Abstract
The bacterial flagellar motor is generally supposed to be a stepping mechanism. The main evidence for this is based on a fluctuation analysis of experiments with tethered bacteria in which rotation frequency was varied by applying an external torque: the variance in time taken for a fixed number of revolutions was found to be essentially proportional to the inverse square of the frequency. This behavior was shown to characterize a Poissonian stepper. Here we present a rigorous kinetic and stochastic analysis of elastic crossbridge stepping in tethered bacteria. We demonstrate that Poissonian stepping is a virtually unachievable limit. To the extent that a system may approach Poissonian stepping it cannot be influenced by an externally applied torque; stepping mechanisms capable of being so influenced are necessarily non-Poissonian and exhibit an approximately inverse cubic dependence. This conclusion applies whatever the torsional characteristics of the tether may be, and contrary to claims, no perceptible relaxation of the tether following each step is found. Furthermore, the inverse square dependence is a necessary but not sufficient condition for Poissonian stepping, since a nonstepping mechanism, which closely reproduces most experimental data, also fulfills this condition. Hence the inference that crossbridge-type stepping occurs is not justified.
INTRODUCTION
It is commonly considered that both the proton-driven bacterial flagellar motor and the proton-translocating ATP synthase are stepping motors (1–3). Although stepping has been directly observed on the ATP synthase with different preparations (4–6), it has only been indirectly inferred in the case of the flagellar motor. Samuel and Berg (7) carried out a fluctuation analysis of the motor rotation and interpreted the results in terms of a Poissonian stepping model. Here we show that this model is incompatible with the experimental procedure used, namely imposing a variable external torque on tethered cells to obtain different rotational frequencies. We further show that the results of the fluctuation analysis can be satisfactorily reproduced by a nonstepping model (8).
The model considered by Samuel and Berg (7) comprises a single, and thus rate-limiting, biochemical step linked to an elementary angular step φ of some unspecified element around the periphery of the rotor (see Fig. 1), which results in an equal increment in rotation angle Δθ. Thus the Poissonian distribution of the biochemical events is directly translated into a Poissonian distribution of steps in rotation angle. The authors showed theoretically that the variance in the time taken for n revolutions at a given rotational frequency f is
 |
(1) |
where A = n/k with k denoting the number of elementary steps per revolution. Since their variance measurements obtained by imposing a variable external torque on tethered Escherichia coli cells conformed with Eq. 1 (see Fig. 5, case 1), they concluded that the observations were consistent with a Poissonian stepping mechanism using ∼400 steps per revolution.
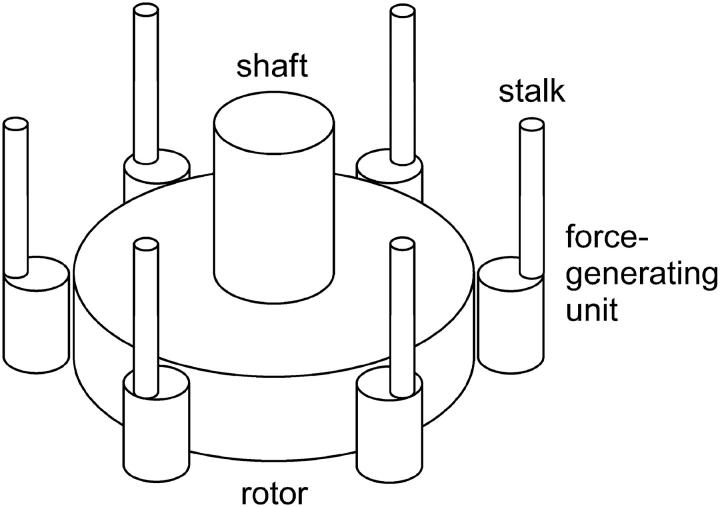
FIGURE 1.
Schematic diagram of the bacterial flagellar motor. The flagellar filament is attached by means of a hook to the shaft, which passes through a bushing in the cell wall (not shown, see Ref. 3). The force-generating units (MotA/MotB subunits), arrayed around the rotor (M and C rings), are permanently fixed to the cell wall by means of the stalks. In the case of the stepping model they are reversibly bound to specific attachment points on the peripheral surface of the rotor (not shown). From time to time one of the units steps to an adjacent attachment point. In so doing the stalk of the unit is elastically deformed, giving rise to a change in the torque exerted on the rotor. The rate constants governing the stepping depend on the driving force for the rotation (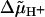 ) and the elastic energy associated with the deformation.
) and the elastic energy associated with the deformation.
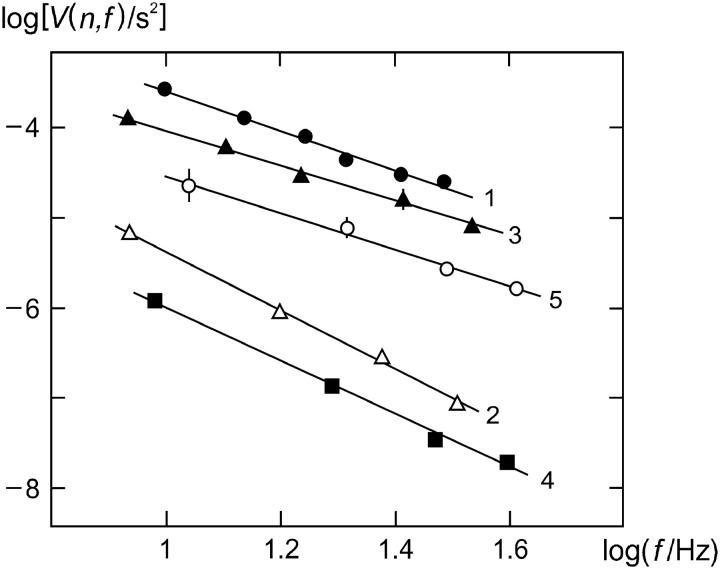
FIGURE 5.
Analysis of V(n,f), the variance in time for n revolutions at rotational frequency f, according to the relation log[V(n,f)] = log A − m log f ; where applicable the average number of elementary steps per revolution 〈k〉 is given (see text). (case 1) Experimental data (Ref. 7), m = 2.22 ± 0.14, 〈k〉 = 414 ± 36; (case 2) bidirectional stepping model, m = 3.28 ± 0.12; (case 3) unidirectional stepping model (α0 varied), m = 1.96 ± 0.08, 〈k〉 = 207 ± 26; (case 4) model of Ryu et al. (1), m = 3.00 ± 0.15; and (case 5) electrostatic model, m = 2.06 ± 0.14. Parameter values as follows: case 2, λ = 0.5, α0 = 200 s−1; case 3, λ = 0, σ = 23.9 nN nm/rad2, α0/s−1 = 500, 750, 1000, 1500, and 2000 (Tex not used); case 4, λ = 0, u = 5, α0 = 1.23 × 105 s−1, for other details see Ryu et al. (1); case 5, u = 11, for other details, see Walz and Caplan (8). Except where stated, u = 8, σ = 0.8 nN nm/rad2, kχ = 0.4 nN nm/rad2, φ = 2π/50, Db = 0.256 rad2/s, d = 20, 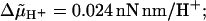 Tex/(nN nm rad−1) = 0, 1, 2, 3; n = 10; and temperature, 22°C. For graphical reasons, curves 2–5 are vertically displaced by −0.8, −0.7, 0, and −0.5 units, respectively. Standard deviations are shown by vertical bars where they exceed the size of the symbols.
Tex/(nN nm rad−1) = 0, 1, 2, 3; n = 10; and temperature, 22°C. For graphical reasons, curves 2–5 are vertically displaced by −0.8, −0.7, 0, and −0.5 units, respectively. Standard deviations are shown by vertical bars where they exceed the size of the symbols.
In the model of Samuel and Berg an increment Δθ can only occur if the unspecified element mentioned above is elastic. For Δθ to equal φ, the rate of relaxation of this elastic element has to be high enough to allow the relaxation to be essentially completed before the next elementary step takes place. Moreover, the effect of an externally applied torque Tex is not taken into account, but it is implicitly assumed that changing Tex yields different frequencies f. To clarify this issue we have analyzed a stepping model in which explicit consideration is given to the relaxation of the stepping units and that of a tether, as well as to the effect of Tex.
ANALYSIS AND SIMULATIONS
Stepping model
The scheme in Fig. 1 shows the salient features of our elastic stepping model, whereas the notation used is defined in Fig. 2. Consider u units, each of which is bound to one of r equally spaced attachment points on the rotor. At a given time t, this brings about an angular displacement ξj(t) of the jth unit from its rest position,
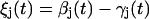 |
(2) |
(see Fig. 2). This unit then exerts a torque
 |
(3) |
on the rotor and simultaneously a torque −Tj(t) on the cell body, where σ is an elasticity coefficient reflecting the deformation of the stalk. The total torque Tel exerted by all units is
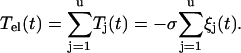 |
(4) |
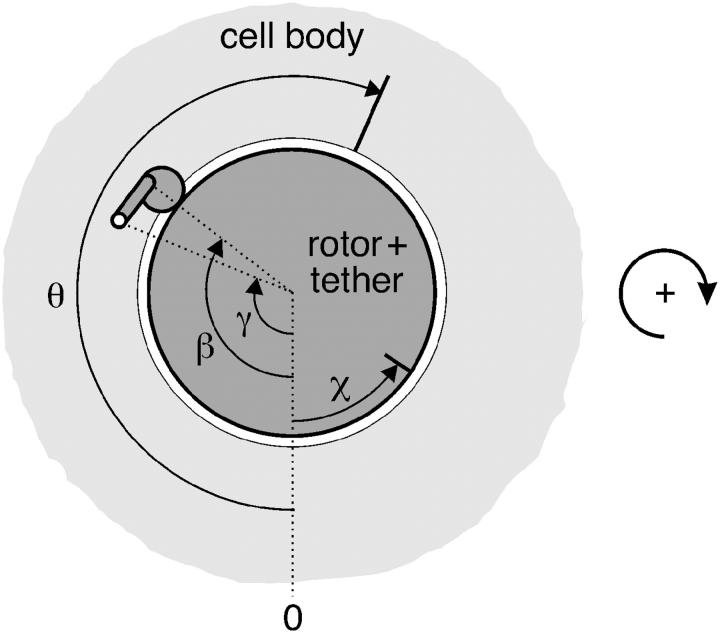
FIGURE 2.
Projection of the motor in a cell which is tethered to a support, viewed from the side of the tethering filament (i.e., from the top in Fig. 1). The dotted line marked “zero” is the reference point on the support with respect to which angles are measured (positive sense indicated on the right, corresponding to the clockwise direction of rotation of the cell body in nonreversing strains). The elastically deformed stalk of one of the units is shown whose attachment points on the rotor and the cell body are represented by the angles β and γ, respectively (subscripts j for jth unit omitted for clarity). Note that θ is the rotation angle of the cell, whereas χ is the torsion angle of the tether (usually negative, as shown).
The rotation rate of the cell body dθ/dt as a result of Tel and a constant externally applied torque Tex is
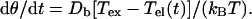 |
(5) |
Here Db denotes the rotational diffusion coefficient of the cell body, kB is Boltzmann's constant, and T the absolute temperature. The torsion rate of the tether dχ/dt as a result of Tel is
 |
(6) |
where kχ is the torsional spring constant of the tether (9) and Dr is, in effect, a rotational diffusion coefficient of the rotor plus tether.
The evolution of θ and χ in time arises from the stepping of one of the units to an adjacent attachment point at a time-point ti and the subsequent relaxation of the displacements ξj during the time-interval Δti = ti+1 − ti (see Appendix for a detailed derivation). The intervals Δti are determined by the rate constants
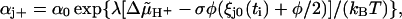 |
(7a) |
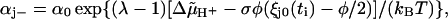 |
(7b) |
describing the stepping of the jth unit, where αj+ and αj− pertain to a step by +φ and −φ, respectively, and φ = 2π/r is the angle between adjacent attachment points (see Appendix). The coefficient λ reflects the position of the transition state in the step, 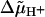 is the electrochemical potential difference for protons driving the rotation, and ξj0(ti) denotes the displacement just before the stepping of the unit.
is the electrochemical potential difference for protons driving the rotation, and ξj0(ti) denotes the displacement just before the stepping of the unit.
Trajectories θ(t) and χ(t) were obtained by Monte Carlo simulation, taking into account exclusion of attachment points due to steric hindrance (see Appendix), and the rotational frequency f was determined as the slope of a linear regression to θ(t). The trajectories θ(t) were evaluated according to the procedure of Samuel and Berg (7), and the variances were fitted to the relation
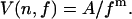 |
(8) |
For a Poissonian stepper, m = 2 (see Eq. 1); moreover, k = 〈tn〉2/[nV(n,f)] at any value of f, where tn is the time taken for n revolutions (7), and an average value 〈k〉 can then be calculated.
Poissonian versus Non-Poissonian behavior
With physically reasonable parameters, which yield the correct frequencies for the actual 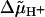 value used (8), our stepping model winds up the tether to an average steady-state angle χss such that its restoring torque kχχss balances the average elastic torque 〈Tel〉 (see Fig. 3 B and Eq. 6). The corresponding frequency f = 2π [dθ/dt]ss is determined by Tex − 〈Tel〉 and Db (Eq. 5), i.e., f can be varied by an externally applied torque Tex, which includes stalling the motor (Fig. 3 A). The variance analysis of this case yields a value of m above 3 (Fig. 5, case 2), which is to be expected since a single rate-limiting step does not occur. Depending on Tex, between 0.7% and 7% of all steps are “back steps”, i.e., pertain to −φ. Two relaxation times, with their associated exponential functions, appear in the equations of the model (Eqs. 20). Simulations were performed of the time courses of the incremental changes θ′(t′) and χ′(t′) (see Appendix, Eqs. 9) and the average displacement 〈ξ〉(t′) = 〈ξ〉(ti) + χ′(t′) − θ′(t′) (Eqs. 12, 18, and 20) in the intervals 0 ≤ t′ ≤ Δti. These revealed that the two relaxation times, τ+ and τ− (Eq. 22), govern the relaxation of the displacements ξj and the torsion of the tether, respectively. Hence, the extent of relaxation during the time intervals Δti is indicated by the values of the functions g±(t′) (Eq. 21) at t′ = Δti. These functions are bounded by the limits 0 and 1 corresponding to zero and complete relaxation, respectively. It is evident from Fig. 4, A and B, that the relaxation of the displacements ξj is rarely complete, while that of the tether is only marginal. Interestingly, we do find m close to 2 with u = 1 (a single stepping unit, not shown), but 〈k〉 = 1540 ± 150 — although in this particular case, k should be equal to 50, and back-stepping ranges between 11% and 32%.
value used (8), our stepping model winds up the tether to an average steady-state angle χss such that its restoring torque kχχss balances the average elastic torque 〈Tel〉 (see Fig. 3 B and Eq. 6). The corresponding frequency f = 2π [dθ/dt]ss is determined by Tex − 〈Tel〉 and Db (Eq. 5), i.e., f can be varied by an externally applied torque Tex, which includes stalling the motor (Fig. 3 A). The variance analysis of this case yields a value of m above 3 (Fig. 5, case 2), which is to be expected since a single rate-limiting step does not occur. Depending on Tex, between 0.7% and 7% of all steps are “back steps”, i.e., pertain to −φ. Two relaxation times, with their associated exponential functions, appear in the equations of the model (Eqs. 20). Simulations were performed of the time courses of the incremental changes θ′(t′) and χ′(t′) (see Appendix, Eqs. 9) and the average displacement 〈ξ〉(t′) = 〈ξ〉(ti) + χ′(t′) − θ′(t′) (Eqs. 12, 18, and 20) in the intervals 0 ≤ t′ ≤ Δti. These revealed that the two relaxation times, τ+ and τ− (Eq. 22), govern the relaxation of the displacements ξj and the torsion of the tether, respectively. Hence, the extent of relaxation during the time intervals Δti is indicated by the values of the functions g±(t′) (Eq. 21) at t′ = Δti. These functions are bounded by the limits 0 and 1 corresponding to zero and complete relaxation, respectively. It is evident from Fig. 4, A and B, that the relaxation of the displacements ξj is rarely complete, while that of the tether is only marginal. Interestingly, we do find m close to 2 with u = 1 (a single stepping unit, not shown), but 〈k〉 = 1540 ± 150 — although in this particular case, k should be equal to 50, and back-stepping ranges between 11% and 32%.
FIGURE 3.
Trajectories of rotation angle θ (A, C) and torsion angle χ (B, D), calculated as described in the Appendix. Parameter values: u = 8 and r = 50 (10), φ = 2π/50 (7.2°), Db = 0.256 rad2/s, corresponding to a frictional drag coefficient 2π kBT/Db = 0.1 nN nm rad−1 Hz−1 (8), d = 20, kχ = 0.4 nN nm/rad2 (9), 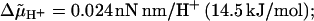 Tex /(nN nm rad−1) = 0 (a), 1.5 (b), −1.53 (c); temperature, 22°C; sampling time, 5 ms; bidirectional stepping model, σ = 0.8 nN nm/rad2, α0 = 200 s−1, λ = 0.5 (A, B); unidirectional stepping model, σ = 23.9 nN nm/rad2, α0 = 500 s−1, λ = 0 (C, D). The relaxation time (ms) for χ (t) in B and the frequency f (Hz) at steady state in A are 13.6 ± 0.7 and 8.7 ± 0.3 (a), 10.2 ± 1.1 and 19.9 ± 0.4 (b), 17.2 ± 0.4 and 0.002 ± 0.2 (c), respectively. The corresponding values for D and C are 41.1 ± 1.6 and 8.6 ± 0.4 (a), 44.4 ± 2.3 and 8.5 ± 0.4 (b), and 42.4 ± 0.6 and 8.8 ± 0.4 (c), respectively.
Tex /(nN nm rad−1) = 0 (a), 1.5 (b), −1.53 (c); temperature, 22°C; sampling time, 5 ms; bidirectional stepping model, σ = 0.8 nN nm/rad2, α0 = 200 s−1, λ = 0.5 (A, B); unidirectional stepping model, σ = 23.9 nN nm/rad2, α0 = 500 s−1, λ = 0 (C, D). The relaxation time (ms) for χ (t) in B and the frequency f (Hz) at steady state in A are 13.6 ± 0.7 and 8.7 ± 0.3 (a), 10.2 ± 1.1 and 19.9 ± 0.4 (b), 17.2 ± 0.4 and 0.002 ± 0.2 (c), respectively. The corresponding values for D and C are 41.1 ± 1.6 and 8.6 ± 0.4 (a), 44.4 ± 2.3 and 8.5 ± 0.4 (b), and 42.4 ± 0.6 and 8.8 ± 0.4 (c), respectively.
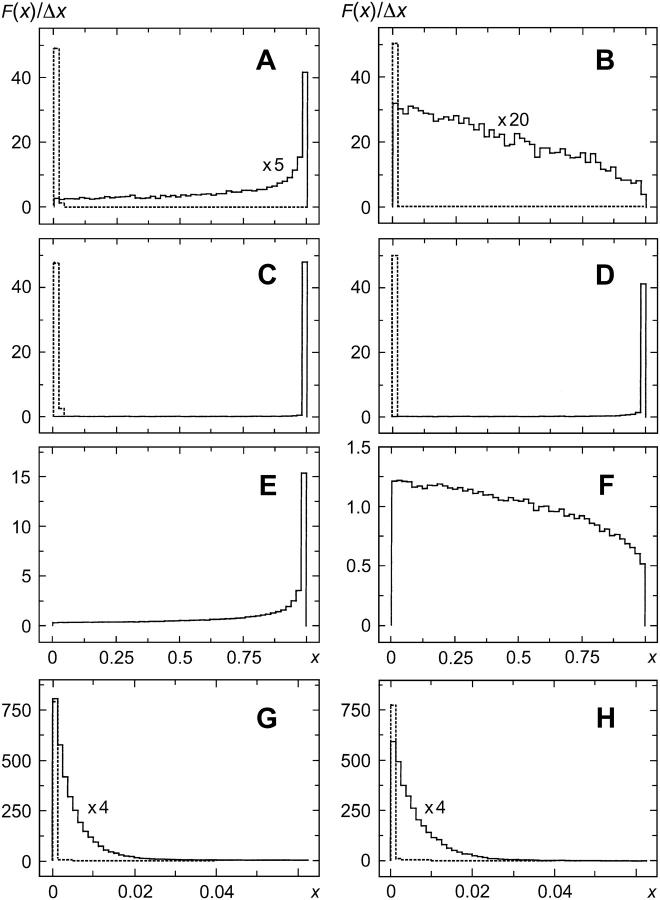
FIGURE 4.
Partition functions (histograms) for values of g± (Eq. 22) and g (Eq. 27) at the end of the time intervals Δti. F(x) denotes the normalized frequency with which values of the variable x were found in the interval from x to x + Δx, x represents g+(Δti) (solid line), g−(Δti) (dotted line) in A–D, G, and H, or g(Δti) in E and F; Δx = 0.02 (A–F) and 0.00125 (G, H). For graphical reasons F(x) for g+(Δti) in A, B, G, and H is multiplied by a factor as indicated. Bidirectional stepping model with Tex = 0 (A) and 3 nN nm/rad (B); unidirectional stepping model with α0 = 500 s−1 (C, E) and 2000 s−1 (D, F) for d = 20 (elastic tether, C and D) and d = 0 (stiff tether, E and F); model of Ryu et al. (1) with Tex = 0 (G) and 40 nN nm/rad (H), in this case F(x) ≡ 0 for x > 0.0625. Other parameter values are given in the legends to Figs. 3 and 5. The relaxation times τ+ (μs) and τ− (ms) are 113 and 44.4 (A, B); 3.96 and 42.1 (C, D); and 175 and 45.8 (G, H), respectively, whereas τb = 87.4 μs (E, F). The mean values of the time intervals Δti (μs) obtained from the simulations are 242 ± 252 (A), 76 ± 79 (B), 286 ± 284 (C), 73 ± 75 (D), 291 ± 297 (E), 73 ± 74 (F), 0.82 ± 0.86 (G), and 1.08 ± 1.11 (H); note that the standard deviations are close to the mean values as required for a Poisson distribution.
Unidirectional stepping should be possible when λ is set to 0, i.e., αj+ = α0 (Eq. 7a), and the elasticity coefficient σ of the stalks is increased until the usually negative term commencing with σ in Eq. 7b becomes large enough to satisfy the condition αj− → 0. Simulations with increasing values of σ revealed that back-steps persist (and even increase in frequency) until a critical value of 22.8 nN nm/rad2 is reached. Upon further increase of σ there is an increasing incidence of steps for which the quantity ξj0(ti) − φ/2 is positive (i.e., αj− → ∞), and αj− for these steps has to be set to 0 to eliminate very large and physically meaningless values of αj−. The critical value of σ causes τb and thus τ+ (Eqs. 17 and 22) to be so small that g+(Δti) is larger than 0.94 (see Fig. 4, C and D), i.e., relaxation of the displacements ξj during the time intervals Δti is essentially complete. On the other hand, a (Eq. 16) and thus q (Eq. 23) approach unity, which in turn causes τ− (Eq. 22) to be so large that g−(Δti) ≤ 0.04, i.e., the tether still does not relax to an appreciable extent. Such a unidirectional stepper, however, is insensitive to an externally applied torque Tex (see Fig. 3 C), and its frequency f is solely determined by α0. Hence, the only possible means of changing f is by direct alteration of α0, which would require changing the temperature rather than imposing an external torque. A simulation of this case indeed yields a value of m close to 2 (Fig. 5, case 3), but the value of 〈k〉 is ∼200 instead of 400 as predicted by Samuel and Berg (10). This arises from the exclusion of steps due to steric hindrance. If this condition is released, m ≈ 2 and 〈k〉 ≈ 400 are obtained (not shown), but almost all αj− values tend to infinity and have to be eliminated. Moreover, a crossing-over of units occurs, and the displacements attain unrealistically high values of up to 12π.
Ryu et al. (1) have proposed a stepping model in which each step comprises three substeps covering 5%, 90%, and 5% of the step interval, respectively, with a corresponding subdivision of 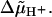 This model is sensitive to Tex despite the condition λ = 0 for the rate constants of all substeps, but Monte Carlo simulations performed with this model yield again an m value of 3 (Fig. 5, case 4).
This model is sensitive to Tex despite the condition λ = 0 for the rate constants of all substeps, but Monte Carlo simulations performed with this model yield again an m value of 3 (Fig. 5, case 4).
Nonstepping model
We have shown (8) that an entirely different model based on electrostatic interactions, in which no attachment points are present and hence no stepping occurs, reproduces the results of a wide variety of different studies, providing two proton channels are assigned to each force-generating unit; biochemical evidence that this is probably the case has since been presented (11,12). The simulations with this model were previously carried out using a deterministic (kinetic) methodology. We have reexamined this model using Monte Carlo simulations (13) and including Brownian motion, thus making fluctuation analysis possible. The results are not only fully in agreement with our earlier simulations, but yield a value of m close to 2 for the simulation of the Samuel and Berg study (7) (Fig. 5, case 5).
DISCUSSION
The stepping model proposed by Ryu et al. (1) incorporates three states per step in which the units are always bound to the attachment points (high duty ratio). It is purely phenomenological, since no mechanistic explanation is provided as to how the transport of protons through the channel of a unit causes its release from an attachment point and its movement to the adjacent point. Moreover, proton movement is described in terms of 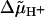 instead of explicitly in terms of proton concentrations and a membrane potential (8,14). The value of the intrinsic rate constant α0 necessary for the simulation of the experimental results gives rise to time intervals Δti, which are at least two orders-of-magnitude smaller than the relaxation times τ+ and τ−. Hence g±(Δti) ≤ 0.04 (Fig. 4, G and H), i.e., there is very little relaxation of the displacements ξj as well as of the tether, and the term σφ (ξj0(ti) − φ /2) in Eq. 7b is rather small. As a consequence, despite the setting of λ = 0, considerable “back stepping” occurs (49% and 34% for Tex = 0 and 40 nN nm rad−1, respectively). In addition, as already noticed by Ryu et al. (1), the model is rather insensitive to the value of σ if chosen in a physically reasonable range, which can be estimated from the elastic parameters of actin filaments (15,16). It is therefore not surprising that this model cannot reproduce the experimentally observed inverse second-power dependence of variance on frequency.
instead of explicitly in terms of proton concentrations and a membrane potential (8,14). The value of the intrinsic rate constant α0 necessary for the simulation of the experimental results gives rise to time intervals Δti, which are at least two orders-of-magnitude smaller than the relaxation times τ+ and τ−. Hence g±(Δti) ≤ 0.04 (Fig. 4, G and H), i.e., there is very little relaxation of the displacements ξj as well as of the tether, and the term σφ (ξj0(ti) − φ /2) in Eq. 7b is rather small. As a consequence, despite the setting of λ = 0, considerable “back stepping” occurs (49% and 34% for Tex = 0 and 40 nN nm rad−1, respectively). In addition, as already noticed by Ryu et al. (1), the model is rather insensitive to the value of σ if chosen in a physically reasonable range, which can be estimated from the elastic parameters of actin filaments (15,16). It is therefore not surprising that this model cannot reproduce the experimentally observed inverse second-power dependence of variance on frequency.
For reasons of consistency we have adopted the approach of Ryu et al. (1) when designing our stepping model. This model can be considered to be a simplified version of the three-state model in which binding, release, and transfer of protons in a channel all take place in one step. Although this simplification reduces the number of possible transitions by a factor of 3, a rate-limiting step still does not appear if physically reasonable parameter values are used. Such a step can only occur if λ is set to zero and the stalks are assumed to be extremely stiff. This, however, eliminates the dependence on 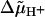 since αj+ = α0 (Eq. 7a) and back steps whose rate constants αj− depend on
since αj+ = α0 (Eq. 7a) and back steps whose rate constants αj− depend on 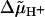 are excluded. On the other hand, for the intrinsic stepping expressed by the rate constant α0, the considerable elastic work involved in the movement of a unit between attachment points (see Eq. 30) would have to be covered by thermal energy, which is already highly unlikely at the critical value of σ and is even less feasible as σ becomes larger. We also find that the presence of Tex does not alter the rotational frequency f (Fig. 3 C, and additional simulations with Tex up to 200 nN nm rad−1), in clear contradiction to what was found experimentally (7,17). Moreover, the unidirectional stepping model cannot reproduce the experimentally observed proportionality between f and the number of units (10). Simulations with u = 4 and 1 yield frequencies which are, respectively, 8% and 17% larger than those for u = 8. The corresponding m values are still close to 2, whereas the 〈k〉 values decrease to ∼140 and 60, respectively. Hence it appears that the model used by Samuel and Berg (7) to explain the observed inverse second-power dependence of variance on frequency is neither physically feasible nor able to reproduce experimental results.
are excluded. On the other hand, for the intrinsic stepping expressed by the rate constant α0, the considerable elastic work involved in the movement of a unit between attachment points (see Eq. 30) would have to be covered by thermal energy, which is already highly unlikely at the critical value of σ and is even less feasible as σ becomes larger. We also find that the presence of Tex does not alter the rotational frequency f (Fig. 3 C, and additional simulations with Tex up to 200 nN nm rad−1), in clear contradiction to what was found experimentally (7,17). Moreover, the unidirectional stepping model cannot reproduce the experimentally observed proportionality between f and the number of units (10). Simulations with u = 4 and 1 yield frequencies which are, respectively, 8% and 17% larger than those for u = 8. The corresponding m values are still close to 2, whereas the 〈k〉 values decrease to ∼140 and 60, respectively. Hence it appears that the model used by Samuel and Berg (7) to explain the observed inverse second-power dependence of variance on frequency is neither physically feasible nor able to reproduce experimental results.
The rotational mobility of the rotor plus tether expressed by the parameter Dr plays only a minor role. Simulations performed with different values of Dr up to the limit of 1280 rad2 s−1, which corresponds to a frictional drag coefficient 2π kBT/Dr = 2 × 10−5 nN nm rad−1 Hz−1 as estimated by Berg (3) for a freely movable rotor in a lipid phase, revealed that Dr merely affects the time required for the system to reach the steady state, whereas the characteristics outlined above are not altered. In particular, the model with the large value of σ required for unidirectional stepping remains insensitive to an externally applied torque Tex even for the largest value of Dr. In fact, the rather stiff elements in this model firmly connect the cell body to the rotor plus tether, which makes them behave as one rigid body. As a consequence, in contrast to the case of the less rigid stalks, Tex (which acts on the cell body) has no effect on the rotational frequency but manifests itself entirely in an altered average steady-state tether angle χss (Fig. 3 D) at which the restoring torque kχ χss is balanced by the average elastic torque 〈Tel〉 and Tex. Thus it would seem that the picture envisaged by Berg (3,18) for a stepping motor cannot hold true. In that picture the stepping of a unit first predominantly winds up the more mobile tether, which subsequently relaxes, thus carrying the cell body forward. However, this postulated relaxation of the tether is imperceptibly small in our simulations (see Fig. 4).
A rigid tether is obtained if Dr is set to zero, and Eq. 20 is then replaced by Eq. 26 (see Appendix). Simulations and variance analyses under this condition yield essentially the same results as with an elastic tether. The m values are slightly decreased by ∼0.2 for the model of Ryu et al. (1) and our stepping model with λ = 0.5 (i.e., bidirectional) and σ = 0.8 nN nm/rad2. They remain at ∼2 for our model with λ = 0 (i.e., unidirectional) and σ ≥ 22.8 nN nm/rad2. However, the function g(Δti) in Eq. 27, which is analogous to g+(Δti) in Eq. 20, adopts all possible values in the interval from 0 to 1 with variable frequencies depending on the value of α0 (Fig. 4, E and F). This indicates that relaxation of the displacements ξj during the time intervals Δti is mostly incomplete under these circumstances, but this criterion seems not to be crucial for m to attain a value close to 2.
Brownian motion was excluded from the simulations with the stepping models in order not to blur the stepping behavior. Including Brownian motion has little effect on the model of Ryu et al. (1) and our model with λ = 0 and σ ≥ 22.8 nN nm/rad2, but decreases m by ∼0.4 if λ = 0.5 and σ = 0.8 nN nm/rad2. It should be mentioned that Brownian motion in a broken motor yields, for all models, m ≈ 3 (not shown), as is experimentally observed.
The experimental finding that the motor in de-energized cells appears to be “locked” (9) may be considered as evidence for a motor with units always bound to attachment points. However, simulations with the stepping models as well as with our electrostatic model at 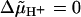 did not show any locking of the motor. But we can reproduce this phenomenon if we assume closure of proton channels upon de-energization, in analogy to the closure of channels in a sodium ion-driven motor (11). Hence it is not the type of interaction between rotor and stator (binding of units or electrostatic interaction) but the restricted proton movement that causes the locking of the motor.
did not show any locking of the motor. But we can reproduce this phenomenon if we assume closure of proton channels upon de-energization, in analogy to the closure of channels in a sodium ion-driven motor (11). Hence it is not the type of interaction between rotor and stator (binding of units or electrostatic interaction) but the restricted proton movement that causes the locking of the motor.
In conclusion, the inverse second-power dependence of variance on frequency (Eq. 1) is not a suitable criterion for stepping; in fact a nonstepping mechanism can comply with this criterion, whereas a stepping mechanism in many cases does not. Hence there is no evidence for the notion (1,3,7) that the flagellar motor operates in a stepwise mode with units that are virtually always engaged (high duty ratio).
APPENDIX A
Motor rotation due to stepping of units and relaxation of elastic elements as well as elastic tether
Let θ′ and χ′ denote the advancement of θ and χ during the relaxation period Δti = ti+1 − ti, respectively, such that
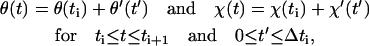 |
(9) |
where
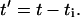 |
(10) |
Since all units are attached to the rotor and the cell body during this period, i.e., a duty ratio of 1 (see Ref. 1),
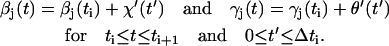 |
(11) |
Hence by means of Eq. 2,
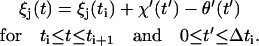 |
(12) |
Recalling that ξj0(ti) denotes displacements just before the stepping of the kth unit occurs, it follows that
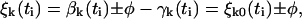 |
(13a) |
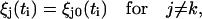 |
(13b) |
where φ = 2π/r. Note that steps of +φ and −φ give rise to positive and negative contributions to the displacement, respectively. Inserting Eqs. 4, 9, 10, 12, and 13 into Eqs. 5 and 6 yields
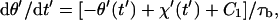 |
(14a) |
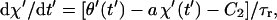 |
(14b) |
with the abbreviations
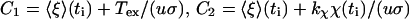 |
(15) |
and
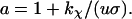 |
(16) |
Here τb and τr are scaling factors denoting, respectively, characteristic rotation times of the cell body and the rotor plus tether, and are defined as
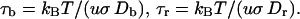 |
(17) |
The quantity 〈ξ〉(ti) is the average displacement just after the kth unit has stepped,
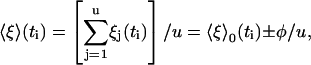 |
(18) |
where
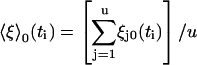 |
(19) |
is the average displacement just before stepping of the unit. The solutions to Eqs. 14 satisfying the boundary conditions θ′(0) = χ′(0) = 0 are found to be
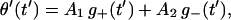 |
(20a) |
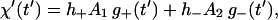 |
(20b) |
where
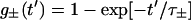 |
(21) |
and the relaxation times τ± are defined as
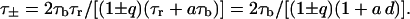 |
(22) |
The quantities d, q, and h± are abbreviations that read
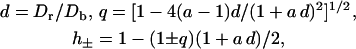 |
(23) |
and the constants A1 and A2 are given by
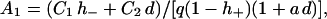 |
(24a) |
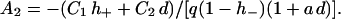 |
(24b) |
Since the ξj values reached at the end of the ith time interval Δti are the ξj0values of the next interval starting at ti+1, it follows from Eq. 12 that
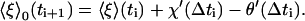 |
(25) |
Special case Dr → 0
This case corresponds to a rigid or motionless tether. Here d → 0, q → 1, h+ → 0, h− → 1 (Eq. 23), τ+ → τb (Eq. 22), and A1 = C1 (Eq. 24a). The term A2 g−(t′) can be shown, by L‘Hopital’s rule, to go identically to zero in this limit. Hence
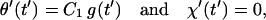 |
(26) |
where
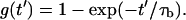 |
(27) |
Kinetics
Following Ryu et al. (1) the rate constants αj+ and αj− describing the stepping of the jth unit are assumed to be determined by an intrinsic rate constant α0 and a Boltzmann factor which comprises the difference in free energy ΔGj± associated with the step and a coefficient λ reflecting the position of the transition state in the step,
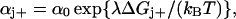 |
(28a) |
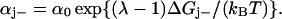 |
(28b) |
ΔGj± is composed of two terms, the free energy which powers the rotation, i.e., 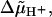 and the difference in elastic energy ΔGj,el between the initial and the final state of the step in the direction that gives rise to a positive contribution to Tel. The elastic energy can be determined by integrating the relation
and the difference in elastic energy ΔGj,el between the initial and the final state of the step in the direction that gives rise to a positive contribution to Tel. The elastic energy can be determined by integrating the relation
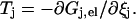 |
(29) |
Hence by means of Eq. 3, and with the boundary condition Gj,el = 0 for ξj = 0,
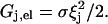 |
(30) |
Since the displacements ξj0(ti) and ξj(ti) represent the initial and final states of a step by +φ, but the final and initial states of a step by −φ, the difference in free energy becomes (see Eq. 30)
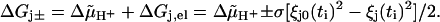 |
(31) |
Inserting Eqs. 13a (with k = j) and 31 into Eqs. 28 then yields Eqs. 7.
Monte Carlo simulations
For the Monte Carlo simulation (13) it is convenient to renumber the rate constants as
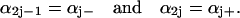 |
(32) |
The order of the renumbered rate constants is not crucial. An alternative numbering yielding the same results is αj = αj− and αu+ j = αj+. The time interval Δti at the time point ti is then given by
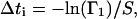 |
(33) |
whereas the index k satisfying the condition
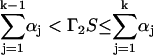 |
(34) |
determines which unit steps, and in which direction. In Eqs. 33 and 34 the quantities Γ1 and Γ2 are random numbers drawn from a unit-interval uniform distribution, and S denotes the sum over all rate constants:
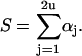 |
(35) |
Since binding of two units to the same attachment point should be excluded for steric reasons, a bookkeeping of occupied attachment points is performed, and the rate constant of a step which would end in an occupied attachment point is set to zero.
Trajectories θ(t) and χ(t) can then be calculated by means of Eqs. 7, 9, and 15–25, starting from the initial conditions t0 = θ (t0) = χ(t0) = 〈ξ〉0(t0) = 0.
APPENDIX B: GLOSSARY
- a
Dimensionless quantity defined by Eq. 16.
- Db
Rotational diffusion coefficient of cell body.
- Dr
Rotational diffusion coefficient of rotor plus tether.
- d, q, h+, h−
Dimensionless quantities defined by Eqs. 23.
- F(x)
Normalized frequency with which values of x occur in the interval x to x + Δx.
- f
Rotational frequency.
- g+, g−, g
Exponential functions associated with τ+, τ−, and τb, respectively (Eqs. 21 and 27).
- k
Number of elementary steps per revolution.
- kB
Boltzmann's constant.
- kχ
Torsional spring constant of tether.
- m
Exponent of f in expression for V: for a Poissonian stepper m = 2.
- n
Number of revolutions.
- r
Number of attachment points.
- T
Absolute temperature.
- Tel
Total torque exerted by all elastic units.
- Tex
Externally applied torque.
- Tj
Torque exerted by jth unit on rotor.
- t
Time.
- u
Number of stepping units.
- V
Variance in time taken for n revolutions at rotational frequency f.
- αj+, αj−
Rate constants specifying the stepping of the jth unit.
- α0
Intrinsic rate constant.
- β
Angle specifying attachment point of stepping unit to rotor.
- Γ1, Γ2
Random numbers drawn from a unit-interval uniform distribution.
- γ
Angle specifying attachment point of stepping unit to cell body.
- ΔGj+, ΔGj−
Free energy differences associated with the jth step.
- ΔGj,el
Elastic energy difference between initial and final state of the jth step.
- Δti
Time interval between steps at time points ti and ti+1.
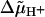
Electrochemical potential difference for protons driving rotation.
- θ
Rotation angle of cell.
- λ
Coefficient reflecting the position of the transition state in a step.
- ξ
Angular displacement of a stepping unit from its rest position.
- σ
Elasticity coefficient of stepping unit.
- τb, τr
Characteristic rotation times of cell body and rotor plus tether, respectively.
- τ+, τ−
Relaxation times governing unit displacements and tether torsion, respectively.
- φ
Elementary angular step.
- χ
Torsion angle of tether.
- χss
Average steady-state torsion angle of tether.
- ′
Denotes an incremental change between steps (e.g., θ′).
References
- 1.Ryu, W. S., R. M. Berry, and H. C. Berg. 2000. Torque-generating units of the flagellar motor of Escherichia coli have a high duty ratio. Nature. 403:444–447. [DOI] [PubMed] [Google Scholar]
- 2.Noji, H., and M. Yoshida. 2001. The rotary machine in the cell, ATP synthase. J. Biol. Chem. 276:1665–1668. [DOI] [PubMed] [Google Scholar]
- 3.Berg, H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19–54. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda, R., H. Noji, K. Kinosita, Jr., and M. Yoshida. 1998. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120° steps. Cell. 93:1117–1124. [DOI] [PubMed] [Google Scholar]
- 5.Pänke, O., K. Gumbiowski, W. Junge, and S. Engelbrecht. 2000. F-ATPase: specific observation of the rotating c-subunit oligomer of EFOEF1. FEBS Lett. 472:34–38. [DOI] [PubMed] [Google Scholar]
- 6.Börsch, M. D., M. Diez, B. Zimmermann, R. Reuter, and P. Gräber. 2002. Stepwise rotation of the γ-subunit of EF0F1-ATP synthase observed by intramolecular single-molecule fluorescence resonance energy transfer. FEBS Lett. 527:147–152. [DOI] [PubMed] [Google Scholar]
- 7.Samuel, A. D. T., and H. C. Berg. 1995. Fluctuation analysis of rotational speeds of the bacterial flagellar motor. Proc. Natl. Acad. Sci. USA. 92:3502–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walz, D., and S. R. Caplan. 2000. An electrostatic mechanism closely reproducing observed behavior in the bacterial flagellar motor. Biophys. J. 78:626–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Block, S. M., D. F. Blair, and H. C. Berg. 1989. Compliance of bacterial flagella measured with optical tweezers. Nature. 338:514–518. [DOI] [PubMed] [Google Scholar]
- 10.Samuel, A. D. T., and H. C. Berg. 1996. Torque-generating units of the bacterial flagellar motor step independently. Biophys. J. 71:918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato, K., and M. Homma. 2000. Functional reconstitution of the Na+-driven polar flagellar motor component of Vibrio alginolyticus. J. Biol. Chem. 275:5718–5722. [DOI] [PubMed] [Google Scholar]
- 12.Braun, T. F., and D. F. Blair. 2001. Targeted disulfide cross-linking of the MotB protein of Escherichia coli: evidence for two H+ channels in the stator complex. Biochemistry. 40:13051–13059. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie, D. T. 1977. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 81:2340–2361. [Google Scholar]
- 14.Hill, T. L. 1977. Free Energy Transduction in Biology. Academic Press, New York.
- 15.Kojima, H., A. Ishijima, and T. Yanagida. 1994. Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro nanomanipulation. Proc. Natl. Acad. Sci. USA. 91:12962–12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuda, Y., H. Yasutake, A. Ishijima, and T. Yanagida. 1996. Torsional rigidity of single actin filaments and actin-actin bond breaking force under torsion measured directly by in vitro micromanipulation. Proc. Natl. Acad. Sci. USA. 93:12937–12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg, H. C., and L. Turner. 1993. Torque generated by the flagellar motor of Escherichia coli. Biophys. J. 65:2201–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg, H. C. 1976. Does the flagellar rotary motor step? In Cell Motility, Cold Spring Harbor Conferences on Cell Proliferation. R. Goldman, T. Pollard, and J. Rosenbaum, editors. Cold Spring Harbor, NY. 47–56.