Abstract
Salicylate is a small amphiphilic molecule which has diverse effects on membranes and membrane-mediated processes. We have utilized micropipette aspiration of giant unilamellar vesicles to determine salicylate's effects on lecithin membrane elasticity, bending rigidity, and strength. Salicylate effectively reduces the apparent area compressibility modulus and bending modulus of membranes in a dose-dependent manner at concentrations above 1 mM, but does not greatly alter the actual elastic compressibility modulus at the maximal tested concentration of 10 mM. The effect of salicylate on membrane strength was investigated using dynamic tension spectroscopy, which revealed that salicylate increases the frequency of spontaneous defect formation and lowers the energy barrier for unstable hole formation. The mechanical and dynamic tension experiments are consistent and support a picture in which salicylate disrupts membrane stability by decreasing membrane stiffness and membrane thickness. The tension-dependent partitioning of salicylate was utilized to calculate the molecular volume of salicylate in the membrane. The free energy of transfer for salicylate insertion into the membrane and the corresponding partition coefficient were also estimated, and indicated favorable salicylate-membrane interactions. The mechanical changes induced by salicylate may affect several biological processes, especially those associated with membrane curvature and permeability.
INTRODUCTION
Salicylate, an active metabolite of aspirin and a popular non-steroidal anti-inflammatory drug (NSAID), has been used for centuries to treat fever, pain, and arthritis (1,2). The drug's main therapeutic function has been attributed to its ability to block the activity of cyclo-oxygenase (COX), which catalyzes the production of prostaglandin from arachidonic acid (2,3). Prostaglandins are important signaling molecules that regulate a number of biological processes and are important in the mammalian immune system. However, several of the main side-effects of salicylate and other NSAIDs are independent of tissue prostaglandin levels and appear not to be mediated by COX (1). These include effects on neutrophil aggregation (4), gastric intestinal (GI) toxicity (5,6), and reversible hearing loss (7,8). GI toxicity is especially noteworthy, because it leads to life-threatening ulcers. In the GI tract, gastric mucosal cells secrete phospholipids which form a hydrophobic barrier that protects the surrounding tissue from the acidic contents of the gut (5,9,10). Lichtenberger and co-workers have accumulated compelling evidence that the mechanism by which salicylate and related NSAIDs lead to ulcer formation is linked to the ability of these molecules to chemically associate with amphiphatic lipids and directly decrease the hydrophobicity of the mucus gel layer (6,11–13).
Consideration of salicylate's chemical structure suggests that salicylate will interact with phospholipids. Salicylate (α-hydroxybenzoic acid) is composed of a nonpolar benzene ring and a polar domain consisting of both carboxyl- and hydroxyl-functional groups (Fig. 1). This amphiphilic structure points to the likelihood that salicylate will penetrate into biomembranes and alter their physical properties. The consequences of salicylate's effects on membrane mechanics have not been characterized and quantified. Membrane mechanical properties are crucial to many cellular functions, such as permeability, shape deformation, adhesion, communication, fusion, and endo-/exocytosis. In addition, experimental evidence is accumulating that membrane elastic properties affect the function of integral membrane proteins, including the kinetics of ion channels (14).
FIGURE 1.
Chemical structures of salicylate (a) and aspirin (b) (ACD/ChemSketch 5.0). Both compounds are benzoic acid derivatives. Aspirin is quickly hydrolyzed to salicylate in an aqueous environment. These amphiphilic molecules interact with membranes, potentially leading to serious side effects.
Biomembranes are held together by hydrophobic forces, which give rise to surface tension (15,16). This cohesive force is opposed by a surface pressure, which balances with the surface tension in the stress-free condition. The difference between the surface tension and the surface pressure defines the membrane tension (17). Both surface tension and surface pressure are determined by lipid packing parameters, such as the surface area and molecular volume of the lipid headgroup and the length of the lipid acyl chain (18,19). When the membrane structure is perturbed by incorporation of amphiphilic molecules, such as salicylate, the lipid packing can be changed, thereby affecting lipid-lipid interactions.
The effects of a number of amphiphilic molecules on membrane mechanics have been investigated. For instance, short-chain alcohols (20,21), bile acids (22), and lysolipids (23) partition into the membrane and affect membrane elasticity, bending stiffness, and lysis tension. In the case of large surfactants, such as bile acids and lysolipids, the effects on the elastic compressibility were attributed to tension-dependent partitioning, i.e., the incorporation of more molecules into the membrane as the membrane is stretched by increases in applied pressure. In addition, small surfactants are predicted to change the bilayer lateral pressure profile, leading to changes in membrane mechanical moduli and thickness (24). In this article we use micropipette aspiration of giant unilamellar lipid vesicles (GUVs) to characterize salicylate's ability to affect mechanical properties of a phosphatidylcholine (lecithin) membrane. We introduce a method to correct micropipette aspiration data for tension-dependent partitioning effects which is generally applicable to experiments with soluble surfactants. A quantitative understanding of the concentration dependence of salicylate's effects on membranes is important for ascertaining the drug's side effects in the physiological range.
We demonstrate that salicylate rapidly enters the membrane and reduces the apparent elastic area compressibility and bending moduli in a dose-dependent manner. Salicylate is especially effective at decreasing bending stiffness, although it has a smaller effect on the elastic area compressibility modulus, especially after accounting for the effects of tension-dependent partitioning. These changes are consistent with a salicylate-induced decrease in membrane thickness. The molecular volume of salicylate in the membrane is estimated to be ∼240 Å3 by comparing the results of a transfer experiment to a “sponge” model developed by Evans et al. (22). The model is consistent with independent results that salicylate molecules exist as dimers in solution (25,26). We also estimated the free energy of transfer to be ∼13.5 kJ/mol and the corresponding partition coefficient to be ∼2.2 by converting our elastic compressibility moduli to surface-tension values (21). Taken together, the results verify that salicylate molecules prefer the membrane's hydrophobic environment over an aqueous one.
Finally, we explored the effects of salicylate on membrane strength by applying dynamic tension spectroscopy (DTS), a technique recently developed by Evans et al. (31). Membrane strength is indicated by the ability of the membrane to resist rupture under high values of applied tension. Evans et al. (31) propose that rupture of membrane is a molecular-dynamic process composed of two steps: an initial formation of a spontaneous defect followed by subsequent formation of an unstable hole (cavitation) that leads the membrane to burst. In this theoretical development, the terms “defect” and “hole” refer to energetic (or thermodynamic) states of the membrane, and do not necessarily correspond to a particular geometry. Under this scheme, the spontaneous defect represents a metastable state which can transition to an unstable hole that leads to membrane failure. Both of these processes have associated energy barriers, and the tension at which the membrane fails depends upon the rate of pressure loading because the rate of tension application changes the probability of the transition from a spontaneous defect to an unstable hole. DTS experiments reveal that salicylate increases the frequency of spontaneous defects and significantly lowers the tension scale for cavitation. The corresponding edge energy of the membrane hole calculated from the tension scale data indicates that salicylate stabilizes membrane holes.
Our mechanical and DTS measurements point to a unified picture in which the primary mechanical result of salicylate partitioning at low concentrations is a reduction in the membrane's bending stiffness. Many undesirable side effects of salicylate could stem from these mechanical effects on the membrane.
MATERIALS AND METHODS
Materials
The phospholipid 1-stearoyl-2-oleoyl-phosphatidyl-choline (SOPC) was purchased from Avanti Polar Lipids (Alabaster, AL). Lipids were dissolved in chloroform and stored at −20°C under nitrogen. Sodium salicylate and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO). Glucose and sucrose were purchased from Fisher Scientific (Pittsburgh, PA).
Methods
Vesicle formation
Vesicles were formed from SOPC using an electroformation procedure (27,28). The SOPC/chloroform solution was spread on platinum electrodes aligned parallel and ∼5 mm apart from each other in a vesicle-making chamber. The chamber was then placed in a desiccator under vacuum for at least 2 h to ensure complete evaporation of chloroform. The dried lipid film was then slowly rehydrated in 200 mM sucrose solution. An alternating AC field with amplitude from 0.5 V to 2 V and frequency from 10 Hz to 1 Hz was then applied to the electrodes. After ∼2 h, SOPC vesicles with sizes between 10 μm and 40 μm in diameter would form and bud off the platinum electrodes. The vesicle solution was then collected in a glass vial and stored at 4°C under nitrogen.
We also formed SOPC GUVs using the traditional rehydration method (29) to ensure that the lipids were not oxidized during the electroformation process. Approximately 25 μl of 0.5 mg/ml SOPC/chloroform was spread on the roughened surface of a Teflon disk and dried under vacuum in a desiccator for at least 2 h. The Teflon disk with SOPC was rehydrated in 200 mM sucrose solution under N2 overnight.
Micropipette aspiration
Microaspiration measurements were conducted on the stage of a Zeiss Axiovert 200M inverted microscope equipped with differential interference contrast (DIC) optics. We found that superior optical quality was achieved by using a Zeiss Plan-Neofluar 40×/0.85 polarized DIC objective (Carl Zeiss, Thornwood, NY). Additional magnification was provided by a 1.6 optovar. Micropipettes used to apply pressure on lipid vesicles were fabricated using a micropipette puller (Sutter P-97, Novato, CA) and then broken cleanly on a custom-made microforge. A 0.02% bovine serum albumin (BSA) solution was then used to coat the pipette. An extensive washing of the coated pipette with both 200 mM glucose solution and distilled water was performed in an effort to eliminate excess BSA on the pipette (30).
The finished pipette with diameter of 8–10 μm was mounted on a micromanipulator coupled to a water-filled reservoir and inserted into the aspiration chamber that contains lipid vesicles suspended in isomolar glucose/salicylate solution (200 mOsM). Pressure loading was achieved by changing the height of a water column mounted on a motorized mechanical slider purchased from Robocylinder (RCP-SA6I-L-600-P, Torrance, CA). The pressure level was calibrated and periodically checked using small vesicular debris that was found in the chamber and was small enough (∼1–2 μm in diameter) to move freely through the aspirating pipette. Unilamellar vesicles with a diameter between 18 μm and 40 μm were chosen for experiments. When negative pressure was applied, a portion of the vesicle would enter the pipette, creating a vesicular projection (Fig. 2 a). After each pressure-loading step, the vesicle was given at least 10 s for salicylate exchange to equilibrate between the vesicle and the bulk solution. This time was chosen because a transfer experiment (fully described below) used to characterize the timecourse of salicylate uptake indicated that it took <10 s for salicylate to complete its uptake into membrane (Fig. 3). After the projection length reached steady state, aspiration images were captured using a Zeiss Axiocam MRm camera. The vesicle and pipette dimensions were measured and analyzed using Zeiss Axiovision 3.0 software. To obtain accurate measurements of the projection length and the sizes of the pipette and vesicle, comparative light intensity plots (31) of the corresponding vesicle images were periodically used to verify the measurements obtained from the scaling software (Fig. 2 b). All measurements were conducted at room temperature and at the original pH of the salicylate/glucose solution. The pH was not manipulated for the concern that additional ions could complicate the results.
FIGURE 2.
(a) DIC images of an aspirated SOPC vesicle at a low tension of 0.01 mN/m (top) and a higher tension of 7.3 mN/m (bottom). (b) Plots of comparative light intensity of the corresponding vesicle images in a. Using the Zeiss Axiovision 3.0 software, the light intensity values of the images along a horizontal line drawn across the center the pipette and vesicle were obtained. These light intensity data were then plotted against distance to indicate the exact location of the edges of the projection inside of the pipette and the tip of the pipette, as well as the edge of the vesicle outside of the pipette. The solid line represents the light intensity pattern at 0.01 mN/m, and the dotted line is the pattern at 7.3 mN/m. The small dip of the solid line at ∼74 μm indicates the edge of the projection in the pipette. The huge variation at ∼80 μm represents the tip of the pipette, where diffraction caused large variations in light intensity. The dip at ∼110 μm is the edge of the vesicle outside of the pipette. The dotted line clearly shows that increasing the tension caused an increase in the projection length inside of the pipette.
FIGURE 3.
Kinetics of salicylate-membrane interaction. The timecourse of salicylate uptake into a SOPC vesicle suspended in 10 mM salicylate was obtained via the modified transfer experiment discussed in the text (for this vesicle, the tension was ∼3.5 mN/m). The open circles indicate the uptake process. As shown, it took ∼8 s for salicylate and lipids to equilibrate. The solid circles demonstrate when salicylate was removed, the projection length returned to its original position. The complete recovery took ∼20 s. Both the original and modified transfer experiments give very similar results for the timecourse of salicylate uptake (data of the original transfer experiments not shown).
The following equations were applied to calculate membrane tension (τ) and apparent fractional area change (αapp) (32,33),
 |
(1) |
 |
(2) |
where A is membrane surface area; ΔA is the change in membrane area resulting from tension; Rv and Rp are radii of the aspirated vesicle and pipette, respectively; L is the vesicle projection length; and ΔL is the change in L resulting from the change in tension. Membrane tension (τ) was then plotted against the fractional area change (αapp). As shown in Fig. 4 a, a typical plot of τ versus αapp is composed of two regions. An initial exponential domain corresponds to low values of applied tension (<0.5 mN/m). In this area the work of the applied tension is predominantly used to decrease the thermally driven out-of-plane fluctuations of the membrane surface (34). This is the reason that the geometrically measured area expansion is designated as an apparent area change. Thermal fluctuations in vesicle shape are observed at room temperature because the bending energy is small and comparable to the thermal energy kBT, where kB is the Boltzmann constant and T is temperature in Kelvin (35). As the fluctuations are smoothed, the τ versus αapp curve makes a transition to a region of linear elastic behavior. The slope of the high-tension domain (>0.5 mN/m) is the apparent compressibility modulus, Kapp (32,33). The initial low-tension region of the plot can be used to calculate the bending modulus kc. Specifically, the slope of the low-tension portion of a plot of ln (τ) versus αapp (Fig. 4 b) is equal to 8πkc/kBT (34).
FIGURE 4.
(a) Plot of tension (τ) versus fractional area change (α) for a representative vesicle. The slope of the high tension region (>0.5 mN/m) is the apparent area compressibility modulus (Kapp). (b) Plot of ln (τ) versus α for another vesicle in which α in the low-tension region was carefully measured. The slope of the low-tension domain of the plot was used to calculate the bending modulus (kc). Plots shown are SOPC in 200 mM glucose (○) and in 10 mM salicylate solution at 200 mOsM (•).
Elastic area compressibility modulus (KA)
The thermodynamic compressibility modulus characterizes the energy associated with changing the area of the membrane. However, in the aspiration experiments, membrane thermal fluctuations are present throughout the entire tension range, causing the measured membrane area dilation to have contributions from smoothing thermal undulations at all tension levels (34). To obtain the elastic compressibility modulus, denoted KA, the fractional area increase resulting from smoothing of membrane fluctuations at each data point i was removed from the measured total area using the model developed by Evans and Rawicz (34) and Rawicz et al. (33). The removed fractional area increase, denoted Δα(i) is equal to
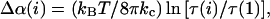 |
(3) |
where τ (1) is initial tension. The tension data for each vesicle was then replotted against the elastic area dilation, denoted αA, where αA(i) = αapp(i) − Δα(i). The elastic area compressibility modulus (KA) is the slope of the τ-versus-αA plot. KA will always be larger than Kapp because the total amount of Δα(i) increases with increasing tension, meaning that the slope of the plot of τ versus αA is greater than that of τ versus αapp.
Transfer experiment
Soluble amphiphiles also complicate the calculation of the actual thermodynamic compressibility modulus. The uptake of a soluble amphiphile into the membrane is a complex kinetic process (23,36). When performing micropipette experiments with soluble amphiphiles, it is important to know whether the given amphiphile reaches equilibrium in the membrane and how long the approach to equilibrium takes. Moreover, important experimental parameters related to tension-dependent partitioning can be derived from these experiments. Salicylate incorporation into the membrane can be measured from the increased projection length of an aspirated vesicle exposed to salicylate. The transfer experiments were first conducted following previously described methods (36,37). Briefly, a chamber containing 200 mOsM glucose (the control chamber) was set side-by-side with another chamber filled with salicylate-glucose solution at the same osmolarity (200 mOsM) on the aspiration stage. Another, much larger micropipette (transfer pipette with diameter of at least 50 μm) containing control solution (200 mM glucose) was inserted into the chamber from the opposite side of the aspirating pipette. An aspirated vesicle was then inserted into the transfer pipette in the control chamber. The entire apparatus was moved to the salicylate chamber containing glucose-salicylate solution. The timecourse of the projection increase was recorded and converted to αapp, and the approach to equilibrium took <10 s. However, we were unable to record the recovery using this standard transfer process. We attribute this to the fact that the transfer pipette contains control solution, and the vesicle projection reaches equilibrium during the transfer process. Thus, the transfer experiment was modified.
The modified transfer experiment utilized the same dual-chamber setup as described above. However, the transfer pipette and the water column linked to the pipette were filled with a salicylate solution, instead of a control solution. The pressure in this pipette (the salicylate pipette) was then calibrated and set to zero in a chamber containing the same salicylate solution (the salicylate chamber). The manipulators holding the two pipettes were prealigned so that a rapid movement of the transfer pipette would place it exactly in front of the vesicle pipette. During the uptake experiment, the salicylate pipette was swiftly moved into the control chamber, the vesicle was inserted ∼50-μm deep into the salicylate pipette and the timecourse of the projection length increase was recorded (Fig. 3). To monitor the projection length recovery, the salicylate pipette was swiftly moved back into the salicylate chamber and the recovery timecourse was recorded. The complete recovery of the vesicle projection in 200 mM glucose (Fig. 3) indicates that the residual salicylate in the control chamber was insignificant. This is expected due to the small volume of solution in the pipette as compared to the large volume of solution in the chamber. To prevent salicylate accumulation in the control chamber, solutions were changed frequently.
Tension dependence of salicylate partitioning
In addition to determining the timecourse of salicylate uptake, the transfer experiments can also be utilized to study the concentration- and tension-dependence of salicylate partitioning. During an aspiration experiment, the membrane is stretched and more salicylate partitions into the membrane, making the salicylate concentration in the membrane tension-dependent. In the extreme limit, KA could vary as a function of tension. Hence the experimentally determined area dilation, αapp(i), at ith tension level will contain a contribution from tension-dependent partitioning of salicylate molecules, designated αpar(i). To account for this, we define a term, αAe(i), which is the actual area increase caused by changes in lipid-lipid cohesion,
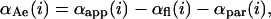 |
(4) |
where αfl(i) is the area increase caused by smoothing of membrane fluctuations.
Thermodynamic theories developed for exchange of surfactants with bilayers can be used to obtain αpar(i). At equilibrium, the chemical potential of salicylate in the membrane is equal to that in the bulk solution (21,22),
 |
(5) |
where 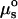 and
and  are the standard chemical potentials in the solution and inside of the membrane; xs and xm represent the mole fractions of the salicylate in solution and in vesicle membrane; Vsal is the molecular volume of the salicylate in the membrane; τ is the membrane tension; and lc is the length of the hydrophobic core (estimated to be ∼3 nm) (21). The term τ/lc is the lateral pressure, whereas the terms ns and nm correspond to the number of salicylate molecules in aggregates inside and outside of the membrane; and are included because salicylate has been shown to form dimers in solution (25,26).
are the standard chemical potentials in the solution and inside of the membrane; xs and xm represent the mole fractions of the salicylate in solution and in vesicle membrane; Vsal is the molecular volume of the salicylate in the membrane; τ is the membrane tension; and lc is the length of the hydrophobic core (estimated to be ∼3 nm) (21). The term τ/lc is the lateral pressure, whereas the terms ns and nm correspond to the number of salicylate molecules in aggregates inside and outside of the membrane; and are included because salicylate has been shown to form dimers in solution (25,26).
Eq. 5 indicates that the size of the salicylate molecule in the membrane will be the operative parameter in determining αpar. Vsal can be experimentally determined from the modified transfer experiment described earlier. An aspirated vesicle in the control solution was transferred into the salicylate pipette at different constant tension levels. The projection length change before and after the transfer at each tension was recorded and converted to fractional area changes. Because salicylate decreases kc (see Experimental Results, below), the uptake of salicylate will add area by increasing membrane thermal undulations. We then removed the area increase associated with membrane undulations to obtain the area increase at a fixed tension level i caused only by salicylate partitioning, designated αpar(i).
Dynamic tension spectroscopy (DTS)
Traditionally, the strength of membranes has been studied by measuring the tension at which the membrane bursts, referred to as the lysis tension (29,38,39). However, the process of membrane lysis is complex. A recently developed technique, dynamic tension spectroscopy (DTS), has been used to explore the intricacies of salicylate's effects on membrane lysis. The rupture process of membranes consists of two steps: spontaneous defect formation followed by cavitation (hole) formation (31). Each process is characterized by a frequency of occurrence (ν) and an associated tension scale (σ). Experimentally four parameters are obtained from DTS: the tension scales of defect and cavitation formation, σδ and σc, and the frequencies of defect and cavitation processes, νδ and νc.
We have implemented this technique, described in detail by Evans et al. (31), in our laboratory. Briefly, using the same microaspiration setup, the motorized mechanical slider was used to apply pressure at various slider speeds. The entire experiment from the beginning to rupture was video-recorded using a CCD camera (Panasonic WV-BL204, Panasonic/Matsushita, Secaucus, NJ). The video file was later converted into image frames using X Video Converter (AoA Media, http://www.aoamedia.com) to determine the time at which the membrane burst. The pressure loading rate, Rσ, was calculated from the slope of the plot of membrane tension versus time (Fig. 5 a). The similar loading rate values (±0.5 mN/m/s) were grouped into bins and corresponding rupture tension measurements were averaged. Fig. 5 b shows a plot of τrupture versus log(Rσ), referred to as a DTS spectrum (31). When all data points were plotted, a very similar pattern to the plot obtained from the averaged data was seen. For the purpose of clarity, the averaged plot was used. Two distinct sections are present in the plot, a low-tension domain and a high-tension domain. The nonlinear, low tension domain represents the energy barrier for cavitation, or pore formation. The linear, high-tension domain represents the energy barrier for forming a spontaneous defect in the membrane. The two domains are described by the following two equations, respectively,
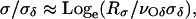 |
(6) |
 |
(7) |
FIGURE 5.
(a) Dependence of membrane rupture on loading rate. The results of experiments on two separate vesicles are shown. The pressure loading rate (Rδ) is the slope of the plot of membrane tension versus time. The experiment was carried out until membrane rupture. For the lower plot, vesicle rupture occurred at a tension 3 mN/m when Rδ = 0.068 mN/m/s. For the upper plot, vesicle rupture occurred at 22 mN/m for Rδ = 7.4 mN/m/s. The results clearly indicate that the tension needed to lyse a vesicle increases as Rδ increases. (b) A dynamic tension spectrum (DTS) of membrane rupture shows two distinct regions. The low-tension regime represents cavitation formation, whereas the high-tension regime indicates defect formation. For SOPC, σδ of defect formation is 3.2 mN/m and σc of cavitation is 197 mN/m.
The above equations were derived from the Markov master equations used to describe the development of membrane rupture (31). Equation 6 applies to spontaneous defect formation, and Eq. 7 governs cavitation formation. In a DTS spectrum, the slope of the linear high-tension domain (loading rates of >10 mN/m/s) is σδ and the extrapolation of the slope line can be used to calculate νδ because  (31). The parameters associated with cavitation, σc and νc, can be obtained by using Eq. 7 to fit the nonlinear, low-tension domain from the loading rates of 0.01 mN/m/s to 3 mN/m/s (31).
(31). The parameters associated with cavitation, σc and νc, can be obtained by using Eq. 7 to fit the nonlinear, low-tension domain from the loading rates of 0.01 mN/m/s to 3 mN/m/s (31).
BSA affects salicylate-membrane interactions
Bovine serum albumin (BSA) is traditionally used in microaspiration experiments to discharge the glass pipette surface (20,29,30). Initially, we performed control experiments with SOPC in 200 mM glucose with 0.02% BSA and found our data of Kapp to be within the acceptable range for SOPC (∼180 mN/m) and to be reproducible (mean ± SD of ∼16 mN/m for 10 vesicles). Moreover, the measured values of Kapp decreased in a dose-dependent manner in high concentrations of salicylate (>5 mM; data not shown). However, at low salicylate concentrations (<1 mM), the measurements were irreproducible. Previous investigators have reported that albumin binds to lipids and prevents membrane leakage (40). Shoemaker and Vanderlick (30) reported that elastic measurements of anionic vesicles were highly variable in the presence of BSA. Furthermore, sodium salicylate binds to BSA in solution and prevents heat coagulation of albumin molecules (41). Therefore, we decided to eliminate BSA in the aspiration chamber and only coat the glass pipette tip and coverslips of the chamber with 0.02% BSA and wash extensively with glucose solution and Millipore water, as discussed by Shoemaker and Vanderlick (30). When conducting studies without BSA in the system, all data became reproducible.
EXPERIMENTAL RESULTS
Apparent area compressibility measurements
We first measured the effect of salicylate on the apparent compressibility modulus of the membrane. A linear behavior in the high-tension domain (>0.5 mN/m) of the plot τ versus αapp demonstrates that the membrane still behaved as linear elastic material in the presence of salicylate. Fig. 6 shows that salicylate decreased Kapp of SOPC in a dose-dependent manner. The range of salicylate concentrations tested was chosen to be 0.5–10 mM. The physiological effects of salicylate resulting from normal aspirin consumption occur at concentrations between 0.l and 2 mM (42). When the blood serum level of salicylate reaches 10 mM, diverse pathological effects occur, including myocardial necrosis (43). The minimum amount of salicylate required to change the apparent membrane compressibility (Kapp) was ∼1 mM. As shown in Fig. 6, 10 mM salicylate decreased Kapp from ∼211 mN/m to ∼138 mN/m (p < 0.05), a ∼40% decrease.
FIGURE 6.
The apparent area compressibility (Kapp) dose-response curve for salicylate-SOPC interaction. Salicylate decreases Kapp of SOPC vesicles in a dose-dependent manner. The concentrations of salicylate tested were 0 mM, 0.5 mM, 1 mM, 5 mM, and 10 mM. Error bars represent the standard deviation of the mean.
Bending rigidity (kc) and elastic area compressibility modulus (KA)
Next, we addressed the issue of whether the changes in Kapp were due to changes in the bending stiffness (kc), the elastic area compressibility modulus (KA), or partitioning. The results of these experiments are shown in Figs. 7 and 8. Salicylate decreased kc in a dose-dependent manner. The control value of kc was measured to be ∼0.9 × 10−19 J, in agreement with earlier measurements for SOPC (21,33). We found that 1 mM salicylate caused a ∼40% decrease in kc to ∼0.6 × 10−19 J. The Kapp was only reduced by ∼10% from 211 mN/m to 191 mN/m at this same concentration. Higher concentrations of salicylate produced further decreases in kc, though by a lesser amount than seen at 1 mM. Qualitative evidence for a dramatic change in the bending stiffness comes from observations of clearly apparent thermal fluctuations when the vesicles were suspended in 10 mM salicylate.
FIGURE 7.
A dose-response curve of the effect of salicylate on membrane-bending modulus (kc) is shown. At low concentrations, salicylate had a dramatic effect on kc.
FIGURE 8.
(a) The effect of salicylate on KA (♦) compared with Kapp (○). The KA curve was obtained by removing the contribution from thermal fluctuations. (b) Salicylate's effects on KA (♦) compared with its effects on the kc (▪).
Following the methods of Rawicz et al. (33), we removed the contribution of thermal fluctuations and calculated the elastic area compressibility modulus (KA) of SOPC exposed to salicylate. When the area increase caused by smoothing of the undulations was removed, the KA of SOPC increased to ∼246 mN/m from Kapp of ∼211 mN/m. This value agrees well with the results of Rawicz et al. (33) and Ly et al. (21). Fig. 8 shows salicylate had less effect on KA at the higher concentrations tested. For example, KA only decreased ∼20% from ∼246 mN/m to ∼194 mN/m in 10 mM salicylate. Because KA was calculated from Kapp by removing the area increase associated with thermal fluctuations, salicylate's small effect on KA indicates that the significant decrease of Kapp is mainly due to a decreased bending rigidity that causes induced membrane fluctuations.
We also repeated microaspiration experiments with GUVs formed using the traditional rehydration method in 10 mM salicylate. The resulting mechanical parameters showed no statistical difference from those extracted from vesicles made via electroformation (Table 1).
TABLE 1.
Effect of salicylate on membrane mechanical properties of SOPC vesicles
| [Sal] (mM) | Kapp (mN/m) | KA (mN/m) | kc (10-19 J) | KAe (mN/m) |
|---|---|---|---|---|
| 0 | 212 ± 23 (17) | 247 ± 32 (7) | 0.90 ± 0.15 (10) | 247 ± 32 (7) |
| 0.5 | 204 ± 19 (11) | |||
| 1 | 191 ± 28* (9) | 227 ± 41* (9) | 0.60 ± 0.19* (6) | 235 ± 44 (9) |
| 5 | 147 ± 33* (23) | 200 ± 40* (6) | 0.54 ± 0.10* (7) | 230 ± 40 (6) |
| 10 | 138 ± 32* (10) | 189 ± 18* (7) | 0.44 ± 0.12* (8) | 222 ± 16* (7) |
| SOPC† (rehydration method) 10 mM Sal | 128 ± 27 (6) | 183 ± 51 (6) | 0.45 ± 0.17 (6) | N/A |
Values are shown as mean ± SD. The numbers in parentheses represent the number of vesicles used during experiments.
Values are statistically different from control, as determined by Student's t-test results obtained at 95% confidence.
Experiments with SOPC GUVs synthesized via rehydration method in 10 mM salicylate. There was no statistical difference when compared to parameters extracted from GUVs made using the electroformation method.
Molecular volume of salicylate in the membrane
To estimate the molecular volume of salicylate, Eq. 5 can be simplified. Assuming that the concentration of salicylate in solution (xs) is constant because vesicle concentration in the chamber was extremely small (in pM) and that measured area increase reflects changes in the salicylate concentration in the membrane, the equation can be derived (22) as
 |
(8) |
where αpar is the area dilation caused solely by partition of salicylate molecules at each ith tension level, xs is the salicylate concentration in solution, and C is a constant. The term of nm/ns is an indicator of a change in the state of molecular aggregation during partitioning. The experiments were performed as discussed in Methods, above, to obtain the area increase caused by tension-dependent salicylate partitioning, designated as αpar. After the membrane fluctuations were removed, the ln αpar versus τ was plotted for 1 mM and 10 mM salicylate (Fig. 9). The slopes of these plots were extrapolated to the tension-free condition to obtain the dependence of αpar(0) on the bulk salicylate concentrations (22). The αpar(0) values at tension-free state for both 1 mM and 10 mM salicylate were used to calculate the ratio of nm/ns that is necessary to deduce the correlation between αpar(0) and the bulk salicylate concentration. The nm/ns value was estimated to be ∼0.5, indicating a pattern of dissociation during insertion. The volume of a salicylate molecule was calculated to be Vsal ∼240 Å3 from the slope of the plots in Fig. 9. The estimated molecular volume for methyl salicylate is ∼200 Å3 (44), indicating that our estimated value of Vsal is reasonable. Together with the calculated value of nm/ns, the molecular volume data imply that salicylate molecules exist as dimers in aqueous solution and dissociate into monomers once in the membrane.
FIGURE 9.
Changes in the area dilation as a result of salicylate partitioning at various tension levels. A vesicle was transferred at constant tension levels into known concentrations of salicylate (1 mM, ▪, and 10 mM, ♦). Projection-length changes before and after transfer were recorded and used to determine αpar as described in the text.
Calculation of the actual elastic compressibility modulus (KAe)
The measured KA values at each bulk salicylate concentration potentially contain a contribution from the continual partitioning of salicylate into the membrane during aspiration. Although this effect is small—at the lysis tension, an increase of ∼5–10% over the initial concentration is expected—it can become significant for surfactants with large packing areas. Because the chemical potential of the solution remains constant when tension in the membrane is changed, Eq. 5 can be written as (21,23)
 |
(9) |
where xm,o is the salicylate concentration in the membrane at zero-tension and xm,i is the membrane salicylate concentration at ith tension. Eq. 9 can be simplified to express the fractional change in the mole fraction of salicylate in the membrane resulting from applied tension,
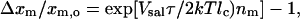 |
(10) |
where Δxm = xm,i − xm,o. Since mole fractions of salicylate are considered to be equivalent to the area dilation, α, and nm = 1 as shown above, the above relation can be written as
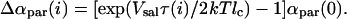 |
(11) |
Equation 11 can then be combined with Eqs. 3 and 4 to give the total change-in-area increase that needs to be removed to estimate the true elastic area dilation αAe(i) = αapp(i) − Δα(i), where
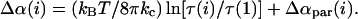 |
(12) |
Using Eqs. 11 and 12, we corrected the area dilation data for all vesicles by removing αpar(i) at each tension level. The tension data were then replotted versus αAe to determine the actual elastic compressibility modulus, designated as KAe. In practice, this usually resulted in an approximately 10 mN/m increase in the measured compressibility modulus for a given vesicle. This modulus reflects contributions from the initial salicylate partitioning at zero-tension. As shown in Table 1, salicylate was found to have a much smaller effect on KAe at all concentrations tested after the tension-dependent partitioning was accounted for. The difference between KAe and KA is small, but increases with the bulk salicylate concentration, as expected from Eq. 11.
The results of our micromechanical experiments are summarized in Table 1.
Kinetics of salicylate interactions with membranes
Salicylate uptake and recovery curves were obtained using the modified transfer experiment. The vesicle was transferred into the salicylate pipette at a tension of ∼1 mN/m and the increase in the projection length was recorded. The fractional area increase (α) was then plotted against time to give the uptake timecourse (Fig. 3). The uptake curve was fitted using the general exponential growth equation, 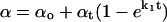 (OriginLab, Northampton, MA), where αo is the initial area dilation before the vesicle was exposed to salicylate, αt is the total increase in α, and k1 is the time constant of salicylate uptake. The recovery curve was fitted with the standard exponential decay function α = αo + Ae−k2t, where A is an arbitrary parameter and k2 is the time constant of salicylate recovery. For the experiment shown in Fig. 3, k1 was ∼0.5 ± 0.06 s−1 and k2 was ∼0.15 ± 0.08 s−1. The time for equilibration of salicylate with the membrane is much faster than that reported for the lysolipid mono-oleoylphosphatidylcholine, where k1 = 0.3 s−1 (23) and for the uptake of bile acids, which require up to ∼30 s to reach equilibrium (22).
(OriginLab, Northampton, MA), where αo is the initial area dilation before the vesicle was exposed to salicylate, αt is the total increase in α, and k1 is the time constant of salicylate uptake. The recovery curve was fitted with the standard exponential decay function α = αo + Ae−k2t, where A is an arbitrary parameter and k2 is the time constant of salicylate recovery. For the experiment shown in Fig. 3, k1 was ∼0.5 ± 0.06 s−1 and k2 was ∼0.15 ± 0.08 s−1. The time for equilibration of salicylate with the membrane is much faster than that reported for the lysolipid mono-oleoylphosphatidylcholine, where k1 = 0.3 s−1 (23) and for the uptake of bile acids, which require up to ∼30 s to reach equilibrium (22).
Dynamic tension spectroscopy
To test the effect of salicylate on membrane strength, we performed dynamic tension spectroscopy (DTS). We verified results obtained by Evans et al. (31) in control conditions and performed experiments in 10 mM salicylate. A total of 120 vesicles were used.
As Fig. 10 shows, 10 mM salicylate clearly shifted the DTS spectrum of an SOPC membrane. The effects were most dramatic in the low-tension domain that represents the cavitation process. The application of 10 mM salicylate reduced σc to ∼50 mN/m from the control value of ∼129 mN/m and reduced νc to ∼1 × 105 s−1 from the control value of ∼1 × 106 s−1. In the high-tension domain the effects were not as dramatic: σδ was practically unchanged in 10 mM salicylate, and νδ showed a modest increase from 0.32 s−1 to 0.98 s−1. The results of the DTS experiments are summarized in Table 2. The shift of the spectrum demonstrates that salicylate increases the frequency of spontaneous defects and also affects membrane strength by lowering the energy barrier of hole formation, hence stabilizing membrane holes.
FIGURE 10.
Dynamic tension spectra (DTS) of SOPC in 200 mM glucose (○) and SOPC with 10 mM salicylate (♦). The solid and dashed lines represent the trends of the data. Salicylate does not affect σδ in the high-tension regime at this concentration. However, σc in the low-tension regime was shifted down from ∼160 mN/m (SOPC) to ∼51 mN/m with 10 mM salicylate.
TABLE 2.
Dynamic tension measurements
| Dynamic tension properties | σδ (mN/m) | νδ (s−1) | σc (mN/m) | νc(s−1) | ɛ (pJ/m) |
|---|---|---|---|---|---|
| SOPC | 3.2 | 0.32 | 129 | 1 × 106 | 14.2 |
| SOPC + 10 mM salicylate | 3.3 | 0.98 | 51 | 1 × 105 | 7.9 |
The tension scale of cavitation formation is dependent upon the edge energy of the unstable cavity, which can be expressed as σc = πɛ2/kBT, where ɛ is the membrane edge energy (31). The ɛ-values of both pure SOPC and SOPC with 10 mM salicylate were calculated and listed in the Table 2 along with tension scale and frequency data. The results indicate that the membrane edge energy was significantly lowered in the presence of salicylate.
DISCUSSION
The underlying mechanisms responsible for the diverse biological effects of salicylate and other NSAIDs are a matter of considerable controversy in the literature. Some maintain that these drugs exert all their biological effects, including therapeutic functions and side effects, as a result of direct interactions with enzymes, such as COX (2,3). However, others assert NSAIDs' side effects are actually caused by nonspecific interactions with biomembranes (1,6,11,45). It should be noted that in Vane's classic article, it was reported that salicylate, which has profound anti-inflammatory activity, was the least potent of the NSAIDs in inhibiting prostaglandin biosynthesis (3), providing evidence for a non-COX-mediated mechanism of action of salicylate and perhaps other drugs of this class. Here, we have demonstrated that physiological concentrations of salicylate can have dramatic effects on the mechanical properties of an SOPC membrane.
Salicylate decreases membrane bending rigidity
One very important point we have established is that salicylate decreases membrane strength by affecting molecular processes associated with the formation of both defects and holes. Often in the biophysical literature the term “pores” is used to designate transport pathways across the membrane without reference to a two-state model of membrane rupture. Since the formation of membrane pores is believed to govern the passive permeability of membranes (46) and plays an important part in phospholipid translocation (47,48), these results imply that salicylate alters the membrane's hydrophobic barrier properties. In particular, we have found that salicylate increases the frequency of occurrence of spontaneous defects. Moreover, salicylate significantly lowers the tension scale of cavitation formation, but leaves the tension scale of defect formation unaffected, indicating that salicylate stabilizes membrane holes without disrupting lipid cohesion. Salicylate's small effect on the defect process agrees with our membrane elasticity results, which demonstrate that salicylate does not significantly decrease the actual elastic compressibility modulus at the concentrations tested. The large downshift in the low-tension domain (related to hole formation) of the DTS spectrum in the presence of salicylate is consistent with our measurements, which show that salicylate decreases membrane bending stiffness, thereby stabilizing holes. The demonstrated decrease in the edge energy of a hole when the membrane was exposed to salicylate (Table 2) provides further support for this argument. In Fig. 11, we demonstrate that our data correlates well with the ɛ-versus-kc data that Evans et al. (31) presented for membranes composed of lipids with different chain length and unsaturation levels. Therefore, both the elasticity and dynamic tension results coincide to show that by lowering the bending stiffness, salicylate stabilizes membrane holes, causing a weaker and likely a more permeable membrane.
FIGURE 11.
The correlation between edge energy of the hole and membrane-bending stiffness. The data points of ɛ versus kc for salicylate (•) were superimposed onto the ɛ-versus-kc plot that Evans et al. (31) obtained for membranes composed of lipids with different chain length and unsaturation levels (○).
The bending and area compressibility moduli of a thin material are related to each other by the relationship  where hm is the thickness of the monolayer and c is a constant (33). The result that salicylate has a large effect on kc and a lesser effect on KAe suggests that salicylate changes the thickness of the membrane. In Fig. 12, the plot (kc/KAe)1/2 versus [Sal] shows that our data are consistent with a dose-dependent thinning of the membrane. We have measured the effect of salicylate on the height of supported monolayers using atomic force microscopy, and our preliminary observations indicate a noticeable thinning of the membrane in the presence of 5 mM salicylate (data not shown).
where hm is the thickness of the monolayer and c is a constant (33). The result that salicylate has a large effect on kc and a lesser effect on KAe suggests that salicylate changes the thickness of the membrane. In Fig. 12, the plot (kc/KAe)1/2 versus [Sal] shows that our data are consistent with a dose-dependent thinning of the membrane. We have measured the effect of salicylate on the height of supported monolayers using atomic force microscopy, and our preliminary observations indicate a noticeable thinning of the membrane in the presence of 5 mM salicylate (data not shown).
FIGURE 12.
Predicted change in bilayer thickness, (kc/KAe)1/2, as result of salicylate incorporation. The estimated changes using experimentally determined values of KAe and kc at different salicylate concentrations are consistent with a salicylate-induced thinning of the membrane. The line is used to indicate the trend.
Interestingly, membranes composed of PC lipids with acyl chains ranging from 13 to 22 carbons and containing up to two double bonds all have a similar KA, but kc varies widely among these membranes; these observations are explained by changes in membrane thickness (33). Coupled with the correlation shown in Fig. 12, these observations suggest that incorporation of salicylate into a PC membrane makes its properties similar to those of a membrane composed of shorter chain and/or polyunsaturated lipids. It is also interesting to note that certain peptides, such as the antimicrobial peptide alamethicin, can also induce pore formation by thinning the membrane and then preferentially inserting into the edges of the membrane pores (49–51). Moreover, alamethicin-induced membrane thinning reaches a plateau when the peptide/lipid ratio becomes larger than a critical value (49), similar to the trend predicted for salicylate (Fig. 12). These considerations emphasize that the correlation between membrane thinning and peptide-induced pore formation reflect an underlying general biophysical mechanism.
Free energy of transfer and partition coefficient
The Gibbs free energy of salicylate adsorption into the membrane can be estimated from our experimental data using a thermodynamic expression well developed for monolayers (52) and recently applied to bilayers (21),
 |
(13) |
where  is the change in free energy associated with the transfer of salicylate from water into the SOPC membrane. The term (dΔγ/dc) represents the change in surface tension as salicylate concentration changes. This term can be estimated by relating the actual compressibility modulus KAe to the surface pressure Π and recalling that surface tension is equal to surface pressure (γ = Π) at the stress-free state (21). Using an idealized polymer-brush model of membrane elasticity, Rawicz et al. (33) predicted that KAe ∼6Π, and thus γ ∼ KAe/6. Based on our data,
is the change in free energy associated with the transfer of salicylate from water into the SOPC membrane. The term (dΔγ/dc) represents the change in surface tension as salicylate concentration changes. This term can be estimated by relating the actual compressibility modulus KAe to the surface pressure Π and recalling that surface tension is equal to surface pressure (γ = Π) at the stress-free state (21). Using an idealized polymer-brush model of membrane elasticity, Rawicz et al. (33) predicted that KAe ∼6Π, and thus γ ∼ KAe/6. Based on our data,  for salicylate partitioning into the membrane is ∼−13.5 ± 3.9 kJ/mol. However, this value should be viewed with caution; although we measured a change in KAe with salicylate concentrations lower than 10 mM, a rigorous t-test did not indicate our values were significant at 95% level.
for salicylate partitioning into the membrane is ∼−13.5 ± 3.9 kJ/mol. However, this value should be viewed with caution; although we measured a change in KAe with salicylate concentrations lower than 10 mM, a rigorous t-test did not indicate our values were significant at 95% level.
Because of the structural similarities between salicylate and benzoic acid, the thermodynamics of salicylate's interaction with the membrane may be comparable to that of benzoic acid. The free energy of hydration 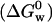 of benzoic acid at 25°C is ∼−16 kJ/mol, and its free energy of solvation in n-octanol
of benzoic acid at 25°C is ∼−16 kJ/mol, and its free energy of solvation in n-octanol 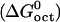 is ∼−30 kJ/mol, and so its free energy of transfer is
is ∼−30 kJ/mol, and so its free energy of transfer is 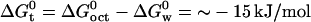 (53). This implies that hydrophobic effects play an important role in salicylate partitioning into the membrane. The difference may arise from the fact that salicylate, with a pKa of ∼3, would exist predominantly as an anion in a nearly neutral solution, which can have unfavorable electrostatic interactions with lipids. Furthermore, the additional hydroxyl group on salicylate may lead to more favorable interactions with the aqueous solution, thus further decreasing
(53). This implies that hydrophobic effects play an important role in salicylate partitioning into the membrane. The difference may arise from the fact that salicylate, with a pKa of ∼3, would exist predominantly as an anion in a nearly neutral solution, which can have unfavorable electrostatic interactions with lipids. Furthermore, the additional hydroxyl group on salicylate may lead to more favorable interactions with the aqueous solution, thus further decreasing 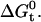
The change in free energy of adsorption can be used to compute the partition coefficient Pmem from the standard relationship 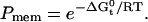 Our value of
Our value of  corresponds to a log (Pmem) ∼2.1. To our knowledge, we are the first to measure the partition coefficient of salicylate into a biological membrane. However, the partition coefficient for sodium salicylate into an octanol/water interface has been reported to be log(Poct) ∼1.5 (54). A recent study reports that partition coefficients for weak organic acids such as salicylate, estimated using octanol-water interfaces (Poct), are generally underestimates of partition coefficients (Pmem) into biological membranes (55). Notably at pH 6 where our experiments were performed, the difference between Pmem and Poct can be approximately an order of magnitude. Thus, our reported partition coefficient agrees with the literature value once this correction is taken into account, which reinforces the accuracy of our estimated value of
corresponds to a log (Pmem) ∼2.1. To our knowledge, we are the first to measure the partition coefficient of salicylate into a biological membrane. However, the partition coefficient for sodium salicylate into an octanol/water interface has been reported to be log(Poct) ∼1.5 (54). A recent study reports that partition coefficients for weak organic acids such as salicylate, estimated using octanol-water interfaces (Poct), are generally underestimates of partition coefficients (Pmem) into biological membranes (55). Notably at pH 6 where our experiments were performed, the difference between Pmem and Poct can be approximately an order of magnitude. Thus, our reported partition coefficient agrees with the literature value once this correction is taken into account, which reinforces the accuracy of our estimated value of 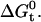
From the transfer experiment, it is tempting to argue that the time constants for salicylate uptake and recovery represent salicylate adsorption and desorption from the membrane. However, it is easy to illustrate that this assumption gives incorrect results. Assuming Keq = k1/k2, the equilibrium constant, Keq, is ∼3.3 ± 1.8. The relationship ΔGads = −RTlnKeq then gives ΔGads ∼3 kJ/mol, and the corresponding log (Pmem) of ∼0.5. These values are clearly too small compared to the literature value of 1.5 for log (Pmem) (54). Thus, the experimentally observed time constants are not indicative of adsorption and desorption processes, but likely also reflect flip-flop and bilayer crossing.
The molecular area and volume of salicylate in an SOPC membrane
The estimated salicylate volume of ∼240 Å3 in the membrane is equivalent to the physical size of one salicylate molecule and corresponds to an estimated cross-sectional area of ∼50 Å2. Since the aromatic ring occupies most of the physical space in a salicylate molecule, benzene can be used for comparison in terms of packing. The cross-sectional area of benzene varies between ∼22 Å2 and ∼69 Å2, depending on the absorbent (56). The packing area of salicylate is between that of bile acids (60 Å2) (22) and lysolipids (35 Å2) (23). However, the interactions of salicylate with the membrane are significantly different. Whereas both bile acids and lysolipids had large effects on membrane elasticity, salicylate showed a small effect on the elastic compressibility. The difference may arise from the finding that salicylate occupies much less volume (∼240 Å3) in the membrane than bile acids and lysolipids (between 1000 Å3 and 2000 Å3). Therefore, molecular volume becomes an important factor in ascertaining the effects of small molecules on membrane elasticity.
Our results that the value of nm/ns is ∼0.5 are consistent with salicylate forming dimers in solution. The idea of salicylate forming dimers in solution is not unusual, since the carboxyl and hydroxyl groups covalently attached on salicylate's aromatic ring can participate in H-bonding with those on the adjacent molecule(s) and establish a dimeric form in solution. There is evidence that salicylate molecules exist as dimers in solution (25), which is purported to explain salicylate's ability to induce micellar chain formation (26). In this NMR study, salicylate molecules in one micelle were shown to form dimers with salicylate molecules in the membranes of neighboring micelles.
Salicylate's membrane-mediated biological activity
Long-term consumption of non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin and salicylate, can lead to undesirable side effects that have serious consequences. Side effects such as gastric intestinal (GI) toxicity are related to the ability of these drugs to interact with the membrane (5,45,57,58). Salicylate and other NSAIDs decrease the hydrophobicity of the membrane layer covering the gastric mucosa, causing the membrane to become more wettable (10,45,57,59,60). The detailed structure of the phospholipid layer covering the GI mucosa is unknown, but the characteristic structure of the GI tract is rugae and pits (61), both of which are highly curved structures. Because salicylate is effective at decreasing membrane bending stiffness, it is likely to disrupt the structural integrity of the phospholipid layer in the GI tract as well as perhaps leading to cavitation formation.
Another non-COX-mediated effect of salicylate is on hearing. The mechanism by which salicylate affects hearing is due to the ability of salicylate to attenuate outer hair cell electromotility (62,63). Some argue that salicylate affects the function of the outer hair cell motor protein prestin by competing with chloride ions for putative binding sites on the molecule (64). However, the growing recognition that protein conformational changes are associated with changes in membrane curvature and mechanical properties (24,65) suggests the possibility that salicylate's ability to dramatically decrease kc may be involved in its effects on electromotility. This interpretation is consistent with the membrane-bending model of electromotility, which proposes that electrically induced changes in nanoscale membrane curvature are involved in electromotility (66).
Finally, it is speculated that salicylate increases the water permeability of biomembranes, including the outer hair cell plasma membrane, by inducing the formation of membrane pores (67). Our DTS finding supports this argument because salicylate acts to lower the energy barrier for forming membrane cavitations, thereby leading to a stabilization of the membrane holes.
CONCLUSION
As a powerful NSAID, salicylate has been used since ancient times to treat a series of inflammatory responses, such as fever, pain, and arthritis. However, NSAIDs, including salicylate, cause serious side effects. The cause of these side effects is a subject of active debate, with some advocating specific drug-enzyme interactions and other arguing for nonspecific drug-membrane interactions. Here, through mechanical studies of micropipette aspiration and dynamic tension spectroscopy, we have demonstrated that salicylate interacts with lecithin membranes and changes their mechanical properties at physiological concentrations. Membranes containing salicylate have lowered bending stiffness, rupture more easily, and are likely to be thinner and more permeable. These mechanical changes induced by salicylate may affect several physiological processes, especially those mediated by membrane proteins.
Acknowledgments
We are grateful to Drs. Lenard Lichtenberger and Huey Huang for insightful discussions and for critiquing a draft of this manuscript. We also thank Dr. Robert Cantor for pointing out important references on surfactant partitioning into bilayer membranes.
This study was supported in part by a grant from the state of Texas Technology Development and Transfer Program.
References
- 1.Weissmann, G. 1991. Aspirin. Sci. Am. 264:84–90. [DOI] [PubMed] [Google Scholar]
- 2.Vane, J. R., Y. S. Bakhle, and R. M. Botting. 1998. Cyclooxgenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 38:97–120. [DOI] [PubMed] [Google Scholar]
- 3.Vane, J. R. 1971. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 231:232–235. [DOI] [PubMed] [Google Scholar]
- 4.Weissmann, G., M. C. Montesinos, M. Pillinger, and B. N. Cronstein. 2002. Non-prostaglandin effects of aspirin III and salicylate: inhibition of integrin-dependent human neutrophil aggregation and inflammation in COX 2- and NF-κ-B (P105)-knockout mice. Adv. Exp. Med. Biol. 507:571–577. [DOI] [PubMed] [Google Scholar]
- 5.Wallace, J. L. 1997. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 112:1000–1016. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenberger, L. M. 2001. Where is the evidence that cyclooxygenase inhibition is the primary cause of nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal injury? Topical injury revisited. Biochem. Pharmacol. 61:631–637. [DOI] [PubMed] [Google Scholar]
- 7.Myers, E. N., and J. M. Bernstein. 1965. Salicylate ototoxicity; a clinical and experimental study. Arch. Otolaryngol. 82:483–493. [DOI] [PubMed] [Google Scholar]
- 8.Jung, T. T., C. K. Rhee, C. S. Lee, Y. S. Park, and D. C. Choi. 1993. Ototoxicity of salicylate, nonsteroidal antiinflammatory drugs, and quinine. Otolaryngol. Clin. North Am. 26:791–810. [PubMed] [Google Scholar]
- 9.Ligumsky, M., E. M. Golanska, D. G. Hansen, and G. L. Kauffman, Jr. 1983. Aspirin can inhibit gastric mucosal cyclo-oxygenase without causing lesions in Rat. Gastroenterology. 84:756–761. [PubMed] [Google Scholar]
- 10.Lichtenberger, L. M. 1995. The hydrophobic barrier properties of gastrointestinal mucus. Annu. Rev. Physiol. 57:565–583. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenberger, L. M., Z. M. Wang, J. J. Romero, C. Ulloa, J. C. Perez, M. N. Giraud, and J. C. Barreto. 1995. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with Zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat. Med. 1:154–158. [DOI] [PubMed] [Google Scholar]
- 12.Goddard, P. J., B. A. Hills, and L. M. Lichtenberger. 1987. Does aspirin damage canine gastric mucosa by reducing its surface hydrophobicity? Am. J. Physiol. 252:G421–G430. [DOI] [PubMed] [Google Scholar]
- 13.Goddard, P. J., Y. C. Kao, and L. M. Lichtenberger. 1990. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 98:361–370. [DOI] [PubMed] [Google Scholar]
- 14.Lundbaek, J. A., P. Birn, A. J. Hansen, R. Sogaard, C. Nielsen, J. Girshman, M. J. Bruno, S. E. Tape, J. Egebjerg, D. V. Greathouse, G. L. Mattice, R. E. Koeppe II, et al. 2004. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of micelle-forming amphiphiles and cholesterol. J. Gen. Physiol. 123:599–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gennis, R. B. 1989. Biomembrane-Molecular Structure and Function. C. R. Cantor, editor. Springer-Verlag, New York.
- 16.Cevc, G., and D. Marsh. 1987. Phospholipid Bilayers: Physical Principles and Models. E.E. Bittar, editor. John Wiley & Sons, New York.
- 17.Evans, E. A., and R. Skalak. 1980. Mechanics and Thermodynamics of Biomembranes. CRC Press, Boca Raton, FL.
- 18.Israelachvili, J. N., D. J. Mitchell, and B. W. Ninham. 1976. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. II. 72:1525–1568. [Google Scholar]
- 19.Israelachvili, J. N., S. Marcelja, and R. G. Horn. 1980. Physical principles of membrane organization. Q. Rev. Biophys. 13:121–200. [DOI] [PubMed] [Google Scholar]
- 20.Hung, V. L., D. E. Block, and M. L. Longo. 2002. Interfacial tension effect of ethanol on lipid bilayer rigidity, stability, and area/molecule: a micropipette aspiration approach. Langmuir. 18:8988–8995. [Google Scholar]
- 21.Ly, H. V., and M. L. Longo. 2004. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 87:1013–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans, E. A., W. Rawicz, and A. Hoffmann. 1995. Lipid bilayer expansion and mechanical disruption in solutions of water-soluble bile acid. In Bile Acids in Gastroenterology: Basic and Clinical Advances. A. Hoffmann, G. Paumgartner, and A. Stiehl, editors. Kluwer Academic, Boston, MA.
- 23.Zhelev, D. V. 1998. Material property characteristics for lipid bilayers containing lysolipid. Biophys. J. 75:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantor, R. S. 1999. Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 76:2625–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein, G. T. M. 1969. Report NYO-3242–30. U.S. Atomic Energy Commission, Washington, DC.
- 26.Rao, U. R., C. Manohar, B. S. Valaulikar, and R. M. Iyer. 1987. Micellar chain model for the origin of the viscoelasticity in dilute surfactant solutions. J. Phys. Chem. 91:3286–3291. [Google Scholar]
- 27.Troiano, G. C., K. J. Stebe, R. M. Raphael, and L. Tung. 1999. The effects of gramicidin on electroporation of lipid bilayers. Biophys. J. 76:3150–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angelova, M. J., S. Soleau, P. Melaeard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by AC electric fields. Kinetics and applications. Prog. Colloid Polym. Sci. 89:127–131. [Google Scholar]
- 29.Needham, D., and R. S. Nunn. 1990. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 58:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoemaker, S. D., and T. K. Vanderlick. 2002. Intramembrane electrostatic interactions destabilize lipid vesicles. Biophys. J. 83:2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans, E., V. Heinrich, F. Ludwig, and W. Rawicz. 2003. Dynamic tension spectroscopy and strength of biomembranes. Biophys. J. 85:2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwok, R., and E. Evans. 1981. Thermoelasticity of large lecithin bilayer vesicles. Biophys. J. 35:637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawicz, W., K. C. Olbrich, T. McIntosh, D. Needham, and E. Evans. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans, E., and W. Rawicz. 1990. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 64:2094–2097. [DOI] [PubMed] [Google Scholar]
- 35.Schneider, M. B., J. T. Jenkins, and W. W. Webb. 1984. Thermal fluctuations of large cylindrical phospholipid vesicles. Biophys. J. 45:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needham, D., and D. V. Zhelev. 1995. Lysolipid exchange with lipid vesicle membranes. Ann. Biomed. Eng. 23:287–298. [DOI] [PubMed] [Google Scholar]
- 37.Olbrich, K., W. Rawicz, D. Needham, and E. Evans. 2000. Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 79:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mui, B. L., P. R. Cullis, E. A. Evans, and T. D. Madden. 1993. Osmotic properties of large unilamellar vesicles prepared by extrusion. Biophys. J. 64:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans, E. A., R. Waugh, and L. Melnik. 1976. Elastic area compressibility modulus of red cell membrane. Biophys. J. 16:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, Y., and G. L. Fletcher. 2000. Efficacy of antifreeze protein types in protecting liposome membrane integrity depends on phospholipid class. Biochim. Biophys. Acta. 1524:11–16. [DOI] [PubMed] [Google Scholar]
- 41.Saleh, A. M., A. T. Florence, T. L. Whateley, and L. K. el-Khordagui. 1989. The effect of hydrotropic agents on the heat coagulation of bovine serum albumin. J. Pharm. Pharmacol. 41:298–301. [DOI] [PubMed] [Google Scholar]
- 42.Sue, Y. J., and M. Shannon. 1992. Pharmacokinetics of drugs in overdose. Clin. Pharmacokinet. 23:93–105. [DOI] [PubMed] [Google Scholar]
- 43.Pena-Alonso, Y. R., M. A. Montoya-Cabrera, E. Bustos-Cordoba, L. Marroquin-Yanez, and V. Olivar-Lopez. 2003. Aspirin intoxication in a child associated with myocardial necrosis: is this a drug-related lesion? Pediatr. Dev. Pathol. 6:342–347. [DOI] [PubMed] [Google Scholar]
- 44.Marcus, Y. 1985. Ion Solvation. John Wiley & Sons, London, UK.
- 45.Lichtenberger, L. M., C. Ulloa, A. L. Vanous, J. J. Romero, E. J. Dial, P. A. Illich, and E. T. Walters. 1996. Zwitterionic phospholipids enhance aspirin's therapeutic activity, as demonstrated in rodent model systems. J. Pharmacol. Exp. Ther. 277:1221–1227. [PubMed] [Google Scholar]
- 46.Monnard, P. A., and D. W. Deamer. 2001. Nutrient uptake by protocells: a liposome model system. Orig. Life Evol. Biosph. 31:147–155. [DOI] [PubMed] [Google Scholar]
- 47.Raphael, R. M., and R. E. Waugh. 1996. Accelerated interleaflet transport of phosphatidylcholine molecules in membranes under deformation. Biophys. J. 71:1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raphael, R. M., R. E. Waugh, S. Svetina, and B. Zeks. 2001. Fractional occurrence of defects in membranes and mechanically driven interleaflet phospholipid transport. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 64:051913. [DOI] [PubMed] [Google Scholar]
- 49.Chen, F. Y., M. T. Lee, and H. W. Huang. 2003. Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys. J. 84:3751–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee, M. T., F. Y. Chen, and H. W. Huang. 2004. Energetics of pore formation induced by membrane active peptides. Biochemistry. 43:3590–3599. [DOI] [PubMed] [Google Scholar]
- 51.Huang, H. W., F. Y. Chen, and M. T. Lee. 2004. Molecular mechanism of peptide-induced pores in membranes. Phys. Rev. Lett. 92:198304. [DOI] [PubMed] [Google Scholar]
- 52.Heywang, C., G. Mathe, D. Hess, and E. Sackmann. 2001. Interaction of GM(1) glycolipid in phospholipid monolayers with wheat germ agglutinin: effect of phospholipidic environment and subphase. Chem. Phys. Lipids. 113:41–53. [DOI] [PubMed] [Google Scholar]
- 53.Perlovich, G. L., S. V. Kurkov, A. N. Kinchin, and A. Bauer-Brandl. 2003. Thermodynamics of solutions. IV. Solvation of ketoprofen in comparison with other NSAIDs. J. Pharm. Sci. 92:2502–2511. [DOI] [PubMed] [Google Scholar]
- 54.Drayton, C. J. 1990. Cumulative subject index and drug compendium. In Comprehensive Medicinal Chemistry. P.G.S.C. Hansch and J.B. Taylor, editors. Pergamon Press, Oxford, UK.
- 55.Escher, B. I., and R. P. Schwarzenbach. 2002. Mechanistic studies on baseline toxicity and uncoupling of organic compounds as a basis for modeling effective membrane concentrations in aquatic organisms. Aquat. Sci. 64:20–35. [Google Scholar]
- 56.McClellan, A. L., and A. H. F. Harnsberger. 1967. Cross-sectional areas of molecules adsorbed on solid surfaces. J. Colloid Interface Sci. 23:577–599. [Google Scholar]
- 57.Morris, G. P., J. L. Wallace, P. L. Harding, E. J. Krausse, and S. J. Lolle. 1984. Correlations between changes in indicators of gastric mucosal barrier integrity at time of exposure to “barrier breakers” and extent of hemorrhagic erosions one hour later. Dig. Dis. Sci. 29:6–11. [DOI] [PubMed] [Google Scholar]
- 58.Abramson, S., and G. Weissmann. 1989. The mechanisms of action of nonsteroidal antiinflammatory drugs. Clin. Exp. Rheumatol. 7(Suppl 3):S163–S170. [PubMed] [Google Scholar]
- 59.Kao, Y. C., P. J. Goddard, and L. M. Lichtenberger. 1990. Morphological effects of aspirin and prostaglandin on the canine gastric mucosal surface. Analysis with a phospholipid-selective cytochemical stain. Gastroenterology. 98:592–606. [DOI] [PubMed] [Google Scholar]
- 60.Giraud, M. N., C. Motta, J. J. Romero, G. Bommelaer, and L. M. Lichtenberger. 1999. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: a possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs). Biochem. Pharmacol. 57:247–254. [DOI] [PubMed] [Google Scholar]
- 61.Siew, S., and M. L. Goldstein. 1981. Scanning electron microscopy of mucosal biopsies of the human upper gastrointestinal tract. Scan. Electron Microsc. 4:173–181. [PubMed] [Google Scholar]
- 62.Kakehata, S., and J. Santos-Sacchi. 1996. Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J. Neurosci. 16:4881–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shehata, W., E. Brownell, and R. Dieler. 1991. Effects of salicylate on shape, electromotility and membrane characteristics of isolated outer hair cells from guinea pig cochlea. Acta Otolaryngol. 111:707–718. [DOI] [PubMed] [Google Scholar]
- 64.Oliver, D., D. Z. He, N. Klocker, J. Ludwig, U. Schulte, S. Waldegger, J. P. Ruppersberg, P. Dallos, and B. Fakler. 2001. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 292:2340–2343. [DOI] [PubMed] [Google Scholar]
- 65.Gov, N., and S. A. Safran. 2004. Pinning of fluid membranes by periodic harmonic potentials. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 69:011101. [DOI] [PubMed] [Google Scholar]
- 66.Raphael, R. M., A. S. Popel, and W. E. Brownell. 2000. A membrane bending model of outer hair cell electromotility. Biophys. J. 78:2844–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morimoto, N., R. M. Raphael, A. Nygren, and W. E. Brownell. 2002. Excess plasma membrane and effects of ionic amphipaths on mechanics of outer hair cell lateral wall. Am. J. Physiol. Cell Physiol. 282:C1076–C1086. [DOI] [PubMed] [Google Scholar]














