Abstract
Polymers play an important role in many biological systems, so a fundamental understanding of their cross-links is crucial not only for the development of medicines but also for the development of biomimetic materials. The biomechanical movements of all mammals are aided by tendon fibrils. The self-organization and biomechanical functions of tendon fibrils are determined by the properties of the cross-links between their individual molecules and the interactions among the cross-links. The cross-links of collagen and proteoglycan molecules are particularly important in tendons and, perhaps, bone. To probe cross-links between tendon molecules, we used the cantilever tip of an atomic force microscope in a pulling setup. Applying higher forces to rat tail tendon molecules with the tip led to a local disruption of the highly organized shell of tendon fibrils and to the formation or an increase of a polymer brush of molecules sticking out of the surface. The cross-linking between these molecules was influenced by divalent Ca2+ ions. Furthermore, the molecules of the polymer brush seemed to bind back to the fibrils in several minutes. We propose that sacrificial bonds significantly influence the tendon fibrils' self-organization and self-healing and therefore contribute to toughness and strength.
INTRODUCTION
Polymerized structures feature heavily in the material sciences and in biology—most structural components of mammals contain polymers of proteins and glycoconjugates. One of the most abundant structural proteins in mammals is collagen, which is present in many tissues including not only tendons but also bone, ligaments, and the basement membrane of the skin. The interactions between individual polymer molecules of tendons, and thus their mechanical properties, are important for the development of new materials based on such biological polymers and for the treatment collagen-related diseases. Elucidating collagen and associated polymer properties is crucial for an understanding of the structure and functional mechanisms of biocomposites, such as bone and cartilage, on a single-molecule and microscopic level.
The basic structural unit of a tendon is the collagen molecule conjoined with proteoglycans (1). Three of the left-handed coiled collagen molecules form a right-handed coiled triple helix, called tropocollagen (2). In the case of type I collagen, the coil consists of two α1 chains and one α2 chain. For the structural organization of tropocollagen into sub- and/or microfibrils, different models are discussed in the literature. However, it is agreed that a number of sub and/or microfibrils form a single collagen fibril. Type I collagen fibrils are the best-organized structures of all collagen types, so most of the research on structure and mechanical properties of collagen has been done on this collagen. Type I collagen fibrils in their native form typically display a banding pattern of ∼67 nm spacing, called the D-period, when visualized with transmission electron microscopy (3,4) or atomic force microscopy (AFM) (5–7). The composition of the structural organization resulting in 67 nm banding is still a source of discussion.
Tendons are described as exceptionally designed for elastic-energy storage and adequately designed for strength and toughness (8). Therefore, the function of collagen fibrils is of general interest for material researchers. Besides collagen, other molecules, like proteoglycans (9), also influence the mechanical properties of tendons. It has been proposed that proteoglycans are important for the gliding in the matrix between fibrils (10). However, little is known about interactions between collagen and proteoglycans. All common models are based on the assumption of laterally homogeneous close-packing of a collagen fibril; we recently showed that a collagen fibril is an inhomogeneous structure composed of a relatively hard shell and a softer core (7). Thus, the mechanical properties of the whole fibril depend on the properties of its shell and core.
To investigate the formation of collagen fibrils and the formation and properties of the cross-links between individual tropocollagens, various experiments have been conducted. M. C. Goh and her group investigated the formation of self-assembled collagen fibrils (11,12). Other groups have used chemicals or enzymes to disassemble collagen fibrils. The development of single molecule force spectroscopy with the AFM (13–17) has given us a new tool to investigate the interaction forces involved in collagen fibril interactions. We previously used the AFM to pull individual molecules out of rat tail tendons (18). The AFM can also be used to study polymer adhesion (16,19).
Here, we show that the tip of the cantilever of an AFM can be used to locally disrupt the surface of a rat tail tendon collagen fibril. We believe that this disruption can, in some cases, lead to a “polymer brush”, or to an increase of a natural brush originating from the tendon, and can also lead to an attachment of molecules to the tip of the cantilever. The mechanical and adhesive properties of the polymer molecules sticking out of tendon fibrils can be probed with the AFM. The adhesion between the individual molecules is influenced by the divalent ion Ca2+ and thus we propose the existence of “sacrificial bonds” (20) between the polymer molecules like the Ca2+-dependent bonds observed by Thompson et al. (21) on bone.
Thompson et al. (21) demonstrated increased energy dissipation in successive pulls of the same molecule of a commercial collagen preparation or of the components of bone if divalent ion Ca2+ were present in a buffer. They attributed this to the formation of sacrificial bonds involving polymer molecules with negatively charged groups and the Ca2+ ions. They did not investigate the probability of picking up one or more molecules under different buffer conditions. And they did not test to see if molecules could be picked up without touching the fibril surface. In this study, an exchange of 40 mM Na+ by 40 mM Ca2+ led to an increased probability of picking up molecules of the whole fibrils. This effect could result from the exchange of mono- against divalent ions, which also led to an increase of the ionic strength from 150 mM to 230 mM.
Here, we show that the sacrificial bonds that we see above tendons are most likely due to cross-linking between the molecules and a recapturing of the tip into a polymer brush, and not due to refolding of individual polymers permanently attached to the tip.
MATERIALS AND METHODS
Tails from rats sacrificed for other experiments were frozen at −20°C typically for weeks before our experiments. Rat tail tendons were removed from thawed rat tails and stored in a buffer containing 150 mM NaCl and 2 mM Tris adjusted to a pH of 7.4 for several hours before sample preparation. Fibrils from these tendons were prepared wet on a glass disk and dried with a stream of filtered air. To properly control preparation, they were then imaged with an MFP3D (Asylum Research, Santa Barbara, CA) or a Multimode Nanoscope IV equipped with PicoForce (Veeco/Digital Instruments, Santa Barbara, CA) in direct current (DC) or alternating current (AC) mode using commercial cantilevers (CSC12, MikroMasch, Portland, OR; NSG11, NT-MDT, Moscow, Russia). The images showed the surface of the collagen fibrils with the typical banding structure as described above. The dried tendons were cut into pieces (∼7 mm long) and both their ends glued to the glass disk with epoxy (2-Ton Clear Epoxy, Devcon, Danvers, MA). The tendons were rehydrated in four different buffers, which all contained 2 mM Tris and were adjusted to a pH of 7.4. The buffers further contained i), 150 mM NaCl, 5 mM EDTA; ii), 150 mM NaCl; iii), 150 mM NaCl, 1 mM CaCl2; and iv), 110 mM NaCl, 40 mM CaCl2. Buffer (i) was used to avoid all effects of divalent ions in the sample or di-water; buffer (iii) resembles the physiological NaCl and CaCl2 concentrations, and buffers (ii) and (iv) were those used in the earlier work on bone (21). The idea was to search for a, perhaps subtle, calcium ion dependence by using one buffer with a much higher than physiological calcium level, 40 mM, and one with a much lower than physiological calcium level, 0 mM. Buffer (ii) had an ionic strength of 150 mM, buffer (iii) of 153 mM, and buffer (iv) of 230 mM. All experiments were conducted at a constant room temperature of 20°C.
The molecule-pulling experiments were conducted using BioLever (Olympus, Tokyo, Japan) and two different AFMs—the MFP3D and the MultiMode PicoForce. Both systems were controlled in the z-position. The spring constant of the cantilevers were thermally calibrated, and the distances (see Fig. 2) were corrected by the deflection of the cantilever.
FIGURE 2.
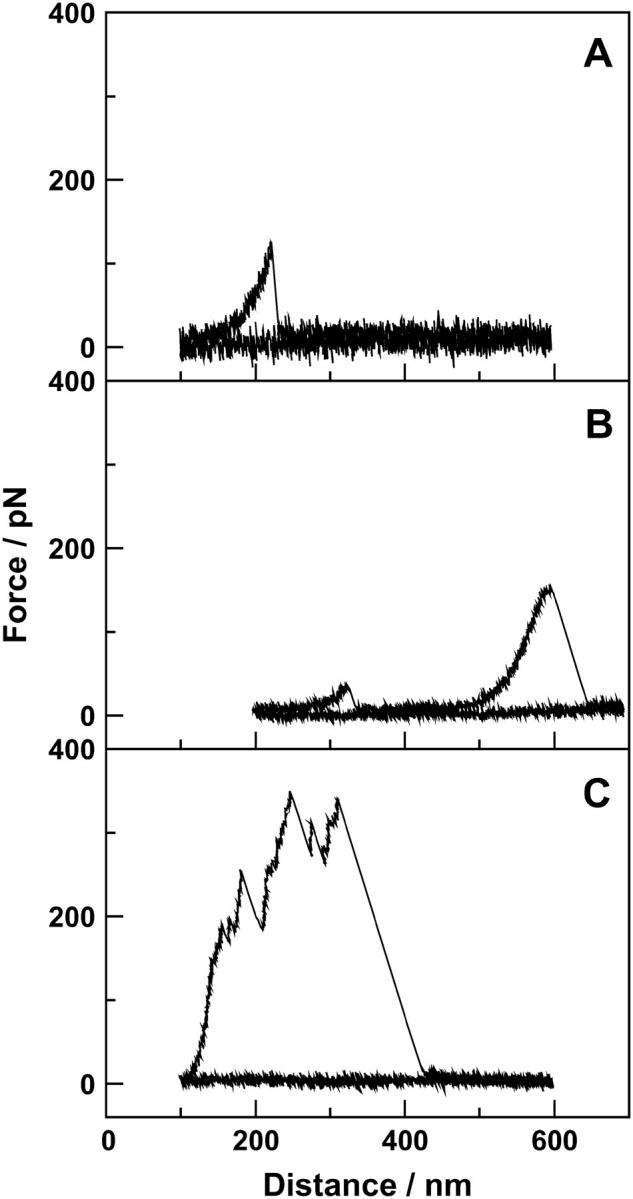
Force spectroscopy of a polymer brush from rat tail tendon fibrils. More than 95% of the curves only show the breakage of one (A) or two (B) bonds between the sample surface and the cantilever tip; ∼5% of the curves show the breakage of a whole bundle of molecules (C). The three curves show experiments on whole (not mechanically smashed) rat tail tendon fibrils with (A) a distance of 100 nm between surface and tip and a dwell time of 3 s in a buffer containing 110 mM NaCl, 40 mM CaCl2, 2 mM Tris, pH 7.4; (B) a distance of 200 nm between surface and tip and a dwell time of 5 s in a buffer containing 110 mM NaCl, 40 mM CaCl2, 2 mM Tris, pH 7.4; and (C) a distance of 100 nm between surface and tip and a dwell time of 10 s in a buffer containing 110 mM NaCl, 40 mM CaCl2, 2 mM Tris, pH 7.4.
The experiments were conducted in the following manner (Fig. 1):
After calibrating the cantilever, we brought the tip into contact with the surface of the tendon, thereby determining the position of the surface in the z direction (Fig. 1, step 1). The force applied on the cantilever was between 2 and 3 nN.
We moved the cantilever far off the surface to break all bonds between molecules and the cantilever tip (Fig. 1, step 2).
Then, we moved the cantilever closer to the surface with a velocity of 1 μm/s and let it hover for a dwell time t with a distance d above the surface (Fig. 1, step 3).
We pulled the cantilever a certain distance away from the surface with a velocity of 1 μm/s while recording the deflection data for the cantilever. The pulling length was selected to break any adhesion between the surface and the cantilever tip (Fig. 1, steps 4, 5, and 6).
We repeated steps c and d for an overall time period of T.
FIGURE 1.
Schematic of the force spectroscopy experiments. The insets show schematic force versus distance curves and the dot labels the position of the tip of the cantilever in the illustrations of the cantilever and sample. The parameter d in the curves is the distance between the tip and the base of the polymer brush. Sizes of cantilever and polymer brush are not to scale: the thickness of the polymer brush is exaggerated for clarity.
All data points reflected the probability that one or more bonds were broken within a block of 50 individual pulling experiments.
The rat tail tendons were used as untreated samples (whole fibril) and as smashed samples (smashed fibril), where the fibrils were mechanically ground with a glass slide.
RESULTS AND DISCUSSION
Fig. 2 shows three examples of force spectroscopy curves from the experiments on rat tail tendon. Thousands were made, but these are good representatives of the three general types of curves observed. Each figure shows two curves: one approach and one pulling. The approach curve is always flat (besides the noise level) and serves as a baseline for the experiment. Fig. 2 A shows a force spectroscopy experiment at a starting point of 100 nm above the surface and a dwell time close to the surface of 3 s. In this case, there is one rupture event at a pulling distance of ∼230 nm. Fig. 2 B shows an experiment with a starting point of 200 nm above the surface and a dwell time of 5 s. As can be seen from the curve, there are two rupture events, one at 330 nm and one at 600 nm pulling distance. The type of curves seen in Fig. 2, A and B, were observed in 95% of experiments. Fig. 2 C shows a force spectroscopy curve with a starting point 100 nm above the surface and a dwell time of 10 s. In this case there were multiple rupture events between 150 and 300 nm pulling distance. This type of curve was only seen in ∼5% of the experiments.
Each of the events seen in Fig. 2, A and B, seems to represent the rupture event of the attachment of single molecules between the surface and fibril, between the tip and fibril, or between two molecules. If the force spectroscopy in Fig. 2, A and B, represents the stretch and rupture of single molecules, the shape of curves corresponds to what is known as the entropic spring—the work done against entropy to stretch the molecule. The model that describes such a spring in the case of proteins is known as the wormlike chain (WLC) model (22). The WLC model has been successfully used to model the entropic force effect for many proteins such as titin (23,24), tenascin (25), bacteriorhodopsin (26), DNA (27), myelin basic protein (28), and protomers of the hexagonally packed intermediate layer (29). For a persistence length of 0.5 nm, we calculated a contour length of 252 and 257 ± 1 nm for the initial events in Fig. 2, A and B, respectively. Because the exact positions where the molecules linked to the surface or to other molecules or where they bound to the tip were not known, the values for the persistence and the contour length should probably not be given too much weight.
Fig. 2 C shows the force curve of an experiment with multiple rupture events in quick succession. This indicates the presence of multiple linkages between entangled molecules and within single cross-linked molecules that were broken before molecules were stretched to complete rupture. The distance between the rupture events in this curve and in curves similar to it did not seem to have any reproducible values, as it does in the case of titin.
Whether it was the linkage between surface and fibril, between fibril and tip, or between two fibrils to rupture is not evident from the curves seen in Fig. 2. It is likely that a few molecules became permanently attached to the tip during the experiments. This was verified through the following control experiment: a cantilever that had been used in the force spectroscopy experiments on tendon was tested on a freshly cleaved mica surface. Rupture events in the force spectroscopy experiments on the mica could be observed showing that some molecules were permanently attached to the tip (experiments not shown here). The molecules were evidently protruding far enough from the tip to attach to the mica surface.
That we are able to see rupture events even though the tip and surface have not been in contact suggests that polymer molecules are protruding from the surface and/or the tip and are able to attach themselves to each other or the opposite surface. The position and the number of rupture events were random, suggesting that a number of molecules were involved in the attachment between surface and tip and that these molecules were sticking out from the surface like a brush.
Fig. 3 shows the result from many curves like the ones in Fig. 2. Here we have plotted the likelihood of rupture events (one or more) and thus the binding probability as function of the hover distance starting at 100 nm above the surface to 900 nm above the surface. We did this with molecules from two different fibril types—whole tendon fibrils in the four different buffers and tendon fibrils smashed with the edge of a glass slide before the force spectroscopy measurements in buffer (ii). Each of the marked points on the curves represents the percentage of 50 curves having one or more rupture events (events similar to the ones shown in Fig. 2). For all five curves, it is evident that the likelihood of rupture events decreased with increasing hover distance to the surface, d, to zero probability when d reached 500 nm. One collagen molecule has a length of ∼300 nm, thus, it is likely not only that single molecules can protrude from the surface but also assemblies of collagen and proteoglycan molecules or that one molecule attaches to the surface and another to the cantilever tip. It is also clear from the experiments that the likelihood for rupture events in the case of smashed fibrils is much larger than for the whole fibrils. The whole fibrils showed an increasing binding probability with increasing amounts of CaCl2: in the presence of EDTA it was lowest and highest for the buffers containing 1 or 40 mM CaCl2. The difference between 1 and 40 mM CaCl2 was not significant, and thus we propose that the physiological concentration of 1 mM was already enough to occupy all important Ca2+ binding sites.
FIGURE 3.
Probability that one or more bonds were formed between molecules and tip decreases with the distance and depends on the presence of Ca2+. The experiments were performed with whole and smashed rat tail tendon collagen fibrils, a surface dwell time of t = 3 s, and four different buffers containing 150 mM NaCl and 5 mM EDTA; 150 mM NaCl and 0 mM CaCl2; 150 mM NaCl and 1 mM CaCl2; and 110 mM NaCl and 40 mM CaCl2. All buffers contain 2 mM Tris at pH 7.4.
To prove that a mechanical treatment of a fibril can lead to the formation of a polymer brush, we used a glass slide to smash the fibrils. Fig. 3 shows that the probability of binding events increased and even at distances of 600 nm binding events were observed.
Fig. 4 shows the binding probability as a function of the dwell time: that is, the time that the tip was kept at a fixed distance from the surface to pick up molecules. The figure supports the polymer brush model: in the range of 0 to 10 s a longer surface dwell time leads to an increase in the binding probability for molecules to bind to the tip and be detected during the next force spectrum. A longer dwell time of 20 s led to a decrease in the binding probability due to a second overlaying effect which will be discussed later. It could not be distinguished whether a saturation of the curve already occurred or not.
FIGURE 4.
Probability that one or more bonds were formed between molecules and tip increases with longer surface dwell times. The experiments were performed using whole rat tail tendon fibrils, a distance between tip and surface of d = 100 nm, and a buffer containing 110 mM NaCl, 40 mM CaCl2, 2 mM Tris, pH 7.4.
To study the polymer brush further, we tried to induce disruption of the tendon fibril locally, similar to what we did with the glass slide on a more extended scale. We disrupted the surface of the fibril locally with the tip itself by forcing the tip into the surface and quickly pulling it out. We repeated this until the surface of the fibril under the tip was damaged. The typical force used was 2–3 nN. The likelihood of rupture events increased with increasing force applied to the fibrils. However, it was not clear if the brush was present on the whole tendon fibril and was only increased by the tip-fibril interaction or if it was induced by the damaging of the fibril surface by the tip.
A related question is what happens if the binding probability studies are conducted for a long period of time over the same disrupted spot. Each marked point in Fig. 5 represents the percentage of 50 curves having one or more rupture events. It is clear that the binding probability decreases as the overall time of the experiment increases. The decrease in the probability (Fig. 5) can be fitted by an exponential function with a time constant of ∼34 ± 6 min. A reason an exponential functions fits to the data cannot be given. However, the observation that the probability decreased has three possible reasons: i), there is a passivation of the tip due to adsorption of molecules from the solution, ii), continuous pulling removes molecules from the fibril brush, or iii), the molecules of the fiber brush bind back to the collagen fibril. To investigate which of the possibilities was responsible for the decrease, the following experiment was performed: after 50 pulling curves, the continuous pulling was stopped for 30 min and then continued. If (ii) was the reason for the decrease of the probability with time, the probability to pick up molecules should be approximately the same for the time point after the 30 min interruption and for the next 50 pulling curves without the interruption. In contrast to this, the assumption (iii) that the molecules of the brush bind back to the fibril predicts that an interruption would not change the run of the exponential curves because the rebinding would constantly take place without doing the pulling experiments. Thus, the probability after the interruption should be comparable to the value observed for the 50 pulling curves at this time point of the experiment with continuous pulling. As can be seen in Fig. 5, the probability to pick up a molecule after the interruption is close to zero. Because all following probabilities were zero or close to zero, for clarity we did not plot these data in Fig. 5. The later possibility (iii) describes the effect we observed; no interruption and an interruption in the pulling experiments led to the same decrease in the probability of binding events. Possibility (i) could also explain the results; however, the control experiments utilizing a used tip on bare mica showing similar rupture events demonstrate that a complete passivation of the tip is unlikely. Thus, the most likely explanation is that the molecules of the tendon can bind back to the fibril, which might be called a “self-healing” of the fibrils. In the case where molecules are permanently attached to the tip of the cantilever, they might bind with an increased number of binding sites to the tip. This “self-healing” supports the idea that sacrificial bonds play an important role in the organization of tendons.
FIGURE 5.
Probability that one or more bonds were formed between molecules and the tip decreases with the overall time of the experiment. One data point reflects the probability within a block of 50 pulls starting with the first pull at the given time. The experiment was performed (▪) with no interruption between the blocks of 50 pulls and (•) with a break of 30 min between the first and the second block. The solid line is an exponential fit to the (▪) data points with a time constant of 34 ± 6 min. The experiments were performed using whole rat tail tendon fibrils, a distance between tip and surface of d = 100 nm, a surface dwell time of t = 10 s, and a buffer containing 110 mM NaCl, 40 mM CaCl2, 2 mM Tris, pH 7.4.
Because tendon is a composite material of collagen fibrils embedded in a proteoglycan-rich matrix (1), it is not clear if the molecules sticking out of its fibrils are collagen or other molecules. Raspanti et al. showed that native collagen fibrils are covered with irregular blobs or with filamentous material (30). It could be that the molecules we pulled consisted solely of this filamentous material, but they were most likely a combination of collagen and the filamentous material that Raspanti et al. interpret as proteoglycan. For the interpretation of our data, this knowledge is not essential because we describe the properties of the molecules of the whole tendon. On the basis of our data, it is not possible to distinguish whether the rupture events originate from intermolecular interactions between molecules from the fibril and molecules attached to the tip or from polymer surface interaction between molecules from the fibril and the surface of the tip. Probably both effects contribute to the observed pulling curves because control experiments showed a permanent binding of molecules to the cantilever tip. One possible way to solve this problem is to glue collagen fibrils to the tip, which requires the development of new experimental procedures.
Ca2+ ions appear to increase cross-linking of the molecules in our measurements as observed previously by Thompson et al. (21) on bone. Thus, the details of the electrostatic interaction between the molecules are important and should be investigated further in systems that are better defined than smashed fibrils. In studies using defined polymers, it was already shown that the electrostatic interactions between polyelectrolytes and substrates is influenced by the line-charge density of the polymer, the charge density of the substrate, and the presence of calcium in the buffer (19,31).
In summary, we have demonstrated new aspects of the nature of sacrificial bond–hidden length systems: specifically that these sacrificial bonds can be formed if polymer brushes are present, even if the tip and sample do not come into contact. Of the two possibilities suggested in an earlier article (20) that introduced the concept of sacrificial bonds, our measurements here favor “…successive release of sacrificial interchain bonds holding a cross-linked multi-chain matrix together” rather than “the successive opening of intrachain loops or folded domains within a single molecule” for the tendons we studied here. The molecules can be conceived of as functioning as nanoscale polymer Velcro: if many elastic strips of Velcro with, for example, loops were attached at one end to a surface (to simulate a polymer brush above a collagen fibril surface) and another surface with hooks (to simulate a tip) was brought nearby, then in the presence of shaking (to simulate thermal motion) the two surfaces could become bound together if they were brought close enough together—even if they did not come into direct contact. And these bonds would break successively as the surfaces were separated, simulating the pulling curves presented here.
A more sophisticated model, to simulate the interactions that could occur between two collagen fibrils coated with polymer brushes, in the presence of calcium ions, would be two surfaces (to simulate the two fibrils), each with many elastic strips of Velcro with, for example, loops (to simulate the polymer brushes) were submerged into a fluid that had many suspended disks with both sides covered with hooks (to simulate the divalent calcium ions). The disks would bind to the loops of the elastic strips and, in some cases, bind together strips dangling from one surface with strips dangling from the other surface—even if the surfaces were never brought into contact. In this model the surfaces would then be bound together with a sacrificial bonds-hidden length mechanism (20), and it would require substantial work to separate them. In the real case of two collagen fibrils in a tendon, this mechanism could provide strong and tough adhesion between the fibrils. We have also shown that tendon seems to heal itself after being disrupted locally—this has clear implications for the understanding of tendon and bone mechanics.
Acknowledgments
We thank Herbert Waite and Daniel E. Morse for helpful discussions. We are grateful to Diane McClure and Amber Griffin of the campus animal resource center for saving rat tails for us from rats sacrificed for other experiments. We thank Simcha Frieda Udwin for her extensive and welcome revisions.
This work was supported by the by the National Institutes of Health under Award No. GM65354, the National Science Foundation, through the MRL Program under Award No. DMR00-80034, the NASA/URETI Bioinspired Materials program under award NCC-1-02037, the USARL Institute for Collaborative Biotechnology (ICB) under award DAAD19-03-D-0004, Veeco, the Danish Research Council (STVF), and the Deutsche Forschungsgemeinschaft (project GU 568/2-1).
Thomas Gutsmann and Tue Hassenkam contributed equally to this work.
Thomas Gutsmann's present address is Research Center Borstel, Division of Biophysics, Parkallee 10, 23845 Borstel, Germany.
Tue Hassenkam's present address is Nano-Science Center, University of Copenhagen, Universitetsparken 5, Bygning D, 2100 København Ø, Denmark.
References
- 1.Scott, J. E. 1991. Proteoglycan: collagen interactions and corneal ultrastructure. Biochem. Soc. Trans. 19:877–881. [DOI] [PubMed] [Google Scholar]
- 2.Ramachandra, G. N., and G. Karthan. 1955. Structure of collagen. Nature. 176:593–595. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt, F. D., C. E. Hall, and M. A. Jakns. 1942. Electron microscopy investigations of the structure of collagens. J. Cell. Comp. Physiol. 20:11–33. [Google Scholar]
- 4.Chapman, J. A., and D. J. S. Hulmes. 1984. Electron microscopy of the collagen fibril. In Ultrastructure of the Connective Tissue Matrix. P. M. Motta and A. Ruggeri, editors. Kluwer Academic Publishers, Boston. 1–33.
- 5.Baselt, D. R., J. P. Revel, and J. D. Baldeschwieler. 1993. Subfibrillar structure of type I collagen observed by atomic force microscopy. Biophys. J. 65:2644–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revenko, I., F. Sommer, D. T. Minh, R. Garrone, and J. M. Franc. 1994. Atomic force microscopy study of the collagen fibre structure. Biol. Cell. 80:67–69. [DOI] [PubMed] [Google Scholar]
- 7.Gutsmann, T., G. E. Fantner, M. Venturoni, A. Ekani-Nkodo, J. B. Thompson, J. H. Kindt, D. E. Morse, D. K. Fygenson, and P. K. Hansma. 2003. Evidence that collagen fibrils in tendons are inhomogeneously structured in a tubelike manner. Biophys. J. 84:2593–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gosline, J., M. Lillie, E. Carrington, P. Guerette, C. Ortlepp, and K. Savage. 2002. Elastic proteins: biological roles and mechanical properties. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cribb, A. M., and J. E. Scott. 1995. Tendon response to tensile stress: an ultrastructural investigation of collagen:proteoglycan interactions in stressed tendon. J. Anat. 187:423–428. [PMC free article] [PubMed] [Google Scholar]
- 10.Puxkandl, R., I. Zizak, O. Paris, J. Keckes, W. Tesch, S. Bernstorff, P. Purslow, and P. Fratzl. 2002. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin, A. C., and M. C. Goh. 2002. Investigating the ultrastructure of fibrous long spacing collagen by parallel atomic force and transmission electron microscopy. Proteins. 49:378–384. [DOI] [PubMed] [Google Scholar]
- 12.Paige, M. F., J. K. Rainey, and M. C. Goh. 2001. A study of fibrous long spacing collagen ultrastructure and assembly by atomic force microscopy. Micron. 32:341–353. [DOI] [PubMed] [Google Scholar]
- 13.Florin, E. L., V. T. Moy, and H. E. Gaub. 1994. Adhesion forces between individual ligand-receptor pairs. Science. 264:415–417. [DOI] [PubMed] [Google Scholar]
- 14.Rief, M., F. Oesterhelt, B. Heymann, and H. E. Gaub. 1997. Single molecule force spectroscopy on polysaccharides by atomic force microscopy. Science. 275:1295–1297. [DOI] [PubMed] [Google Scholar]
- 15.Clausen-Schaumann, H., M. Seitz, R. Krautbauer, and H. E. Gaub. 2000. Force spectroscopy with single bio-molecules. Curr. Opin. Chem. Biol. 4:524–530. [DOI] [PubMed] [Google Scholar]
- 16.Senden, T. J., J.-M. di Meglio, and P. Auroy. 1998. Anomalous adhesion in adsorbed polymer layers. Eur. Phys. J. B. 3:211–216. [Google Scholar]
- 17.Janshoff, A., M. Neitzert, Y. Oberdorfer, and H. Fuchs. 2000. Force spectroscopy of molecular systems-single molecule spectroscopy of polymers and biomolecules. Angew. Chem. Int. Ed. Engl. 39:3212–3237. [DOI] [PubMed] [Google Scholar]
- 18.Gutsmann, T., G. E. Fantner, J. H. Kindt, M. Venturoni, S. Danielsen, and P. K. Hansma. 2004. Force spectroscopy of collagen fibers to investigate their mechanical properties and structural organization. Biophys. J. 86:3186–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seitz, M., C. Friedsam, W. Jostl, T. Hugel, and H. E. Gaub. 2003. Probing solid surfaces with single polymers. ChemPhysChem. 4:986–990. [DOI] [PubMed] [Google Scholar]
- 20.Smith, B. L., T. E. Schäffer, M. Viani, J. B. Thompson, N. A. Frederick, J. Kindt, A. Belcher, G. D. Stucky, D. E. Morse, and P. K. Hansma. 1999. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature. 399:761–763. [Google Scholar]
- 21.Thompson, J. B., J. H. Kindt, B. Drake, H. G. Hansma, D. E. Morse, and P. K. Hansma. 2001. Bone indentation recovery time correlates with bond reforming time. Nature. 414:773–776. [DOI] [PubMed] [Google Scholar]
- 22.Bustamante, C., J. F. Marko, E. D. Siggia, and S. Smith. 1994. Entropic elasticity of lambda-phage DNA. Science. 265:1599–1600. [DOI] [PubMed] [Google Scholar]
- 23.Rief, M., M. Gautel, F. Oesterhelt, J. M. Fernandez, and H. E. Gaub. 1997. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 276:1109–1112. [DOI] [PubMed] [Google Scholar]
- 24.Oberhauser, A. F., P. K. Hansma, M. Carrion-Vazquez, and J. M. Fernandez. 2001. Stepwise unfolding of titin under force-clamp atomic force microscopy. Proc. Natl. Acad. Sci. USA. 98:468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberhauser, A. F., P. E. Marszalek, H. P. Erickson, and J. M. Fernandez. 1998. The molecular elasticity of the extracellular matrix protein tenascin. Nature. 393:181–185. [DOI] [PubMed] [Google Scholar]
- 26.Oesterhelt, F., D. Oesterhelt, M. Pfeiffer, A. Engel, H. E. Gaub, and D. J. Muller. 2000. Unfolding pathways of individual bacteriorhodopsins. Science. 288:143–146. [DOI] [PubMed] [Google Scholar]
- 27.Smith, S. B., L. Finzi, and C. Bustamante. 1992. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science. 258:1122–1126. [DOI] [PubMed] [Google Scholar]
- 28.Mueller, H., H. J. Butt, and E. Bamberg. 1999. Force measurements on myelin basic protein adsorbed to mica and lipid bilayer surfaces done with the atomic force microscope. Biophys. J. 76:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller, D. J., W. Baumeister, and A. Engel. 1999. Controlled unzipping of a bacterial surface layer with atomic force microscopy. Proc. Natl. Acad. Sci. USA. 96:13170–13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raspanti, M., T. Congiu, and S. Guizzardi. 2001. Tapping-mode atomic force microscopy in fluid of hydrated extracellular matrix. Matrix Biol. 20:601–604. [DOI] [PubMed] [Google Scholar]
- 31.Friedsam, C., B. A. Del Campo, U. Jonas, H. E. Gaub, and M. Seitz. 2004. Polymer functionalized AFM tips for long-term measurements in single-molecule force spectroscopy. ChemPhysChem. 5:388–393. [DOI] [PubMed] [Google Scholar]






