mM β-mercaptoethanol, 1 mM EDTA, 10% glycerol). Purified GST-Us11C and PKR-FLAG proteins (1 μg each) were sequentially added to the resin-bound MBP or MBP-3 in amylose binding buffer and placed on a rotating wheel for 1 h at 4°C. After binding, the resin was washed six times with 500 μl of amylose binding buffer containing 1% Triton X-100. The ability of PKR-FLAG to interact with MBP or MBP-3 was analyzed by Western blotting for FLAG.
Expression and purification of PACTΔ1 from E. coli.
The protein-coding region for PACTΔ1, which is missing PACT domain 1, was subcloned into pET15b (Novagen) to generate pet15b-PACTΔ1. This results in an in-frame fusion of correct PACT coding sequence to the histidine tag. The expression vector was transformed into BL21(DE3) cells containing a plasmid overexpressing thioredoxin to enhance protein solubility (45). The bacteria were grown overnight, transferred to a larger culture volume, and grown for 3 to 4 h. The culture was shifted to room temperature, isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a final concentration of 0.5 mM, and bacteria were grown for 12 h. The culture was harvested at 8,000 rpm for 10 min in a Beckman JA10 rotor. Cells were washed once in ice-cold phosphate-buffered saline (PBS) and then resuspended in 20 ml of lysis buffer (500 mM NaCl, 50 mM NaH2PO4, 20 mM imidazole, 10% glycerol, 0.1% NP-40, complete protein inhibitors, 5 mM β-mercaptoethanol)/liter. Cells were lysed by passing them twice through a French press (1,000 lb/in2), and the lysate was cleared by spinning at 15,000 × g for 20 min. The supernatant was mixed with 10 ml of Ni-NTA agarose and incubated for 1 h at 4°C on a spinning wheel. After binding, the beads were washed twice in buffer A (500 mM NaCl, 50 mM NaH2PO4, 20 mM imidazole, 10% glycerol [pH 8.0]) and packed into a column. The column was washed with 100 ml of buffer A and 100 ml of buffer B (buffer A at pH 6.0). His-PACTΔ1 was eluted with 30 ml of elution buffer (150 mM l-histidine in buffer A, pH 6.8). The eluted protein was concentrated by placing it into dialysis tubing (10,000 molecular weight cutoff) covered with dry polyethylene glycol (PEG 20000; Fluka) at 4°C for 1 to 2 h. The protein was further purified by gel filtration (200 mM NaCl) using a Superdex 75 matrix (Pharmacia) at a constant flow rate of 0.5 ml/min. After the final elution off the column, the protein preparation was treated with micrococcal nuclease to remove any contaminating dsRNA (33). To ensure that any and all dsRNA was digested, the protein was heat inactivated by boiling and tested for residual dsRNA in PKR activation assays in vitro. Purified PACTΔ1 protein was stored at −80°C until use.
Apoptosis assay.
HT1080 cells growing on glass coverslips in 6-well dishes were cotransfected with pCI-Us11 and pcDNA3-FLAG PACT or pcDNA3-FLAG PACTΔ1. At 6 h after transfection, the cells were treated with 50 ng of actinomycin D/ml. Cells were fixed in 4% methanol-free formaldehyde 24 h after transfection. A terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) assay using the Apoptosis Detection System (Promega) was performed according to the manufacturer's protocol. After a 2× SSC wash (1× SCC is 0.15 M NaCl plus 0.015 M sodium citrate) and rinses in PBS, blocking buffer (10% goat serum and 3% bovine serum albumin in Tris-buffered saline-Tween 20) was placed on the cells for 10 min at room temperature. Cells were stained using mouse anti-FLAG (M5) monoclonal antibody at a 1:1,000 dilution for 45 min at room temperature. After three 5-min washes in PBS, cells were stained with goat anti-mouse immunoglobulin G-Texas Red conjugate (Molecular Probes) at a 1:1,500 dilution for 45 min at room temperature. After three more 5-min washes in PBS, the cells were mounted on glass slides in Vectashield with DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories) and examined under a fluorescence microscope. For quantitation of apoptosis, at least 300 protein-expressing cells were scored for TUNEL positivity.
RESULTS
Inhibition of PACT-mediated PKR activation by Us11.
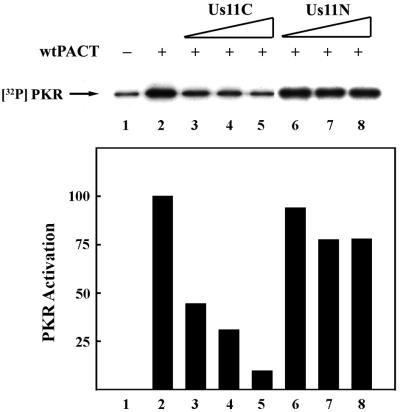
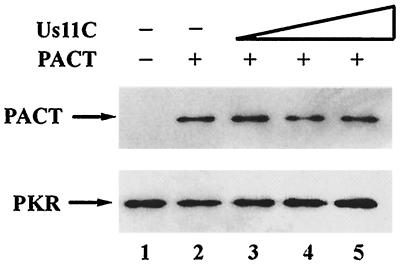
Us11 is known to inhibit PKR activation by dsRNA (4, 38). We tested the effects of Us11 on PKR activation by the alternative activator PACT by measuring in vitro autophosphorylation of PKR (Fig. 1). For this purpose, PACT, Us11, and PACT mutants expressed in E. coli and purified to homogeneity by affinity chromatography were incubated with PKR and radiolabeled ATP. Full-length Us11 inhibited PKR activation by either dsRNA or PACT (data not shown). Dose-dependent inhibition of PACT-mediated PKR activation was also observed for Us11C, the C-terminal half of Us11 (Fig. 1, lanes 1 to 5), but not for Us11N, the N-terminal half (Fig. 1, lanes 6 to 8). These results established that the carboxyl-terminal half of Us11 can block PKR activation by PACT.
FIG. 1.
Us11C, but not Us11N, can block PKR activation by PACT. The effects of increasing concentrations of two purified bacterially expressed Us11 mutants, Us11N containing residues 1 to 87 and Us11C containing residues 88 to 161, on wt PACT-mediated PKR activation in vitro were tested. Purified Us11 mutant protein, followed by wt PACT, was added to PKR immunoprecipitated from HT1080 cells and incubated for 20 min in the presence of [γ-32P]ATP. PKR phosphorylation was monitored on an SDS-PAGE gel by using autoradiography. Lane 1, activity buffer; lanes 2 to 8, 2 ng of wt PACT; lanes 3 and 6, 0.1 μg of purified truncated Us11; lanes 4 and 7, 0.3 μg of purified truncated Us11; lanes 5 and 8, 1.0 μg of purified truncated Us11. PhosphorImager analysis was done to quantify the PKR activation level. The background PKR activation level (lane 1) was subtracted from all values. The amount of radioactivity in PKR activated by wt PACT alone (lane 2) was considered 100%, and the values for other treatments are presented as percentages of that value.
Characteristics of Us11 binding to PKR and PACT.
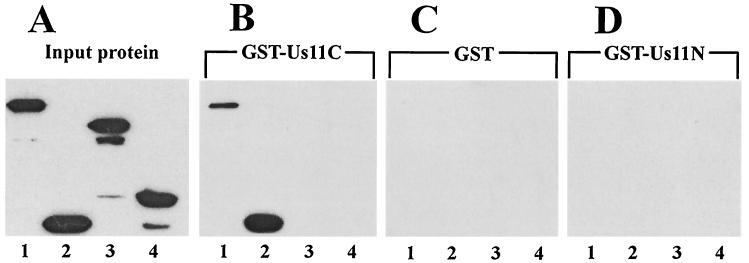
In the next series of experiments, protein-protein interactions between Us11C and PKR or PACT and its mutants were assayed. GST-tagged Us11C protein was used in a GST pulldown assay to test its interaction with PKR. FLAG-tagged wild-type (wt) PKR or different PKR deletion mutants were expressed in HT1080 cells, and the cell extracts were mixed with GST or GST-Us11C for potential interactions. Wt PKR, PKR 1-170, PKR 171-551, and PKR 360-551 were present in large quantities in the respective extracts (Fig. 2A, lanes 1 to 4). As expected, none of these proteins interacted with GST alone (Fig. 2C). When incubated with GST-Us11C, only wt PKR and PKR 1-170 were retained on the glutathione-agarose beads (Fig. 2B, lanes 1 to 4). In contrast, none of the proteins interacted with GST-Us11N (Fig. 2D). These results clearly showed that the interaction between Us11 and PKR is mediated by the C-terminal half of Us11 and the N-terminal 170 residues of PKR. The same region of PKR is known to be responsible for dsRNA binding as well as for dsRNA-independent protein-protein interactions among various members of the PKR family of dsRNA-binding proteins (34).
FIG. 2.
Us11C binds to PKR residues 1 to 170. GST pulldown assays were used to measure the binding of GST-tagged Us11C to PKR and PKR deletion mutants. FLAG-tagged PKR and PKR mutants were transfected into HT1080 cells, and cell extracts were prepared. The cell extracts were mixed with either purified GST or GST-Us11C protein in binding buffer, and GST-containing protein was pulled down using glutathione-Sepharose 4B. The PKR-FLAG protein constructs interacting with the GST-containing protein were analyzed by Western blotting with FLAG antibody. In panels B to D, the amount of extracts used was twice that used in panel A. (A) Input wt PKR and mutant proteins used to measure Us11C binding. The major band in each lane shows the position of the protein. (B to D) PKR mutant proteins interacting with GST-Us11C (B), GST (C), and GST-Us11N (D). Lanes 1, full-length PKR 1-551 (K296R); lanes 2, PKR 1-170; lanes 3, PKR 171-551; lanes 4, PKR 360-551.
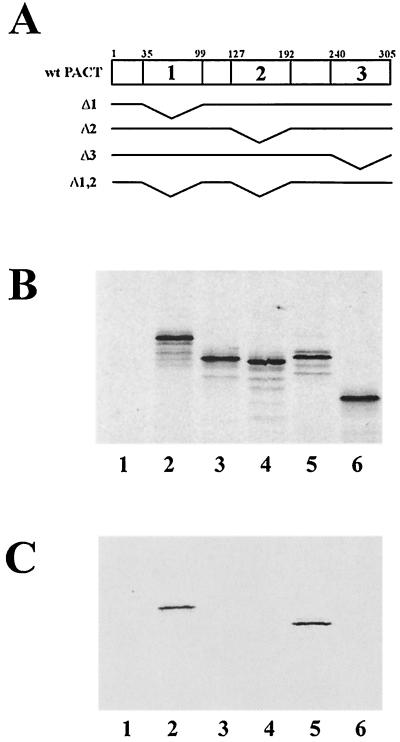
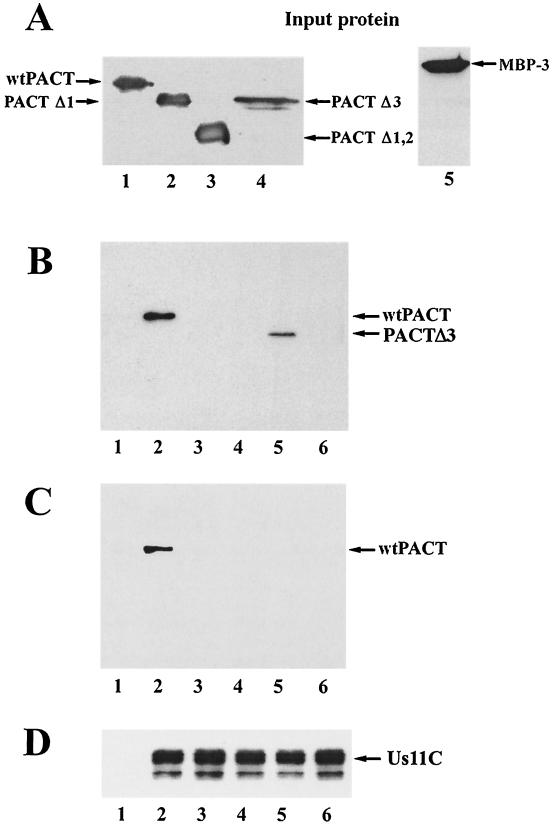
Interactions between Us11C and PACT or its mutants (Fig. 3A) were studied next. Radiolabeled PACT or its mutants were synthesized by in vitro translation (Fig. 3B) and used in a GST pulldown assay. Wt PACT interacted with GST-Us11C but not with GST (Fig. 3C, lanes 1 and 2). Among the PACT mutants missing one or more of its three domains, only PACTΔ3 (Fig. 3C, lane 5), missing domain 3, could interact with Us11C, indicating that both domains 1 and 2, but not 3, of PACT are necessary for Us11C interaction. The same conclusions were confirmed by another experiment using purified bacterially expressed PACT mutants. Wt PACT, PACTΔ1, PACTΔ1,2 (missing domains 1 and 2), PACTΔ3, and MBP-3 were expressed in bacteria and purified by affinity chromatography (Fig. 4A). Wt PACT (Fig. 4B and C, lanes 2) and PACTΔ3 (Fig. 4B, lane 5) were pulled down by GST Us11C, but the other mutants of PACT failed to interact with it.
FIG. 3.
In vitro translated PACTΔ3, but not Δ1, Δ2, or Δ1,2, bind Us11C. GST pulldown assays were used to measure the binding of PACT and PACT mutants to GST-tagged Us11C. 35S-labeled wt PACT and PACT mutants were translated in vitro. Three microliters of the reticulocyte lysate containing PACT or PACT mutant was mixed with 1 μg of GST or GST-Us11C in binding buffer. Glutathione-Sepharose 4B was used to pull down GST-containing protein, and the proteins interacting with it were detected by gel electrophoresis and autoradiography. (A) Maps of human wt PACT protein and deletion constructs. Wt PACT has three domains: PKR interaction domains 1 and 2 and PKR activation domain 3. The small numbers on top indicate amino acid residue numbers. PACTΔ1 is missing PACT amino acid residues 35 to 99, Δ2 is missing PACT amino acid residues 127 to 192, Δ3 is missing PACT amino acid residues 240 to 305, and Δ1,2 is missing PACT amino acid residues 35 to 99 and 127 to 192. (B) Proteins that were translated in vitro (1 μl of lysate/lane). Lane 1, pcDNA3 vector; lane 2, wt PACT; lane 3, PACTΔ1; lane 4, PACTΔ2; lane 5, PACTΔ3; lane 6, PACTΔ1,2. (C) Proteins that interacted with the GST-tagged protein. Lane 1, GST and wt PACT; lanes 2 to 6, GST-US11C; lane 2, wt PACT; lane 3, PACTΔ1; lane 4, PACTΔ2; lane 5, PACTΔ3; lane 6, PACTΔ1,2.
FIG. 4.
Bacterially expressed PACT and PACTΔ3, but not Δ1,2, Δ1, or MBP-3, bind Us11C. GST pulldown assays were used to measure the binding of PACT and PACT mutants to GST-tagged Us11C. One microgram of purified PACT or PACT mutant protein was mixed with 1 μg of purified GST-Us11C in high-salt buffer. Glutathione-Sepharose 4B was used to pull down GST-Us11C, and the proteins interacting with it were analyzed. (A) Purified PACT proteins (1 μg each), representing those that were tested for GST-Us11C binding. Lanes 1 to 4, histidine-tagged PACT proteins Western blotted with anti-histidine antibody; lane 1, wt PACT; lane 2, PACTΔ1; lane 3, PACTΔ1,2; lane 4, PACTΔ3; lane 5, MBP-3 Western blotted with anti-PACT domain 3 antibody. (B to D) Proteins pulled down with glutathione-Sepharose 4B and Western blotted with anti-histidine antibody (B), anti-PACT domain 3 antibody (C), and anti-GST antibody (D). In panels B to D, all lanes except lanes 1 contained GST-Us11C. The additional proteins were as follows: wt PACT, PACTΔ1, PACTΔ1,2, PACTΔ3, and MBP-3 (lanes 1); wt PACT (lanes 2); PACTΔ1 (lanes 3); PACTΔ1,2 (lanes 4); PACTΔ3 (lanes 5); and MBP-3 (lanes 6)
Us11C does not disrupt PACT binding to PKR.
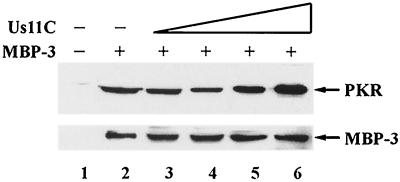
Because Us11C could bind to both PKR and PACT, we wondered whether it could block the binding of PACT to PKR and thus inhibit PKR activation. The results presented in Fig. 5 and 6 demonstrate that this was not the case. Binding of PACT to PKR was not inhibited by increasing concentrations of Us11C (Fig. 5). Under the conditions of this assay, the interaction of PACT with PKR was mediated by domains 1 and 2 of PACT (36). However, for PKR activation, domain 3 needs to interact with PKR. To measure this interaction, in the absence of the much stronger interaction with domains 1 and 2, we examined the effects of Us11C on the binding of MBP-3 to PKR. The binding of MBP-3 to PKR was unimpaired by the presence of increasing concentrations of Us11C (Fig. 6). Thus, it appears that Us11C does not interfere with PKR-PACT interactions mediated either by domains 1 and 2 or by domain 3 of PACT.
FIG. 5.
Us11C does not prevent PACT binding to PKR. Shown are the effects of increasing concentrations of purified Us11C on wt PACT interaction with PKR. PKR was immunoprecipitated from an extract of HT1080 cells treated with beta interferon by using PKR monoclonal antibody/protein G-Sepharose. The final two washes of immobilized PKR were done with a low-salt buffer. Us11C and then PACT were added to the immobilized PKR, followed by incubation for 15 min and then extensive washing with buffer. PACT interaction with PKR was analyzed by Western blotting using a domain 3-specific PACT antibody. All lanes contained PKR and lanes 2 to 5 contained 1 μg of PACT. In addition, 0.3 (lane 3), 1.0 (lane 4), and 2.4 (lane 5) μg of Us11C were added. The bottom panel shows the stripped Western blot reprobed with anti-PKR antibody.
FIG. 6.
Us11C does not displace MBP-3 from PKR. Shown are the effects of increasing concentrations of purified Us11C on MBP-3 interaction with PKR. PKR was immunoprecipitated from pcDNA3-PKR-FLAG-transfected HT1080 cells by using anti-FLAG-agarose. After changing to a low-salt buffer, PKR was eluted off the beads with excess FLAG peptide. Purified Us11C and then purified PKR were added to amylose resin-immobilized MBP-3. The proteins were incubated for 1 h and washed extensively with low-salt buffer. The proteins interacting with MBP-3 were analyzed by Western blotting for PKR using FLAG antibody. Lanes 1 to 6, PKR; lanes 2 to 6, 1.0 μg of purified MBP-3; lane 3, 0.1 μg of purified Us11C; lane 4, 0.3 μg of purified Us11C; lane 5, 1.0 μg of purified Us11C; lane 6, 2.4 μg of purified Us11C. The bottom panel shows the stripped Western blot reprobed with anti-PACT domain 3 antibody.
Critical need for PKR interaction, but not PACT interaction, for the action of Us11.
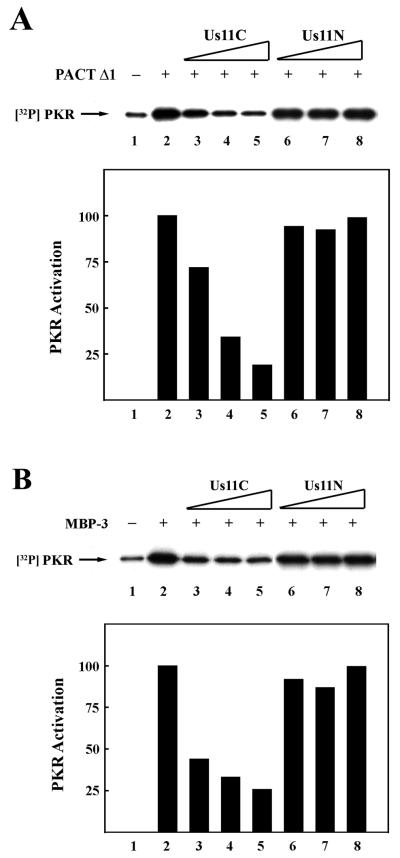
Because Us11C could interact with both PACT and PKR, either of these interactions could potentially cause an inhibition of PKR activation by PACT. To distinguish these possibilities, we used PACT mutants which could not bind Us11C to activate PKR. Although Us11C could not bind to PACTΔ1 (Fig. 3 and 4), it could still inhibit PKR activation by this PACT mutant (Fig. 7A, lanes 1 to 5). Similarly, activation of PKR by MBP-3 was increasingly inhibited by increasing concentrations of Us11C but not of Us11N (Fig. 7B). These results clearly showed that the interaction of Us11C with PKR, not with PACT, was the critical requirement for its ability to inhibit PACT-mediated activation of PKR.
FIG. 7.
(A) Us11C inhibits PKR activation by PACTΔ1. The effects of increasing concentrations of Us11C or Us11N on PACTΔ1-mediated PKR activation in vitro were tested. Purified Us11 mutant protein, followed by PACTΔ1, was added to PKR immunoprecipitated from HT1080 cells and incubated in the presence of [γ-32P]ATP. PKR autophosphorylation was measured by electrophoresis and autoradiography. Lane 1, activity buffer; lanes 2 to 8, 2 ng of PACTΔ1; lanes 3 and 6, 0.1 μg of purified truncated Us11; lanes 4 and 7, 0.3 μg of purified truncated Us11; lanes 5 and 8, 1.0 μg of purified truncated Us11. PhosphorImager analysis was done to quantify the PKR activation level. The background PKR activation level (lane 1) was subtracted from all values. The amount of radioactivity in PKR activated by PACTΔ1 alone (lane 2) was considered 100%, and the values for other treatments are presented as percentages of that value. (B) Us11C inhibits PKR activation by MBP-3. The effects of increasing concentrations of Us11C or Us11N on MBP-3-mediated PKR activation invitro were tested. Purified Us11 mutant protein, followed by MBP-3, was added to PKR immunoprecipitated from HT1080 cells and incubated in the presence of [γ-32P]ATP. PKR autophosphorylation was measured as described above. Lane 1, activity buffer; lanes 2 to 8, 2 ng of MBP-3; lanes 3 and 6, 0.1 μg of purified truncated Us11; lanes 4 and 7, 0.3 μg of purified truncated Us11; lanes 5 and 8, 1.0 μg of purified truncated Us11. PhosphorImager analysis was done to quantify the PKR activation level. The background PKR activation level (lane 1) was subtracted from all values. The amount of radioactivity in PKR activated by MBP-3 alone (lane 2) was considered 100%, and the values for other treatments are presented as percentages of that value.
Inhibition of PACT-mediated apoptosis by Us11.
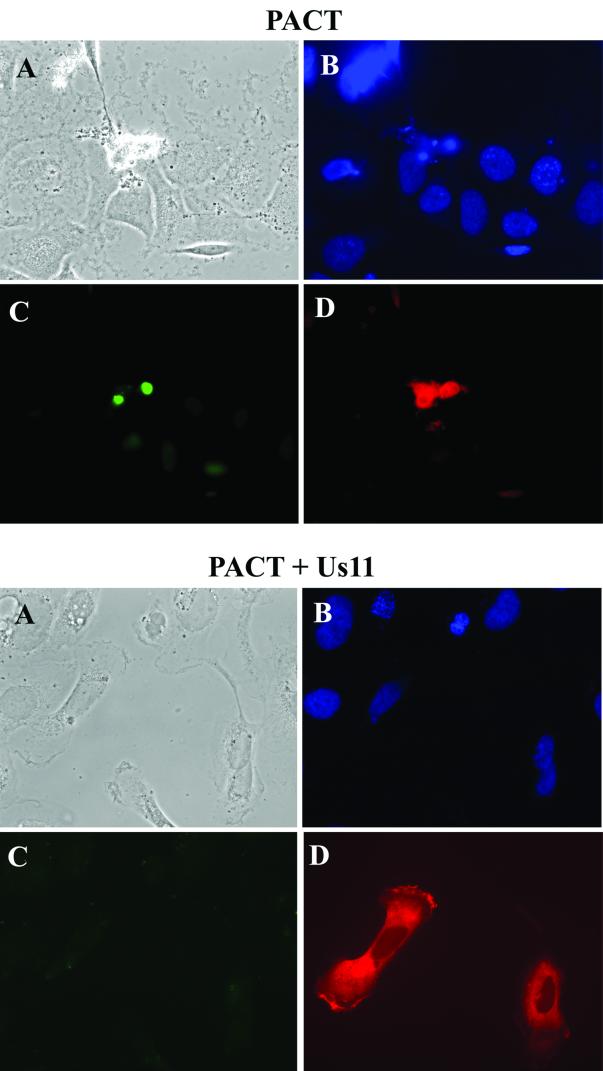
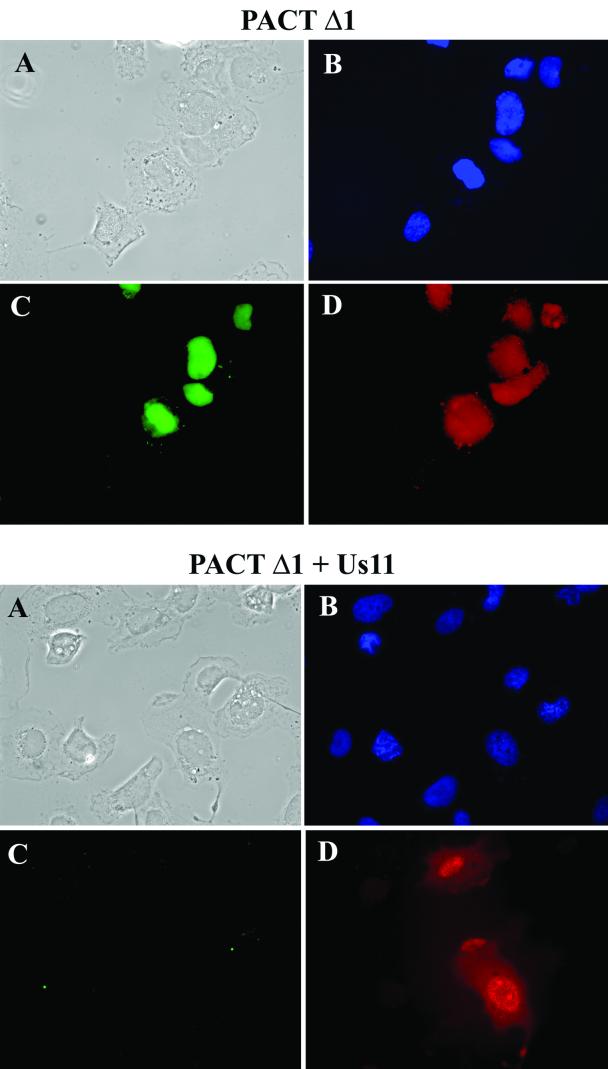
The experiments discussed above clearly showed that in vitro activation of PKR by PACT was blocked by Us11. In the next set of experiments, we examined whether this was also true in vivo. We have previously reported that PKR activation by PACT in vivo causes cellular apoptosis (36). To test the effects of Us11 on the apoptotic process, Us11 and PACT were coexpressed in PKR-containing cells and apoptosis was monitored by TUNEL assay. As expected, expression of PACT caused cellular apoptosis (Fig. 8, upper four panels). However, coexpression of Us11 blocked the process (Fig. 8, lower four panels). This effect was specific because Us11 did not block the apoptotic effects of the chemical camptothecin (data not shown). Similar to the results in vitro, Us11 also inhibited apoptosis caused by PACTΔ1, which does not bind to Us11 (Fig. 9). A quantitative analysis, in which 300 wt PACT- or PACTΔ1-expressing cells were scored for TUNEL positivity, showed that Us11 was equally effective against wt PACT and PACTΔ1. When Us11 was coexpressed with the former, the number of apoptotic cells was reduced from 233 to 15; when it was coexpressed with the latter, the number of apoptotic cells was reduced from 237 to 41 (the nonspecific background was 25 cells, an amount which was subtracted from all values). Thus, the in vivo apoptosis results confirmed the conclusion that the interaction of Us11 with PKR, not with PACT, is critical for the observed Us11 actions on PKR activation.
FIG. 8.
Us11 inhibits PACT-mediated apoptosis. TUNEL and immunofluorescence assays were performed with HT1080 cells transfected with wt PACT alone (top four panels) or cotransfected with Us11 and wt PACT (bottom four panels). (A) Phase-contrast micrographs; (B) DAPI staining, showing nuclei; (C) TUNEL staining, showing green-fluorescing cells undergoing apoptosis; (D) PACT-expressing cells revealed by their red immunofluorescence.
FIG. 9.
Us11 inhibits PACTΔ1-mediated apoptosis. TUNEL and immunofluorescence assays were performed with HT1080 cells transfected with PACTΔ1 alone (top four panels) or cotransfected with Us11 and PACTΔ1 (bottom four panels). (A) Phase-contrast micrographs; (B) DAPI staining, showing nuclei; (C) TUNEL staining, showing green-fluorescing cells undergoing apoptosis; (D) PACT-expressing cells revealed by their red immunofluorescence.
DISCUSSION
Many viral gene products can prevent activation of cellular PKR kinase and inhibit the action of the activated enzyme. HSV-1 encodes at least two such proteins, γ34.5 and Us11 (4, 19, 28, 29). γ34.5 mutant viruses are profoundly attenuated for neurovirulence in animals and cannot complete their lytic program in many cultured cells due to the premature cessation of protein synthesis (7—9). The γ34.5 polypeptide antagonizes PKR action by causing dephosphorylation of phosphorylated eIF-2α (19). Expression of Us11 as a viral immediate-early protein prevents the shutdown of translation that occurs in cells infected with a γ34.5 mutant virus (29). Us11 accomplishes this by preventing PKR activation (4, 29, 38).
Before this study, all viral inhibitors of PKR, including Us11, had been investigated in the light of PKR activation by dsRNA. Our results demonstrated that Us11 is equally effective against the action of PACT on PKR. Moreover, the same domain of Us11, the C-terminal domain, is responsible for blocking the actions of both activators (37). Analysis of protein-protein interactions between Us11, PKR, and PACT indicated that the binding of Us11 to PKR was crucial in order to prevent PACT activation of PKR. Us11C bound to the N-terminal DD of PKR. This domain has previously been shown to mediate homomeric and heteromeric interactions among proteins of the PKR family (34). Members of this family are all dsRNA-binding proteins, and their association with dsRNA is also mediated by the same domain. However, the protein and dsRNA-binding properties are independent of each other because PKR mutants, which are devoid of one property but not the other, have been generated (34, 35). Us11 bound to the DD of PKR and similarly interacted with the cognate region of PACT containing the two dsRNA-binding domains. However, unlike dsRNA or PKR, which can bind to PACT missing either domain 1 or domain 2 but not both, Us11 required the presence of both domains 1 and 2 of PACT in order to bind to it. It is likely that the same is true for Us11 interaction with PKR as well, i.e., both dsRNA binding and dimerization motifs present in the N-terminal domain of PKR are required for Us11 interactions. If this general pattern holds true, then the structural requirements for viral Us11 interactions with PKR, PACT, and other dsRNA-binding proteins are quite distinct from those required for mutual interactions among these cellular factors.
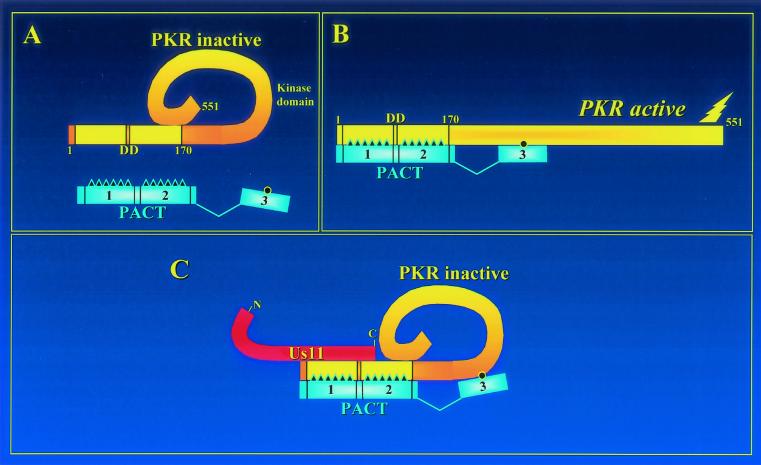
It was surprising that the binding of Us11 to PKR did not affect its interaction with PACT; PACT binding to PKR through any of its three domains was maintained in the presence of Us11. These observations have led to the formulation of the model shown in Fig. 10. It is known that PKR activation is achieved by a conformational change of the protein (12). Structural studies have indicated that the C-terminal domain of PKR interacts with its N-terminal domain (30), keeping it in a closed inactive conformation (Fig. 10A). PACT domains 1, 2, and 3 all interact with PKR. The first two domains interact with the N-terminal domain of PKR, whereas domain 3 interacts with an as-yet-undefined region of PKR (36). It is the interaction of PACT domain 3 with PKR that activates it by changing its conformation (Fig. 10B). Binding of Us11C to the N-terminal domain of PKR prevents this conformational switch by PACT domain 3 (Fig. 10C). Because PACT domains 1 and 2, dsRNA, and Us11C all bind to the same region of PKR, it was conceivable that Us11C outcompeted the others for binding, but this was not the case. Mechanistically, moreover, this issue is irrelevant because only domain 3 of PACT could activate PKR and even this process was blocked by the binding of Us11C to a distant region of PKR. Thus, it seems that Us11C functions by blocking the conformational change of PKR required for its activation by either dsRNA, which binds to the same region as Us11C, or PACT domain 3, which binds to a different region.
FIG. 10.
Model of Us11 inhibition of PACT-mediated PKR activation. (A) PKR contains the DD at its amino terminus and the kinase domain at its carboxyl terminus. PACT has three domains: PKR interaction domains 1 and 2 and PKR activation domain 3. (B) Domain 3 of PACT binds weakly to an undefined site of PKR and changes its conformation, leading to PKR activation. (C) The C-terminal half of Us11 binds to the PKR DD and prevents PACT domain 3 from changing PKR conformation, resulting in an inactive PKR. Although PACT domain 3 cannot activate PKR in the presence of Us11, domain 3 can still bind to PKR.
The above-described model was verified not only by the in vitro PKR autophosphorylation results but also by the in vivo PACT-mediated apoptosis results. PACT-mediated apoptosis requires the presence of PKR and an external stress. Us11C blocked this process. Moreover, the apoptotic action of PACTΔ1, which does not bind Us11C, was also blocked by Us11C, confirming the conclusion that the critical event is the interaction of Us11 with PKR, not with PACT. It remains to be seen whether the observed interaction of Us11 with PACT affects its PKR-independent cellular functions, if any.
Acknowledgments
We thank Judy Drazba for advice on fluorescence microscopy, Mark Kader for experimental assistance, and Karen Toil for secretarial help.
This work was supported by National Institutes of Health grants CA-62220 and CA-68782 to G.C.S. G.A.P. was supported, in part, by training grant DK-07678.
REFERENCES
- 1.Benkirane, M., C. Neuveut, R. F. Chun, S. M. Smith, C. E. Samuel, A. Gatignol, and K. T. Jeang. 1997. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. EMBO J. 16:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bischoff, J. R., and C. E. Samuel. 1985. Mechanism of interferon action. The interferon-induced phosphoprotein P1 possesses a double-stranded RNA-dependent ATP-binding site. J. Biol. Chem. 260:8237-8239. [PubMed] [Google Scholar]
- 3.Carpick, B. W., V. Graziano, D. Schneider, R. K. Maitra, X. Lee, and B. R. G. Williams. 1997. Characterization of the solution complex between the interferon-induced, double-stranded RNA-activated protein kinase and HIV-I trans-activating region RNA. J. Biol. Chem. 272:9510-9516. [DOI] [PubMed] [Google Scholar]
- 4.Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus Us11 protein effectively compensates for the γ134.5 gene if present before the activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620-8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassady, K. A., and M. Gross. 2002. The herpes simplex virus type 1 Us11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J. Virol. 76:2029-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to γ134.5, a gene non-essential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 8.Chou, J., and B. Roizman. 1992. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5 mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu, W. M., D. Ostertag, Z. W. Li, L. Chang, Y. Chen, Y. Hu, B. Williams, J. Perrault, and M. Karin. 1999. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11:721-731. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, P. A., M. Schwemmle, J. Schickinger, K. Hilse, and M. J. Clemens. 1991. Binding of Epstein-Barr virus small RNA EBER-1 to the double-stranded RNA-activated protein kinase DAI. Nucleic Acids Res. 19:243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens, M. J., and A. Elia. 1997. The double-stranded RNA-dependent protein kinase PKR: structure and function. J. Interferon Cytokine Res. 17:503-524. [DOI] [PubMed] [Google Scholar]
- 13.Cosentino, G. P., S. Venkatesan, F. C. Serluca, S. R. Green, M. B. Mathews, and N. Sonenberg. 1995. Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proc. Natl. Acad. Sci. USA 92:9445-9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuddihy, A. R., A. H. Wong, N. W. Tam, S. Li, and A. E. Koromilas. 1999. The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene 18:2690-2702. [DOI] [PubMed] [Google Scholar]
- 15.D'Acquisto, F., and S. Ghosh. 2001. PACT and PKR: turning on NF-κB in the absence of virus. Sci. STKE 89:1-4. [DOI] [PubMed] [Google Scholar]
- 16.Davies, M. V., H. W. Chang, B. L. Jacobs, and R. J. Kaufman. 1993. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 67:1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green, S. R., and M. B. Mathews. 1992. Two RNA-binding motifs in the double-stranded RNA-activated protein kinase, DAI. Genes Dev. 6:2478-2490. [DOI] [PubMed] [Google Scholar]
- 18.Gunnery, S., A. P. Rice, H. D. Robertson, and M. B. Mathews. 1990. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded-RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 87:8687-8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by the double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imani, F., and B. L. Jacobs. 1988. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 σ3 protein. Proc. Natl. Acad. Sci. USA 85:7887-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iordanov, M. S., J. M. Paranjape, A. Zhou, J. Wong, B. R. Williams, E. F. Meurs, R. H. Silverman, and B. E. Magun. 2000. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol. Cell. Biol. 20:617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito, T., M. Yang, and W. S. May. 1999. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J. Biol. Chem. 274:15427-15432. [DOI] [PubMed] [Google Scholar]
- 23.Kawagishi-Kobayashi, M., J. B. Silverman, T. L. Ung, and T. E. Dever. 1997. Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2α. Mol. Cell. Biol. 17:4146-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar, A., J. Haque, J. Lacoste, J. Hiscott, and B. R. Williams. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc. Natl. Acad. Sci. USA 91:6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, A., Y. L. Yang, V. Flati, S. Der, S. Kadereit, A. Deb, J. Haque, L. Reis, C. Weissmann, and B. R. Williams. 1997. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 16:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, T. G., J. Tomita, A. G. Hovanessian, and M. G. Katze. 1990. Purification and partial characterization of a cellular inhibitor of the interferon-induced protein kinase of Mr 68,000 from influenza virus-infected cells. Proc. Natl. Acad. Sci. USA 87:6208-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maclean, C. A., F. J. Rixon, and H. S. Marsden. 1987. The products of gene Us11 of herpes simplex virus type 1 are DNA-binding and localize to the nucleoli of infected cells. J. Gen. Virol. 43:1921-1937. [DOI] [PubMed] [Google Scholar]
- 28.Mohr, I., and Y. Gluzman. 1996. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 15:4759-4766. [PMC free article] [PubMed] [Google Scholar]
- 29.Mulvey, M., J. Poppers, A. Ladd, and I. Mohr. 1999. A herpesvirus ribosome associated, RNA-binding protein confers a growth advantage upon mutants deficient in a GADD34-related function. J. Virol. 73:3375-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanduri, S., B. W. Carpick, Y. Yang, B. R. Williams, and J. Qin. 1998. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J. 17:5458-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel, C. V., I. Handy, T. Goldsmith, and R. C. Patel. 2000. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase PKR. J. Biol. Chem. 275:37993-37998. [DOI] [PubMed] [Google Scholar]
- 32.Patel, R. C., and G. C. Sen. 1992. Identification of the double-stranded RNA-binding domain of the human interferon-inducible protein kinase. J. Biol. Chem. 267:7671-7676. [PubMed] [Google Scholar]
- 33.Patel, R. C., and G. C. Sen. 1998. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 17:4379-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel, R. C., P. Stanton, N. M. McMillan, B. R. Williams, and G. C. Sen. 1995. The interferon-inducible double-stranded RNA-activated protein kinase self-associates in vitro and in vivo. Proc. Natl. Acad. Sci. USA 92:8283-8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel, R. C., P. Stanton, and G. C. Sen. 1996. Specific mutations near the amino terminus of double-stranded RNA-dependent protein kinase (PKR) differentially affect its double-stranded RNA binding and dimerization properties. J. Biol. Chem. 271:25657-25663. [DOI] [PubMed] [Google Scholar]
- 36.Peters, G. A., R. Hartmann, J. Qin, and G. C. Sen. 2001. Modular structure of PACT: distinct domains for binding and activating PKR. Mol. Cell. Biol. 21:1908-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters, G. A., J. Poppers, I. Mohr, and G. C. Sen. 2001. PACT-mediated PKR activation is blocked by HSV-1 Us11 protein. J. Interferon Cytokine Res. 21(Suppl. 1):S129. [Google Scholar]
- 38.Poppers, J., M. Mulvey, D. Khoo, and I. Mohr. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74:11215-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein Us11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roller, R. J., L. L. Monk, D. Stuart, and B. Roizman. 1996. Structure and function in the herpes simplex virus 1 RNA-binding protein Us11: mapping of the domain required for ribosomal and nucleolar association and RNA binding in vitro. J. Virol. 70:2842-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romano, P. R., M. T. Garcia-Barrio, X. Zhang, Q. Wang, D. R. Taylor, F. Zhang, C. Herring, M. B. Mathews, J. Qin, and A. G. Hinnebusch. 1998. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2α kinases PKR and GCN2. Mol. Cell. Biol. 18:2282-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 43.Williams, B. R. 1999. PKR, a sentinel kinase for cellular stress. Oncogene 18:6112-6120. [DOI] [PubMed] [Google Scholar]
- 44.Wong, A. H., N. W. Tam, Y. L. Yang, A. R. Cuddihy, S. Li, S. Kirchhoff, H. Hauser, T. Decker, and A. E. Koromilas. 1997. Physical association between STAT1 and the interferon-inducible protein kinase PKR and implications for interferon and double-stranded RNA signaling pathways. EMBO J. 16:1291-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasukawa, T., C. Kanei-Ishii, T. Maekawa, J. Fujimoto, T. Yamamoto, and S. Ishii. 1995. Increase of solubility of foreign proteins in Escherichia coli by coproduction of the bacterial thioredoxin. J. Biol. Chem. 270:25328-25331. [DOI] [PubMed] [Google Scholar]
- 46.Zamanian-Daryoush, M., T. H. Mogensen, J. A. DiDonato, and B. R. Williams. 2000. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol. Cell. Biol. 20:1278-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]