Abstract
A novel pathway of adeno-associated virus (AAV) replication marked by the assembly of circular monomer duplex intermediates (cAAV) has been recently discovered. In the present report we identify a single AD domain of the inverted terminal repeat as a minimal origin of cAAV replication. A small internal palindrome (BB′), necessary for optimal Rep-inverted terminal repeat interaction, does not contribute to the efficiency of cAAV replication, while the terminal resolution site is an essential cis-acting element. Furthermore, recombinant cAAV vectors that encompass only the AD domain replicate exclusively in a circular form and no detectable linear duplex replicative intermediates are generated, suggesting that both pathways of AAV replication are independent and can be separated. In addition, we show that cAAVs are efficient templates for encapsidation of single-stranded DNA genomes, an observation that assigns a biological role for these novel replication species. Together, these findings shed new light on the current model of AAV replication and packaging.
Adeno-associated virus type 2 (AAV) is a parvovirus nonpathogenic to humans with a genome of approximately 4.7 kb (39). The AAV genome consists of two open reading frames that encode regulatory (Rep) and structural capsid (Cap) proteins flanked by 145-bp inverted terminal repeats (ITR) (39). These ITRs are the only cis-acting elements necessary for virus replication and encapsidation. Recombinant AAVs (rAAV) that do not contain any endogenous coding regions efficiently propagate when Rep and Cap are provided in trans (2, 3). In nature, a secondary infection with helper virus, e.g., adenovirus, is necessary to trigger a productive infection. AAV genomes then undergo replication followed by assembly of infectious virions containing single-stranded DNA (ssDNA) of either positive or negative polarity (2, 4). Adenovirus genes implicated in AAV replication have been identified and include E1A, E1B, E4orf6, E2A, and VA RNA (7, 20, 21, 31, 32). Similar to provirus in latently infected cells, AAV genomes can be efficiently rescued from a recombinant cis plasmid following transient transfection into human cells (33-35). The necessary helper functions can be delivered either by adenovirus infection or by transfecting a plasmid encoding a minimal set of adenovirus helper genes (10, 16).
Events of AAV lytic infection are described by a commonly accepted self-priming strand displacement model (1). The first 125 nucleotides of AAV termini include elements capable of forming a T-shaped duplex structure (A′-B′-B-C′-C-A) and are followed by a unique 20-bp D-sequence (3, 45). The Rep gene encodes four proteins that are synthesized from the same open reading frame via the use of alternate promoters and splicing (2, 39). Two of these proteins (Rep78 and Rep68) possess site-specific and strand-specific endonuclease activity. They bind to the Rep-binding site (Rbs) mapped to the tetrameric GAGC repeat of the A stem of the ITR and cleave it at the terminal resolution site (trs), positioned between the A and D elements (5, 19, 37, 38). A tip of the BB′ palindrome contains RBE′, a cis-acting element essential for optimal Rep-specific activity (5). During replication, the terminus folds on itself and serves as a primer to initiate a leading-strand synthesis. At the elongation step, the complementary strand is displaced and may serve as an independent second replication template. The result of this first round of DNA synthesis is a linear duplex replication form monomer (Rfm) with a covalently closed hairpin on one end. Rep-mediated nicking of the original strand then creates a 3′-OH primer, and the hairpin is extended. If nicking and subsequent ITR repair do not occur before the second round of replication is initiated on an opposite newly formed 3′ end, then continued DNA synthesis leads to formation of a replication form dimer (Rfd), which can be organized head-to-head or tail-to-tail, but never head-to-tail (18, 48). The model also predicts that linear duplex structures are intermediates of packaging. Using these as a template, the other two Rep proteins (Rep52 and Rep40) generate single-stranded progeny genomes (9) which are then encapsidated into preformed capsids (23, 27).
Recently, however, another AAV replication pathway marked by the assembly of circular duplex monomer genomes (cAAV) was identified (26). These circular species may constitute as much as 10% of monomer duplex intermediates of both wild-type (wt) and recombinant AAV, although on occasion these structures are barely detectable (references 26 and 28 and the present report). The circularization point (the so-called TRT domain) of cAAV contains a single copy of the ITR flanked by two D elements (D-A′-B′-B-C′-C-A-D) (13, 26). cAAV can either replicate along the accepted strand displacement pathway following resolution of the TRT domain (defined here as a conventional pathway) or by a mechanism that preserves the integrity of the circular conformation (alternative pathway) (26). Despite identification of these structures during AAV lytic infection, their function mainly remains unknown. Does cAAV require the same cis-acting elements for replication as linear duplex genomes? Can the two pathways proceed independently from each other? What is the biological significance of circular duplex intermediates, not only for replication but also for the AAV life cycle in general? To address these issues, we have constructed a series of cAAV plasmids containing various deletions in the TRT domain and analyzed the effect of these alterations on AAV replication and packaging in cell culture.
In this report we identify and characterize the minimal ITR sequence necessary and sufficient to support cAAV replication. Interestingly, a small internal palindrome (BB′) known to comprise an additional Rep-binding element (RBE′) necessary for optimal Rep-ITR interaction (5) does not contribute to the efficiency of cAAV replication, while the trs is an essential cis-acting element. Furthermore, rAAVs harboring only the AD domain replicate exclusively in a circular form and no linear duplex intermediates are assembled. To our knowledge, this is the first evidence that the conventional and alternative pathways of AAV replication are indeed independent and can be completely separated. Equally important, these studies revealed that cAAV genomes with the AD domain are efficient templates for the packaging of ssDNA as well. This ability to package AAV genomes via the alternative pathway suggests a biological function of these novel replicative intermediates. Together, our findings allowed us to propose a new dimension to the commonly accepted model of AAV lytic infection.
MATERIALS AND METHODS
Construction of mutant cAAV vectors.
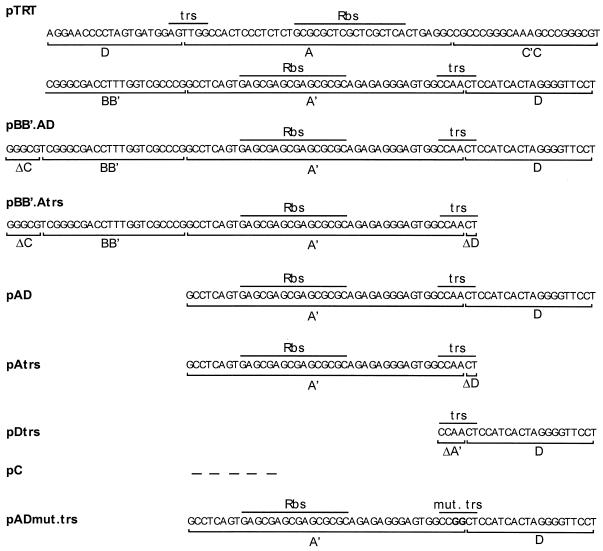
The structures of recombinant cAAV genomes are schematically presented in Fig. 1. All plasmids of this set harbor an enhanced green fluorescent protein (EGFP) under the control of the cytomegalovirus (CMV) promoter (Clontech), as well as different ITR sequences derived from the TRT domain. pTRT contains an intact TRT element consisting of a single ITR flanked by two D sequences (13, 26). This element was derived from a cAAV clone captured by using a bacterial trapping technique from cells during AAV lytic replication and appears to represent a wild-type ITR circularization point. pTRT is similar to pTRT.EGFPori described elsewhere (26) but contains TRT in a flop orientation, which makes it virtually indistinguishable from a 165-bp ITR sequence in the plasmid pDD-2 previously described (49). The original ITR sequence of the corresponding linear vector was derived from psub201 and contains a 13-bp deletion in the A region (34). The TRT domain is identical to the ITR junction fragment found in cAAVs assembled during latent infection in vivo (12, 13). All the deletion mutants were derived from this construct by replacing the TRT domain with PCR-amplified fragments containing different ITR elements. pBB′.AD has only the BB′ half of the hairpin and 5 bp of the CC′ portion of the hairpin followed by single A and D elements. pBB′.Atrs is similar to the previous construct but has the D sequence deleted, except for the nucleotides that comprise the trs. pAD contains only A and D elements. pAtrs is a derivative of pAD, which has most of the D element removed while leaving the trs intact. pDtrs contains a single D sequence and part of the A stem to complement trs. pC is a control vector that does not contain any AAV sequence. PCR was performed with high-fidelity Advantage Genomic Polymerase Mix (Clonetech), and the integrity of each construct was confirmed by sequencing. Importantly, all the cAAVs have the size of a wt virus and are approximately 4.4 to 4.6 kb in length. This permits testing of these constructs as templates for rescue-independent packaging.
FIG. 1.
Schematic representation of cAAV constructs containing deletions in the TRT domain. Major ITR elements including Rep binding site (Rbs) and terminal resolution site (trs) are indicated.
Models of rAAV propagation.
The first model represents a helper virus-free rAAV production method and involves cotransfection of a cis-acting plasmid with an adenovirus-AAV helper plasmid that harbors adenovirus genome fragments encoding only E2A, E4, and VA RNA as well as AAV genes for Rep and Cap. The plasmids (total, 2 μg of DNA; 1:3 ratio) were transfected into 70 to 80% confluent 293 cells in 35-mm-diameter culture wells by using FuGene 6 (Roche). Cells were harvested 72 h posttransfection. To ensure that the findings in this study are not limited to this model, a second classical method for rAAV production was used as well. Briefly, subconfluent 293 cells in 35-mm-diameter culture wells were first infected with Ad5 (multiplicity of infection, 5) for 2 h and then cotransfected with a vector plasmid and pRep.Cap (total, 2 μg of DNA; 1:2 ratio). The latter contains an XbaI/XbaI fragment from psub201 (34) encoding Rep and Cap proteins. Cells were harvested when advanced cytopathic effect developed, usually 48 h posttransfection.
Isolation of Hirt DNA.
Cells seeded in a 35-mm-diameter culture well were harvested, washed with phosphate-buffered saline, and divided into two equal portions for extraction of extrachromosomal DNA and preparation of virus crude lysates. Low-molecular-weight DNA was extracted by the Hirt method (17) with minor modifications. Cells were resuspended in 450 μl of lysis buffer (10 mM Tris-HCl [pH 8.0], 10 mM EDTA, 100 μg of proteinase K/ml) and then lysed by adding sodium dodecyl sulfate (0.6% final concentration). The reaction was then incubated for 2 h at 37°C. After overnight precipitation at 4°C with 1.1 M NaCl, cellular debris were pelleted at 16,000 × g for 30 min and DNA was extracted with phenol:chloroform:isoamyl alcohol (25:24:1) and then chloroform:isoamyl alcohol (24:1). Following ethanol precipitation in the presence of glycogen (final concentration, 30 μg/ml; Roche), the DNA pellet was washed with 70% ethanol, dried, and resuspended in 40 μl of Tris-EDTA buffer containing DNase-free RNase (final concentration, 1 μg/ml; Roche).
Preparation of AAV crude lysate.
The other half of the cells harvested from a 35-mm-diameter dish was resuspended in 500 μl of virus lysis buffer (20 mM Tris, 150 mM NaCl). Following brief sonication the samples were subjected to one freeze-thaw cycle. After removal of cell debris by centrifugation at 3,000 × g for 10 min, the cleared lysates were stored at −80°C.
Analysis of AAV replication intermediates by Southern blotting.
Hirt DNA (10% of total yield from a 35-mm-diameter dish) was digested in a 20-μl reaction volume with various restriction enzymes overnight. Samples were resolved on a 0.8% agarose gel, transferred to a nylon membrane (Hybond-N+; Amersham), and hybridized to a [32P]dCTP random-primer-labeled probe against the CMV.
Assay for site-specific integration.
293 cells were transfected with a Rep-expressing plasmid or pUC19 in 35-mm-diameter plates. Six hours posttransfection, cells were washed and infected with AAV.TRT or AAV.AD (20% of total crude lysate from a 35-mm-diameter dish). After 12 h, media were replaced and cells were incubated for an additional 60 h. Then, cells were harvested and genomic DNA was extracted by using a Qiagen genomic DNA extraction kit.
Integration of ITR-flanked DNA in the AAVS1 site was determined by nested PCR using primer pairs that flank the 5′ or 3′ end of the rAAV genome and AAVS1 site chromosome junction. Primers SM 38 (Ori, 3′ end of rAAV; 5′-TAGTCCTGTCGGGTTTCGCCAC), SM 40 (CMV promoter, 5′ end of rAAV; 5′-CAAGTGGGCAGTTTACCGTA), and SM 33 (AAVS1; 5′-GCGCGCATAAGCCAGTAGAG) (30) were used for the first round of PCR amplification with 500 ng of genomic DNA. The reaction was performed by using touchdown PCR and HotStar Taq polymerase (Qiagen). One percent of the first reaction was subjected to a second amplification by using nested primers SM 20 (Ori, 3′ end of rAAV; 5′-CCACCTCTGACTTGAGCGTC) or SM 39 (CMV promoter, 5′ end of rAAV; 5′-TGGCGTTACTATGGGAACAT) and SM 34 (AAVS1; 5′-ACAATGGCCAGGGCCAGGCAG). Ten percent of the amplification product was resolved on 1.5% agarose gel in duplicates, transferred to a nylon membrane (Hybond-N+; Amersham), and hybridized to AAVS1 or AAV ITR-specific probes. Junction fragments containing both 5′ and 3′ parts of rAAV genome were also subcloned into pCR2.1 (Invitrogen). Sequencing was performed by The Rockefeller University DNA sequencing laboratory by using M13 forward and M13 reverse universal primers.
RESULTS
AD element is the origin of cAAV replication.
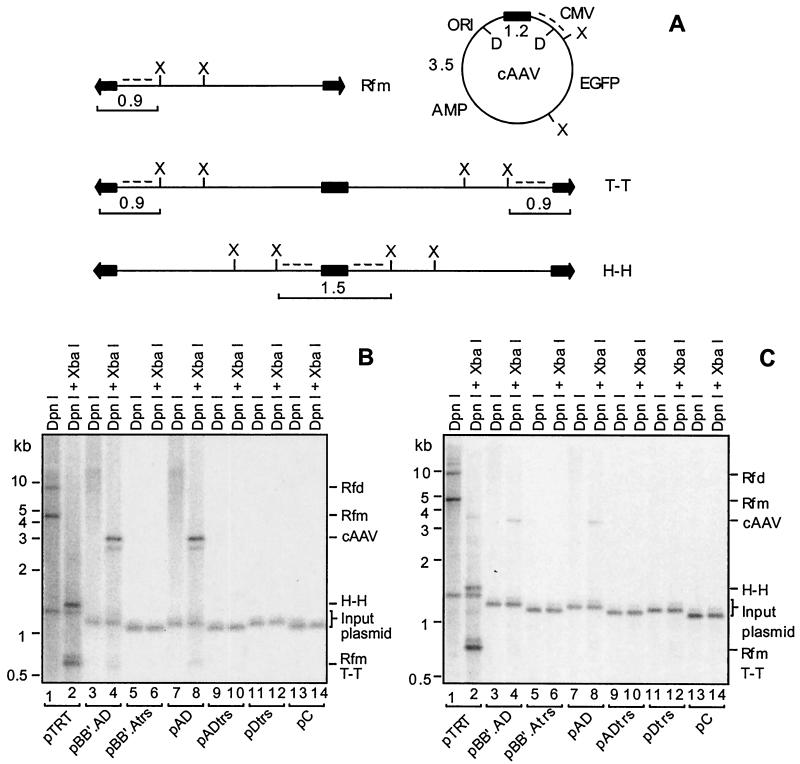
Replication of cAAV constructs containing various deletions in the TRT domain was assayed by using two different models of rAAV propagation in cell culture. Hirt DNA samples digested with DpnI or DpnI and XbaI were resolved on a neutral agarose gel. These enzymes would unambiguously distinguish between different replicative intermediates as well as input plasmid DNA. DpnI selectively cleaves methylated input plasmid but is inactive against templates that have undergone at least one round of replication in mammalian cells. As shown in Fig. 2A, digestion of unreplicated plasmid with DpnI followed by hybridization with a CMV promoter probe is expected to reveal a band of approximately 1.2 kb. When digested with XbaI, Rfm and tail-to-tail RFd should release two ITR fragments which differ in the conformation of the DNA ends. A fragment containing a linear, double-stranded end runs at 0.9 kb, while a fragment containing a hairpin end migrates at 0.8 kb. Head-to-head RFd are expected to liberate a 1.5-kb band. Finally, circular AAV structures are predicted to produce a unique 3.3- to 3.5-kb fragment following XbaI digestion, depending upon the size of the ITR element. As indicated above, DpnI should not cleave any of the replicative forms.
FIG. 2.
Southern blot analysis of replication of cAAV genomes containing deletions in ITRs. (A) Restriction maps of predicted replicative intermediates: a linear monomer (Rfm), circular monomer (cAAV), head-to-head dimer (Rfd, H-H), and tail-to-tail dimer (Rfd, T-T). ITRs are represented by arrows, while the TRT domain is shown as a black box. Other domains include CMV promoter-enhancer (CMV), EGFP cDNA, beta-lactamase gene (Amp), and bacterial origin of replication (Ori). Positions of XbaI (X) and DpnI (D) sites are indicated. The sizes of the fragments liberated following XbaI cleavage and recognized by the CMV promoter-specific probe (dotted line) are shown next to corresponding structures. The position of a 1.2-kb fragment released by DpnI from input plasmids is also indicated. (B) Replication of constructs shown in Fig. 1 following cotransfection with an adenovirus-AAV helper plasmid into 293 cells. Hirt DNA was extracted 72 h posttransfection, and 5% of the total yield from a 35-mm-diameter culture well was digested with DpnI alone or DpnI and XbaI. Samples were resolved on a 0.9% agarose gel, and the blots were hybridized with a 32P-labeled CMV promoter probe. The relative migration of 1-kb size markers is shown to the left of the blot. The AAV replicative intermediates as well as the input plasmid are indicated along the right side of the blot. (C) Replication of the same cAAV constructs after cotransfection with pRep.Cap into adenovirus-infected 293 cells. Cells were harvested 48 h posttransfection, and samples were analyzed as described for the blot in panel B.
The Southern blot data are presented in Fig. 2B and C. DpnI-digested samples for pTRT have revealed a banding profile that is characteristic for AAV lytic replication, including linear duplex Rfm and Rfd (lane 1). It should be noted that cAAV species are not always detected during replication of pTRT in a helper-free system (Fig. 2B, lane 2) but can be readily recognized when adenovirus is used to provide helper functions (Fig. 2C, lane 2) or when a conventional cis plasmid is used as a template (see Fig. 4, lane 2). When the CC′ hairpin and a second copy of the AD sequence were removed (pBB′.AD), dramatic changes in the replication profile were observed. No liner duplex intermediates were clearly detected (Fig. 2B and C, lane 3); instead the plasmid replicated apparently exclusively in a circular form as evidenced by the release of the unique 3.3-kb fragment following XbaI digestion. The absence of intact circular forms in samples cleaved with DpnI alone is likely due to cAAV migration in multiple conformations (e.g., supercoiled and relaxed), which would limit concentration at any one location in a gel. Alternatively, cAAV replicative intermediates could exist as large, poorly resolved heat-to-tail concatemers that would yield only the distinct 3.3-kb band following XbaI cleavage. Equal intensities of the 1.2-kb bands liberated by DpnI from input DNA in each lane suggests that these findings are not a result of variabilities in transfection efficiency, sample loading, or transfer during blotting.
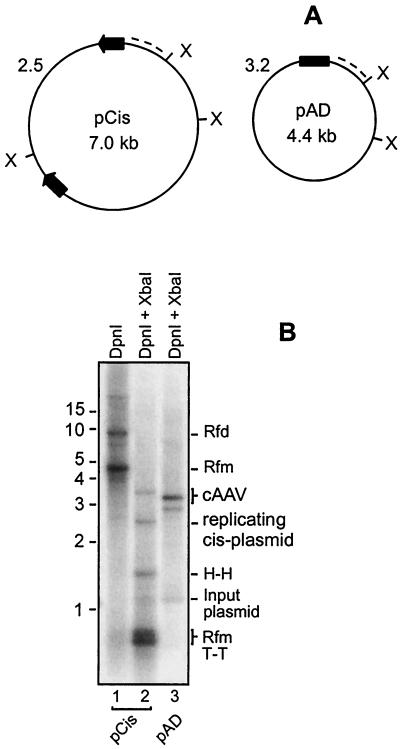
FIG. 4.
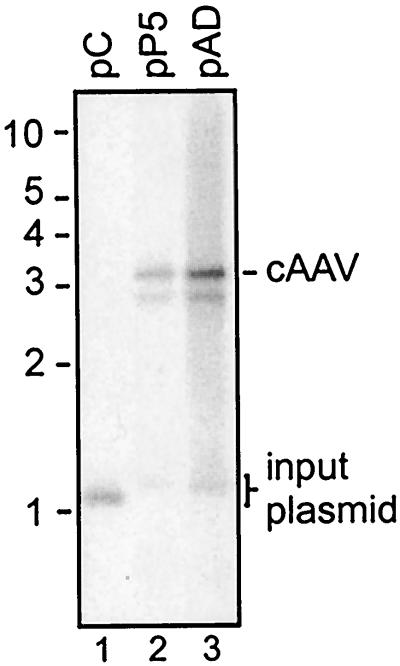
Comparison of replication of pCis and pAD. (A) Schematic representation of pCis and pAD. Positions of XbaI sites (X) are indicated. ITRs of pCis are drawn as arrows and the AD domain of pAD is represented by a box. XbaI cleavage of DpnI-resistant circular species followed by hybridization with a CMV promoter-specific probe (dotted line) is expected to produce 2.5- and 3.2-kb bands for pCis and pAD, respectively. cAAVs assembled during pCis replication are similar in size and structure to pAD, except that they contain the TRT domain. Since pCis would generate the TRT domain that is slightly larger than the AD domain, cAAVs derived from pCis would produce a band of 3.5 kb instead of 3.2 kb. (B) pCis and pAD were assayed for replication as described in the legend to Fig. 2B. Note that linear forms are present in pCis replication but absent in pAD replication. Replicative intermediates are the same as those shown in Fig. 1 and are labeled along the right side of the blot.
We next set out to determine if the D element was essential for the alternative replication pathway. pBB′.Atrs lacks 18 bp of this 20-bp sequence while retaining 2 bp from the trs (Fig. 1). As can be seen in Fig. 2B and C, lanes 5 and 6, no DpnI-resistant material was detected, indicating that the D element is a critical region in the origin of cAAV replication. In order to more precisely localize the minimal 5′ end of the ITR sequence, we removed the BB′ hairpin in pAD. This small internal palindrome has been shown to comprise a cis-acting element (RBE′) important for optimal origin function of the ITR (5). Surprisingly, this alteration did not impair cAAV replication (lane 8). However, deletion of the D element from this construct (pAtrs) completely abolished replication, an observation consistent with the previous finding of the importance of this domain (lanes 9 and 10). Finally, we addressed the involvement of the A sequence in this pathway. Construct pDtrs retains the complete D element and 4 bp from the trs in the A sequence but lacks the rest of this element including the Rbs (Fig. 1). As shown in Fig. 2B and C, lanes 11 and 12, this mutation was deleterious for cAAV replication. Vector pC, which does not contain any AAV sequence, was included as a negative control (lanes 13 and 14). Thus, cis elements required for replication of cAAV can be assigned to a single AD domain of the ITR. The experiments also revealed that that the conventional and alternative pathways are indeed independent and can be completely separated.
Intact trs is essential for cAAV replication.
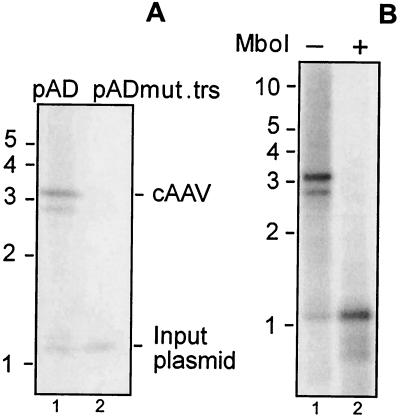
While the AAV replication pathway that is marked by an assembly of linear duplex intermediates requires a functional trs (19), the latter may not be necessary for the replication of cAAV. To test this hypothesis we introduced two point mutations into the trs. Nicking normally occurs between the TT residues in the trs, and these were replaced by two CC residues (Fig. 1). This alteration is expected to block endonuclease reaction mediated by Rep (6). When assayed for replication in 293 cells, this construct produced significantly less DpnI-resistant species than did a control pAD plasmid (Fig. 3A). Equal intensities of the 1.2-kb bands released after DpnI cleavage serve as a control for equal transfection efficiency and gel loading in this experiment. Thus, trs is necessary for efficient replication of AAV via the alternative pathway.
FIG. 3.
Replication of pAD. (A) The trs was mutated in pAD as shown in Fig. 1, and the resulting construct, pADmut.trs, was analyzed for replication as described in the legend of Fig. 2B. pAD was included as a positive control. Bands corresponding to DpnI-resistant cAAVs as well as DpnI-sensitive input plasmids are shown to the right of the blot. (B) pAD undergoes more than one round of DNA synthesis. A Hirt DNA sample from the experiment described in the legend to Fig. 2B corresponding to pAD was digested with XbaI and DpnI with or without MboI. DNA was analyzed as described in the legend to Fig. 2B by using a CMV promoter probe.
cAAVs undergo more than one round of DNA synthesis during replication.
The extent of plasmid DNA replication in mammalian cells can be assayed by resistance to DpnI and MboI. DpnI is active towards templates that have adenosines methylated in the GATC recognition sequence. In contrast, MboI cleaves the same site only if both strands are unmethylated. Since such methylation is performed only in dam+ bacteria but not mammalian cells, sensitivity to DpnI and resistance to MboI indicate that the plasmid has not replicated. Following two rounds of replication, both DNA strands become unmethylated and the plasmid will be DpnI-resistant and MboI-sensitive. We analyzed pAD replication products from the same experiment as that described for Fig. 2B. Hirt DNA samples were digested with XbaI to release a 3.2-kb band unique for cAAV and then with DpnI or DpnI and MboI. As presented in Fig. 3B, all DpnI-resistant species were also MboI-sensitive (compare lanes 1 and 2). Indeed, a 3.2-kb band corresponding in mobility to replicated pAD was completely converted to a 1.2-kb fragment positioned between two DpnI/MboI sites (Fig. 2A). This finding establishes that pAD replication products are the result of more than one round of DNA synthesis.
Substitution of ITR with the AD domain enhances cAAV replication.
We found that cAAV replication with pTRT as a template is less efficient than that with conventional cis plasmids, which harbor a rAAV genome with two complete ITRs separated by a stuffer sequence. This vector (pCis) contains the same non-AAV sequence as pAD and has two intact ITRs derived from psub201 (34) separated by a stuffer sequence (Fig. 4A). As shown in Fig. 4B, cAAVs were assembled far more efficiently during pCis propagation than during replication of pTRT (compare Fig. 4B, lane 2 and Fig. 2B, lane 2). Similar intensities of the 1.2-kb bands released after DpnI cleavage of input plasmid serve as a control for equal transfection efficiency and gel loading in this experiment (compare lanes 2 and 3 in Fig. 4B). Densitometry analysis of this blot established that cAAV intermediates constitute approximately 10% of linear duplex structures (Rfm). This observation confirms a previous report that cAAV is reproducibly identified during AAV replication. (26). Equally important, this experiment revealed that a substitution of ITRs with a single AD domain enhances replication of cAAV (compare lanes 2 and 3 in Fig. 4B) while eliminating generation of linear forms. This effect may be underestimated given the fact that pAD contains only one copy of this element while pCis has two AD copies in opposite orientations. Thus, these results further corroborate that elimination of the more efficient primary replication pathway leaves the alternative circular pathway as the only option for replication.
Circular intermediates are efficient templates for AAV packaging.
All of the cAAV constructs used in this study have a size of approximately 4.4 to 4.6 kb, so they could be packaged without rescue if such rescue-independent encapsidation is possible. Production of infectious virions was directly assayed on 293 cells by limiting dilution of crude cell lysates. These lysates were prepared from a portion of the samples that were used for the replication assay presented in Fig. 2B. This permits conformation of equal plasmid transfection efficiencies of the plasmids by Southern blotting and allows us to correlate replication profiles and packaging.
As shown in Table 1, relatively high numbers of EGFP-positive cells were observed for some samples 24 h postinfection (Table 1). As might be expected, there was a clear association between the ability to replicate and the ability to generate infectious virions. This is best illustrated by comparing replication profiles of pTRT, pBB′.AD, and pAD (Fig. 2B) with the corresponding infectious particle titers (Table 1). No EGFP-positive cells were found for the other constructs including the negative control. For clarity, we will use an AAV prefix to indicate virus, while prefix p will refer to a corresponding plasmid; e.g., AAV.AD is a virus produced by pAD. Virtually no difference between AAV.BB′.AD and AAV.AD titers was found, an observation consistent with a similar efficiency of replication of the corresponding plasmids (compare lanes 4 and 8 in Fig. 2B). This once again establishes that the BB′ palindrome is dispensable for AAV replication once a switch to a different replication pathway has occurred.
TABLE 1.
Circular AAV replication and packaging
| Construct | Rfm and Rfda | cAAVa | Virus yieldb (IU) |
|---|---|---|---|
| pTRT | + | + | 2.6 × 102 |
| pBB′ AD | − | + | 1.2 × 104 |
| pBB′ Atrs | − | − | 0 |
| pAD | − | + | 1.1 × 104 |
| pAtrs | − | − | 0 |
| pDtrs | − | − | 0 |
| pC | − | − | 0 |
As determined by Southern blot analysis shown in Fig. 2B. +, presence; −, absence.
Total yield from a 35-mm-diameter plate.
pAD packages single-stranded genomes.
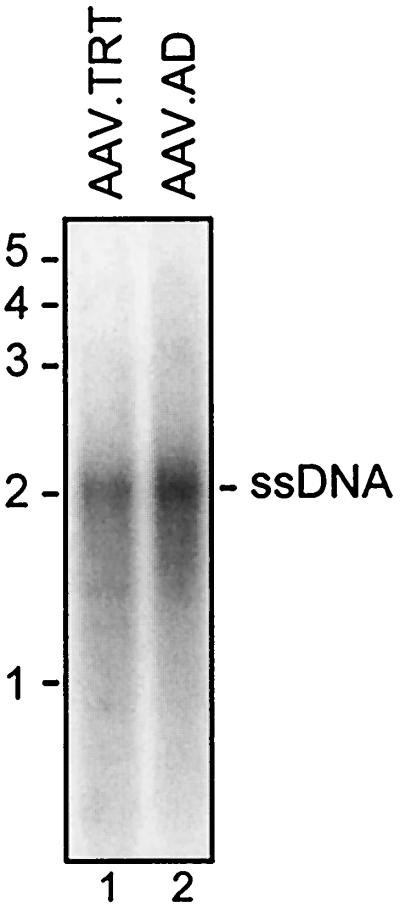
To examine the structure of packaged AAV.AD genomes, crude lysates were extensively digested with DNase I, and virion DNA was then extracted, resolved on a neutral agarose gel, and analyzed by Southern blotting. As presented in Fig. 5, a 2.2-kb band corresponding in size to ssDNA was released from AAV.AD virions. AAV.TRT was included as a positive control. Thus, the result established that virions produced by pAD indeed contain full-length ssDNA.
FIG. 5.
Southern blot analysis of encapsidated AAV genomes. Viral stocks of AAV.AD and AAV.TRT as a positive control (50% of total yield from a 35-mm-diameter plate) were extensively digested with DNase I, and ssDNA was extracted and separated on a neutral 1% agarose gel. The blot was hybridized with a CMV promoter-specific probe.
AAV.AD retains the ability for site-specific integration.
Since AAV.AD contains only a truncated single copy of ITR, we tested the ability of this domain to target site-specific recombination. For this purpose we applied a technique used by other groups (30) that is based on PCR amplification of AAV-AAVS1 junctions from genomic DNA. To distinguish between unidirectional and bidirectional integration, we used sets of nested primers for both 5′ and 3′ ends of the vector sequence. Infections were performed in 293 cells in the presence or absence of Rep provided by transient plasmid transfection. AAV.TRT was included as a positive control.
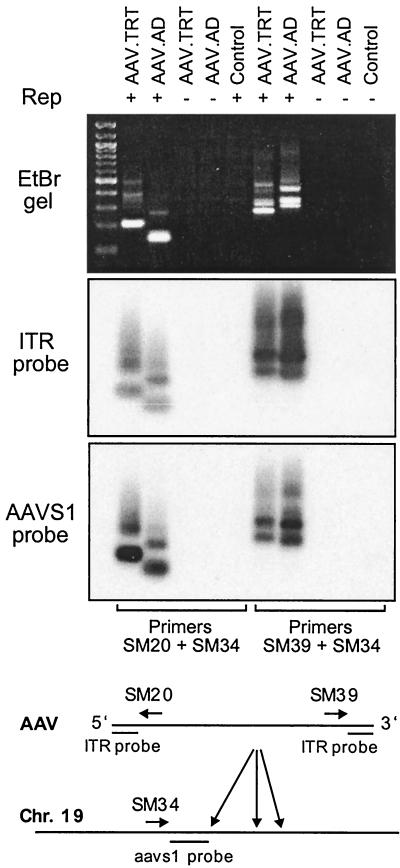
As shown in Fig. 6, specific DNA bands were amplified from 293 cells infected with both AAV.TRT and AAV.AD. The product appears as a smear with multiple bands, which probably reflects the heterogeneity of junction species in a population of transduced cells. Note that no signal was detected in mock-infected cells or cells infected with viruses in the absence of Rep. To confirm the nature of the amplified product, duplicate blots were hybridized with ITR- or AAVS1-specific probes. There is a good correspondence of the hybridization signals between these two blots, further suggesting that the fragments indeed include both AAV and AAVS1 sequences. Equally important, this experiment revealed the ability of AAV.AD genome to integrate in either orientation despite the polarity of the AD domain in a vector plasmid. The PCR products containing both 5′ and 3′ termini of AAV.AD were also subcloned into pCR2.1 (Invitrogen) and sequenced. While the analysis revealed the presence of both AAVS1 and AAV.AD sequences in all the clones analyzed, extensive deletions both within the AD domain and in the integration site were detected (data not shown). This finding, however, is consistent with other reports on rAAV integration marked by rearrangements of an integration site and viral termini (24, 40). Taken together, these results establish that a single AD domain in the context of a virion genome serves as an efficient signal for Rep-mediated site-specific recombination.
FIG. 6.
Site-specific integration of AAV.AD. 293 cells were infected with AAV.AD or AAV.TRT (positive control) in the presence or absence of Rep. Genomic DNA was extracted 72 h postinfection and subjected to nested PCR. Mock-infected cells were included as a negative control. PCR products were analyzed on an ethidium bromide gel (top) and duplicate Southern blots (bottom), which were analyzed using 32P-labeled ITR-specific or AAVS1-specific probes. A 100-bp ladder was loaded into the first lane. Viruses used for the assay are indicated along the top of the gel. The AAV genome, AAVS1 integration site, and positions of primers and probes are schematically represented at the bottom.
Rep P5 promoter is another origin of cAAV replication.
We have identified a minimal ITR sequence that encompasses Rbs, trs, and the D element as an origin of an alternative pathway of AAV replication. With that in mind, we turned our attention to other sequences, known to contain such elements. One of the best-characterized Rep-binding elements is mapped to the AAV endogenous P5 promoter. Indeed, it has been found to be involved in amplification of integrated Rep-Cap sequences in HeLa cells (8, 29, 41) as well as to enhance the propagation of wtAAV itself (42). Considering the high homology between the AD domain and the P5 promoter, we speculated that all these phenomena are examples of the alternative replication pathway described here. To test this hypothesis, plasmid pP5 was created by substituting the 60-bp AD domain in pAD with an 86-bp NlaIII fragment from psub201 (34) containing the P5 promoter (nucleotides 238 to 324 of AAV-2) (Fig. 7). This element was inserted in a direct orientation, i.e., the same orientation as in psub201. pP5, pC (a negative control), and pAD (a positive control) were transfected into 293 cells along with a full complement of helper functions and then assayed for replication and packaging.
FIG. 7.
Comparison of pP5 and pAD replication. pP5 and pAD were assayed for replication as described in the legend to Fig. 2B. pC was included as a negative control. Note the absence of linear duplex intermediates in lanes 1 and 2. Replicating cAAV and input plasmids are indicated.
We were surprised to discover that the replication profile of pP5 was virtually indistinguishable from that of pAD. In fact, as shown in Fig. 7, both plasmids replicated exclusively in a circular form and no linear duplex intermediates were detected (compare lanes 2 and 3). Even more remarkable, pP5 was a template for packaging as well, albeit approximately fivefold less efficiently than pAD. This can be illustrated by comparing functional AAV titers produced by pAD and pP5 (Table 2). Note that both vectors demonstrated a similar level of increase in transduction efficiency by a secondary infection with adenovirus. In summary, the results establish that cis signals for cAAV replication and packaging are not limited to the AD domain of the ITR but may include other homologous sequences, e.g., the P5 promoter.
TABLE 2.
Packaging of pP5
As determined by Southern blot analysis shown in Fig. 8. +, presence; −, absence.
Total yield from a 35-mm-diameter plate.
DISCUSSION
AAV lytic replication appears to proceed along two different pathways marked by the assembly of linear or circular duplex intermediates. While the origin of the conventional pathway has been well characterized, cis-acting elements that direct replication of circular viral genomes are unknown. Previous studies of cis-acting elements mediating liner AAV replication used duplex wt genomes in the context of a recombinant plasmid (44, 45). These studies have helped to identify the major ITR elements that direct AAV replication along the conventional pathway (44-46). However, AAV propagation in these experiments was rescue dependent, since encapsidation required excision of AAV genomes from recombinant plasmids. Even if rescue-independent packaging was possible, it would have remained undetected since the size of the AAV cis-plasmids considerably exceeds the packaging capacity of AAV (3). In addition, wtAAV carries at least one cis-acting replication element outside the ITR that is mapped to the P5 promoter of the rep gene (29, 42). In the present report we show that this element is in fact an efficient origin of circular, but not linear, AAV replication (Fig. 7). Another sequence in proximity to the endogenous promoter P19 may also be involved in replication, considering its high Rep-binding potential (25) and existence of a functional trs (47). Thus, mutational analysis of ITRs in the context of wt genomes cannot unambiguously identify any ITR sequence as a functional origin of cAAV replication. Moreover, another possible cis element, derived from pBR322 and possessing a strong Rep-binding activity, lies within non-AAV DNA of psub201 (25). In order to eliminate this as a variable, we removed a pBR322-derived Rep-binding element and constructed a series of plasmids that do not contain any AAV sequences other than ITRs. These modifications facilitated the conclusive demonstration that the 60-bp AD sequence is both an origin of cAAV replication and a packaging signal.
The identified cis-acting replication element encompasses the A stem and the D sequence with an intact trs and apparently does not require any other ITR domains or 5′ untranslated region (UTR). Of interest, the BB′ hairpin, known to contain a cis signal (RBE′) essential for an efficient Rep-ITR interaction (5), does not enhance cAAV replication (Fig. 2). In attempting to understand this observation, it is important to recognize that RBE′ is not necessary for Rep-catalyzed trs cleavage. Instead, it is required for unwinding of the ITR by the Rep helicase and formation of a trs stem-loop nicking intermediate composed of annealed A and D elements. Indeed, when a wt ITR substrate was substituted with a template which has a preformed trs stem-loop, RBE′ was no longer necessary for the endonuclease reaction to occur (5). Such hairpins are unstable in linear substrates, but in superhelical circular molecules these secondary structures are thermodynamically favored. Analysis of purified cAAV genomes assembled during lytic replication in vivo has revealed that, at least in part, these species are not only represented by covalently closed circular molecules but also are in fact supercoiled (26). Therefore, in cAAV the trs cruciform extrusion may no longer depend on Rep helicase activity. As a result, Rep interaction with the BB′ hairpin would not be essential for efficient trs cleavage. This may explain why the AD domain is the only ITR sequence necessary and sufficient to initiate replication in the context of a circular superhelical replicative intermediate.
Our data indicate that cAAV undergoes rescue-independent encapsidation, a process that is not contingent upon resolution of a Holliday-like ITR structure, such as that necessary for excision of linear duplex intermediates from plasmid DNA or cell chromatin. This finding is consistent with earlier observations that monomer size plasmids containing a portion of ITR with 5′ UTR produce infectious virions (K. J. Fisher, personal communications). As evident from Table 1, cAAV constructs with a single AD domain (pBB′AD and pAD) produce infectious virions at a level comparable to that of circular intermediates with intact ITRs (pTRT), despite the fact that they replicate via different pathways. Preliminary analysis revealed that encapsidated virion genomes consist of ssDNA of wt size. An accepted model of AAV transduction has implied that second-strand synthesis requires self-priming from a hairpin of a terminal repeat (14, 15). Intriguingly, we have found that virions with a single AD domain transduce cells efficiently, despite the absence of terminal hairpins. This may suggest that other mechanisms of generating double-stranded transcription templates are operating. Further studies of the molecular basis of AAV.AD transduction should shed light on a general mechanism with relevance to AAV biology.
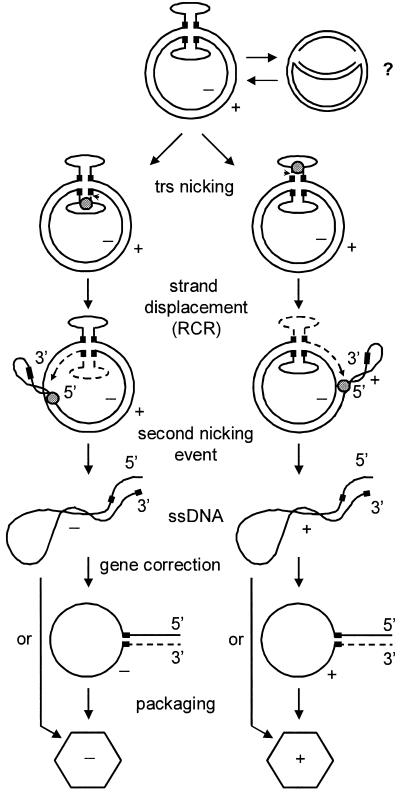
To explain replication of cAAV and production of ssDNA genomes without generating liner duplex intermediates, we propose a model outlined in Fig. 8. Under conditions permissive for AAV lytic replication, Rep binds to the A stem and initiates a rolling-circle replication (RCR) by nicking the trs. This is followed by an extension of the newly generated 3′ end and a simultaneous strand displacement, events that probably involve a helicase activity of small Rep proteins. Given the fact that in the TRT domain there are two AD elements in opposite orientations and Rep endonuclease activity is strand specific, both strands, positive and negative, can be nicked and displaced. After the replication fork has completed a full circle, the displaced strand is cleaved by Rep, known to contain both ssDNA binding and nicking activities (36). Our model predicts that the resulting ssDNA genomes would have a complete ITR only on the 5′ end, while the 3′ end will carry only one D element. The missing ITR, however, can be repaired via a gene correction mechanism described for AAV by Samulski et al. (35). This mechanism has been suggested to occur during normal AAV replication in vivo and allows AAV to efficiently repair extensive deletions within one ITR provided that the other terminus is intact. This event involves a formation of a single-stranded panhandle intermediate in which both D elements being inverted have annealed to initiate a repair DNA synthesis. The resulting progeny ssDNA genomes will have wt termini on both ends and can then be packaged into preformed capsids similar to genomes generated by linear duplex templates (23, 27). Alternatively, in the absence of ITR repair, truncated genomes are encapsidated, thus accounting for the generation of interfering particles. In the Fig. 8 cartoon we displayed only the episodes of the alternative pathway. Should the TRT resolution antecede the initiation of cAAV replication, a linear duplex intermediate is liberated and the replication proceeds along the more efficient conventional pathway (26).
FIG. 8.
Model of cAAV replication and encapsidation. Circular genomes are depicted with hairpins extruded. Black boxes on each of the DNA strands designate the D sequence. Small gray circle, Rep protein complexes bound to the A stem; dashed line, nascent leading strand. Positions of trs are indicated by arrowheads. See text for discussion.
There are several notable features of this model. Progeny strands are synthesized continuously by movement of the replication complex around the circular template, a mechanism known as RCR (11, 22). RCR has been applied to AAV lytic replication as a mechanism for resolution of dimer intermediates (36). Here we propose that RCR is in fact an essential pathway responsible for the propagation of cAAV. In attempting to understand the events of the alternative pathway of AAV replication, it is useful to recognize similarities with other genetic systems. In particular, cAAV replication shows a striking resemblance to that of certain bacteriophages (50). First, the infecting bacteriophage ssDNA is converted to a double-stranded circular intermediate. Then a virus-encoded protein (Rep analog) introduces a nick in the positive strand and initiates RCR. After one round of replication is completed, the displaced strand is cleaved and the ends are ligated, thus providing a template for further rounds of replication. In contrast to these bacteriophages, which have only one nicking site, cAAV has two such sites in opposite orientations, thereby facilitating displacement and encapsidation of both positive and negative strands. It appears that the episodes of a novel pathway of replication that we describe in this report are not limited to AAV but rather are shared by a number of viruses, prokaryotic and eucaryotic replicons (11, 22). This is further supported by the discovery by Smith and Kotin (36) of RCR initiator protein-like activity of Rep78 and the presence of the two conserved amino acid sequence RCR-associated motifs (motifs 2 and 3) that are also found in the initiator proteins of other plasmids and viruses, such as pUB110 (plasmid of gram-positive bacteria), φX174 (ssDNA bacteriophage), TYLCV (tomato yellow leaf curl geminivirus), and a human parvovirus B19 (36). This raises the possibility that the pathway of AAV replication that involves the assembly of circular genomes may have appeared earlier in evolution while a more efficient self-priming mechanism evolved later.
In this study we found that the AD domain is not the only cis element that can direct cAAV replication and packaging. Instead, other homologous sequences, e.g., AAV endogenous P5 promoter, appear to have similar activity (Fig. 7). This region has been shown to possess a high affinity to Rep (25) and contain a functional trs (47). When combined with the observation that it is involved in integrated rep-cap gene amplification (8, 29, 41) and is responsible for more efficient propagation of wtAAV than that of recombinant vectors (42), a common theme emerges. We hypothesize that these phenomena are examples of the alternative pathway of AAV replication described here and may be explained by the model presented in Fig. 8. In fact, when a so-called CARE element, containing a P5 promoter sequence, was subcloned into a vector plasmid, the latter replicated exclusively in a circular form (29). In addition, applying a similar experimental design but using a vector of wt size (4.4 kb), we found that this element is a signal for encapsidation as well (Table 2). Intriguingly, our model predicts that AAVS1, a natural wtAAV integration site on the human chromosome 19, which encompasses both RBS and trs, should also direct cAAV replication. Consistent with this hypothesis, early studies using an in vitro replication assay with purified Rep proteins have revealed that a plasmid containing this integration locus (P1) replicated as an open circular molecule (43).
An important question that remains to be elucidated, however, is the role of the D sequence in AAV replication and encapsidation. Studies conducted by other groups have identified the D sequence as an essential replication element as well as a packaging signal (44-46). Our data also lend support to these findings, since deletion of the D element completely abolished cAAV propagation (Fig. 2). However, there is little homology between the ITR D element and a sequence downstream from the trs of the P5 promoter. Still, pP5 replicated and produced infectious virions at levels comparable to that of pAD (Fig. 7 and Table 2). Further detailed mutational analysis of the D element, P5 promoter, as well as the P1 replication element from the AAVS1 integration locus may identify the minimal cis-acting signal and eventually shed light on the role of this domain.
Acknowledgments
We are grateful to Thomas Shenk for his helpful suggestions during work on the manuscript. We also thank Krishna J. Fisher, Tara Scully, and Lorita Dudus for sharing preliminary data relevant to this project and helpful discussions.
REFERENCES
- 1.Berns, K. I. 1990. Parvovirus replication. Microbiol. Rev. 54:316-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns, K. I., and R. A. Bohenzky. 1987. Adeno-associated viruses: an update. Adv. Virus. Res. 32:243-306. [DOI] [PubMed] [Google Scholar]
- 3.Berns, K. I., and C. Giraud. 1996. Adeno-associated virus (AAV) vectors in gene therapy. Springer, Berlin, Germany.
- 4.Berns, K. I., R. M. Kotin, and M. A. Labow. 1988. Regulation of adeno-associated virus DNA replication. Biochim. Biophys. Acta 951:425-429. [DOI] [PubMed] [Google Scholar]
- 5.Brister, J. R., and N. Muzyczka. 2000. Mechanism of Rep-mediated adeno-associated virus origin nicking. J. Virol. 74:7762-7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brister, J. R., and N. Muzyczka. 1999. Rep-mediated nicking of the adeno-associated virus origin requires two biochemical activities, DNA helicase activity and transesterification. J. Virol. 73:9325-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter, B. J., C. J. Marcus-Sekura, C. A. Laughlin, and G. Ketner. 1983. Properties of an adenovirus type 2 mutant, Ad2dl807, having a deletion near the right-hand genome terminus: failure to help AAV replication. Virology 126:505-516. [DOI] [PubMed] [Google Scholar]
- 8.Chadeuf, G., D. Favre, J. Tessier, N. Provost, P. Nony, J. Kleinschmidt, P. Moullier, and A. Salvetti. 2000. Efficient recombinant adeno-associated virus production by a stable rep-cap HeLa cell line correlates with adenovirus-induced amplification of the integrated rep-cap genome. J. Gene Med. 2:260-268. [DOI] [PubMed] [Google Scholar]
- 9.Chejanovsky, N., and B. J. Carter. 1989. Mutagenesis of an AUG codon in the adeno-associated virus rep gene: effects on viral DNA replication. Virology 173:120-128. [DOI] [PubMed] [Google Scholar]
- 10.Collaco, R. F., X. Cao, and J. P. Trempe. 1999. A helper virus-free packaging system for recombinant adeno-associated virus vectors. Gene 238:397-405. [DOI] [PubMed] [Google Scholar]
- 11.del Solar, G., R. Giraldo, M. J. Ruiz-Echevarria, M. Espinosa, and R. Diaz-Orejas. 1998. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 62:434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan, D., P. Sharma, J. Yang, Y. Yue, L. Dudus, Y. Zhang, K. J. Fisher, and J. F. Engelhardt. 1998. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J. Virol. 72:8568-8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan, D., Z. Yan, Y. Yue, and J. F. Engelhardt. 1999. Structural analysis of adeno-associated virus transduction circular intermediates. Virology 261:8-14. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari, F. K., T. Samulski, T. Shenk, and R. J. Samulski. 1996. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 70:3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher, K. J., G. P. Gao, M. D. Weitzman, R. DeMatteo, J. F. Burda, and J. M. Wilson. 1996. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 70:520-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm, D., A. Kern, K. Rittner, and J. A. Kleinschmidt. 1998. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 9:2745-2760. [DOI] [PubMed] [Google Scholar]
- 17.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 18.Hong, G., P. Ward, and K. I. Berns. 1994. Intermediates of adeno-associated virus DNA replication in vitro. J. Virol. 68:2011-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Im, D. S., and N. Muzyczka. 1990. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell 61:447-457. [DOI] [PubMed] [Google Scholar]
- 20.Janik, J. E., M. M. Huston, K. Cho, and J. A. Rose. 1989. Efficient synthesis of adeno-associated virus structural proteins requires both adenovirus DNA binding protein and VA I RNA. Virology 168:320-329. [DOI] [PubMed] [Google Scholar]
- 21.Janik, J. E., M. M. Huston, and J. A. Rose. 1981. Locations of adenovirus genes required for the replication of adenovirus-associated virus. Proc. Natl. Acad. Sci. USA 78:1925-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan, S. A. 1997. Rolling-circle replication of bacterial plasmids. Microbiol. Mol. Biol. Rev. 61:442-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King, J. A., R. Dubielzig, D. Grimm, and J. A. Kleinschmidt. 2001. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 20:3282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotin, R. M., R. M. Linden, and K. I. Berns. 1992. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 11:5071-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarty, D. M., D. J. Pereira, I. Zolotukhin, X. Zhou, J. H. Ryan, and N. Muzyczka. 1994. Identification of linear DNA sequences that specifically bind the adeno-associated virus Rep protein. J. Virol. 68:4988-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musatov, S. A., T. A. Scully, L. Dudus, and K. J. Fisher. 2000. Induction of circular episomes during rescue and replication of adeno-associated virus in experimental models of virus latency. Virology 275:411-432. [DOI] [PubMed] [Google Scholar]
- 27.Myers, M. W., and B. J. Carter. 1980. Assembly of adeno-associated virus. Virology 102:71-82. [DOI] [PubMed] [Google Scholar]
- 28.Ni, T. H., W. F. McDonald, I. Zolotukhin, T. Melendy, S. Waga, B. Stillman, and N. Muzyczka. 1998. Cellular proteins required for adeno-associated virus DNA replication in the absence of adenovirus coinfection. J. Virol. 72:2777-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nony, P., J. Tessier, G. Chadeuf, P. Ward, A. Giraud, M. Dugast, R. M. Linden, P. Moullier, and A. Salvetti. 2001. Novel cis-acting replication element in the adeno-associated virus type 2 genome is involved in amplification of integrated rep-cap sequences. J. Virol. 75:9991-9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palombo, F., A. Monciotti, A. Recchia, R. Cortese, G. Ciliberto, and N. La Monica. 1998. Site-specific integration in mammalian cells mediated by a new hybrid baculovirus-adeno-associated virus vector. J. Virol. 72:5025-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson, W. D., and H. Westphal. 1981. A cascade of adenovirus early functions is required for expression of adeno-associated virus. Cell 27:133-141. [DOI] [PubMed] [Google Scholar]
- 32.Richardson, W. D., and H. Westphal. 1984. Requirement for either early region 1a or early region 1b adenovirus gene products in the helper effect for adeno-associated virus. J. Virol. 51:404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samulski, R. J., K. I. Berns, M. Tan, and N. Muzyczka. 1982. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc. Natl. Acad. Sci. USA 79:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samulski, R. J., L. S. Chang, and T. Shenk. 1987. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 61:3096-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samulski, R. J., A. Srivastava, K. I. Berns, and N. Muzyczka. 1983. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell 33:135-143. [DOI] [PubMed] [Google Scholar]
- 36.Smith, R. H., and R. M. Kotin. 2000. An adeno-associated virus (AAV) initiator protein, Rep78, catalyzes the cleavage and ligation of single-stranded AAV ori DNA. J. Virol. 74:3122-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder, R. O., D. S. Im, and N. Muzyczka. 1990. Evidence for covalent attachment of the adeno-associated virus (AAV) rep protein to the ends of the AAV genome. J. Virol. 64:6204-6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder, R. O., R. J. Samulski, and N. Muzyczka. 1990. In vitro resolution of covalently joined AAV chromosome ends. Cell 60:105-113. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava, A., E. W. Lusby, and K. I. Berns. 1983. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J. Virol. 45:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surosky, R. T., M. Urabe, S. G. Godwin, S. A. McQuiston, G. J. Kurtzman, K. Ozawa, and G. Natsoulis. 1997. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J. Virol. 71:7951-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tessier, J., G. Chadeuf, P. Nony, H. Avet-Loiseau, P. Moullier, and A. Salvetti. 2001. Characterization of adenovirus-induced inverted terminal repeat-independent amplification of integrated adeno-associated virus rep-cap sequences. J. Virol. 75:375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tullis, G. E., and T. Shenk. 2000. Efficient replication of adeno-associated virus type 2 vectors: a cis-acting element outside of the terminal repeats and a minimal size. J. Virol. 74:11511-11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urcelay, E., P. Ward, S. M. Wiener, B. Safer, and R. M. Kotin. 1995. Asymmetric replication in vitro from a human sequence element is dependent on adeno-associated virus Rep protein. J. Virol. 69:2038-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, X. S., S. Ponnazhagan, and A. Srivastava. 1996. Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions. J. Virol. 70:1668-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, X. S., S. Ponnazhagan, and A. Srivastava. 1995. Rescue and replication signals of the adeno-associated virus 2 genome. J. Mol. Biol. 250:573-580. [DOI] [PubMed] [Google Scholar]
- 46.Wang, X. S., K. Qing, S. Ponnazhagan, and A. Srivastava. 1997. Adeno-associated virus type 2 DNA replication in vivo: mutation analyses of the D sequence in viral inverted terminal repeats. J. Virol. 71:3077-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, X. S., and A. Srivastava. 1997. A novel terminal resolution-like site in the adeno-associated virus type 2 genome. J. Virol. 71:1140-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward, P., and K. I. Berns. 1991. In vitro rescue of an integrated hybrid adeno-associated virus/simian virus 40 genome. J. Mol. Biol. 218:791-804. [DOI] [PubMed] [Google Scholar]
- 49.Xiao, X., W. Xiao, J. Li, and R. J. Samulski. 1997. A novel 165-base-pair terminal repeat sequence is the sole cis requirement for the adeno-associated virus life cycle. J. Virol. 71:941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zinder, N. D., and K. Horiuchi. 1985. Multiregulatory element of filamentous bacteriophages. Microbiol. Rev. 49:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]