Abstract
Adult T-cell leukemia (ATL) cells contain integrated human T-cell leukemia virus type 1 (HTLV-1) proviruses. Although the exact sequence of events leading to the development of ATL remains incompletely resolved, expression of the integrated HTLV-1 long terminal repeat (LTR) is likely required at some point during the process of T-cell transformation. While much has been learned about the regulated expression of transiently transfected LTR reporter plasmids, an analysis of factors required for expression of chromosomally integrated HTLV-1 LTR has not been done. Here, we have constructed CHOK1 and HeLa cells that contain an integrated HTLV-1 LTR-luciferase gene. Using these cells, we have compared the requirements for activation of transiently transfected versus stably integrated HTLV-1 LTR. We observed different requirements for CREB, p300, and P/CAF in the expression of transiently transfected versus stably integrated HTLV-1 LTR. Furthermore, with dominant-negative mutants of CREB, p300, and P/CAF, we found that activation of integrated HTLV-1 LTR by an ambient stress signal, UV-C, proceeds through a path mechanistically distinct from that used by viral oncoprotein, Tax. Our findings point to additional complexities in the regulated expression of HTLV-1 proviruses compared with those hitherto revealed through transfection studies.
Human T-cell leukemia virus type 1 (HTLV-1) is the causative agent of adult T-cell leukemia (ATL) (21, 22, 40, 47). The virus infects T cells and, after reverse transcription of its genome, integrates into cellular chromosomes. All HTLV-infected individuals are thus life-long virus carriers. Statistically, the cumulative risk of developing ATL is estimated to be 1 to 4% per 20 years after infection (52). This pattern of disease progression suggests that while viral infection might be the initiating trigger, additional intracellular events occurring over an extended duration contribute ultimately to ATL development (4, 10, 12, 15, 16, 26, 28, 29, 34, 37, 45, 46, 49). Presumably, at some point after virus infection of the cell, expression from the integrated provirus is required to initiate the transformation process.
Because integration into the host chromosome is an obligatory step in the HTLV-1 life cycle, understanding how the viral long terminal repeat (LTR) is regulated when formatted in a chromatin-associated context is important for elucidating the biology of the provirus. Unlike transiently transfected plasmids, the provirus is a surrogate cellular transcriptional unit. It, like eukaryotic cellular DNA, is wrapped with histone (H2A, H2B, H3, and H4) and nonhistone proteins to form nucleosomes (13), which compose the basic units of chromatin. Indeed, it has been shown for human immunodeficiency virus type 1 (HIV-1) that an integrated provirus by virtue of being assembled into functional chromatin (14) differs physically from a transfected viral plasmid. Chromatin-remodeling histone acetyltransferases (HATs) p300 and P/CAF have been found to be critical for the expression of HIV-1 provirus (7, 22, 33). Hence, an emerging notion suggests that expression of integrated human retroviruses requires cellular factors different from those used for transiently transfected plasmids.
The virally encoded 40-kDa Tax protein is a key activator of the HTLV-1 LTR (17). Tax is a transcription factor that potently activates HTLV-1 expression through three copies of 21-bp cyclic AMP-response elements (CREs) (9, 18, 24, 25) found in the LTR. In model cell culture systems, expression of Tax is sufficient for transforming human and rodent cells (19, 42-45, 52, 53). However, it is noteworthy that many in vivo ATL cells do not synthesize detectable Tax protein (39). Hence, in ATL cells, some degree of physiological expression from the provirus likely occurs through a Tax-independent pathway or pathways. However, this does not exclude that when fully transformed, ATL cells may not need to express HTLV at all and that Tax may function primarily to initiate transformation without being required to maintain transformation.
Studies with transiently transfected reporter plasmids have persuasively shown that three cellular transcription factors, CRE binding protein (CREB), p300/CREB-binding protein (CBP), and p300/CBP-associated factor (P/CAF), play crucial roles in HTLV-1 LTR-directed expression (1, 5, 8, 10, 27, 30, 32, 54, 57). Moreover, the HTLV-1 Tax protein has been found to bind each of these factors independently (20, 27, 47). Despite these insights, no study has addressed whether these findings from transiently transfected LTR plasmids also apply to stably integrated HTLV-1 LTR. Here, for both human (HeLa) and rodent (CHOK1) cells, we report that the expression of stably integrated and transiently transfected HTLV-1 LTRs has different requirements for CREB, p300, and P/CAF. We also show that activation of stably integrated HTLV-1 LTR by an environmental stress signal (i.e., UV-C) proceeds in a manner mechanistically distinct from that used by Tax for activation.
MATERIALS AND METHODS
Cell lines.
CHOK1 and HeLa cells were propagated at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and l-glutamine. Stable transfectants were maintained in the medium described above plus 500 μg of G418 per ml (Geneticin; Invitrogen, Carlsbad, Calif.).
Plasmids.
pHpX (36), pRSV-neo (41), and the HTLV-1 LTR-luciferase (LTR-luc) plasmid pHTLV-luc (31) were described previously. pHOOK-1 was obtained from Invitrogen (Carlsbad, Calif.), and pCMV-βGAL was obtained from Clontech (Palo Alto, Calif.). pCI-P/CAF deletion mutants were produced by digestion with the appropriate restriction enzymes followed by in-frame ligation. Other plasmids were kind gifts from R. H. Goodman (pRSV-CREB and pRSV-KCREB) (55) and Y. Nakatani (Flag-tagged plasmids pCI-p300, pCI-p300ΔHAT, pCI-P/CAF, and pCI-P/CAF ΔHAT).
Construction of stable cell lines.
CHOK1 and HeLa cells were cotransfected with 10 μg of pHTLV-luc plasmid and 1 μg of pRSV-neo plasmid by using Lipofectamine (Invitrogen). Two days after transfection, cells were replated into medium containing 500 μg of G418 per ml. After 14 days of G418 selection, colonies were isolated with cloning cylinders. Independent colonies were confirmed for integrated HTLV-1 LTR-luc by luciferase assay and Southern hybridization.
Transient transfection and luciferase assay.
For assay of transiently transfected LTR, cells were transfected with Lipofectamine with Plus reagent (Invitrogen). For assay of stably integrated LTR, we used two pools of cells consisting of equal numbers of cells from three independently selected cell clones. A typical transfection included 0.2, 1, or 3 μg of pRSV-CREB, pCI-p300, or pCI-P/CAF with 0.1 μg of pCMV-βGAL and 3 μg of pHOOK. Cells were harvested 48 h after transfection. Successfully transfected HTLV-1 LTR-luc cells were selected with pHOOK according to the manufacturer's protocol. Briefly, cells were harvested and incubated with Capture-Tec beads (Invitrogen) at 37°C for 30 min. After incubation, cells were washed and selected for HOOK-positive cells by using a Capture-Tec magnetic stand (Invitrogen, Carlsbad, Calif.). Luciferase activity was measured with an Optocomp II luminometer (MGM Instruments, Hamden, Conn.). Normalization was based on β-galactosidase activity measured with Galacto-Star (Tropix, Bedford, Mass.). All luciferase values represent averages ± standard deviations from at least three independent transfections. Statistically signficant values were assessed with the t test.
Southern hybridization.
Genomic DNA was extracted from cells and restricted with EcoRI. Ten micrograms of digested DNA was electrophoresed and blotted onto nylon membrane. Hybridization was performed with a radiolabeled probe, which consisted of the luciferase open reading frame (NcoI-XbaI fragment) excised from pGL3-Basic vector (Promega, Madison, Wis.).
Western blotting.
Whole-cell extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to polyvinylidene difluoride membrane by semidry electroblotting (Millipore, Bedford, Mass.) at 1.2 mA/cm2 for 1 h. Membranes were incubated for 1 h with primary antibody, washed, and incubated for another hour with the appropriate alkaline phosphatase-conjugated secondary antibody (Tropix). Proteins were visualized by chemiluminescence (Tropix) according to the manufacturer's protocol.
UV-C irradiation.
For UV-C irradiation, cells after removal of culture medium were exposed in situ directly to UV (100 J/m2 at a wavelength of 254 nm) by using UV-Stratalinker (Stratagene, La Jolla, Calif.). Culture medium was immediately replaced after irradiation.
RESULTS
Construction of human and hamster cells that contain integrated HTLV-1 LTR-luc.
Previous studies on the activation of the HTLV-1 LTR have relied largely on transient transfection of LTR plasmids into cells. Transiently introduced LTR plasmid has a conformation different from integrated proviruses in infected or transformed cells. Because we wished to understand how integrated viral LTRs might be transcriptionally regulated, we constructed human and hamster cells that contain stably integrated HTLV-1 LTR-luc reporter.
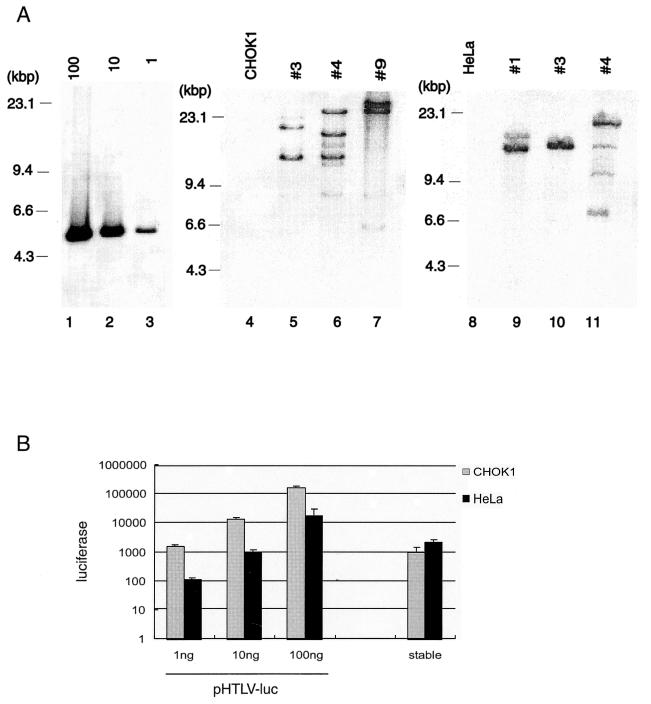
We obtained several clonal CHOK1 and HeLa integrants after cotransfection of CHOK1 and HeLa cells with pHTLV-luc plus pRSV-neo followed by selection with G418. G418-resistant clones were first screened for integrated pHTLV-luc by luciferase assay (not shown); the cells were then characterized by Southern hybridization (Fig. 1A). Based on Southern blottings, we selected three independent HeLa and CHOK1 clones that have integrated between two and four copies of pHTLV-luc (Fig. 1A). In order to minimize biases stemming from particular cellular integration sites, all subsequent assays were conducted with cell pools that contained equal numbers of cells from either the three HeLa integrants or the three CHOK1 integrants. Individual cell clones were propagated separately in order to maintain long-term homogeneity. Cells were pooled in equal numbers immediately prior to use in transfections. For ease of terminology, throughout the paper, pooled HeLa clones will be referred to as “HeLa-luc cells,” and pooled CHOK1 clones will be termed “CHOK1-luc cells.”
FIG. 1.
Southern blotting analyses of stably integrated HTLV-1 LTR-luc in CHOK1 and HeLa cells. (A) Three independently cloned CHOK1 and HeLa cells with a stably integrated HTLV-1 LTR-luc gene are shown. The autoradiographs compare luciferase-specific hybridzation signals from parental cell DNAs (CHOK1, lane 4; HeLa, lane 8) and DNAs from three independently selected CHOK1 (lanes 5 to 7) or HeLa (lanes 9 to 11) integrants. Lanes 1 to 3 show reconstruction controls in which 100, 10, or 1 copy of linearized pGL3-basic plasmid (4.8 kbp) was mixed with EcoRI-digested CV-1 genomic DNA. (B) Comparisons of luciferase activities from transiently transfected pHTLV-luc plasmid and stably integrated pHTLV-luc. One, 10, or 100 ng of pHTLV-luc was transiently transfected into CHOK1 or HeLa cells. Transiently transfected cells were compared to two pools of CHOK1 and HeLa integrants for luciferase activity as described in Materials and Methods. Absolute luciferase values were normalized to β-galactosidase activity from a cotransfected pCMV-βGAL plasmid.
Next, we compared luciferase activity in HeLa-luc or CHOK1-luc cells to that in HeLa or CHOK1 cells that had been transfected transiently with pHTLV-luc. As a preface to these experiments, we first queried the amount of transfected plasmid DNA that would achieve basal activities approximate to that found for the stable cell pools. Figure 1B compares luciferase expression from transiently transfected CHOK1/HeLa cells to that of similar numbers of CHOK1-luc and HeLa-luc cells. We found that CHOK1 cells transiently transfected with 1 ng of pHTLV-luc produced luciferase activity roughly equivalent to that from CHOK1-luc cells, and HeLa cells transiently transfected with 10 ng of pHTLV-luc yielded luciferase activity comparable to that from HeLa-luc cells. For subsequent experiments, unless otherwise indicated, when comparing CHOK1-luc or HeLa-luc to transiently transfected CHOK1 or HeLa cells, we routinely transfected 1 or 10 ng of pHTLV-luc, respectively.
Roles for CREB, p300, and P/CAF in the activation of integrated HTLV-1 LTR.
The HTLV-1 LTR contains three copies of CRE (9, 25). Although much is known as to how transiently transfected LTR plasmids respond to CREB, p300, and P/CAF, a comparable characterization of such requirements for stably integrated HTLV-1 LTR has not been done. To examine this issue, we introduced CREB, p300, or P/CAF expression vector into HeLa-luc or CHOK1-luc cells. We compared these transfections to CHOK1 or HeLa cells transiently cotransfected with pHTLV-luc plus CREB, p300, or P/CAF vector. Responsiveness of either the stably integrated LTR reporter or the transiently transfected LTR reporter to CREB, p300, or P/CAF was quantitated by luciferase assays.
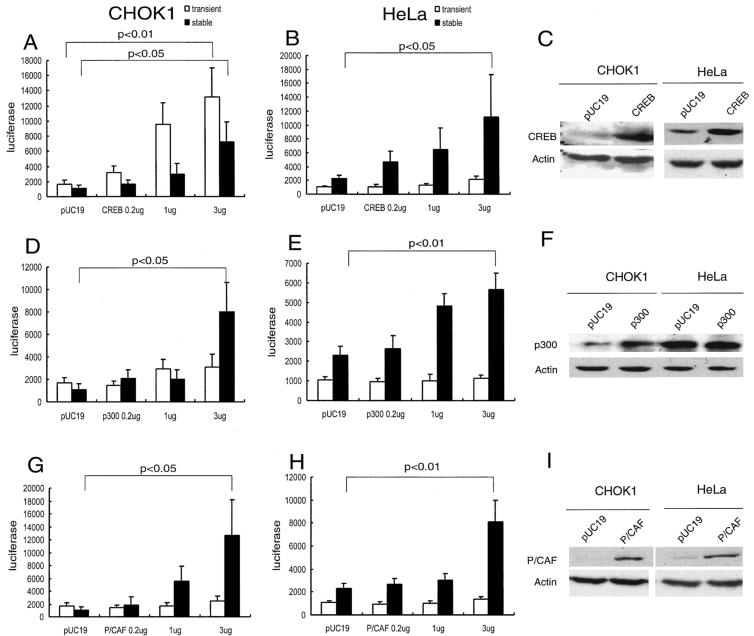
In the CHOK1 background, transiently transfected LTR-luc was activated by CREB (3μg) to approximately 7× over the basal level (Fig. 2A, open bars). In contrast, neither p300 (3 μg) nor P/CAF (3 μg) activated the transiently transfected LTR-luc by more than 0.5× above the value achieved with pUC19 (Fig. 2D and G, open bars). Interestingly, when stably integrated LTR-luc was examined, CREB, p300, and P/CAF activated luciferase expression by more than 6×, 8×, and 12× over basal levels, respectively (Fig. 2A, D, and G, solid bars). Thus, in CHOK1 cells, p300 and P/CAF show different activation capacities for stably integrated versus transiently transfected LTR-luc.
FIG. 2.
Activation of transiently transfected and stably integrated HTLV-luc by CREB, p300, and P/CAF. Transfections were performed with CHOK1 (A, D, and G) and HeLa (B, E, and H) cells. To assess the expression of transiently transfected reporter plasmid (open bars), CHOK1 cells were transfected with 1 ng of pHTLV-luc, and HeLa cells were transfected with 10 ng of pHTLV-luc. Additionally, 0.1 μg of pCMV-βGAL (as a transfection control) and 0.2, 1, or 3 μg of CREB (A), or p300 (D), or P/CAF (G) were cotransfected as indicated. To assess the expression of integrated HTLV-1 LTR-luc (filled bars), CHOK1-luc or HeLa-luc cells were transfected with 3 μg of pHOOK plus 0.1 μg of pCMV-βGAL and 0.2, 1, or 3 μg of CREB (B), p300 (E), or P/CAF (H). Forty-eight hours after transfection, luciferase activities were measured. Luciferase values are averages ± standard deviations from three or more independent transfections. Statistical significance at the indicated P values was verified with the one-tailed t test. Panels C, F, and I show Western blotting of endogenous (pUC19 lanes) CREB (C), p300 (F), and P/CAF (I) in CHOK1 and HeLa cells or the total amounts of CREB, p300, and P/CAF after transfection of 3 μg of the respective expression plasmid.
We next examined CREB, p300, and P/CAF functions in HeLa cells. Here, CREB, p300, and P/CAF augmented the expression of stably integrated LTR-luc by approximately 5×, 3×, and 4×, respectively (Fig. 2B, E, and H, solid bars). Neither p300 nor P/CAF showed any significant activity on transiently transfected LTR-luc (Fig. 2E and H). These results are similar to the counterpart transfections in CHOK1 cells (Fig. 2D and G). On the other hand, whereas CREB activated expression of transiently transfected LTR-luc in CHOK1 cells by 7×, in HeLa cells, CREB had a negligible impact on transiently introduced LTR-luc plasmid (Fig. 2B).
To investigate why the HTLV-1 LTR appeared to be slightly more responsive to exogenously-introduced CREB, p300, and P/CAF in CHOK1 than HeLa cells, we verified the endogenous expression of CREB, p300, and P/CAF in both cells (Fig. 2C, F, and I) by Western blotting. We observed that endogenous amounts of CREB, p300, and P/CAF were relatively higher in HeLa cells than in CHOK1 cells (see pUC19 lanes in Fig. 2C, F, and I). This suggests that the variation in cell-type responsiveness could be explained by differences in endogenous expression of factors rather than by different factor requirements.
The HAT domain of p300, but not P/CAF, is required for activation of integrated HTLV-1 LTR.
In both CHOK1 and HeLa cells, p300 and P/CAF activated stably integrated HTLV-1 LTR (Fig. 2). Because p300 and P/CAF are HATs, and because HAT activity has been shown to be important for the expression of nucleosomally organized genes, we wondered whether activation of integrated LTR-luc by p300 and P/CAF requires their histone-acetylating capacities. To address this issue, we compared p300 and P/CAF to their loss-of-function (histone acetylation) mutants, p300ΔHAT and P/CAFΔHAT. When mutant and wild-type proteins were compared side-by-side in CHOK1 cells on stably integrated LTR-luc, both p300 and P/CAF wild-type proteins behaved similarly, increasing luciferase expression by 8 to 12×, respectively, over that seen with pUC19 (Fig. 3A). In contrast, the ΔHAT mutants of p300 and P/CAF behaved differently. p300ΔHAT failed to activate stably integrated LTR-luc, while P/CAFΔHAT activated expression to a level comparable to that achieved with wild-type P/CAF (Fig. 3A).
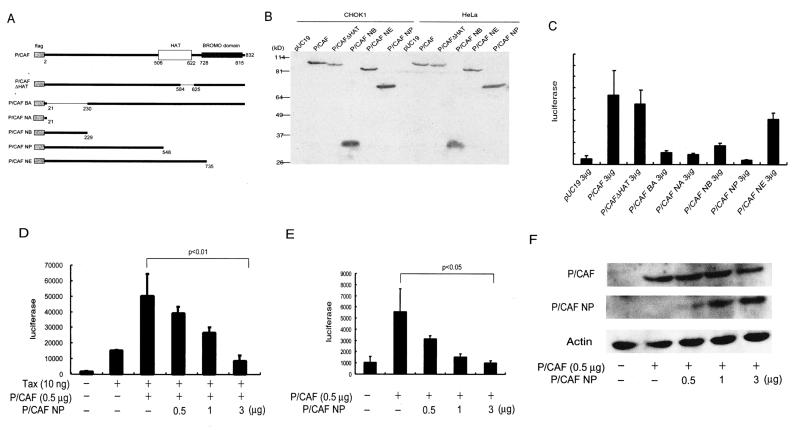
FIG. 3.
The HAT domain from P/CAF is dispensable for the activation of integrated HTLV-luc. (A) CHOK1-luc cells were transfected with 3 μg of pUC19, p300, p300ΔHAT, P/CAF, or P/CAFΔHAT, as indicated. Luciferase activities were measured 48 h after transfection. (B) Transfections were performed as described for panel A, except that each transfection additionally included 10 ng of a Tax expression plasmid. Values are averages ± standard deviations from three independent transfections.
We next repeated the transfections in the presence of the HTLV-1 Tax protein (Fig. 3B). In this setting, a difference between p300ΔHAT and P/CAFΔHAT was also observed. Whereas P/CAFΔHAT, like P/CAF, supported Tax activation of stably integrated LTR-luc, p300ΔHAT, unlike p300, was distinctly deficient in supporting Tax activation (Fig. 3B). In fact, the level of luciferase expression seen with Tax plus p300ΔHAT was lower than that observed with Tax alone (Fig. 3B), suggesting that the p300ΔHAT protein, more than a loss-of-function mutant, has a dominant-negative phenotype. This observed dominant-negative effect of p300ΔHAT is specific, since no reduction in expression of an internally cotransfected pCMV-βGAL plasmid was seen (data not shown). Collectively, the results indicate that the HAT domain of P/CAF, but not p300, is not needed for the activation of integrated HTLV-1 LTR.
Analyses with dominant-negative mutants of roles for CREB, p300, and P/CAF in Tax activation of stably integrated HTLV-1 LTR.
As presented above, overexpression of exogenously transfected CREB, p300, or P/CAF modulated the expression of stably integrated HTLV-1 LTR (Fig. 2 and 3). These overexpression findings, however, do not conclusively address how endogenous CREB, p300, and P/CAF might be physiologically important for Tax activation of integrated HTLV LTR. Experiments with dominant-negative mutants could, however, provide an assessment of endogenous cell factor function. In this regard, the KCREB protein has been previously characterized as a dominant-negative form of CREB (56). In contrast, dominant-negative forms of p300 and P/CAF have not been clearly described in the literature.
Our transfection results in Fig. 3B suggested that p300ΔHAT might be a dominant-negative mutant of p300. Indeed, this phenotype for p300ΔHAT was further confirmed in additional assays (data not shown) (see Fig. 5). On the other hand, the behavior of P/CAFΔHAT was inconsistent with that of a dominant-negative P/CAF (Fig. 3B). Hence, to identify a dominant-negative form of P/CAF, we next constructed and tested five additional deletion mutants: BA, NA, NB, NP, and NE (Fig. 4A). In CHOK1 and HeLa cells, each of these mutants was expressed to levels comparable to those of wild-type P/CAF (Fig. 4B; data not shown for BA and NA). When the mutants were tested for activation of luciferase in CHOK1-luc cells, four of the five (BA, NA, NB, and NP; Fig. 4C) exhibited loss of function. These four were tested for dominant-negative P/CAF activity against wild-type P/CAF. Among the mutants, P/CAF NP showed a strong dominant-negative effect against P/CAF in either the presence (Fig. 4D) or absence (Fig. 4E) of Tax. To verify that P/CAF NP's activity reflects dominant-negative interference with P/CAF function and not a disturbance in P/CAF expression, we directly checked P/CAF protein levels by Western blotting. Indeed, overexpression of P/CAF NP did not perturb intracellular levels of P/CAF protein (Fig. 4F). Similar behaviors of p300ΔHAT on p300 function and expression were also observed (data not shown).
FIG. 5.
Dominant-negative effects of CREB, p300, and P/CAF mutant on Tax activation of integrated HTLV-1 LTR-luc. CHOK1-luc (A, C, and E) and HeLa-luc (B, D, and F) cells were transfected with pTax (10 ng) (48) plus pHOOK (3 μg) plus pCMV-βGAL (0.1 μg) with increasing amounts of KCREB (0.5, 1, 3, and 5 μg [A and B]), p300ΔHAT (0.5, 1, 3, and 5 μg [C and D]), or P/CAF NP (0.5, 1, 3, and 5 μg [E and F]). Total amounts of transfected DNA were normalized with pUC19. Each series of transfections was repeated three or more times. Luciferase activities were assayed 48 h after transfection. Luciferase activities in lanes 1 and 5 are significantly different at the indicated P values (P < 0.01 and P < 0.05). (G) Tax expression was not affected by p300ΔHAT or P/CAF NP. Pooled CHOK1-luc cells were transfected with pTax (48) and p300ΔHAT or P/CAF NP as in the previous panels, followed by Western blotting with rabbit polyclonal anti-Tax. Antiactin antibody was used to verify equivalent sample loadings.
FIG. 4.
Definition of regions within P/CAF that contribute to the activation of the HTLV-1 LTR. (A) Schematic depictions of six P/CAF deletion mutants: ΔHAT, BA, NA, NB, NP, and NE. The full-length Flag-tagged P/CAF protein is shown at top with the HAT and BROMO domains indicated. (B) Efficient expression of transfected P/CAF and P/CAF mutants in CHOK1 and HeLa cells. Cells were transfected with 3 μg of Flag-tagged pCI-P/CAF or the indicated deletion mutant followed by Western blotting with anti-Flag. The NA mutant, due to its small size, could not be detected on this gel. The BA mutant was not assayed in this experiment. (C) Activation of integrated HTLV-luc by P/CAF and P/CAF mutants. Luciferase values are averages ± standard deviations from three independent transfections of pooled CHOK1-luc cells with the indicated P/CAF plasmids. (D and E) Dominant-negative activity of P/CAF NP. Pooled CHOK1-luc cells were transfected with pHOOK (3 μg) plus pCMV-βGAL (0.1 μg) plus pCI-P/CAF (0.5 μg) with increasing amounts of P/CAF NP (0.5, 1, and 3 μg), either with (D) or without (E) Tax. The total amounts of transfected plasmid were normalized with pUC19. Luciferase values are averages ± standard deviations from three independent transfections. A significant difference in luciferase activities for lanes 3 and 6 was seen (P < 0.01). (F) P/CAF NP does not perturb P/CAF expression. Pooled CHOK1 HTLV-luc cells were transfected as described for panel E, followed by Western blotting with anti-P/CAF antibody. Antiactin antibody verified equivalent sample loadings.
Armed with dominant-negative proteins, KCREB, p300ΔHAT, and P/CAF NP, we asked next how endogenous CREB, p300, and P/CAF contributed to Tax activation of integrated HTLV-1 LTR in CHOK1-luc and HeLa-luc cells. In dose-titrated transfections in CHOK1-luc and HeLa-luc cells, KCREB, p300ΔHAT, and P/CAF NP each separately suppressed (albeit to different extents) Tax activation of integrated LTR (Fig. 5A and B). Slightly better suppression was achieved by the dominant-negative mutants in CHOK1-luc cells than in HeLa-luc cells. This is consistent with the smaller endogenous amounts of CREB, p300, and P/CAF in CHOK1 compared to HeLa cells (Fig. 2C, F, and I). Despite slightly lower magnitudes, suppression by dominant-negative proteins in HeLa-luc cells was significant (P < 0.05 and P < 0.01), and both p300ΔHAT and P/CAF NP behaved comparably to the better-characterized KCREB dominant-negative protein (56). To confirm that the effects of our dominant-negative p300ΔHAT and P/CAF NP were not trivially due to suppressed expression of transfected pTax, we checked Tax protein levels directly by Western blotting. The finding of invariant Tax expression (Fig. 5G) supports the proposed dominant-negative effects of p300ΔHAT and P/CAF NP (Fig. 5A to F).
The dominant-negative results (Fig. 5) suggest a complex interplay between CREB, p300, P/CAF, and Tax. Two interpretations emerge. First, because each dominant-negative protein effectively suppressed luciferase expression, it is unlikely that CREB, p300, and P/CAF provide redundant functions for each other in Tax-LTR activation. Second, although Tax independently binds CREB, p300, or P/CAF in vitro, the dominant-negative findings argue that the relevant intracellular interaction is between Tax and a multiprotein complex of CREB plus p300 plus P/CAF. Bilateral interactions of Tax plus CREB, Tax plus p300, or Tax plus P/CAF in themselves would appear to be insufficient for functional activation.
Distinct roles for CREB, p300, and P/CAF in UV-C activation of integrated HTLV-1 LTR.
Tax expression is undetectable in many in vivo ATL cells. Hence, it is important to understand how integrated LTR might express via Tax-independent routes. In this regard, the HTLV-1 LTR has been found to be induced by a variety of environmental stresses (2, 3, 55). Since UV-C is a prevalent ambient insult, we asked whether UV-C would activate chromosomally integrated HTLV-1 LTR, and whether such activation is mechanistically similar to or different from Tax LTR induction.
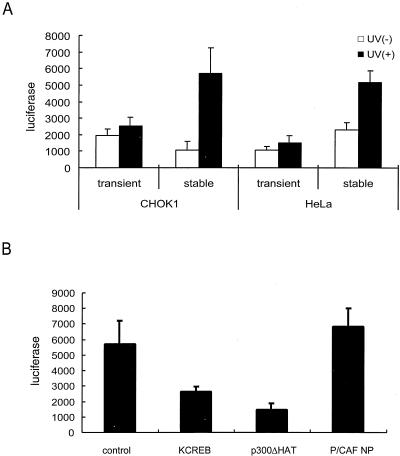
We treated transiently transfected HeLa/CHOK1 and stably integrated HeLa-luc/CHOK1-luc cells with UV-C (254-nm wavelength; Fig. 6A). Cells were assayed for luciferase activity 24 h later, and parallel activities were compared. Interestingly, we found that transfected pHTLV-luc was not activated by UV-C treatment. In contrast, in both HeLa-luc and CHOK1-luc cells, UV-C increased the luciferase expression from stably integrated HTLV-1 LTR (Fig. 6A). These results provide another illustration of differential regulation of transiently introduced reporter plasmid versus stably integrated LTR-luc.
FIG. 6.
Induction of HTLV-1 LTR expression by UV-C irradiation. (A) Activation of HTLV-1 LTR by UV-C. CHOK1 and HeLa cells transiently transfected with or stably integrated for pHTLV-luc plasmid were irradiated or mock irradiated with 100 J of UV-C m2 (254-nm wavelength). After 24 h, cells were harvested and assayed for luciferase. (B) Suppression of UV-C induced LTR expression by dominant-negative mutants of CREB, p300, or P/CAF. Prior to UV-C irradiation, CHOK1 HTLV-luc cells were transfected with KCREB, p300ΔHAT, or P/CAF NP for 24 h. Irradiated cells were assayed for luciferase activity 24 h later. All values are averages ± standard deviations from three independent transfections.
To understand better the factors that influence UV-C induction, we next asked whether UV-C utilizes CREB, p300, and P/CAF, in the same manner as Tax in activating integrated HTLV-1 LTR. In dominant-negative challenges, we reproducibly observed that KCREB and p300ΔHAT, but not P/CAF NP, suppressed LTR activation by UV-C (Fig. 6B). This lack of requirement for P/CAF distinguishes UV-C activation of stably integrated LTR-luc mechanistically from that of Tax (Fig. 5).
DISCUSSION
Expression from the HTLV-1 LTR has been studied extensively in short-term transfection of plasmid reporters into cells (6, 20, 27, 30). In these transient assays, regulatory roles for Tax, CREB, p300, and P/CAF have been well described. However, because transiently transfected plasmids are not known to achieve the same nucleosome structure as stably integrated genes, current understandings based on transient findings may not wholly or accurately reflect the regulation of stably integrated HTLV-1 LTR. Indeed for HIV-1, it has been documented that transcription from integrated versus transiently introduced retroviral LTRs does not proceed via identical paths (35). HIV-1 and HTLV-1 LTRs have divergent sequences and are modulated differently (e.g., Tat binds directly a TAR RNA target, while Tax functionally recognizes CRE-DNA motifs). Because no extant study has systematically addressed how chromosomally integrated HTLV-1 LTR is regulated, we sought to examine this issue. Here, with respect to CREB, p300, and P/CAF, we show that the activation requirements for transiently transfected and stably integrated HTLV-1 LTR are different (Fig. 2). As a further illustration of differential regulation, we found that stably integrated LTR-luc, but not transiently transfected LTR-luc, responded to UV-C induction (Fig. 6).
Our present work offers an initial step toward understanding better the activation of integrated HTLV-1 provirus. For our experiments, we have attempted to minimize potential cell type and integration-specific biases. Our findings are based on two separate pools of CHOK1 and HeLa cells, which contain stably integrated HTLV-1 LTR-luc. Each pool contained an equal number of cells from three independently cloned cell lines (Fig. 1). Additional findings from similarly integrated Rat1 and CV1 cells (data not shown) are further consistent with the HeLa-luc and CHOK1-luc data. Hence, while we cannot exclude that integration sites not present in our cell lines could yield divergent results, our present findings are representative of readouts averaged over seven to eight different HeLa and CHOK1 integration sites. A less extensive examination of other pools of clones (data not shown) provides results fully supportive of our current data.
Our CHOK1-luc and HeLa-luc findings confirm and extend previous hypotheses on Tax, p300, and P/CAF interaction derived from transiently transfected HTLV-1 LTR plasmids (20, 27). In agreement with Jiang et al.'s finding with transfected LTR plasmid, we found that P/CAFΔHAT has a HAT-independent activity on stably integrated HTLV-1 LTR (Fig. 3). However, whereas Jiang et al. (27) proposed that overexpressed P/CAF or p300 can each independently support Tax activation of the LTR, our dominant-negative results (Fig. 5) point to an alternative conclusion. With regard to endogenous P/CAF and p300 expressed at physiological levels inside cells, our findings suggest that a multiprotein complex that includes CREB plus p300 plus P/CAF is required for Tax activation of integrated LTR contains. Interestingly, this simultaneous requirement for both P/CAF and p300 at the integrated LTR, while not seen by Jiang et al. (27) in their transfection assay, is partly reflected by results from by Harrod et al. in a separate study of transiently transfected LTR-CAT plasmid (20).
In the course of our experiments, we unexpectedly observed that UV-C activated stably integrated HTLV-1 LTR, but not transiently transfected LTR plasmid. In analyzing this property of UV-C more carefully, we found that this ambient stress signal utilized CREB, p300, and P/CAF differently from Tax (Fig. 6). In particular, Tax LTR activation was abrogated by dominant-negative P/CAF NP mutant (Fig. 5), while UV-C LTR activation was not affected by P/CAF NP (Fig. 6). Hence, unlike Tax, the UV-C environmental stress stimulus does not use P/CAF in its activation of integrated provirus. Further work is needed to define whether this observation also applies to other stress stimuli. It also remains to be determined whether a not-yet-identified factor substitutes for P/CAF or whether the CREB-p300 combination without P/CAF is wholly sufficient for UV-C activity on the HTLV-1 LTR. Pending further results, the current data support the idea that other, not-yet-characterized, pathways that can activate expression of integrated HTLV provirus likely exist.
It is curious why two HATs (i.e., p300 and P/CAF) are frequently needed at many different promoters (11, 23, 38, 50, 51). One view is that the two activities provide redundancy. Another view is that the two HATs when combined in multiple arrangements produce a combinatorial array of activation specificities. Regarding this viewpoint, we previously found that the HIV-1 Tat protein needed both p300 and P/CAF to activate integrated HIV-1 LTR, but the HAT domain of p300 was dispensable (7). Here, Tax also required both p300 and P/CAF for activation of stably integrated HTLV-1 LTR, but it is the HAT domain of P/CAF that was not needed. This juxtaposition of generally similar but yet distinctly different regulatory requirements between HIV-1 and HTLV-1 provides another example of the evolutionary similarities or divergences in mechanisms used by the two human retroviruses.
REFERENCES
- 1.Adya, N., and C.-Z. Giam. 1995. Distinct regions in human T-cell lymphotropic virus type I Tax mediate interactions with activator protein CREB and basal transcription factors. J. Virol. 69:1834-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, J. M., G. C. Newbound, M. Oglesbee, J. N. Brady, and M. D. Lairmore. 1997. The cellular stress response enhances human T-cell lymphotropic virus type 1 basal gene expression through the core promoter region of the long terminal repeat. J. Virol. 71:741-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, J. M., M. J. Oglesbee, A. V. Trevino, D. J. Guyot, G. C. Newbound, and M. D. Lairmore. 1995. Enhanced human T-cell lymphotropic virus type I expression following induction of the cellular stress response. Virology 208:816-820. [DOI] [PubMed] [Google Scholar]
- 4.Ballard, D. W., E. Bohnlein, J. W. Lowenthal, Y. Wano, B. R. Franza, and W. C. Greene. 1988. HTLV-I tax induces cellular proteins that activate the B element in the IL-2 receptor α gene. Science 241:1652-1655. [DOI] [PubMed] [Google Scholar]
- 5.Bantignies, F., R. Rousset, C. Desbois, and P. Jalinot. 1996. Genetic characterization of transactivation of the human T-cell leukemia virus type 1 promoter: binding of Tax to Tax-responsive element 1 is mediated by the cyclic AMP-responsive members of the CREB/ATF family of transcription factors. Mol. Cell. Biol. 16:2174-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnhart, M. K., L. M. Connor, and S. J. Marriott. 1997. Function of the human T-cell leukemia virus type 1 21-base-pair repeats in basal transcription. J. Virol. 71:337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benkirane, M., R. F. Chun, H. Xiao, V. V. Ogryzko, B. H. Howard, Y. Nakatani, and K. T. Jeang. 1998. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J. Biol. Chem. 273:24898-24905. [DOI] [PubMed] [Google Scholar]
- 8.Bodor, J., W. Walker, E. Flemington, A.-L. Spetz, and J. F. Habener. 1995. Modulation of Tax and PKA-mediated expression of HTLV-I promoter via cAMP response element binding and modulator proteins CREB and CREM. FEBS Lett. 377:413-418. [DOI] [PubMed] [Google Scholar]
- 9.Brady, J., K.-T. Jeang, J. Duvall, and G. Khoury. 1987. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J. Virol. 61:2175-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brauweiler, A., P. Garl, A. A. Franklin, H. A. Giebler, and J. K. Nyborg. 1995. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J. Biol. Chem. 270:12814-12822. [DOI] [PubMed] [Google Scholar]
- 11.Chan, H. M., and N. B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363-2373. [DOI] [PubMed] [Google Scholar]
- 12.Duyao, M. P., D. J. Kessler, D. B. Spicer, C. Bartholomew, J. L. Cleveland, M. Siekevitz, and G. E. Sonenshein. 1992. Transactivation of the c-myc promoter by human T cell leukemia virus type 1 Tax is mediated by NF-κB. J. Biol. Chem. 267:16288-16291. [PubMed] [Google Scholar]
- 13.Elgin, S. C. 1990. Chromatin structure and gene activity. Curr. Opin. Cell Biol. 2:437-445. [DOI] [PubMed] [Google Scholar]
- 14.El Kharroubi, A., G. Piras, R. Zensen, and M. A. Martin. 1998. Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter. Mol. Cell. Biol. 18:2535-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii, M., I. Sassone-Corsi, and I. M. Verma. 1988. c-fos promoter trans-activation by the tax 1 protein of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 85:8526-8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii, M., T. Niki, T. Mori, T. Matsuda, M. Matsui, N. Nomura, and M. Seiki. 1991. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene 6:1023-1029. [PubMed] [Google Scholar]
- 17.Fujisawa, J. I., M. Seiki, M. Sato, and M. Yoshida. 1986. A transcriptional enhancer sequence of HTLV-I is responsible for trans-activation mediated by p40x of HTLV-I. EMBO J. 5:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giam, C. Z., and Y. L. Xu. 1989. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J. Biol. Chem. 264:15236-15241. [PubMed] [Google Scholar]
- 19.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrod, R., Y. L. Kuo, Y. Tang, Y. Yao, A. Vassilev, Y. Nakatani, and C. Z. Giam. 2000. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax and a multi-histone acetyltransferase/activator-enhancer complex. J. Biol. Chem. 275:11852-11857. [DOI] [PubMed] [Google Scholar]
- 21.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollsberg, P., and D. A. Hafler. 1993. Seminars in medicine of the Beth Israel Hospital, Boston. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N. Engl. J. Med. 328:1173-1182. [DOI] [PubMed] [Google Scholar]
- 23.Hottiger, M. O., and G. J. Nabel. 1998. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J. Virol. 72:8252-8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeang, K. T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-kappa B. Cytokine Growth Factor Rev. 12:207-217. [DOI] [PubMed] [Google Scholar]
- 25.Jeang, K.-T., I. Boros, J. Brady, M. Radonovich, and G. Khoury. 1988. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J. Virol. 62:4499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeang, K. T., S. G. Widen, O. J. Semmes, and S. H. Wilson. 1990. HTLV-1 transactivator protein, Tax, is a trans-repressor of the human-polymerase gene. Science 247:1082-1084. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, H., H. Lu, R. L. Schiltz, C. A. Pise-Masison, V. V. Ogryzko, Y. Nakatani, and J. N. Brady. 1999. PCAF interacts with Tax and stimulates Tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 19:8136-8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93:81-91. [DOI] [PubMed] [Google Scholar]
- 29.Kasai, T., Y. Iwanaga, H. Iha, and K. T. Jeang. 2002. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J. Biol. Chem. 277:5187-5193. [DOI] [PubMed] [Google Scholar]
- 30.Kashanchi, F., J. F. Duvall, R. P. S. Kwok, J. R. Lundblad, R. H. Goodman, and J. N. Brady. 1998. The coactivator CBP stimulates human T-cell lymphotropic virus type 1 Tax transactivation in vitro. J. Biol. Chem. 273:34646-34652. [DOI] [PubMed] [Google Scholar]
- 31.Kibler, K. V., and K.-T. Jeang. 2001. CREB/ATF-dependent repression of cyclin A by human T-cell leukemia virus type 1 Tax protein. J. Virol. 75:2161-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwok, R. P. S., M. E. Laurance, J. R. Lundblad, P. S. Goldman, H.-M. Shih, L. M. Connor, S. J. Marriott, and R. H. Goodman. 1996. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature 380:642-646. [DOI] [PubMed] [Google Scholar]
- 33.Marzio, G., K. Verhoef, M. Vink, and B. Berkhout. 2001. In vitro evolution of a highly replicating, doxycycline-dependent HIV for applications in vaccine studies. Proc. Natl. Acad. Sci. USA 98:6342-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Migione, T.-S., J.-X. Lin, A. Cereseto, J. C. Mulloy, J. J. O'Shea, G. Franchini, and W. J. Leonard. 1995. Constitutively activated Jak-STAT pathway in T-cells transformed with HTLV-1. Science 269:79-81. [DOI] [PubMed] [Google Scholar]
- 35.Miller, S. C., A. Taylor, K. Watanabe, K. Mok, and F. M. Torti. 1997. Regulation of NF-kappaB and HIV-1 LTR activity in mouse L cells by ultraviolet radiation: LTR trans-activation in a nonirradiated genome in heterokaryons. Exp. Cell Res. 230:9-21. [DOI] [PubMed] [Google Scholar]
- 36.Nerenberg, M., S. H. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237:1324-1329. [DOI] [PubMed] [Google Scholar]
- 37.Neuveut, C., K. G. Low, F. Maldarelli, I. Schmitt, F. Majone, R. Grassmann, and K.-T. Jeang. 1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 18:3620-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogryzko, V. V., R. L. Schlitz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 39.Okazaki, S., R. Moriuchi, N. Yosizuka, K. Sugahara, T. Maeda, I. Jinnai, M. Tomonaga, S. Kamihira, and S. Katamine. 2001. HTLV-1 proviruses encoding non-functional TAX in adult T-cell leukemia. Virus Genes 23:123-135. [DOI] [PubMed] [Google Scholar]
- 40.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pozzatti, R., R. Muschel, J. Williams, R. Padmanabhan, B. Howard, L. Liotta, and G. Khoury. 1986. Primary rat embryo cells transformed by one or two oncogenes show different metastatic potentials. Science 232:223-227. [DOI] [PubMed] [Google Scholar]
- 42.Pozzati, R., J. Vogel, and G. Jay. 1990. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol. Cell. Biol. 10:413-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratner, L., T. Portis, M. Robek, J. Harding, and W. Grossman. 2000. Studies of the immortalizing activity of HTLV type 1 Tax, using an infectious molecular clone and transgenic mice. AIDS Res. Hum. Retrovir. 16:1647-1651. [DOI] [PubMed] [Google Scholar]
- 44.Rosin, O., C. Koch, I. Schmitt, O. J. Semmes, K. T. Jeang, and R. Grassmann. 1998. A human T-cell leukemia virus Tax variant incapable of activating NF-kappaB retains its immortalizing potential for primary T-lymphocytes. J. Biol. Chem. 273:6698-6703. [DOI] [PubMed] [Google Scholar]
- 45.Ross, T. M., M. Narayan, Z.-Y. Fang, A. C. Minella, and P. L. Green. 2000. Human T-cell leukemia virus type 2 Tax mutants that selectively abrogate NFκB or CREB/ATF activation fail to transform primary human T cells. J. Virol. 74:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santiago, F., E. Clark, S. Chong, C. Molina, F. Mozafari, R. Mahieux, M. Fujii, N. Aximi, and F. Kashanchi. 1999. Transcriptional up-regulation of the cyclin D2 gene and acquisition of new cyclin-dependent kinase partners in human T-cell leukemia virus type 1-infected cells. J. Virol. 73:9917-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scoggin, K. E. S., A. Ulloa, and J. K. Nyborg. 2001. The oncoprotein Tax binds the SRC-1-interacting domain of CBP/p300 to mediate transcriptional activation. Mol. Cell. Biol. 21:5520-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semmes, O. J., and K.-T. Jeang. 1992. Mutational analysis of human T Tax: regions necessary for function determined using 47 mutant proteins. J. Virol. 66:7183-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siekevitz, M., M. Feinberg, N. Holbrook, F. Wong-Staal, and W. C. Greene. 1987. Activation of interleukin 2 and interleukin 2-receptor (tac) promoter expression by the transactivator (tat) gene product of human T-cell leukemia virus type 1. Proc. Natl. Acad. Sci. USA 84:5389-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spilianakis, C., J. Papamatheakis, and A. Kretsovali. 2000. Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes. Mol. Cell. Biol. 20:8489-8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tajima, K., and T. Kuroishi. 1985. Estimation of rate of incidence of ATL among ATLV (HTLV-I) carriers in Kyushu, Japan. Jpn. J. Clin. Oncol. 15:423-430. [PubMed] [Google Scholar]
- 53.Tanaka, A. C., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type 1 in vitro. Proc. Natl. Acad. Sci. USA 87:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tie, F., N. Adya, W. C. Greene, and C.-Z. Giam. 1996. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J. Virol. 70:8368-8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torgeman, A., Z. Ben-Aroya, A. Grunspan, E. Zelin, E. Butovsky, M. Hallak, M. Lochelt, R. M. Flugel, E. Livneh, M. Wolfson, I. Kedar, and M. Aboud. 2001. Activation of HTLV-I long terminal repeat by stress-inducing agents and protection of HTLV-I-infected T-cells from apoptosis by the viral tax protein. Exp. Cell Res. 271:169-179. [DOI] [PubMed] [Google Scholar]
- 56.Walton, K. M., R. P. Rehfuss, J. C. Chrivia, J. E. Lochner, and R. H. Goodman. 1992. A dominant repressor of cyclic adenosine 3′,5′-monophosphate (cAMP)-regulated enhancer-binding protein activity inhibits the cAMP-mediated induction of the somatostatin promoter in vivo. Mol. Endocrinol. 6:647-655. [DOI] [PubMed] [Google Scholar]
- 57.Yin, M. J., and R. B. Gaynor. 1996. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol. Cell. Biol. 16:3156-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]