Abstract
Membrane lysis caused by antibiotic peptides is often rationalized by means of two different models: the so-called carpet model and the pore-forming model. We report here on the lytic activity of antibiotic peptides from Australian tree frogs, Maculatin 1.1, Citropin 1.1, and Aurein 1.2, on POPC or POPC/POPG model membranes. Leakage experiments using fluorescence spectroscopy indicated that the peptide/lipid mol ratio necessary to induce 50% of probe leakage was smaller for Maculatin compared with Aurein or Citropin, regardless of lipid membrane composition. To gain further insight into the lytic mechanism of these peptides we performed single vesicle experiments using confocal fluorescence microscopy. In these experiments, the time course of leakage for different molecular weight (water soluble) fluorescent markers incorporated inside of single giant unilamellar vesicles is observed after peptide exposure. We conclude that Maculatin and its related peptides demonstrate a pore-forming mechanism (differential leakage of small fluorescent probe compared with high molecular weight markers). Conversely, Citropin and Aurein provoke a total membrane destabilization with vesicle burst without sequential probe leakage, an effect that can be assigned to a carpeting mechanism of lytic action. Additionally, to study the relevance of the proline residue on the membrane-action properties of Maculatin, the same experimental approach was used for Maculatin-Ala and Maculatin-Gly (Pro-15 was replaced by Ala or Gly, respectively). Although a similar peptide/lipid mol ratio was necessary to induce 50% of leakage for POPC membranes, the lytic activity of Maculatin-Ala and Maculatin-Gly decreased in POPC/POPG (1:1 mol) membranes compared with that observed for the naturally occurring Maculatin sequence. As observed for Maculatin, the lytic action of Maculatin-Ala and Maculatin-Gly is in keeping with the formation of pore-like structures at the membrane independently of lipid composition.
INTRODUCTION
A wide range of antibiotic peptides and toxins exert their action by inducing alterations in the hydrophobic-hydrophilic seal of cell membranes. The lytic effect is related to the particular amino acid sequence of the peptide and the characteristic composition of the membrane (1–8). Primarily, two different mechanisms of membrane permeation or lysis have been proposed: i), peptides that acquire a helix conformation across the lipid bilayer and consequently form size-defined permeating structure named as pores (1,3,4,9); and ii), peptides that remain tightly bound to the membrane interface and promote bilayer damage via detergent-like or carpet-like mechanism (2,5,7). Maculatin 1.1, Citropin 1.1, and Aurein 1.2 are antimicrobial chemically related peptides from Australian tree frogs (see Table 1 for peptide amino acid sequences). These peptides have antimicrobial activity mainly against Gram positive bacteria (10–12). We recently demonstrated the preferential interaction of Maculatin and Citropin with anionic phospholipids by using the lipid monolayer approach. It was proposed that these peptides adopt a helical conformation at the interface (13). Additional data suggest a pore-forming lytic behavior for the longer sequence Maculatin and a carpeting mechanism for the shorter Citropin (14–16). Maculatin, Citropin, and Aurein, mainly adopt an α-helical conformation in model membrane environments such as oriented lipid bicelles (17). To further characterize the interaction of these peptides with membranes, we have studied the lytic action of Maculatin 1.1, Maculatin-Gly (Pro-15 substituted by Gly), Maculatin-Ala (Pro-15 substituted by Ala), Citropin 1.1, and Aurein 1.2 on lipid bilayers composed of pure phospholipids and phospholipid mixtures by using fluorescence spectroscopy and confocal microscopy. Usually the leakage experiments using fluorescence spectroscopy of carboxyfluorescein-filled large unilamellar vesicles (LUVs) lack information about the mechanism leading to probe release. To gain a deeper insight about the lytic action of the peptide we developed a novel experimental strategy that allows direct tracking, using confocal microscopy, of giant unilamellar vesicles (GUVs) filled with different molecular weight (MW) and water soluble fluorophores (Alexa488-dextran 10,000 MW; Alexa546-maleimide or Alexa633-maleimide, 1300 MW) (18). The real-time tracking of GUVs filled with the different fluorophores permits direct observation of the microscopic scenario and provides visual information that can be related to the leakage mechanism. The combination of GUV technology and fluorescence microscopy previously used to study lipid-lipid interactions (19,20) is used here to give new insights into lipid-peptide interactions and lytic mechanisms.
TABLE 1.
Peptide primary structure
| Maculatin 1.1: | GLFGVLAKVAAHVVPAIAEHF-NH2 |
| Maculatin-Gly: | GLFGVLAKVAAHVVGAIAEHF-NH2 |
| Maculatin-Ala: | GLFGVLAKVAAHVVAAIAEHF-NH2 |
| Citropin 1.1: | GLFDVIKKVASVIGGL-NH2 |
| Aurein 1.2: | GLFDIKKVASVIGGL-NH2 |
MATERIAL AND METHODS
Materials
POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) and POPG (1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]) were purchased from Avanti Polar Lipids (Birmingham, AL) and were used without further purification. Maculatin 1.1, Maculatin-Gly, Maculatin-Ala (Pro-15 replaced by Gly or Ala, respectively), Citropin 1.1, and Aurein 1.2 were synthesized by Mimotopes Pty (Melbourne, Australia) from l-amino acids via standard N-α-Fmoc methods. The purity of the peptides, as judged by high-pressure liquid chromatography and mass spectroscopy, was >90%. The primary structure of the peptides is summarized in Table 1. 5,6-Carboxyfluorescein was purchased from Eastman Kodak (Rochester, NY) and Sigma Biochemicals (St. Louis, MO). DiI C18 (1,1′-dioctadecyl-3,3,3′,3′- tetramethylindocarbocyanine perchlorate), Alexa488-dextran, Alexa543-maleimide, and Alexa633-maleimide were purchased from Molecular Probes (Eugene, OR).
Preparation of carboxyfluorescein-loaded LUVs
Large unilamellar vesicles were prepared by freeze-thaw and extrusion through polycarbonate filters (pore diameter 100 nm) (21) in an extrusion device from Avestin (Ottawa, Canada). The method produces vesicles of ∼93 nm according to light scattering measurements (22). Briefly, carboxyfluorescein 90-mM solution was generated by dissolving the proper amount of the fluorescent marker in Tris-HCl 20 mM, NaCl 150 mM, EDTA 1-mM buffer (pH 7.4 adjusted with NaOH 1 M). A 10-mg/ml lipid (POPC or POPC/POPG 1:1 mol ratio) solution in chloroform/methanol (2:1) was dried under a stream of nitrogen followed by, at least, 4 h high vacuum drying. The lipid film was rehydrated with the carboxyfluorescein 90-mM buffer, freeze-thawed for five cycles, and extruded 10 times. The free carboxyfluorescein was removed by passing the liposome suspension through a Sephadex G-100 column (Amersham-Pharmacia Biotech, Uppsala, Sweden). To ascertain the lipid concentration after the passing of the LUVs through the column, phospholipid phosphorous was measured by the modified microprocedure of Barlett (23).
Measure of carboxyfluorescein leakage from LUVs
The release of carboxyfluorescein dye from the POPC or POPC/POPG LUVs after peptide exposure was measured using SLM Aminco 4800C and Spex Fluoromax-3 (Jovin Yvon Horiba, Tokyo, Japan) spectrofluorometers at excitation and emission wavelengths of 480 and 510 nm, respectively. Fluorescence intensity was continuously recorded after the desired amount of peptide solution was added to a 1-ml cuvette (under continuous stirring) containing the carboxyfluorescein-loaded LUVs. Leakage is expressed as a percentage relative to the total amount of dye released by addition of 1% of Triton X-100, which represented 100% leakage, as described by Weinstein et al. (24). The error was <5% (experiments were performed at least three times). The experiments were performed maintaining the concentration of osmotic active particles in the same proportion inside and outside of vesicles. The time of presented release measuring was 5 min.
Preparation and visualization of GUVs
GUVs were prepared using the electroformation method, originally developed by Angelova and Dimitrov (25,26). A homemade temperature-controlled chamber was used as previously described (27,28). Briefly, 3 μl lipid stock solution (0.2 mg/ml in chloroform with or without the fluorescent probe DiIC18 0.5 mol%) was spread on each platinum electrode. The chamber was then put into a vacuum chamber overnight to remove any remaining trace of organic solvent. Solutions of sucrose/Alexa488-dextran 1 μM/Alexa546-maleimide 10 μM, sucrose/Alexa488-dextran 1 μM/Alexa633-maleimide 10 μM or sucrose/carboxyfluorescein 5 μM with an overall osmolarity of 150 mOsM (measured with an Advanced Instruments model 3D3 osmometer, Norwood, MA) were equilibrated to temperatures above the lipid phase transition and then added to the chamber covering the Pt electrodes (0.4 ml final volume). Immediately after buffer addition, the platinum wires were connected to a function generator (Digimess FG 100) and a low-frequency alternating field (sinusoidal wave function with a frequency of 10 Hz and amplitude of 1 V) was applied for 120 min. The AC field was turned off and the GUVs were harvested from the chamber. To remove the fluorescent solution from outside the vesicles, the GUVs were washed several times in an iso-osmolar solution of glucose in 15-ml centrifuge tubes or passed through a Sephadex G-100 column.
Aliquots of GUVs suspended in glucose (0.3 ml) were added to an eight-well plastic chamber (Lab-tek Brand Products, Naperville, IL). Due to the density difference between the sugar solutions inside and outside the vesicles the GUVs precipitate at the bottom of the chamber, which facilitates observation in the inverted confocal microscope. The chamber was located in an inverted confocal microscope (Zeiss LSM 510 META, Jena, Germany) for observation. The vesicles formed by this procedure had diameters ranging from 15 to 50 μm. The excitation wavelengths were 488 nm (for Alexa488-dextran and carboxyfluorescein), 543 nm (for Alexa546-maleimide or DiI C18) and/or 633 nm (for Alexa633-maleimide). An iso-osmolar solution of peptide was injected into the chamber (reaching a peptide/lipid mol ratio of ∼8) and a time-series scan was started to follow the peptide-vesicle interaction. The experimental data are representative of at least three experiments, where several vesicles were followed as a function of time after peptide injection.
All leakage experiments were quantitatively analyzed using Metamorph software by taking the change in fluorescence intensity as the average gray value measured for each micrograph of Alexa488-dextran and Alexa546-maleimide inside GUVs after peptide exposure (18).
RESULTS
Carboxyfluorescein leakage from LUVs after peptide exposure
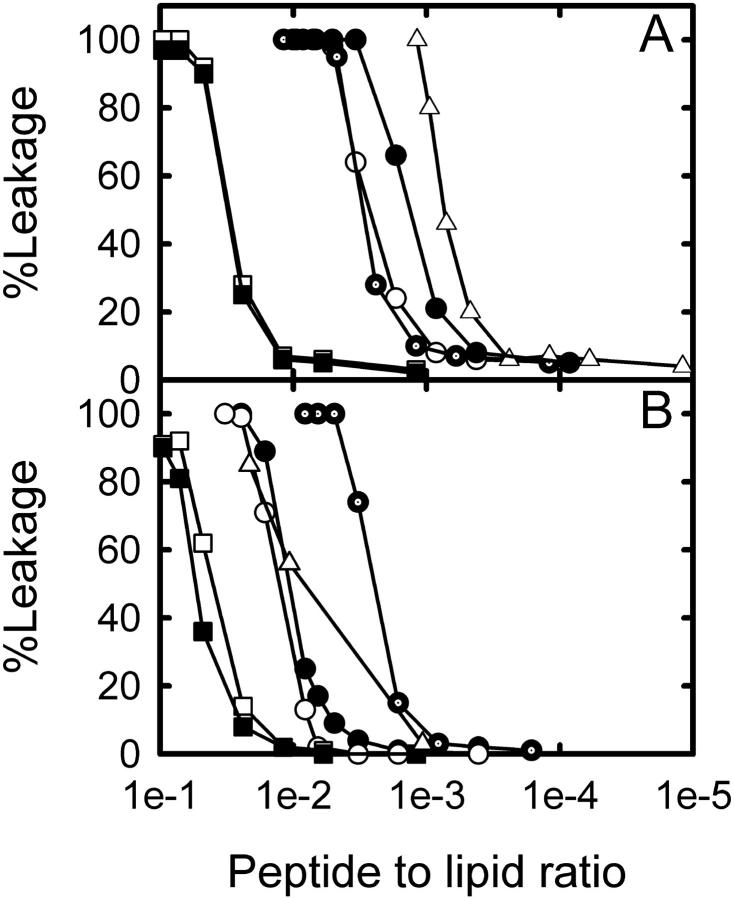
The leakage of POPC LUVs filled with carboxyfluorescein, after the vesicles were exposed to different peptide concentrations, is shown in Fig. 1. Undoubtedly the peptides alter the hydrophobic/hydrophilic seal of LUV membranes allowing permeation of carboxyfluorescein. As can be observed in Fig. 1, Maculatin, Maculatin-Gly, and Maculatin-Ala (peptide sequences are given in Table 1) result in 50% dye leakage at a similar peptide/lipid mol ratio. However, a slightly higher activity is evident for Maculatin-Ala with POPC vesicles. Citropin and Aurein also provoke dye leakage but the lipid/peptide mol ratio for 50% of carboxyfluorescein release was noticeably higher than that observed for the Maculatin-related peptides (Fig. 1 A). Also, Fig. 1 shows the lytic behavior of melittin, a well-known lytic pore-forming peptide from bee venom, which was used as a control. The peptide/lipid mol ratio obtained for 50% of leakage induced by melittin is similar to that previously reported by Gómara et al. (29). We further observed that melittin showed a decrease in the extent of leakage in POPC/POPG composed LUVs compared with POPC LUVs (Fig. 1), which also was reported previously (29).
FIGURE 1.
Effect of antimicrobial peptides on LUVs permeability. Percent of carboxyfluorescein release from LUVs after 5 min peptide addition. Maculatin 1.1 (dotted circles), Maculatin-Gly (open circles), Maculatin-Ala (solid circles), Citropin (solid squares), Aurein (open squares), and melittin (triangles). (A) LUVs composed of POPC; (B) LUVs composed of POPC/POPG (1:1 mol ratio). The peptide/lipid mol ratio was referred to the total amount in the cuvette of lipid and peptide (bound and unbound to LUVs).
For LUVs composed of POPC/POPG (1:1 mol ratio) an interesting difference in the lytic behavior of Maculatin peptides was observed (Fig. 1 B). Even though the peptide/lipid mol ratio to produce 50% of carboxyfluorescein leakage for Maculatin was similar in POPC/POPG and POPC LUVs, the “Pro-15 mutant” peptides (Table 1) have 10-fold lower activities in the POPC/POPG (Fig. 1 B) mixture compare to POPC membranes (Fig. 1 A). This feature points out the importance of the Pro residue for the lytic action of the wild-type Maculatin.
Aurein and Citropin, however, show a slight difference in their lytic activity against LUV membranes composed of either pure POPC or POPC/POPG lipid mixture (see Fig. 1). Against anionic vesicles, the peptides seem to be a little less active compared with POPC LUVs (Fig. 1). This difference was also seen for the well-known highly cationic peptide melittin (Fig. 1), but the difference was more pronounced probably due to the presence of more positive charges for this peptide (30,31). It is also interesting to note that Citropin and Aurein peptides require a higher concentration in comparison with Maculatin peptides to affect the integrity of the membrane.
Confocal microscopy of fluorescently loaded GUVs after peptide exposure
The incorporation of both fluorophores, Alexa488-dextran (MW 10,000) and Alexa546-maleimide (or Alexa633-maleimide; MW ∼ 1300), inside the GUVs is very useful to directly observe the time course of any effect on membrane permeability change (leakage of probes from inside of GUVs; see Ambroggio et al. (18)). All the experiments were carried out at a peptide/lipid mol ratio equivalent to obtain 100% of carboxyfluorescein release as described above (>0.05; see Fig. 1). As control, in absence of peptide, the fluorescent content was not spontaneously released even after 24 h of vesicle formation. The changes reported correspond to single or several vesicles analyzed in a determined optical field. The overall effect in the vesicle population was >90% for all peptides studied. It is necessary to remark that the time analysis was obtained from experiments done in a special chamber where it is not possible to perform continuous stirring (to allow rapid homogenization of the peptide after injection) and the lag time before the leakage start could be erroneously overestimated. Stirring inside the chamber will generate movement of the GUVs and will hamper the potential to perform time-series experiments on single vesicles.
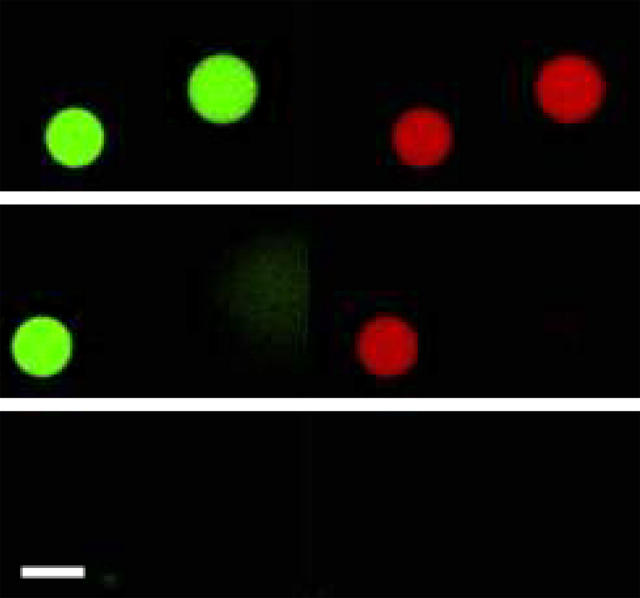
The effects of Maculatin, Maculatin-Gly, and Maculatin-Ala on GUVs filled with the two fluorescent markers are shown in Fig. 2. As can be seen from the fluorescent images, when POPC GUVs are exposed to Maculatin peptides (which have a longer amino acid sequence than Citropin and Aurein; Table 1), the low molecular weight probe Alexa546-maleimide first leaked out of the vesicles without significant leakage of the high molecular weight probe Alexa488-dextran. This last observation is confirmed in a quantitative manner in Fig. 3. Control experiments in the absence of peptide are also given in the insert of Fig. 3 in which no change in fluorescence intensity was observed for a similar period of time. Therefore, we exclude any significant photobleaching of the fluorescent probes demonstrating that the observed decrease in fluorescence intensity is provoked by the action of the peptides interacting with GUVs. The results shown in Figs. 2 and 3 can be mechanistically interpreted as being due to the presence of pore-like structures at the membrane since the overall three-dimensional structure and integrity of the GUVs are preserved after peptide exposure. As controls, similar experiments were performed for the well-known pore-forming peptide melittin (supporting information files), which exhibited similar results to those observed for Maculatin peptides.
FIGURE 2.
Direct visualization of lytic action by pore forming antimicrobial peptides. Confocal microscopy of POPC GUVs, filled with Alexa488-dextran (green) and Alexa546-maleimide (red), exposed to Maculatin 1.1 5 μM. Time course of the effect of lipid-peptide interaction on bilayer permeability after peptide addition. Left (green) and right (red) panels show the differential leakage of the fluorophores. (Top) 0 s, peptide addition; (middle) 580 s, 50% Alexa546-maleimide fluorescence intensity; (bottom) 670 s, <1% Alexa546-maleimide fluorescence intensity. The same behavior was observed for either Maculatin-Gly or Maculatin-Ala 5 μM interacting with POPC GUVs. See Fig. 3 for a clearer picture of the time course of dye release. Scale bar, 32 μm.
FIGURE 3.
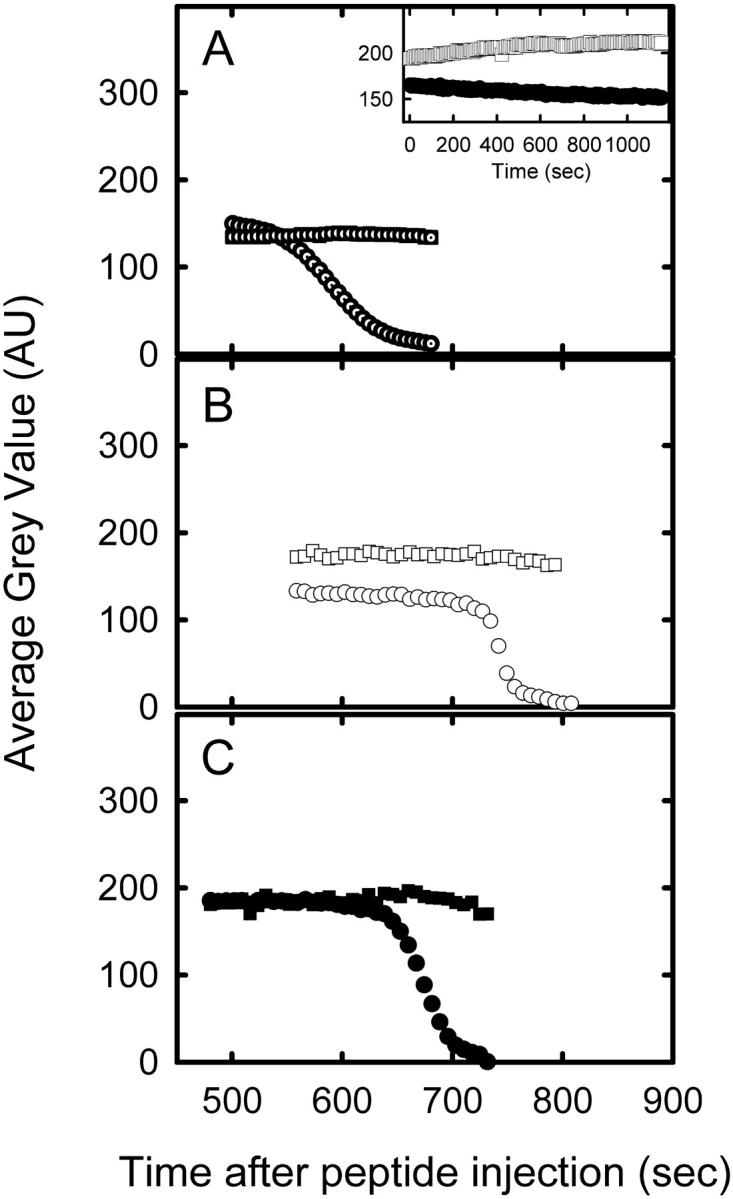
Dye release kinetics from inside GUVs after the exposure to antimicrobial peptides. Relative fluorescence leakage from POPC GUVs filled with Alexa488-dextran (squares) and Alexa546-maleimide (circles) after exposure to 5 μM peptide of: Maculatin 1.1 (A, dotted symbols), Maculatin-Gly (B, open symbols), and Maculatin-Ala (C, solid symbols). The remaining fluorescence intensity was determined in each micrograph using Metamorph software to quantify the average gray value of Alexa488-dextran and Alexa546-maleimide inside the GUVs after exposure to the peptides. (A, insert) Control experiments: time-dependent fluorescence intensity in absence of peptide of Alexa488-dextran (squares) and Alexa546-maleimide (circles) (see Materials and Methods).
Fig. 4 shows the same experiments as described above but for Citropin and Aurein. From these data it is clear that these peptides have a different lytic behavior compared with those observed for Maculatin peptides. GUVs exposed to Citropin or Aurein were completely destroyed showing the simultaneous leakage of both dyes (no sequential).
FIGURE 4.
Direct visualization of lytic action by carpet mode induced by antimicrobial peptides. Confocal microscopy of POPC GUVs, filled with Alexa488-dextran (green) and Alexa546-maleimide (red), exposed to 5 μM of Aurein. Time course of the effect of lipid-peptide interaction on membrane permeability. (Top) Peptide addition, 0 s; (middle) partial damage, 1162 s; (bottom) complete damage of GUVs, 1191 s). Left (green, high molecular weight fluorescent probe) and right (red, low molecular weight fluorescent probe). A similar behavior is observed for 5 μM of Citropin. Scale bar, 25 μm.
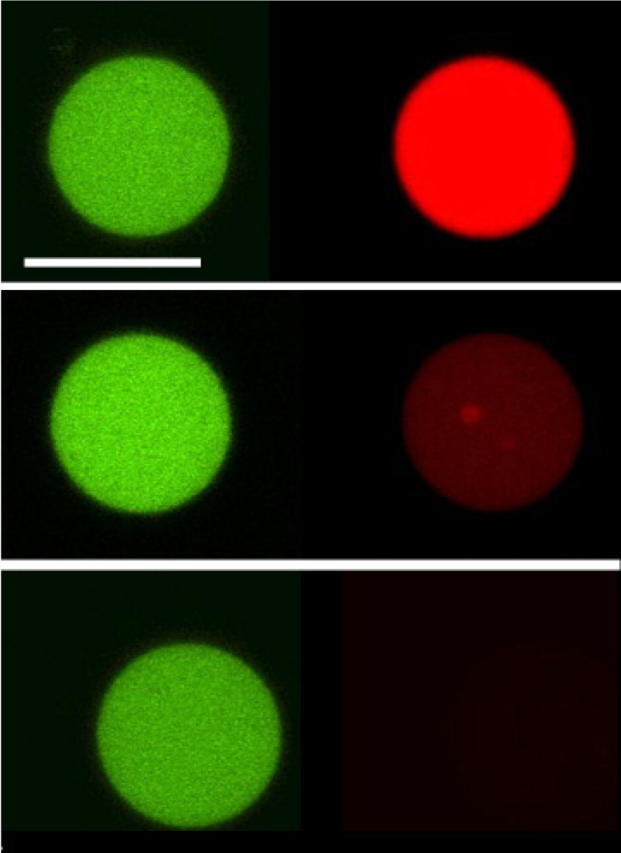
To confirm that the long-range membrane integrity was not altered by the addition of pore-like forming peptides, the DiIC18 probe was incorporated into GUVs as an indicator of membrane bilayer structure. Fig. 5 shows micrographs of POPC GUVs labeled with DiIC18 and loaded with Alexa488-dextran and Alexa633-maleimide. After Maculatin exposure (Fig. 5 A) GUV's membrane remains intact and the high molecular weight probe is retained inside the vesicle, confirming the experimental observations shown in Fig. 2. Conversely, when GUVs were exposed to either Aurein or Citropin the membrane was completely disintegrated with the simultaneous loss of fluorescence from the three dyes (complete membrane disruption; see Fig. 5 B).
FIGURE 5.
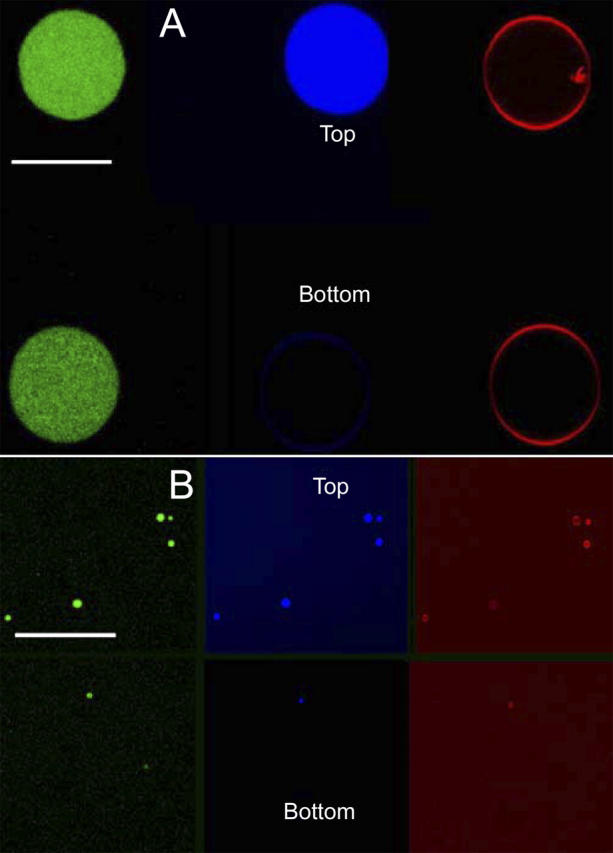
Direct visualization of membrane integrity after addition of pore-like forming and carpet acting antimicrobial peptides. Confocal microscopy of DiI-C18 containing POPC GUVs filled with Alexa488-dextran (green) and Alexa633-maleimide (blue), exposed to 5 μM of: Maculatin 1.1 (A; a similar behavior was observed for either Maculatin-Ala or Maculatin-Gly), Citropin (B; a similar behavior was observed for Aurein). Effect of lipid-peptide interaction from the time, 0 s (from top to bottom) after injection of the peptide. Left (green) and middle (blue) panels show the differential leakage of the fluorophores. Right (red) panel shows that the membrane shape is not altered during the leakage process by the Maculatin peptides (A). In contrast, panel B shows the complete disintegration of several vesicles after peptide addition. Scale bar, 95 μm.
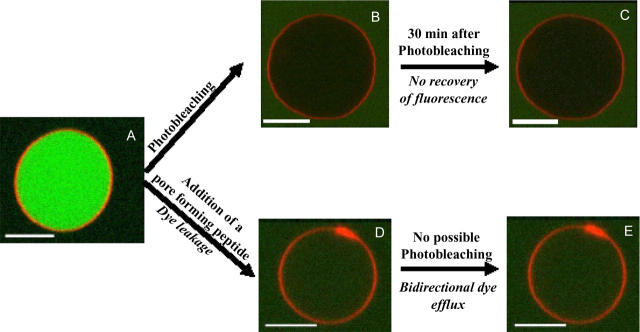
A POPC GUV labeled with DiIC18 and loaded with carboxyfluorescein is shown in Fig. 6. We observed that the carboxyfluorescein probe experienced strong photobleaching when it was irradiated continuously with the excitation (laser) source (compare Fig. 6, A and B). As it is schematized in Fig. 6 C there was not recovery of fluorescence after photobleaching confirming the hydrophobic/hydrophilic seal of the lipid bilayer. After addition of Maculatin, the background was visible inside the vesicle (Fig. 6 D). Photobleaching was not inducible after peptide addition, indicating a bidirectional exchange of dye inside and outside the vesicle (no loss of fluorescence can be induced; compare Fig. 6 D with Fig. 6 E). A similar effect was observed for Maculatin-Ala, Maculatin-Gly, or melittin (Supplementary Material).
FIGURE 6.
Bidirectional flux of carboxyfluorescein in POPC GUVs after exposure to pore-like forming antimicrobial peptides. Confocal microscopy of DiI-C18 labeled POPC GUVs, filled with carboxyfluorescein 5 μM before (A), and after (D) exposure to Maculatin 1.1 or Maculatin-Gly or Maculatin-Ala 5 μM. Immediate recovery of fluorescence background after site-directed carboxyfluorescein photobleaching (induced by the laser in the microscope) in the interior of the vesicle after exposure to the Maculatin peptides (D and E). (Control) GUVs that were not exposed to the peptides do not recover the fluorescence background (C) after site-directed photobleaching of carboxyfluorescein in the GUV interior (B). A similar behavior was observed for 5 μM of Maculatin-Ala, Maculatin-Gly, and melittin. Scale bar, 10 μm.
DISCUSSION
The effect of antibiotic peptides on model membranes has been widely reported in the literature. Two different mechanisms of membrane lysis are generally accepted for these peptides: the so-called carpet mechanism and the pore-forming mechanism (1–5,7,9). From the results of fluorescence experiments in cuvette, it is clear that all of the peptides tested modify the permeability of POPC and POPC/POPG bilayers (Fig. 1). Even though this information clearly reflects a lytic action induced by the peptides studied, the data do not provide direct information about any particular lytic mechanism. Therefore, we developed a novel experimental approach to allow observation of peptide-induced differential leakage of different molecular-size fluorescent markers loaded into single vesicles (18). Previously, Ladokhin et al. have described the differential release of coencapsulated fluorescent markers of different size from LUVs using fluorescence spectroscopy provoked by the pore-forming melittin peptide (30). In our experiments, we have been able to directly visualize differential release by using confocal fluorescence microscopy.
Citropin and Aurein peptides
GUVs are completely destroyed by addition of the shorter sequence peptides, Citropin and Aurein. In these experiments the fluorescence intensity from both probes, regardless of their molecular size, was instantly decreased to zero at a specific time with simultaneous rupture of the membrane. We attribute this behavior to the particular lytic mechanism, i.e., the carpeting model. As proposed by Separovic and co-workers, Citropin and Aurein may interact with the lipid bilayer by partial insertion but their lengths do not allow for complete spanning of the membrane with subsequent formation of a pore-like structure (17). This partial insertion model is reasonable because the short sequence peptides cannot cross the bilayer due to the mismatch between the bilayer thickness and the peptide length. Fluorescence experiments in cuvettes indicate that, in contrast to the Maculatin peptides, a high peptide/lipid ratio is needed for Aurein and Citropin to achieve dye leakage. This fact leads us to speculate that the amount of peptide molecules required to form a critical structure to disrupt the membrane is larger for peptides acting in the carpeting mode than for those peptides that are lytic by a pore-like mechanism (if similar partition coefficients are considered).
Maculatin peptides
From the studies carried out with the Maculatin natural occurring sequence or with its mutants, we conclude that the peptides induce an alteration in the permeation properties of the GUV membranes, most likely by a pore-forming mechanism. This fact was clearly demonstrated using confocal microscopy studies, and was reflected by the rapid loss in fluorescence intensity of the small molecular weight fluorescent probe whereas the fluorescence intensity from the high molecular weight probe trapped within the GUVs remained almost constant (Fig. 2). Importantly, a similar behavior to that observed for Maculatin was obtained when fluorescently labeled GUVs were exposed to the pore-forming melittin peptide in agreement with previously reported data obtained from other techniques for this peptide (29,30). Our data also show that the shape of the GUV and the long-range membrane organization do not seem to be altered when pores are formed by Maculatin and its related peptides, indicating a metastable peptide-lipid interaction when these peptides are inserted into the membrane. Also, the diameter of the GUVs was not substantially altered after peptide interaction (as it can be appreciated in Fig. 2 or Fig. 5). As reported previously for other lytic peptides, peptide insertion into the membrane, and consequent pore formation, can induce alterations in the elastic properties of the membrane or changes in membrane order (32–35). Although our results do not provide information about the mechanical properties or lateral order of the lipid membrane, we believe that such changes ought to occur during pore formation to compensate for peptide insertion, resulting in a similar three-dimensional structure of the GUVs after peptide addition. Additionally, as illustrated in Fig. 6, we observed that laser-induced photobleaching of the dye inside the GUVs was only possible before peptide addition, which indicates a relative fast bidirectional flow of the probe throughout the pores (i.e., no photobleaching was possible after peptide exposure indicating the simultaneous in and out flow of the dye through the membrane).
An interesting point is that Maculatin has similar lytic activity (translated into a comparable peptide/lipid mol ratio) when LUVs are composed of either POPC or POPC/POPG but when Pro-15 is replaced by Gly or Ala, the lytic potency is significantly reduced in anionic vesicles. This characteristic suggests that proline in the Maculatin sequence should play a key role in the antibiotic activity allowing the peptide to adopt an optimal amphiphilic conformation at the interface leading to membrane damage (15,36). A slight difference in the leakage of carboxyfluorescein-loaded POPC LUVs was observed for the Maculatin-Ala peptide, which was slightly more active than the Gly-15 mutant. This enhanced activity can be due to the more hydrophobic nature of the Ala (compared with Gly) or, possibly, reflects subtle changes in peptide conformation at the interface. Additionally Gly substitution can provoke a more flexible structure and for this reason the peptide may lose its optimal amphiphilic configuration when interacting with membranes.
In summary, we have directly visualized differences in the two models of lytic action of antibiotic peptides proposed by several authors (1–9). The disparity in the length of the peptide sequence (assuming 1.5 Å per residue when the peptide is adopting an ideal α-helical structure, see Ambroggio et al. and others (13,18), a fluid lipid bilayer has an average hydrophobic width of ∼40 Å see Hristova and White (37) and the appropriate amphiphilicity may be the reason for this difference. The longer sequence peptides are able to adopt a transbilayer configuration allowing probably for peptide oligomerization at the interface and, consequently, the formation of pore-like structures, preserving the bilayer shape. Although the shorter sequence peptides are able to interact with the membrane, there is a mismatch between the peptide length and the bilayer thickness, which prevents pore formation but leads to membrane destabilization.
Finally, our experimental strategy can aid in the determination of the average pore size by using a series of different molecular weight probes. Experiments are in progress within our lab to address this issue.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
The authors thank Dr. D. M. Jameson for critical reading of the manuscript.
This work was supported by grants from CONICET, FONCYT (PICT 0609228), SECYT-UNC, Agencia Córdoba Ciencia, and Ministerio de Salud de la Nacion (Carrillo Oñativia fellowship). Research in the laboratory of L.A.B. is funded by a grant from SNF, Denmark (21-03-0569) and the Danish National Research Foundation (which supports MEMPHYS-Center for Biomembrane Physics).
References
- 1.Matsuzaki, K. 1998. Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim. Biophys. Acta. 1376:391–400. [DOI] [PubMed] [Google Scholar]
- 2.Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta. 1462:55–70. [DOI] [PubMed] [Google Scholar]
- 3.Hara, T., Y. Mitani, K. Tanaka, N. Uematsu, A. Takakura, T. Tachi, H. Kodama, M. Kondo, H. Mori, A. Otaka, F. Nobutaka, and K. Matsuzaki. 2001. Heterodimer formation between the antimicrobial peptides magainin 2 and PGLa in lipid bilayers: a cross-linking study. Biochemistry. 40:12395–12399. [DOI] [PubMed] [Google Scholar]
- 4.Chen, F. Y., M. T. Lee, and H. W. Huang. 2003. Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys. J. 84:3751–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papo, N., and Y. Shai. 2003. Exploring peptide membrane interaction using surface plasmon resonance: differentiation between pore formation versus membrane disruption by lytic peptides. Biochemistry. 42:458–466. [DOI] [PubMed] [Google Scholar]
- 6.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature. 415:389–395. [DOI] [PubMed] [Google Scholar]
- 7.Mani, R., J. J. Buffy, A. J. Waring, R. I. Lehrer, and M. Hong. 2004. Solid-state NMR investigation of the selective disruption of lipid membranes by protegrin-1. Biochemistry. 43:13839–13848. [DOI] [PubMed] [Google Scholar]
- 8.Lehrer, R. I. 2004. Primate defensins. Nat. Rev. Microbiol. 2:727–738. [DOI] [PubMed] [Google Scholar]
- 9.Huang, H. W., F. Y. Chen, and M. T. Lee. 2004. Molecular mechanism of peptide-induced pores in membranes. Phys. Rev. Lett. 92:198304. [DOI] [PubMed] [Google Scholar]
- 10.Rozek, T., K. L. Wegener, J. H. Bowie, I. N. Olver, J. A. Carver, J. C. Wallace, and M. J. Tyler. 2000. The antibiotic and anticancer active aurein peptides from the Australian bell frogs Litoria aurea and Litoria raniformis: the solution structure of aurein 1.2. Eur. J. Biochem. 267:5330–5341. [DOI] [PubMed] [Google Scholar]
- 11.Wegener, K. L., P. A. Wabnitz, J. A. Carver, J. H. Bowie, B. C. Chia, J. C. Wallace, and M. J. Tyler. 1999. Host defence peptides from the skin glands of the Australian blue mountains tree-frog Litoria citropa. Solution structure of the antibacterial peptide citropin 1.1. Eur. J. Biochem. 265:627–637. [DOI] [PubMed] [Google Scholar]
- 12.Rozek, T., R. J. Waugh, S. T. Steinborner, J. H. Bowie, M. J. Tyler, and J. C. Wallace. 1998. The maculatin peptides from the skin glands of the tree frog Litoria genimaculata: a comparison of the structures and antibacterial activities of maculatin 1.1 and caerin 1.1. J. Pept. Sci. 4:111–115. [DOI] [PubMed] [Google Scholar]
- 13.Ambroggio, E. E., F. Separovic, J. Bowie, and G. D. Fidelio. 2004. Surface behaviour and peptide-lipid interactions of the antibiotic peptides, Maculatin and Citropin. Biochim. Biophys. Acta. 1664:31–37. [DOI] [PubMed] [Google Scholar]
- 14.Balla, M. S., J. H. Bowie, and F. Separovic. 2004. Solid-state NMR study of antimicrobial peptides from Australian frogs in phospholipid membranes. Eur. Biophys. J. 33:109–116. [DOI] [PubMed] [Google Scholar]
- 15.Niidome, T., K. Kobayashi, H. Arakawa, H. Hatakeyama, and H. Aoyagi. 2004. Structure-activity relationship of an antibacterial peptide, maculatin 1.1, from the skin glands of the tree frog, Litoria genimaculata. J. Pept. Sci. 10:414–422. [DOI] [PubMed] [Google Scholar]
- 16.Chia, C. S., J. Torres, M. A. Cooper, I. T. Arkin, and J. H. Bowie. 2002. The orientation of the antibiotic peptide maculatin 1.1 in DMPG and DMPC lipid bilayers. Support for a pore-forming mechanism. FEBS Lett. 512:47–51. [DOI] [PubMed] [Google Scholar]
- 17.Marcotte, I., K. L. Wegener, Y. H. Lam, B. C. Chia, M. R. de Planque, J. H. Bowie, M. Auger, and F. Separovic. 2003. Interaction of antimicrobial peptides from Australian amphibians with lipid membranes. Chem. Phys. Lipids. 122:107–120. [DOI] [PubMed] [Google Scholar]
- 18.Ambroggio, E. E., D. H. Kim, F. Separovic, C. J. Barrow, K. J. Barnham, L. A. Bagatolli, and G. D. Fidelio. 2005. Surface behavior and lipid interaction of Alzheimer beta-amyloid peptide 1–42: a membrane-disrupting peptide. Biophys. J. 88:2706–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagatolli, L. A. 2003. Thermotropic behavior of lipid mixtures studied at the level of single vesicles: giant unilamellar vesicles and two-photon excitation fluorescence microscopy. Methods Enzymol. 367:233–253. [DOI] [PubMed] [Google Scholar]
- 20.Bagatolli, L. A. 2003. Direct observation of lipid domains in free standing bilayers: from simple to complex lipid mixtures. Chem. Phys. Lipids. 122:137–145. [DOI] [PubMed] [Google Scholar]
- 21.Mayer, L. D., M. J. Hope, and P. R. Cullis. 1986. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta. 858:161–168. [DOI] [PubMed] [Google Scholar]
- 22.Nolan, V., M. Perduca, H. L. Monaco, B. Maggio, and G. G. Montich. 2003. Interactions of chicken liver basic fatty acid-binding protein with lipid membranes. Biochim. Biophys. Acta. 1611:98–106. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett, G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466–468. [PubMed] [Google Scholar]
- 24.Weinstein, J. N., R. D. Klausner, T. Innerarity, E. Ralston, and R. Blumenthal. 1981. Phase transition release, a new approach to the interaction of proteins with lipid vesicles. Application to lipoproteins. Biochim. Biophys. Acta. 647:270–284. [DOI] [PubMed] [Google Scholar]
- 25.Angelova, M. I., and D. S. Dimitrov. 1986. Liposome electroformation. Faraday Discuss. Chem. Soc. 81:303–311. [Google Scholar]
- 26.Angelova, M. I., S. Soleau, P. Meleard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external fields. Kinetics and application. Prog. Colloid Polym. Sci. 89:127–131. [Google Scholar]
- 27.Bagatolli, L. A., and E. Gratton. 1999. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys. J. 77:2090–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagatolli, L. A., and E. Gratton. 2000. A correlation between lipid domain shape and binary phospholipid mixture composition in free standing bilayers: a two-photon fluorescence microscopy study. Biophys. J. 79:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomara, M. J., S. Nir, and J. L. Nieva. 2003. Effects of sphingomyelin on melittin pore formation. Biochim. Biophys. Acta. 1612:83–89. [DOI] [PubMed] [Google Scholar]
- 30.Ladokhin, A. S., M. E. Selsted, and S. H. White. 1997. Sizing membrane pores in lipid vesicles by leakage of co-encapsulated markers: pore formation by melittin. Biophys. J. 72:1762–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladokhin, A. S., and S. H. White. 2001. ‘Detergent-like’ permeabilization of anionic lipid vesicles by Melittin. Biochim. Biophys. Acta. 1514:253–260. [DOI] [PubMed] [Google Scholar]
- 32.Lau, W. L., D. S. Ege, J. D. Lear, D. A. Hammer, and W. F. DeGrado. 2004. Oligomerization of fusogenic peptides promotes membrane fusion by enhancing membrane destabilization. Biophys. J. 86:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachar, M., and O. M. Becker. 2000. Protein-induced membrane disorder: a molecular dynamics study of melittin in a dipalmitoylphosphatidylcholine bilayer. Biophys. J. 78:1359–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longo, M. L., A. J. Waring, and D. A. Hammer. 1997. Interaction of the influenza hemagglutinin fusion peptide with lipid bilayers: area expansion and permeation. Biophys. J. 73:1430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longo, M. L., A. J. Waring, L. M. Gordon, and D. A. Hammer. 1998. Area expansion and permeation of phospholipid membrane bilayers by influenza fusion peptides and Melittin. Langmuir. 14:2385–2395. [Google Scholar]
- 36.Chia, C. S., J. A. Carver, T. D. Mulhem, and J. H. Bowie. 2000. Maculatin 1.1, an anti-microbial peptide from the Australian tree frog, Litoria genimaculata solution structure and biological activity. Eur. J. Biochem. 267:1894–1908. [DOI] [PubMed] [Google Scholar]
- 37.Hristova, K., and S. H. White. 1998. Determination of the hydrocarbon core structure of fluid dioleoylphosphocholine (DOPC) bilayers by x-ray diffraction using specific bromination of the double-bonds: effect of hydration. Biophys. J. 74:2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.