Abstract
We present a membrane-staining dye, di-4-ANEPPDHQ, which differentiates liquid-ordered phases from liquid-disordered phases coexisting in model membranes under both linear and nonlinear microscopies. The dye's fluorescence emission spectrum is blue-shifted 60 nm in liquid-ordered phases compared with liquid-disordered phases, and shows strong second harmonic generation in the liquid-disordered phase compared with the liquid-ordered phase. The ease of staining and the ability of this single dye to detect both phases, should lead to broad applications in biophysical studies of lipid domains in model membranes and cells.
The concept of lipid domains, or rafts, as organizing centers for localized signaling pathways in membranes (1) has achieved significant prominence in cell biology. Model membrane studies of coexisting lipid phases help provide a biophysical basis for these phenomena. Several imaging methods have been developed to distinguish lipid domains of different phases. Most optical probes used in model membranes, such as lissamine rhodamine-DPPE, distinguish the two phases by preferential partitioning (2). They are usually mixed into the lipids before the membrane is formed, so they are not candidate probes for rafts in cells. In cell studies, the probes used to mark rafts are usually antibodies or toxins, which bind to membrane components that concentrate in rafts, such as caveolin and GM1. Although this approach probes for clustering of putative raft molecules, it does not probe the physical environment that defines rafts. Laurdan is a fluorescent dye that is sensitive to solvent polarity and has been used in both model and cell membranes (3,4). It distinguishes the two phases by both an emission spectrum shift and a photoselection effect. Laurdan is an ultraviolet-excited dye, and to circumvent its propensity to photobleach under single-photon excitation, it is usually imaged with two-photon excitation fluorescence (TPF) microscopy (5).
Here, we introduce a novel optical probe for lipid phases, di-4-ANEPPDHQ, an environmentally sensitive styryl dye that was originally developed to detect transmembrane potential changes (6). The probe allows us to visualize model membranes with both linear and nonlinear microscopies, using single-photon excitation fluorescence (SPF), TPF, and second harmonic generation (SHG). Between the liquid-ordered and -disordered phases, di-4-ANEPPDHQ shows a 60-nm emission spectrum shift for fluorescence, as well as a very large intensity difference for SHG.
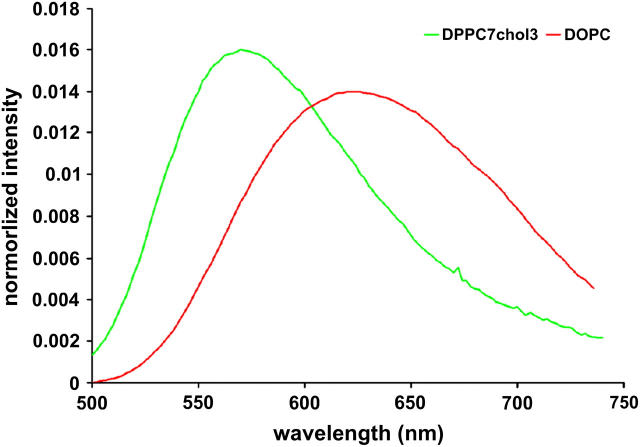
We made model membranes in either a liquid-ordered or liquid-disordered phase, stained them with di-4-ANEPPDHQ and measured the emission spectrum for each phase. Large unilamellar vesicles (LUVs) of pure 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) form a disordered phase at 20°C. LUVs of a mixture of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and cholesterol in a 7:3 molar ratio form an ordered phase at 20°C. We stained the LUVs with 1 μM di-4-ANEPPDHQ in the vesicle suspensions and measure the emission spectrum at 475-nm excitation. In Fig. 1, we show that the emission peak in the ordered phase LUVs is at 570 nm compared with 630 nm in the disordered phase LUVs.
FIGURE 1 .
Emission spectra of di-4-ANEPPDHQ in LUVs of pure DOPC (red) and LUVs of 7:3 DPPC/cholesterol (green).
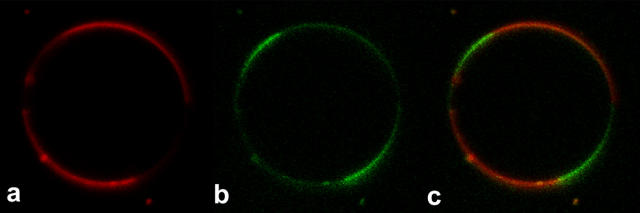
After we discovered this property of di-4-ANEPPDHQ, we imaged giant unilamellar vesicles (GUVs) of a mixture of DPPC, DOPC, and cholesterol in a 2:2:1 molar ratio that is known to form separated liquid-ordered and liquid-disordered phase domains at room temperature (2). DPPC and cholesterol are more concentrated in the ordered phase domains, whereas DOPC is more concentrated in the disordered phase domains. Fig. 2 shows confocal fluorescence images of a GUV stained with 10 μM di-4-ANEPPDHQ and excited at 488 nm. There are four domains in this optical slice. Two domains show greater fluorescence on the green channel whereas the other two show greater fluorescence on the red channel. At 10 μM di-4-ANEPPDHQ, the concentration of dye in the membrane is estimated at 4 mol% relative to lipid. We observed similar patterns with GUVs composed of brain sphingomyelin/egg phosphatidylcholine/cholesterol in a 2:2:1 molar ratio (data not shown), representing a more natural lipid mixture.
FIGURE 2 .
Confocal fluorescence images of a GUV (2:2:1 DPPC/DOPC/cholesterol) stained with 1 μM di-4-ANEPPDHQ. (a) Long-pass 650-nm channel; (b) band-pass 500–530-nm channel; (c) merge of panels a and b.
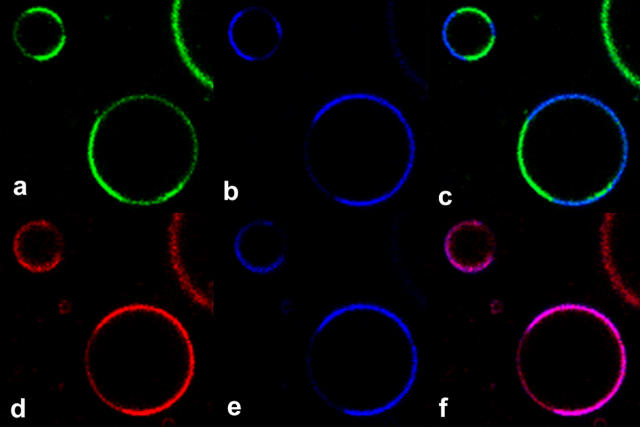
Figs. 1 and 2 show data collected using linear SPF spectroscopy and microscopy; nonlinear TPF and SHG may also be used to image phase domains as indicated by this dye. Fig. 3 shows TPF and SHG images from phase-separated GUVs, stained with 10 μM di-4-ANEPPDHQ and excited at 910 nm. The red and green TPF images clearly correspond to the SPF images, with each phase domain showing either more green or more red fluorescence. The SHG signal is greater in the domain corresponding to red TPF; this suggests that di-4-ANEPPDHQ has a greater SHG signal in the liquid-disordered phase. The SHG signal also shows a greater contrast between the two domains (ranging from 5:1 to 7:1) than either emission wavelength excited by TPF (ranging from 2:1 to 3:1).
FIGURE 3 .
TPF (red and green) and SHG (blue) images of GUVs with both phases stained with di-4-ANEPPDHQ. (a) TPF from the 515–565-nm emission channel; (b) SHG signal taken simultaneously with a; (c) merge of a and b; (d) TPF from the 650–700-nm emission channel; (e) SHG signal taken simultaneously with d; (f) merge of d and e.
We conclude that di-4-ANEPPDHQ partitions into both liquid-ordered and liquid-disordered phase domains in model membranes and senses the environmental difference between the two phases. The difference can be observed with both linear and nonlinear microscopies. For both SPF and TPF, di-4-ANEPPDHQ's emission spectrum is blue-shifted in the liquid-ordered phase compared with the liquid-disordered phase, whereas SHG is greater in the liquid-disordered phase. Furthermore, the dye is water soluble yet binds to lipid membranes with high affinity, and is therefore easily loaded into membranes without requiring surfactants such as Pluronic or other intermediates such as cyclodextrin. Free dye in solution provides negligible background signals because it only shows significant fluorescence and SHG after binding to membranes. The dye has already proven successful for live cell measurements of transmembrane potential (6). All of these features should also make di-4-ANEPPDHQ a good probe for rafts in cells, as will be demonstrated in a full article.
METHODS
Vesicle preparation
GUVs are developed using electric field oscillation (2,7). GUVs are stained by 10 μM di-4-ANEPPDHQ in water after development. LUVs are made from 2.5 mg/ml hydrated lipid suspensions in water using extrusion (Avanti Polar Lipids, Alabaster, AL).
Spectroscopy
LUV suspensions are diluted in water to ∼0.8 mg/ml, and stained with di-4-ANEPPDHQ at 2 μM in water. Spectra are measured by a Fluorolog 1681 spectrometer (JY Horiba, Edison, NJ) using 475-nm excitation.
Confocal fluorescent images are obtained with a Zeiss (Jena, Germany) LSM 510 microscope. The fluorescence is excited with a 488-nm argon laser and collected with a plan-Apochromat 63× 1.4 N.A. oil-immersion DIC objective. The two emission channels are LP 650 nm and BP 500–530 nm.
TPF and SHG
Nonlinear signals are excited at 910 nm by a Mira 900 (Coherent, Santa Clara, CA) Ti:sapphire ultrafast laser pumped by a 10-W Verdi (Coherent) solid-state laser. The laser light is circularly polarized by a combination of a half-waveplate (CVI Laser, Albuquerque, NM) and a quarter-waveplate (CVI Laser). A Fluoview (Olympus America, Melville, NY) scanning system directs the laser light into the sample through an IR-Achroplan 40× 0.8 N.A. water-immersion objective (Zeiss) on an Axioskop microscope (Zeiss). TPF is collected back through the objective and a band-pass filter (either 540/50 nm or 675/50 nm) and detected by a photomultiplier tube R3896 (Hamamatsu, Hamamatsu, Japan). The SHG is collected forward through a 0.9 N.A. condenser and a band-pass filter onto a photon-counting head H7421-40 (Hamamatsu). Signals from both TPF and SHG channels are input to the Fluoview control unit (Olympus) (8).
Acknowledgments
The authors thank Enrico Gratton's laboratory and Sarah Keller's laboratory for suggestions and assistance.
This study is funded by the National Institutes of Health (grant No. EB001963).
References
- 1.Brown, D., and J. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68:533–544. [DOI] [PubMed] [Google Scholar]
- 2.Veatch, S. L., and S. L. Keller. 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietrich, C., L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Gratton. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaus, K., E. Gratton, E. P. W. Kable, A. S. Jones, I. Gelissen, L. Leonard Kritharides, and W. Jessup. 2003. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc. Natl. Acad. Sci. USA. 100:15554–15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagatolli, L. A., S. A. Sanchez, T. Hazlett, and E. Gratton. 2003. Giant vesicles, laurdan, and two-photon fluorescence microscopy: evidence of lipid lateral separation in bilayers. Methods Enzymol. 360:481–500. [DOI] [PubMed] [Google Scholar]
- 6.Obaid, A. L., L. M. Loew, J. P. Wuskell, and B. M. Salzberg. 2004. Novel naphthylstyryl-pyridium potentiometric dyes offer advantages for neural network analysis. J. Neurosci. Methods. 134:179–190. [DOI] [PubMed] [Google Scholar]
- 7.Angelova, M. I., S. Soleau, P. Meleard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields: kinetics and applications. Prog. Colloid Polym. Sci. 89:127–131. [Google Scholar]
- 8.Millard, A. C., L. Jin, M.-D. Wei, J. P. Wuskell, A. Lewis, and L. M. Loew. 2004. Sensitivity of second harmonic generation from styryl dyes to transmembrane potential. Biophys. J. 86:1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]