Abstract
The multifunctional genome-linked protein (VPg) of Potato virus A (PVA; genus Potyvirus) was found to be phosphorylated as a part of the virus particle by a cellular kinase activity from tobacco. Immunoprecipitation, immunolabeling, and immunoelectron microscopy experiments showed that VPg is exposed at one end of the virion and it is accessible to protein-protein interactions. Substitution Ser185Leu at the C-proximal part of VPg reduces accumulation of PVA in inoculated leaves of the wild potato species Solanum commersonii and delays systemic infection, which is not observed in tobacco plants. Our data show that kinases of S. commersonii differentially recognize the VPg containing Ser or Leu at position 185, whereas both forms of VPg are similarly recognized by tobacco kinases. Taken together, our data imply that the virion-bound VPg may interact with host proteins and that phosphorylation of VPg may play a role in the VPg-mediated functions during the infection cycle of potyviruses.
Potato virus A (PVA; genus Potyvirus) has a monopartite, single-stranded, messenger-polarity RNA genome of 9,565 or 9,567 nucleotides that codes for a polyprotein that is subsequently processed up to 10 proteins by three viral proteinases (21, 36). The viral genome-linked protein (VPg) of PVA is found in virions (34); its significance is not known. However, studies of other potyviruses, such as Tobacco vein mottling virus (TVMV), show that the linkage of VPg to the 5′ end of viral RNA is required for infectivity (32) and that degradation of VPg linked to the RNA in virions of Tobacco etch virus (TEV) (16) and Plum pox virus (PPV) (39) alters viral infectivity.
The RNA of potyviruses is not capped, and, therefore, initiation of translation, including binding of the initiation factor eIF4E to the m7G cap (28), cannot proceed in the conventional way. Instead, an alternative mechanism has been suggested in which eIF(iso)4E interacts with the VPg (44). This interaction is necessary for the infectivity of Turnip mosaic virus in host plants (26, 27). After initiation of infection, VPg may also act as a primer for viral RNA synthesis, analogous to the VPg-primed RNA synthesis in poliovirus (35). Consistent with this possibility, an interaction between VPg and the RNA-dependent RNA polymerase (NIb) of potyviruses has been shown in vitro (11) and in yeast (17).
Plant viruses encode movement proteins (MP) to facilitate the transport of viral genomes from cell to cell via plasmodesmata and over long distances via phloem (reviewed in references 7, 23-25, and 41). However, the form in which potyviruses are transported in plants is not known. Since certain amino acid substitutions in the coat protein (CP) of TEV prevent virion assembly and also interfere with viral cell-to-cell movement, transport of potyviruses as virions has been proposed (9, 10). VPg is the other viral protein present in virions, and while it seems unable to dilate plasmodesmata between mesophyll cells in some study systems (40), it is a determinant of cell-to-cell movement for TVMV in certain virus isolate-host genotype combinations (33). Also, involvement of VPg in vascular movement of TEV (42), and PVA (15, 37, 38) has been reported, but the mechanism by which it mediates the movement of potyviruses is not yet known.
We have previously shown that the recombinant VPg of PVA is phosphorylated in vitro by a cellular protein kinase activity from tobacco (18). In this study, we extend the previous studies by showing that the VPg bound to virions can also be phosphorylated. The data indicate that VPg is bound and exposed at one end of the virion, being accessible to protein-protein interactions. Furthermore, the kinases of tobacco and a wild potato species can differentially recognize the VPg of PVA, a finding that is consistent with the different abilities of VPg to support vascular movement and accumulation of PVA in these two hosts. These findings provide novel insight to the possible transport mechanisms of potyviruses.
MATERIALS AND METHODS
Plants and viruses.
The growth conditions for tobacco plants (Nicotiana tabacum cv. SR1) (18) and for the plants of the wild potato species Solanum commersonii (clone C1) (38) have been described previously.
PVA isolate B11 (21, 36) was propagated in the tobacco plants. One gram of homogenized PVA-infected leaf material diluted with 4 ml of distilled water was used as an inoculum. Tobacco plants were mechanically inoculated by rubbing the virus onto the lower leaves with Carborundum as an abrasive. PVA infection was detected by immunoblotting with anti-CP immunoglobulin G (IgG) (Bioreba). Infected leaves were used for virus purification as previously described (5, 13), and the virus preparation was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to verify its purity and quality.
Plasmid construction, expression of recombinant VPg, and purification of fusion proteins.
Constructs for protein expression were made as described previously (29). The VPg-encoding sequence was amplified by PCR from infectious cDNAs of PVA and cloned into the pQE-30 vector (Qiagen), allowing isopropyl-1-thio-d-galactopyranoside-inducible expression and immobilized metal-affinity chromatography purification of the proteins on Ni2+-nitrilotriacetic acid agarose (Qiagen). Protein purification was carried out under denaturing conditions as described by Merits et al. (29). Purified VPg proteins were refolded by a rapid dialysis procedure previously shown to recover the RNA binding activity of PVA proteins (29, 30).
SDS-PAGE and immunoblotting.
Protein samples were loaded on SDS-12.5% polyacrylamide gels. Either gels were stained with Coomassie brilliant blue R-250 (Sigma), or proteins were transferred electrophoretically to polyvinylidine difluoride membranes (Immobilon P; Millipore Corp.). The proteins used in tryptic phosphopeptide mapping were visualized by Ponceau S staining of the membranes. Radioactively labeled VPgs were visualized on the membranes with a PhosphorImager (Fuji) and Tina 2.09c software (Raytest). For Western analysis, the CP and VPg protein blots were incubated 60 min with rabbit polyclonal anti-VPg antibodies (diluted 1:2,000 in phosphate-buffered saline [PBS]) or anti-CP IgGs (diluted 1:5,000; Bioreba), respectively, and detected with alkaline phosphatase- or peroxidase-conjugated antirabbit or antimouse antibodies (diluted 1:5,000 in PBS; Sigma), respectively.
In vitro phosphorylation.
Phosphorylation was measured as the incorporation of radioactivity from [γ-33P]ATP into purified substrate proteins. Redivue [γ-33P]ATP (2,500 Ci/mmol) was obtained from Amersham Pharmacia Biotech. Freshly prepared total protein extracts from tobacco and S. commersonii were prepared and used as the source of kinase activity as described previously (18). Phosphorylation reactions were performed at room temperature for 30 min in a total volume of 22 μl. The reaction mixture contained 0.5 μM [γ-33P]ATP (2,500 Ci/mmol), 3 μg of recombinant VPg, ca.1 μg of total protein extract, 25 mM HEPES (pH 7.4), and 5 mM MnCl2. The reaction was terminated by adding 5× SDS-PAGE sample buffer, followed by boiling for 5 min. Target protein phosphorylation was further analyzed by SDS-PAGE.
Phosphorylation of VPg in virions was assayed with a modified reaction mixture containing purified virions in a final concentration of 2.4 μg/μl, 3 μM [γ-33P]ATP (2,500 Ci/mmol), 10 mM HEPES, (pH 7.4), 4 mM MnCl2, and 12 μl of total plant protein extract in a total volume of 262 μl. The amount of VPg used in the reaction was approximately 250 ng. The phosphorylated VPg-RNA complexes were purified from the particles by extracting RNA with LiCl. Equal volumes of particles and 4 M LiCl were mixed and incubated at −20°C overnight. Precipitated RNA was washed with 70% ethanol and solubilized in water. After RNase A treatment of viral RNA, the proteins were analyzed by SDS-PAGE.
Immunoprecipitation of virions with anti-VPg antibodies.
Fully expanded leaves of PVA-infected and mock-inoculated tobacco plants were cut into small pieces, and the middle ribs were removed. Leaf pieces of equal sizes were homogenized with a mortar and a pestle in equal volume of NET buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM EDTA) containing 0.2% Triton X-100 (Sigma). The lysates were cleared by centrifugation and diluted (1:10) with NET buffer. Presoaked protein A-Sepharose (Amersham Pharmacia Biotech) was added at 1% (wt/vol) to the diluted lysates, and this mixture was then incubated at 4°C for 1.5 h to remove the proteins nonspecifically bound to the protein A-Sepharose, the mixture was spun at 3,200 × g (4°C) for 5 min, and protein A-Sepharose was removed. Mouse anti-CP IgG (Bioreba) or polyclonal rabbit anti-VPg antiserum (30 μl/2 ml) was added to the lysates, which were then incubated with agitation overnight. The protein-antibody complexes were then allowed to interact with protein A-Sepharose at 4°C for 2 h, followed by centrifugation at 3,200 × g (4°C) for 10 min. Supernatant was removed, and the protein A-Sepharose pellet washed twice with NET containing Triton X-100 and then twice with NET alone. Immunoprecipitates were analyzed by SDS-PAGE, and PVA CP was visualized by Coomassie staining.
Purified virions were immunoprecipitated as described above for the plant extracts. Recombinant six-His-tagged PVA CP was included as a negative control, and equal amounts (120 μg) of six-His-tagged CP and PVA particles were used. Proteins precipitated with the polyclonal rabbit anti-VPg antibodies or the polyclonal antibodies to the cylindrical inclusion protein (CI) of PVA (both antisera kindly provided by A. Merits) were analyzed by SDS-PAGE, and the presence of CP in the immunoprecipitates was verified by Western blot analysis with mouse anti-CP IgG.
Immunogold labeling and IEM.
Formvar-supported and carbon-coated copper grids were incubated on droplets of diluted purified virus at room temperature. As a control, some grids were also incubated on droplets of virus-like particles obtained by expressing PVA CP in bacterial cells [Escherichia coli strain M15(pREP4)]. Anti-VPg antibodies and preimmune IgGs were diluted 1:300 in Dulbecco's medium containing PBS (pH 7.4), 5 mM MgCl2, 7 mM CaCl2, and 2% bovine serum albumin (BSA). Grids were incubated on droplets of the antibody solutions for 60 min, washed three times with Dulbecco's medium, incubated with 15-nm-particle-diameter protein A-gold conjugate (Department of Cell Biology, Utrecht School of Medicine) for 60 min, and washed three times with PBS and once with distilled water to remove the excess salt. Negative staining with neutral aqueous 3% uranyl acetate solution was used to visualize the complexes formed by the virus, antibody, and protein A. Virion-antibody complexes were visualized by immunoelectron microscopy (IEM) with a JEOL 1200 EX II electron microscope at 60 kV.
Tryptic phosphopeptide mapping.
Tryptic phosphopeptides were mapped essentially as described previously (3). Phosphorylated VPgs were resolved by SDS-PAGE (12.5% polyacrylamide), electroblotted, and detected on Ponceau S-stained membranes. The bands corresponding to VPg (containing 3 to 4 μg of the protein) were excised from the filter and digested with trypsin in situ (sequencing grade; Promega) in 50 mM NH4HCO3 containing 10% acetonitrile, at 37°C overnight. The samples were desalted by repeated lyophilization and solubilization. The tryptic peptide mixture was dissolved in 128 mM NH4HCO3 (pH 8.9) and applied as spots to a 20- by 20-cm cellulose thin-layer chromatography (TLC) plate (Merck). An HTLE-7000 thin-layer electrophoresis (TLE) system (C.B.S. Scientific Company) was used for separation of the peptides in the first dimension. Electrophoresis was performed at 1,000 V for 24 min in ammonium carbamate (Merck) buffer at the concentration of 128 mM (pH 9.1). The plate was air dried and subjected to separation in the second dimension by chromatography in n-butyl alcohol-pyridine-acetic acid-water (15:10:3:12 [vol/vol/vol/vol]). The plate was air dried, and phosphopeptides were visualized by autoradiography with Biomax MR film (Kodak) or by a phosphorimager (Fuji).
Phosphoamino acid analysis.
Phosphoamino acid analysis was carried out essentially as described previously (4). Radioactive peptides were rescued from the TLE/TLC plate into a pH 1.9 buffer (distilled water-acetic acid-formic acids at 900:78:22.4 [vol/vol/vol]). The peptides were lyophilized twice and subjected to hydrolysis in 6 M HCl (110°C, 60 min). The hydrolysates were lyophilized and mixed with nonlabeled phosphoamino acid standards (0.6 μg/μl). The samples were subjected to two-dimensional TLE: the first dimension in a buffer with pH 1.9 (distilled water-acetic acid-formic acid at 900:78:22.4 [vol/vol/vol]) at 1,600 V for 40 min and the second dimension in a buffer with pH 3.5 (distilled water-acetic acid-pyridine at 945:50:5 [vol/vol/vol]) at 1,400 V for 20 min. Phosphoamino acid standards were visualized with 2% ninhydrin in ethanol, and radioactive amino acids were detected from autoradiograms.
RESULTS
VPg bound to virions is phosphorylated.
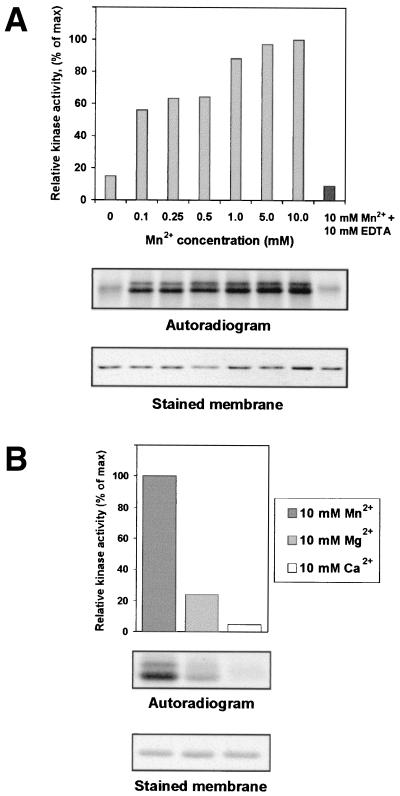
We have reported previously that recombinant PVA VPg was phosphorylated by a yet uncharacterized protein kinase activity from tobacco (18). The results of this study showed that the protein kinase activity is stimulated following an increase in Mn2+ concentration from 0.1 to 10 mM (Fig. 1A). When free Mn2+ was removed in a 1:1 molar complex with EDTA, the kinase activity remained at the original level (Fig. 1A). Comparison of phosphorylation at a 10 mM concentration of Mn2+, Mg2+, or Ca2+ revealed that the kinase activity has a preference for Mn2+ over Mg2+ and apparently is not dependent on Ca2+ (Fig. 1B). These results on VPg are similar to those previously obtained with analysis of the tobacco kinase activity phosphorylating the CP of PVA (18).
FIG. 1.
Phosphorylation of PVA VPg by plant protein kinase activity is stimulated by Mn2+. (A) Increasing concentrations of manganese were introduced into assays containing bacterially expressed PVA VPg, total protein kinase activity from leaves of N. tabacum, and [γ-33P]ATP. Phosphoproteins were separated by SDS-PAGE and transferred to membranes, and their positions were identified by staining with Ponceau S. Radioactivity associated with phosphoproteins was compared by PhosphorImager densitometry and plotted against Mn2+ concentration. In a control experiment, manganese was removed from phosphorylation reaction in a 1:1 molar complex with EDTA. (B) Effect of Mn2+, Mg2+, or Ca2+ on phosphorylation of PVA VPg. Proteins were assayed for phosphorylation in a reconstituted system containing plant enzymes, [γ-33P]ATP, and 10 mM Mn2+, Mg2+, or Ca2+. Proteins were subjected to SDS-PAGE and transferred to membranes. Autoradiograms of phosphorylated proteins are shown together with stained membranes.
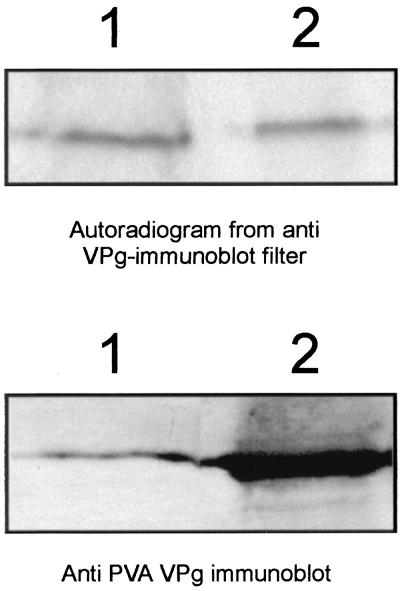
Phosphorylation of the VPg bound to PVA virions was tested with the kinase activity of a total protein extract from tobacco leaves. Three independent phosphorylation reactions were carried out, including dialyzed recombinant VPg as a positive control. Subsequently, viral RNA was extracted from virions to concentrate the VPg (covalently attached to RNA) and to remove the excess of CP. RNase-treated samples were analyzed by probing protein gel blots with the anti-VPg antiserum and subjected to autoradiography. Results showed that the VPg extracted from virions (Fig. 2, lane 1) and the recombinant VPg produced in E. coli (Fig. 2, lane 2) were phosphorylated by the kinase activity from tobacco. Superimposition of the radioactively labeled bands shown in Fig. 2 (lower panel) on the bands observed on protein gel blots verified that the labeled protein was VPg. Thus, these data indicated that the VPg attached to viral RNA in virions was phosphorylated and that the virion-associated VPg can interact with plant protein kinases in vitro.
FIG. 2.
PVA VPg is phosphorylated when packaged into virions. PVA particles were phosphorylated with a kinase activity from tobacco leaf extracts, and VPg-RNA complexes were isolated from PVA particles by the LiCl method and treated with RNase A. The virus-derived VPg (lane 1) and the recombinant VPg (lane 2) expressed in E. coli and used as a control in the phosphorylation experiment were subjected to SDS-PAGE and blotted onto a membrane, and phospohorylation was verified by autoradiography (upper panel). VPg was detected with anti-VPg antibodies to compare the blotted amounts of VPg (lower panel).
Virions are immunoprecipitated with anti-VPg antibodies.
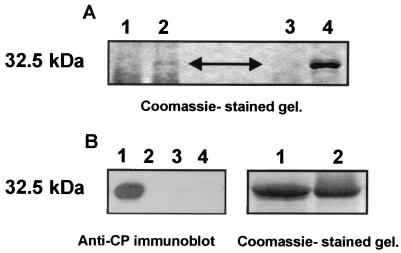
Virions of PVA were immunoprecipitated from leaf extracts of the PVA-infected tobacco plants with anti-CP IgGs or an anti-VPg antiserum. The anti-CP IgGs readily precipitated the virions, as indicated by the high yield of PVA CP (Fig. 3A, lane 4), whereas no protein of similar size was precipitated from the extracts of mock-inoculated plants (Fig. 3A, lane 3). Immunoprecipitation with the anti-VPg antibodies also resulted in a band corresponding to the size of PVA CP (Fig. 3A, lane 2). Again, the band was not observed in the negative controls (extracts from the mock-inoculated plants) (Fig. 3A, lane 1). These data suggested that the anti-VPg antibodies could be used to immunoprecipitate virions.
FIG. 3.
PVA particles can be immunoprecipitated with anti-VPg antibodies. (A) Immunoprecipitation of PVA particles from infected plant material. The protein extracts derived from PVA-infected and mock-inoculated tobacco plants were mixed with anti-VPg antiserum or anti-CP IgG. The antigen-antibody complexes were collected with protein A beads and washed with NET buffer (see Materials and Methods). The immunoprecipitated proteins were resolved by SDS-PAGE and visualized by Coomassie staining. Lanes: 1, extract from a mock-inoculated control plant immunoprecipitated with anti-VPg antiserum; 2, extract from a PVA-infected plant immunoprecipitated with anti-VPg antiserum; 3, extract from a mock-inoculated control plant immunoprecipitated with anti-CP IgG; 4, extract from a PVA-infected plant immunoprecipitated with anti-CP IgG. The position of CP is indicated by an arrowhead, and its molecular mass (32.5 kDa) is indicated to the left. (B) Immunoprecipitation of purified PVA particles and recombinant CP. Purified PVA particles were immunoprecipitated with anti-VPg antiserum. Bacterially expressed CP was used as a control to rule out possible nonspecific interaction of anti-VPg antibodies or Sepharose A with PVA CP. Anti-CI antiserum was used as an additional control. Immunoprecipitated proteins were resolved by SDS-PAGE, blotted to a nylon membrane, and detected with anti-CP IgG by the ECL enhanced chemiluminescence method. Lanes: 1, PVA virions immunoprecipitated with anti-VPg antiserum; 2, six-His-tagged CP immunoprecipitated with anti-VPg antiserum; 3, PVA virions immunoprecipitated with anti-CI antiserum; 4, six-His-tagged CP immunoprecipitated with anti-CI antiserum. The panel to the right represents a Coomassie-stained gel, showing that equal amounts of CP (lane1) and virions (lane2) were subjected to immunoprecipitation. The molecular mass of CP (32.5 kDa) is indicated to the left.
To further verify that the anti-VPg antibodies indeed could immunoprecipitate virions, purified virions of PVA were tested. Results were positive for the virions (Fig. 3B, lane 1), whereas the bacterially expressed recombinant CP of PVA was not precipitated with the anti-VPg antibodies, ruling out nonspecific interactions between the anti-VPg antibodies and the CP (Fig. 3B, lane 2). An anti-CI antiserum was used as an additional control, and it did not immunoprecipitate either virions or the recombinant CP of PVA (Fig. 3B, lanes 3 and 4). Thus, results showed that the virions of PVA were immunoprecipitated specifically with the anti-VPg and anti-CP antibodies.
Anti-VPg antibodies recognize one end of the virion.
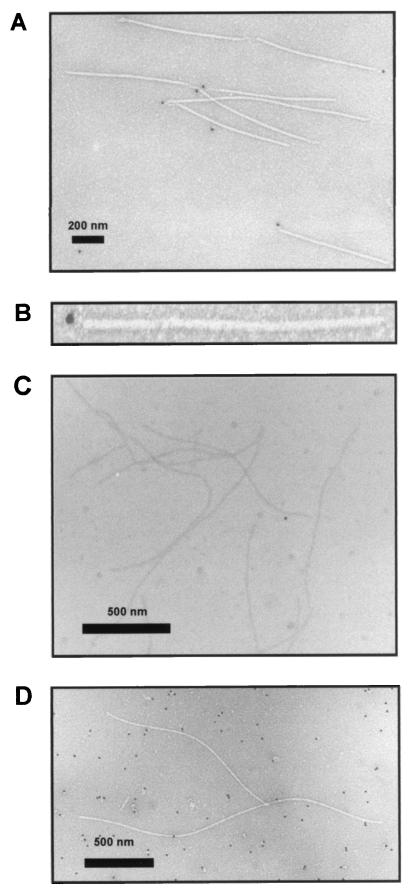
Localization of VPg in the virions of PVA was studied by IEM with anti-VPg antibodies and a protein A complex conjugated with a gold label. The protein A-gold conjugate interacts with antirabbit antibodies. The signals of gold labeling were observed only at one end of the virion (Fig. 4A), and one or two gold particles (diameter 15 nm) were observed per virion (see Fig. 4B for an enlargement). In no case were gold particles detected at both ends of a virion, nor were they bound to any other part of the virion. Preimmune serum was tested for labeling the virions, and no signals were observed, which ruled out nonspecific interaction of the IgGs with the virion's end or VPg (Fig. 4C). The virus-like particles produced following expression of recombinant PVA CP in the bacterial cells were used as an additional control (Fig. 4D). These virus-like particles do not contain PVA RNA or VPg. No labeling of the virus-like particles was observed with the anti-VPg antibodies (Fig. 4D), but background signals randomly distributed across the area viewed were observed, probably due to antibody binding to bacterial proteins. This was expected, since the recombinant VPg used for antibody production had been expressed in and purified from E. coli. Taken together, the results indicated a polar localization of VPg in PVA virions and, consistent with the immunoprecipitation experiments, showed that the VPg bound to virions is exposed to protein-protein interactions. These data are consistent with those from previous studies indicating that VPg is linked to the 5′ end of potyviral RNA (32).
FIG. 4.
Detection of PVA VPg in virions by IEM. The complexes of anti-VPg antibodies and virion-associated VPg were visualized with gold-conjugated protein A. The size of the gold particles is 15 nm. (A) The complexes of gold-conjugated protein A and anti-VPg antibodies are detected at only one end of the virion (bar, 200 nm). (B) Magnified view of a labeled PVA particle. (C) Preimmune IgGs or gold-conjugated protein A does not bind directly to the virions (particles not treated with anti-VPg antibodies; bar, 500 nm). (D) Virus-like particles, which contain neither viral RNA nor VPg, are not recognized by anti-VPg antibodies (bar, 500 nm).
Comparison of the phosphorylation patterns of bacterially expressed wild-type and mutated VPgs.
Since the virion-bound VPg was found to be accessible to interactions with host protein kinases, it also appeared possible that the virion-bound VPg could have putative functions during the infection cycle and that phosphorylation of VPg could be a mechanism regulating such functions.
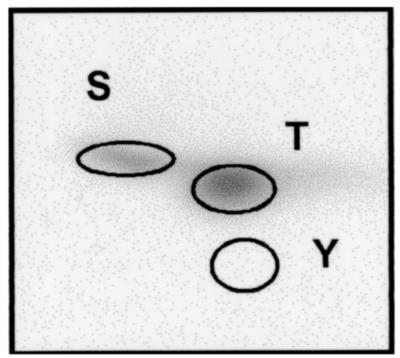
The substrate specificity of the host kinases phosphorylating viral movement-related proteins, such as the MP of tobamoviruses (8, 14, 20) and the CP of potyviruses (PVA and PPV) (12, 18), has been described previously. In this study, we analyzed the substrate specificity of the kinase or kinases involved in phosphorylation of PVA VPg. Recombinant VPg of the PVA wild-type strain B11 (designated as B11wt) was subjected to phosphoamino acid analysis. It was phosphorylated in vitro by using the kinase activity from tobacco leaves and hydrolyzed, and the amino acids were separated by two-dimensional TLE. The results showed that VPg was phosphorylated on threonine and serine residues (Fig. 5), which in turn indicated that phosphorylation was carried out by a Ser/Thr-specific protein kinase or kinases. The specific threonine and serine residues that were phosphorylated, however, were not determined in this study.
FIG. 5.
Phosphoamino acid composition of PVA VPg. An autoradiogram shows the results from phosphoamino acid analysis of 33P-labeled VPg assayed by two-dimensional TLE. The positions of phosphoamino acid markers are indicated by circles. S, phosphoserine; T, phosphothreonine; Y, phosphotyrosine.
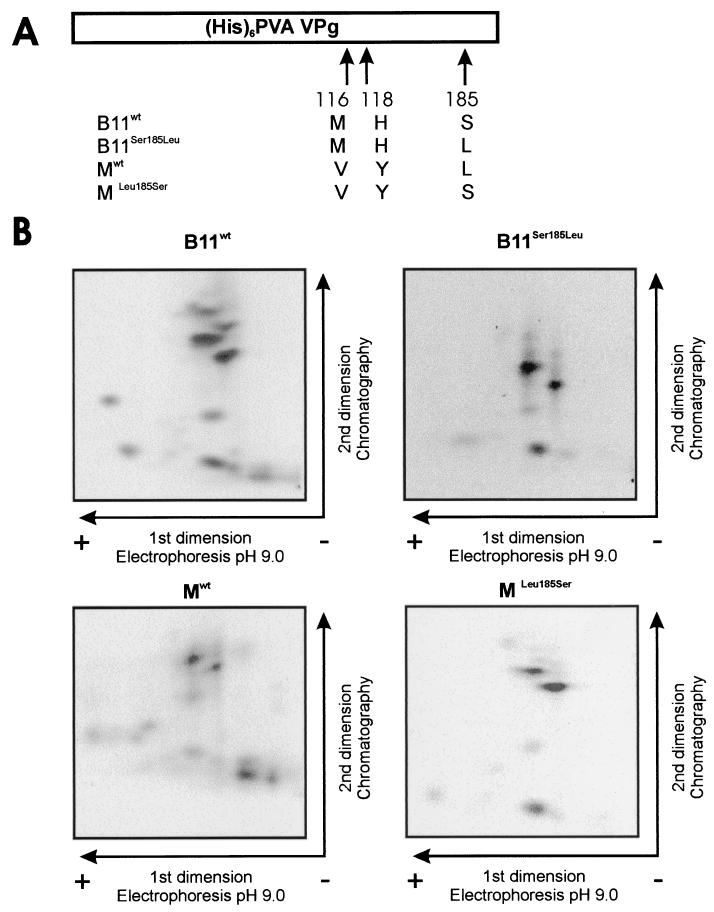
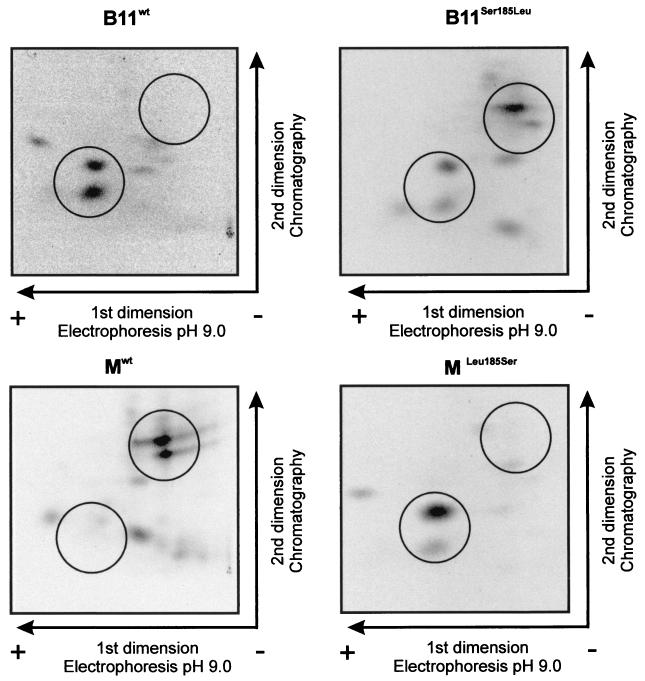
The PVA strain B11-M is a chimeric virus derived from strain B11 (37). These two strains are identical, except for three amino acids that differ within the VPg (Val116Met, Tyr118His, and Leu185Ser, respectively) and one that differs within the 6K2 protein (Met5Val) (37). Some of these amino acid substitutions influence accumulation or phloem loading of PVA in the inoculated leaves of S. commersonii, a model plant for studies of PVA (38). One of them is Ser185Leu at the C terminus of VPg, reducing the accumulation of PVA in infected cells (38). Since VPg was found to be phosphorylated by Ser/Thr-kinase(s), we hypothesized that Ser185 could be a phosphorylation site for a Ser-specific protein kinase and that a Ser185Leu substitution could result in a phosphorylation-deficient mutant VPg that is unable to support normal levels of virus accumulation. Another hypothesis was that the Ser185Leu substitution could alter the conformation of VPg, which in turn could affect recognition of VPg by host kinases. To test the first hypothesis mentioned, four different VPg molecules were produced in E. coli. Two of them corresponded to the VPg of strains B11 and B11-M (B11wt and Mwt, respectively), and two others were mutated to substitute the amino acid at position 185 (B11Ser185Leu and MLeu185Ser, respectively). Phosphorylation of these four recombinant proteins was tested with the total protein extracts from tobacco and S. commersonii as sources of kinase activity. The kinase activity from tobacco resulted in similar phosphorylation patterns for all four VPgs (Fig. 6B), indicating that the tobacco kinase or kinases responsible for phosphorylation of VPg do not distinguish between the different VPg molecules, regardless of Ser or Leu at position 185, or the two other amino acid differences at positions 116 and 118. However, the results obtained with the kinase activity from S. commersonii were different. While the phosphorylation pattern of Mwt was similar according to the kinase activity from S. commersonii and tobacco, the patterns for the three other forms of VPg appeared to be different, depending on the source of kinase activity (Fig. 7). The phosphorylation patterns of MLeu185Ser and B11wt resembled each other. The phosphorylation pattern of B11Ser185Leu appeared to be a combination of the patterns of the three aforementioned VPgs. The positions of peptides with patterns that differ are circled in Fig. 7.
FIG. 6.
Similar phosphorylation patterns obtained for the VPgs of PVA strains B11 and B11-M by using kinase activity from tobacco. (A) Schematic map of the recombinant VPg molecules. The amino acid differences between VPgs B11wt, Mwt, B11Ser185Leu, and MLeu185Ser and the positions of the changed amino acids in VPg are indicated. (His)6, six-His tag. (B) Recombinant VPgs B11wt, Mwt, B11Ser185Leu, and MLeu185Ser were phosphorylated in the presence of 5 mM Mn 2+ in vitro with a total protein extract of tobacco as the kinase source. Phosphorylated proteins were purified by SDS-PAGE, blotted onto nylon membrane, and visualized with Ponceau S. VPg was digested on the membrane with trypsin, and the released peptides were lyophilized and separated on TLE/TLC plates. Radioactive 33P-labeled peptides were autoradiographically visualized.
FIG. 7.
Different phosphorylation patterns obtained for the VPgs of PVA strains B11 and B11-M by using kinase activity from S. commersonii. The recombinant VPg proteins and phosphorylation assay were those described in the legend to Fig. 6, except that the kinase source was the total protein extract derived from S. commersonii. The positions at which the differences in the phosphorylation patterns obtained with potato-derived kinase are most obvious are indicated by circles.
DISCUSSION
The form in which potyviruses are transported in plants is not yet solved. Since VPg is needed for development of systemic infection in several potyvirus-host combinations (15, 22, 33, 37, 38, 42), it is likely to interact with host components during the viral movement process. VPg is found in several forms in virus-infected plants. It is the N-proximal portion of the NIa protein synthesized as part of the polyprotein. Subsequently, NIa is cleaved from the polyprotein by its C-proximal proteinase domain (NIa-Pro), and the VPg domain is separated from the proteinase domain by processing of a suboptimal cleavage site between these two domains (6, 31). Virus replication requires the proteolytic processing of the suboptimal site between the VPg and NIa-Pro domains, which takes place in the cytoplasmic pool of the NIa (6). As indicated by its name, a portion of the VPg pool becomes covalently linked to the newly synthesized viral genomic RNA strands and remains in encapsidated virions. In PVA, VPg is found in virions only in the processed form (34). In the view of the previously suggested involvement of virions (9, 10) and the demonstrated role of VPg in potyvirus transport, Nicolas et al. (33) proposed that VPg may be exposed in virions. In this study, evidence has now been provided that the VPg in virions can be phosphorylated by a host kinase activity. In addition, our data show that the virions of PVA can be immunoprecipitated with anti-VPg antibodies and that VPg is detected at one end of the virion by IEM. These data imply that the virion-bound VPg could interact with host proteins and be involved in the viral transport in plants.
The VPg of PVA is phosphorylated in vitro by a kinase activity from tobacco (18). Our data presented in this study extend previous findings showing that the kinase activity prefers Mn2+ over Mg2+ and is not dependent on Ca2+. The cation preference of the kinase activity responsible for VPg phosphorylation, therefore, resembles the previously reported cation preference of the kinases that phosphorylate the CP of PVA and the movement protein (MP) of TMV (18). However, competition assays carried out in the previous studies have established that the kinase or kinases phosphorylating VPg are different from the kinase or kinases responsible for phosphorylation of the PVA CP and the TMV MP (18). In light of these data, it may be hypothesized that initiation of infection in cells may be regulated via phosphorylation of VPg. For example, a few studies have shown that translatability of ribonucleoprotein complexes and virions is regulated by phosphorylation (2, 19) and, consequently, phosphorylation of VPg could trigger virion disassembly and the subsequent translation process in potyviruses.
VPg influences the accumulation and transport of PVA in plants of the wild potato species S. commersonii (38). In this study, we have obtained evidence that the amino acid substitutions that have a major impact on the ability of VPg to support viral accumulation and/or movement in S. commersonii also have a major impact on phosphorylation of VPg. An amino acid substitution, His118Tyr, in the central part of VPg in the strain B11 of PVA enhances phloem loading and vascular transport as well as viral accumulation in infected cells. In contrast, an additional substitution, Ser185Leu, in the C-terminal part of VPg reduces viral accumulation in inoculated leaves and delays systemic infection. These amino acid substitutions have no detectable effect on the accumulation or phloem loading of strain B11 in tobacco plants (1). In this study, the phosphorylation patterns of the different forms of VPg were studied in vitro by using recombinant proteins and the kinase activities derived from S. commersonii and tobacco. The kinase or kinases derived from tobacco phosphorylated the different recombinant VPg molecules in an indistinguishable manner (Fig. 6). In contrast, the kinase or kinases from S. commersonii produced three distinct phosphorylation patterns, depending on the form of VPg (Fig. 7). The phosphorylation patterns were similar for the two VPg molecules (B11wt and MLeu185Ser) containing a serine residue at position 185 and which can accumulate to high titers in the inoculated leaves of S. commersonii (38). On the other hand, the phosphorylation patterns of the two other VPg molecules (Mwt and B11Ser185Leu) with a leucine residue at position 185 contained two phosphopeptides not observed in the aforementioned VPgs. The corresponding PVA strains containing leucine at position 185 in VPg tend to accumulate to a lower titer in the inoculated leaves of S. commersonii (38). Taken together, the differences observed in the phosphorylation patterns correlate with the phenotypic differences in S. commersonii, which suggests, but does not prove, that phosphorylation of VPg could have functional significance for the virus-host interactions. Another possibility is that differences in the phosphorylation patterns reflect changes in the conformation of the VPg caused by the amino acid substitutions. The conformational changes could then influence recognition of VPg by the host kinases on one hand and by some other, putative, host factors involved in virus movement on the other hand. Nevertheless, our data show that the host factors in S. commersonii recognize the VPg differently from those in tobacco plants.
Evidence for the functional importance of protein phosphorylation at the different stages of plant viral infection cycle is becoming increasingly apparent. Phosphorylation of TMV MP regulates the viral cell-to-cell trafficking (43). The TMV RNA that encodes a mutated MP devoid of C-terminal phosphorylation is infectious in Nicotiana benthamiana, but not in Nicotiana tabacum. In addition, phosphorylation affects the intracellular localization and stability of tobamoviral MP (20) and it is involved in conversion of the translation-incompetent TMV movement intermediates to the translation-ready state (19). Translational activation of encapsidated Potato virus X RNA due to CP phosphorylation has also been reported (2). Therefore, phosphorylation of the VPg, which is an integral part of virions, may regulate specific interactions between VPg and host or viral factors involved in viral replication and/or movement, as well as in virion assembly or dissociation.
Acknowledgments
We thank Sarah Butcher for critical reading of the manuscript, Sanna Peltola for help in growing experimental plants, Eija Jokitalo for help in the IEM experiments, and Mart Saarma for valuable advice and discussions. The EM unit of the Institute of Biotechnology is acknowledged for providing the facilities for the IEM studies.
This work was financially supported by the Academy of Finland (grants 40934, 51981, 52265, and 53862), the Finnish National Technology Agency (grant 40723/00), the Center for International Mobility (for K.I.), and the Swedish Forestry and Agricultural Research Council (SJFR/Formas, grants 32.0667/97 and 301.0663/00).
REFERENCES
- 1.Andrejeva, J., Ü. Puurand, A. Merits, F. Rabenstein, L. Järvekülg, and J. P. T. Valkonen. 1999. Potyvirus helper component-proteinase and coat protein (CP) have coordinated functions in virus-host interactions and the same CP motif affects virus transmission and accumulation. J. Gen. Virol. 80:1133-1139. [DOI] [PubMed] [Google Scholar]
- 2.Atabekov, J. G., N. P. Radionova, O. V. Karpova, S. V. Kozlowsky, V. K. Novikov, and M. V. Arkhipenko. 2001. Translational activation of encapsidated potato virus X RNA by coat protein phosphorylation. Virology 286:466-474. [DOI] [PubMed] [Google Scholar]
- 3.Blume-Jensen, P., C. Wernsted, C.-H. Heldin, and L. Rönnstrand. 1995. Identification of the major phosphorylation sites for protein kinase C in kit/stem cell factor receptor in vitro and in intact cells. J. Biol. Chem. 270:14192-14200. [DOI] [PubMed] [Google Scholar]
- 4.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-148. [DOI] [PubMed] [Google Scholar]
- 5.Browning, I. A., R. Burns, E. L. George, and M. Darling. 1995. Development and evaluation of ELISA assays incorporating monoclonal antibodies for the detection of potato A potyvirus. EPPO Bull. 25:259-268. [Google Scholar]
- 6.Carrington, J. C., R. Haldeman, V. V. Dolja, and M. A. Restrepo-Hartwig. 1993. Internal cleavage and trans-proteolytic activities of the VPg-proteinase (NIa) of tobacco etch potyvirus in vivo. J. Virol. 67:6995-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrington, J. C., K. D. Kasschau, S. K. Mahajan, and M. C. Schaad. 1996. Cell-to-cell and long-distance transport of viruses in plants. Plant Cell 8:1669-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citovsky, V., B. G. McLean, J. Zupan, and P. Zambryski. 1993. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 7:904-910. [DOI] [PubMed] [Google Scholar]
- 9.Dolja, V. V., R. Haldeman, N. L. Robertson, W. G. Dougherty, and J. C. Carrington. 1994. Distinct functions of capsid protein in assembly and movement of tobacco etch potyvirus. EMBO J. 13:1482-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolja, V. V., R. Haldeman-Cahill, M. C. Schaad, A. E. Montgomery, K. A. Vandenbosch, and J. C. Carrington. 1995. Capsid protein involved in cell-to-cell and long-distance movement of tobacco etch virus. Virology 206:1007-1016. [DOI] [PubMed] [Google Scholar]
- 11.Fellers, J., J. Wan, Y. Hong, G. B. Collins, and A. G. Hunt. 1998. In vitro interactions between a potyvirus-encoded, genome-linked protein and RNA-dependent RNA polymerase. J. Gen. Virol. 79:2043-2049. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Fernández, M. R., E. Camafeita, P. Bonay, E. Méndez, J. P. Albar, and J. A. García. 2001. The capsid protein of a plant single-stranded RNA virus is modified by O-linked N-acetylglucosamine. J. Biol. Chem. 277:135-140. [DOI] [PubMed] [Google Scholar]
- 13.Fribourg, C. E., and J. Nakashima. 1984. Characterization of a new potyvirus from potato. Phytopathology 74:1363-1369. [Google Scholar]
- 14.Haley, A., T. Hunter, P. Kilberstis, and D. Zimmern. 1995. Multiple serine phosphorylation sites on the 30 kDa TMV cell-to-cell movement protein synthesized in tobacco protoplasts. Plant J. 8:715-724. [DOI] [PubMed] [Google Scholar]
- 15.Hämäläinen, J. H., T. Kekarainen, C. Gebhardt, K. N. Watanabe, and J. P. T. Valkonen. 2000. Recessive and dominant resistance interfere with the vascular transport of Potato virus A in diploid potatoes. Mol. Plant-Microbe Interact. 13:402-412. [DOI] [PubMed] [Google Scholar]
- 16.Hari, V. 1981. The RNA of tobacco etch virus: further characterization and detection of protein linked to RNA. Virology 112:391-399. [DOI] [PubMed] [Google Scholar]
- 17.Hong, Y., K. Levy, J. F. Murphy, P. G. Klein, J. G. Shaw, and A. G. Hunt. 1995. A potyvirus-encoded polymerase interacts with the viral coat protein and VPg in yeast cells. Virology 214:159-166. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov, K., P. Puustinen, A. Merits, M. Saarma, and K. Mäkinen. 2001. Phosphorylation down-regulates the RNA-binding function of the coat protein of potato virus A. J. Biol. Chem. 276:13530-13540. [DOI] [PubMed] [Google Scholar]
- 19.Karpova, O. V., N. P. Rodinova, K. I. Ivanov, S. V. Kozolovsky, Y. L. Dorokhov, and J. G. Atabekov. 1999. Phosphorylation of tobacco mosaic virus movement proteins abolishes its translation repressing ability. Virology 216:20-24. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami, S., H. S. Padgett, D. Hosokawa, Y. Okada, R. N. Beachy, and Y. Watanabe. 1999. Phosphorylation and/or presence of serine 37 in the movement protein of tomato mosaic tobamovirus is essential for intracellular localization and stability in vivo. J. Virol. 73:6831-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kekarainen, T., A. Merits, I. Oruetxebarria, M.-L. Rajamäki, and J. P. T. Valkonen. 1999. Comparison of the complete sequences of five different isolates of Potato virus A (PVA), genus Potyvirus. Arch. Virol. 144:2355-2366. [DOI] [PubMed] [Google Scholar]
- 22.Keller, K. E., I. E. Johansen, R. R. Martin, and R. O. Hampton. 1998. Potyvirus genome linked protein (VPg) determines pea seed-borne mosaic virus pathotype-specific virulence in Pisum sativum. Mol. Plant-Microbe Interact. 11:124-130. [DOI] [PubMed] [Google Scholar]
- 23.Lazarowitz, S. G., and R. N. Beachy. 1999. Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell 11:535-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, J-Y., and W. Lucas. 2001. Phosphorylation of viral movement proteins—regulation of cell to cell trafficking. Trends Microbiol. 9:5-7. [DOI] [PubMed] [Google Scholar]
- 25.Lee, J.-Y., B.-C. Yoo, and W. J. Lucas. 2000. Parallels between nuclear-pore trafficking of information molecules. Planta 210:177-187. [DOI] [PubMed] [Google Scholar]
- 26.Lellis, A. D., K. D. Kasschau, S. E. Whitham, and J. C. Carrington. 2002. Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for elF(iso)4E during potyvirus infection. Curr. Biol. 12:1046-1051. [DOI] [PubMed] [Google Scholar]
- 27.Léonard, S., D. Plante, S. Wittman, N. Daigneault, M. G. Fortin, and J. F. Laliberté. 2000. Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J. Virol. 74:7730-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKendrick, L., V. P. Pain, and S. J. Morley. 1999. Translation initiation factor 4E. Int. J. Biochem. Cell Biol. 31:31-35. [DOI] [PubMed] [Google Scholar]
- 29.Merits, A., D. Guo, and M. Saarma. 1998. VPg, coat protein and five non-structural proteins of potato A potyvirus bind RNA in a sequence-unspecific manner. J. Gen. Virol. 79:3123-3127. [DOI] [PubMed] [Google Scholar]
- 30.Merits, A., D. Guo, L. Järvekülg, and M. Saarma. 1999. Biochemical and genetic evidence for interaction of potato A potyvirus encoded proteins P1 and P3 with proteins of putative replication complex. Virology 263:15-22. [DOI] [PubMed] [Google Scholar]
- 31.Merits, A., M.-L. Rajamäki, P. Lindholm, P. Runeberg-Roos, T. Kekarainen, P. Puustinen, K. Mäkeläinen, J. P. T. Valkonen, and M. Saarma. 2002. Proteolytic processing of potyviral proteins and polyprotein processing intermediates in insect and plant cells. J. Gen. Virol. 83:1211-1221. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, J. F., P. G. Klein, A. G. Hunt, and J. G. Shaw. 1996. Replacement of the tyrosine residue that links a potyviral VPg to the viral RNA is lethal. Virology 220:535-538. [DOI] [PubMed] [Google Scholar]
- 33.Nicolas, O., S. W. Dunnington, L. F. Gotow, T. P. Pirone, and G. M. Helmann. 1997. Variations in the VPg protein allow a potyvirus to overcome va gene resistance in tobacco. Virology 237:452-459. [DOI] [PubMed] [Google Scholar]
- 34.Oruetxebarria, I., D. Guo, A. Merits, K. Mäkinen, M. Saarma, and J. P. T. Valkonen. 2001. Identification of the genome-linked protein in virions of Potato virus A, with comparison to other members in the genus Potyvirus. Virus Res. 73:103-112. [DOI] [PubMed] [Google Scholar]
- 35.Paul, A., J. H. van Boom, D. Filipov, and E. Wimmer. 1998. Protein primed RNA synthesis by purified poliovirus RNA polymerase. Nature 393:280-284. [DOI] [PubMed] [Google Scholar]
- 36.Puurand, Ü., K. Mäkinen, L. Paulin, and M. Saarma. 1994. The nucleotide sequence of potato virus A genomic RNA and its sequence similarities with other potyviruses. J. Gen. Virol. 75:457-461. [DOI] [PubMed] [Google Scholar]
- 37.Rajamäki, M.-L, and J. P. T. Valkonen. 1999. The 6K2 protein and the VPg of potato virus A are determinants of systemic infection in Nicandra physailoides. Mol. Plant-Microbe Interact. 12:1074-1081. [DOI] [PubMed] [Google Scholar]
- 38.Rajamäki, M.-L., and J. P. T. Valkonen. 2002. Viral genome-linked protein (VPg) controls accumulation and phloem-loading of a potyvirus in inoculated potato leaves. Mol. Plant-Microbe Interact. 15:138-149. [DOI] [PubMed] [Google Scholar]
- 39.Riechmann, J. L., S. Lain, and J. A. Garcia. 1989. The genome linked protein and 5′ RNA sequence of plum pox Potyvirus. J. Gen. Virol. 70:2785-2789. [DOI] [PubMed] [Google Scholar]
- 40.Rojas, M. R., F. M. Zerbini, R. F. Allison, R. L. Gilbertson, and W. J. Lucas. 1997. Capsid protein and helper component-proteinase function as potyvirus cell-to-cell movement proteins. Virology 237:283-295. [DOI] [PubMed] [Google Scholar]
- 41.Santa Cruz, S. 1999. Phloem transport of viruses and macromolecules—what goes in must come out. Trends Microbiol. 6:237-241. [DOI] [PubMed] [Google Scholar]
- 42.Schaad, M. C., A. D. Lellis, and J. C. Carrington. 1997. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long-distance movement. J. Virol. 71:8624-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waigmann, E., M.-H. Chen, R. Bachmaier, S. Ghosroy, and V. Citovsky. 2000. Regulation of plasmodesmal transport by phosphorylation of tobacco mosaic virus cell-to-cell movement protein. EMBO J. 19:4875-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wittmann, S., H. Chatel, M. G. Fortin, and J.-F. Laliberté. 1997. Interaction of the viral protein genome linked of turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso)4E of Arabidopsis thaliana using the yeast two-hybrid system. Virology 234:84-92. [DOI] [PubMed] [Google Scholar]