Abstract
The extraordinary climbing skills of gecko lizards have been under investigation for a long time. Here we report results of direct measurement of single spatula forces in air with varying relative humidities and in water, by the force-distance method using an atomic force microscope. We have found that the presence of water strongly affects the adhesion force and from analysis of our results, we have demonstrated that the dominant force involved is the capillary force.
The climbing ability of gecko lizards has attracted human attention for more than two millennia. The total clinging force produced by a Tokay gecko can be >20 N (1), a strong force for an animal with a body weight of ∼43 g and an average 227 mm2 of footpad area. The gecko footpad areas are covered with hundreds of thousands of setae with a density (2) of 5300/mm2. Each seta is branched into hundreds of spatulas with dimensions of ∼100 nm, as shown in Fig. 1 (bottom left and right). This configuration allows the spatulas to follow the surface topography. Assuming that all spatulas are in contact with the surface, the adhesion force contribution of individual seta and spatula is ∼20 μN and tens of nanonewtons, respectively. Numerous attempts have been made to study and understand the nature of the adhesive force between the spatulas and the surface (3) as well as the effect of spatula orientation (4) but the complex structure of a gecko seta has made it difficult to determine how many spatulas are in instantaneous physical contact with the sensor. Nevertheless it is crucial to understand the nature of gecko adhesion force to manufacture gecko mimicking devices (5). In general, the total force between two surfaces in close proximity consists of up to 11 components (6), including the van der Waals, dipole, and capillary forces. However, discrimination among the individual force components presents a considerable challenge especially for weak surface interaction forces such as the van der Waals force, because it is typically accompanied by stronger forces such as the capillary force in air or a dipole force in water. To determine the amplitude of the van der Waals force, the measurement has to be done either in a liquid or completely dry (high vacuum) environment to eliminate the capillary force (7). Due to the fact that in any natural habitat the relative humidity (RH) is always at least 10%, it is possible that the capillary force plays a role in the gecko's consistently impressive adhesion. In this contribution we report results of direct measurement of force between a single spatula and a tipless AFM cantilever by the force distance method in a controlled fluid environment to determine the nature of gecko's adhesion force.
FIGURE 1.
Images of spiny-tailed house gecko and its seta. (Top) A close-up photograph of the gecko on a glass-covered mirror used in this study. At the bottom are scanning electron microscope images of gecko setae showing the tree-like structure with a magnification of 900 times (left) and 8500 times (right).
The atomic force microscope (AFM), introduced ∼20 years ago (8), has brought about new opportunities to study surface and material properties at the subnanometer scale as well as to enable the study of interaction forces between two objects in a controlled environment by the force-distance method with sensitivity in the piconewton range (9). It was previously used to determine the adhesion force between a spider leg seta and the flattened tip of a silicon nitride AFM cantilever (10).
In our experiment, a fresh single gecko toe from a spiny-tailed house gecko (Hemidactylus frenatus) was glued with an epoxy resin to a magnetic sample plate of an AFM (Multimode, Veeco, Santa Barbara, CA) equipped with a tipless cantilever, with the gecko setae facing up. Because the spatula's heights are uneven (see Fig. 1, bottom right), we can expect one spatula to come into contact with the cantilever first. As the vertical sensitivity of the AFM is better than 0.1 nm, the probability of contacting two setae simultaneously without distinguishing between them is negligible. Furthermore, employing statistical methods eliminates this possible problem. It leads to a conclusion that we can test a multisetae sample as described above and get identical results as those from a single seta, as demonstrated by our experiment (results are not shown in this article). Working with a whole gecko toe instead of individual spatulas greatly simplifies sample preparation and makes it feasible to mount the sample into the liquid cell of the AFM.
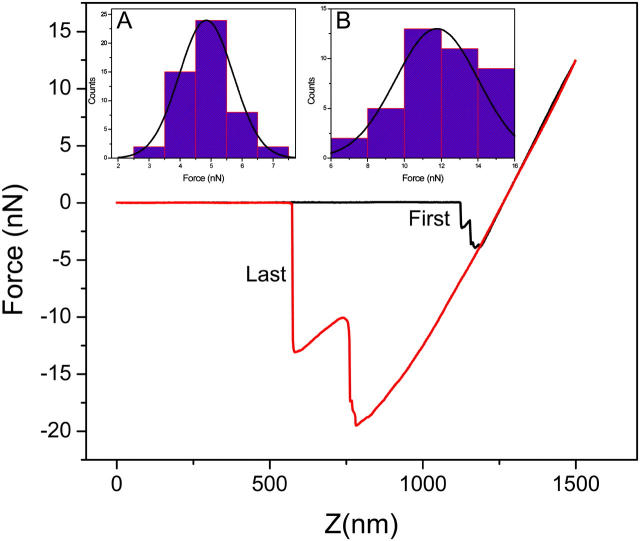
Fig. 2 shows a typical force-distance measurement of gecko spatulas with the curve exhibiting a saw-tooth pattern. The force magnitude of a saw tooth is related to the force contribution of individual spatulas through a complex interdependent network of effective spatula stalk springs. However, the first (during contact) and last (during release) saw-tooth data, shown in Fig. 2, represent isolated events.
FIGURE 2.
Force-distance curve measured with a silicon cantilever with a spring constant of 0.1 N/m, in air with RH of 70%. Histograms of forces measured with hydrophilic (inset A) and hydrophobic (inset B) silicon cantilevers (hydrophobic surface). The black and red lines are the extending curve and retracting curve, respectively.
It is possible that more than one spatula may have almost the same height and contact or release from the cantilever surface simultaneously. In such a case, the measured adhesion force would be significantly stronger. More than 50 measurements were carried out in each experiment and the measured adhesion forces are plotted in a histogram (see Fig. 2, insets) to find the most probable value, distinguishing multispatula adhesion results from the more frequent single spatula event. The measured adhesion force for a single spatula can be deduced by this analysis although there are hundreds of spatulas on each seta.
As the variation in force with surface hydrophobicity is a major feature of capillary forces (11), modification of the cantilever hydrophobicity is a conventional way to determine its amplitude. We have measured the cantilever-gecko force interaction with silicon cantilevers with surface water contact angles of 30° (hydrophilic) and 110° (hydrophobic). The mean adhesion force derived from histograms (shown in Fig. 2) was 11.8 and 4.9 nN, respectively, which suggests that the dominating component of the gecko force is the capillary force as the amplitude of van der Waals force decreases instead of increases with the increase in water between the surface and the spatula (7).
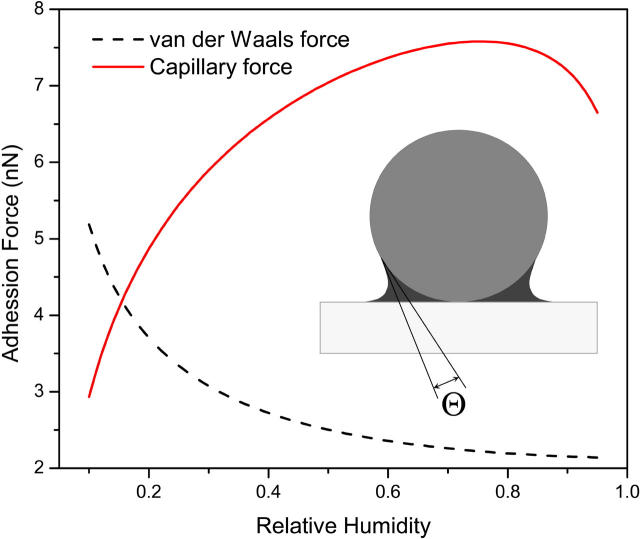
We have also calculated the magnitude of both van der Waals (12) and capillary forces (13) for a sphere/plane geometry (see inset of Fig. 3) to compare their contributions as a function of RH (see Fig. 3). As long as the value of RH is >16%, the capillary force dominates. It should be noted that the calculation assumed interaction between two atomically flat surfaces. As van der Waals force is a short distance force while the capillary water bridge between two surfaces is not significantly affected by the roughness, deviation from perfect flatness would cause a drop in the amplitude of van der Waals force. Thus, in a real environment the van der Waals contribution is lower than calculated.
FIGURE 3.
Computer simulation of the adhesion forces between a silicon nitride sphere with the radium R of 15 nm and a mica, at RH varied from 0.10 to 0.95. The contact angles of Si3N4 and mica are 60° and 0°, respectively. The simulation configuration is shown in the inset.
Force-distance measurements at different RH were performed to confirm behavior predicted by the calculation. A measurement was first taken at normal laboratory conditions with RH of 70%, which yielded a mean adhesion force of 11.8 nN. The sample was then purged with dry N2 for 20 min, which caused the adhesion force to drop to 4.4 nN. Finally, the sample was exposed to wet N2 for 15 min, during which the mean force rose to 6.2 nN. The results confirmed that decreasing RH decreases the force (70% RH → dry N2) and increasing the RH increases the force (dry N2 → wet N2). These observations verify that the adhesion force versus RH shows the same trend as calculated forces. All mean values of gecko's adhesion force as well as the standard deviations (mean ± SD) are listed in Table 1.
TABLE 1.
Gecko adhesion forces
| Contact angle | Environment | Force (nN) | Mean ± SD (nN) |
|---|---|---|---|
| 30° | Dry N2 | 4.4 | 0.8 |
| 30° | 70% RH | 11.8 | 2.2 |
| 30° | Wet N2 | 6.2 | 1.2 |
| 110° | 70% RH | 4.9 | 0.9 |
| N.A. | Water | 1.8 | 0.5 |
It has been accepted that the dominant adhesion force between silicon nitride AFM cantilever and a mica surface is the capillary force (14). As a control experiment, the interaction between a silicon nitride AFM cantilever and a fresh mica surface was conducted under the same conditions as the experiments with the gecko seta, i.e., in different RH and under deionized water. Because both systems (gecko spatula—tipless cantilever and the silicon nitride AFM cantilever—mica) showed identical trends, i.e., adhesion forces in air are proportional to the RH, we can assume that the nature of forces in both systems is the same.
The van der Waals force increases in a lower RH environment because there is less screening from water (7), so we conclude here that the van der Waals force does not play a dominating role in the gecko's adhesion.
It should be noted that the sample might change its position relative to the cantilever while blowing N2. However, the statistical method adopted in the data analysis removes random error and we report the most probable force value of a single spatula rather any specific spatula (see histograms in Fig. 2).
When the sample was immersed into water, the adhesion force decreased to <20% of its original value (see Table 1). This eliminates the possibility that the H-bond force could play a major role because its amplitude does not decrease with increase of water (15). This confirms that the dominant force in an ambient air environment is the capillary force.
The experimental results show that the force between gecko spatula and an AFM cantilever exhibits behavior consistent with an adsorbed surface water layer. As long as there is the presence of surface water, capillary forces will exist. The only exception is when the gecko setae were completely submerged in water where the adhesion force dropped to ∼2 nN. However, even in this case there are other forces that might be stronger than the van der Waals force, such as the double-layer force, the hydration force, and the hydrophobic force (7).
As a simple way to illustrate the importance of the capillary force, a live gecko was allowed to climb a “dry” vertical surface. Once sprayed with water, the gecko was unable to adhere to the surface. This technique of spraying geckos with water to remove them from vertical surfaces is well known to zoologists. It shows that the adhesive force of a gecko is significantly reduced in the absence of capillary forces. Van der Waals force may still play a role, but in ambient air the capillary forces dominate.
In summary, we have measured the adhesion force produced by an individual gecko spatula. As the gecko force was influenced by the surface hydrophobicity as well as the presence of water, we conclude that the dominating component of the adhesion force is the capillary force. This finding epitomizes one of many intriguing natural phenomena that can be adapted to improve the technological know-how of humans, such as development of an artificial gecko mimicking devices.
Acknowledgments
The authors are grateful for the support from the Institute of Bioengineering and Nanotechnology, Singapore as well as the A-STAR, Singapore.
References
- 1.Irschick, D. J., C. C. Austin, K. Petren, R. N. Fisher, J. B. Losos, and O. Ellers. 1996. A comparative analysis of clinging ability among pad-bearing lizards. Biol. J. Linn. Soc. 59:21–35. [Google Scholar]
- 2.Ruibal, R., and V. Ernst. 1965. The structure of the digital setae of lizards. J. Morphol. 117:271–294. [DOI] [PubMed] [Google Scholar]
- 3.Autumn, K., M. Sitti, Y. A. Liang, A. M. Peattie, W. Hansen, S. Sponberg, T. W. Kenny, T. Fearing, J. N. Israelachvili, and R. J. Full. 2002. Evidence of van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA. 99:12252–12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autumn, K., Y. A. Liang, S. T. Hsieh, W. Zesch, W. P. Chan, T. W. Kenny, R. Fearing, and R. J. Full. 2000. Adhesive force of a single gecko foot-seta. Nature. 405:681–685. [DOI] [PubMed] [Google Scholar]
- 5.Geim, A. K., S. V. Dubonos, I. V. Grigorieva, K. S. Novosedlov, A. A. Zhukov, and S. Y. Shapoval. 2003. Microfabricated adhesive mimicking gecko foot-seta. Nat. Mater. 2:461–463. [DOI] [PubMed] [Google Scholar]
- 6.Israelachvili, J. N. 1992. Intermolecular and Surface Forces, 2nd Ed. Academic Press, New York.
- 7.Cappella, B., and G. Dieter. 1999. Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 34:1–104. [Google Scholar]
- 8.Binnig, G., C. F. Quate, and C. Gerber. 1986. Atomic force microscope. Phys. Rev. Lett. 56:930–933. [DOI] [PubMed] [Google Scholar]
- 9.Frederix, P. L. T. M., T. Akiyama, U. Staufer, C. Gerber, D. Fotiadis, D. J. Müller, and A. Engel. 2003. Atomic force bio-analytics. Curr. Opin. Chem. Biol. 7:641–647. [DOI] [PubMed] [Google Scholar]
- 10.Kesel, A. B., A. Martin, and T. Seidl. 2004. Getting a grip on spider attachment: an AFM approach to microstructured adhesion in anthropods. Smart Mater. Struct. 13:512–518. [Google Scholar]
- 11.Fujihira, M., D. Aoki, Y. Okabe, H. Takano, J. Frommer, Y. Nagatani, and F. Sakai. 1996. Effect of capillary force on friction force microscopy: a scanning hydrophilicity microscope. Chem. Lett. (Jpn.). 7:499–500. [Google Scholar]
- 12.Wan, F.-T., D. T. Smith, and B. R. Lawn. 1992. Fracture and contact adhesion energies of mica mica, silica silica, and mica silica interfaces in dry and moist atmospheres. J. Am. Ceram. Soc. 75:667–676. [Google Scholar]
- 13.Orr, F. M., L. E. Scriven, and A. P. Rivas. 1975. Pendular rings between solids: meniscus properties and capillary force. J. Fluid Mech. 67:723–742. [Google Scholar]
- 14.Thundat, T., X. Y. Zheng, G. Y. Chen, and R. J. Warmack. 1993. Role of relative humidity in atomic force microscopy imaging. Surf. Sci. Lett. 294:L939–L943. [Google Scholar]
- 15.Joesten, M. D., and L. J. Schaad. 1974. Hydrogen Bonding. Dekker, New York.