Abstract
RNA silencing is a natural defense mechanism against genetic stress factors, including viruses. A mutant hordeivirus (Barley stripe mosaic virus [BSMV]) lacking the γb gene was confined to inoculated leaves in Nicotiana benthamiana, but systemic infection was observed in transgenic N. benthamiana expressing the potyviral silencing suppressor protein HCpro, suggesting that the γb protein may be a long-distance movement factor and have antisilencing activity. This was shown for γb proteins of both BSMV and Poa semilatent virus (PSLV), a related hordeivirus. Besides the functions in RNA silencing suppression, γb and HCpro had analogous effects on symptoms induced by the hordeiviruses. Severe BSMV-induced symptoms were correlated with high HCpro concentrations in the HCpro-transgenic plants, and substitution of the γb cistron of BSMV with that of PSLV led to greatly increased symptom severity and an altered pattern of viral gene expression. The efficient systemic infection with the chimera was followed by the development of dark green islands (localized recovery from infection) in leaves and exemption of new developing leaves from infection. Recovery and the accumulation of short RNAs diagnostic of RNA silencing in the recovered tissues in wild-type N. benthamiana were suppressed in HCpro-transgenic plants. These results provide evidence that potyviral HCpro and hordeivirus γb proteins contribute to systemic viral infection, symptom severity, and RNA silencing suppression. HCpro's ability to suppress the recovery of plants from viral infection emphasizes recovery as a manifestation of RNA silencing.
The first observations of posttranscriptional gene silencing, or RNA silencing, were made more than 10 years ago. At that time, the phenomenon was described as “cosuppression” of endogenous genes caused by expression of homologous sequences in transgenic plants (43, 66). RNA silencing is no longer associated only with transgenic plants but is known as a natural defense system against genetic stress factors, such as viruses and transposable elements, in multicellular eukaryotic organisms (5, 6, 11, 20, 35, 39, 68, 70, 73). RNA silencing is characterized by rapid and specific degradation of cytoplasmic RNAs. The accumulation of small 21- to 25-nucleotide RNA fragments (small interfering RNAs [siRNAs]) originating from the target sequence is diagnostic of RNA silencing (24, 37). The most potent inducer of RNA silencing is double-stranded RNA (dsRNA) (5, 6, 11, 20, 35, 39, 63, 68, 70, 73). The onset of RNA silencing is followed by a propagation phase during which a systemic signal is delivered from the tissues undergoing silencing and is transported to other parts of the plant where homologous RNA molecules will be silenced. The nature of the signal is yet unknown, but the signaling pathway follows the transport of macromolecules and viruses through plasmodesmata between cells and via phloem over long distances (20, 53, 64).
The expression of virus-derived transgenic mRNA sequences in plants triggers degradation of homologous viral RNAs in the transgenic tissues, resulting in high levels of virus-specific resistance (5, 34, 35, 39, 68, 70, 73). In some cases, plants are initially susceptible to the virus and develop a systemic viral infection, but resistance is later induced in developing leaves, which appear to be virus free and unsusceptible to new infections with the same virus (12, 51). The steady-state levels of transgene-derived mRNA and homologous viral RNA are either greatly reduced or undetectable in recovered leaves (60). Similarities between the recovery phenotypes observed in transgenic and nontransgenic plants suggest that RNA silencing is a natural antiviral mechanism (12, 51). For example, siRNAs derived from the virus are detected in leaves recovered in both wild-type (wt) and transgenic plants (24, 62). Silencing-induced RNA (isRNA) in nontransgenic virus-infected plants may originate from dsRNA intermediates of replicating RNA viruses or from overlapping mRNAs of different polarity transcribed from the genomic components of DNA viruses.
Localized recovery, or dark green islands (DGIs), may develop on chlorotic, virus-infected leaves. In DGIs, no viral nucleic acids and proteins are detected, and DGIs are resistant to infection with the same virus (3). Leaves of Nicotiana benthamiana develop DGIs after systemic infection with Potato A potyvirus (PVA), isolate TamMV. In N. benthamiana plants transformed with the coat protein (CP)-encoding region of TamMV, DGIs resemble those observed in nontransformed plants and contain greatly reduced amounts of TamMV and transgene-derived mRNA, in contrast to the surrounding chlorotic tissue (40). Also, in tobacco plants (Nicotiana tabacum), silencing of the gene NtRDRP1 that encodes an RNA-dependent RNA polymerase (a host factor required for RNA silencing) (13, 42), prevents the formation of DGIs after infection with Tobacco mosaic tobamovirus (TMV) (74). These data suggest that RNA silencing is sporadically triggered in some cells of the developing leaves, resulting in localized recovery and development of DGIs (16, 21, 40).
Plant viruses have evolved to suppress and/or circumvent RNA silencing using proteins that show a high degree of diversity at the sequence level (2, 4, 9, 22, 36, 49, 71, 72). Viral silencing suppressors probably have different molecular targets in the host because they seem to interfere with different parts of the silencing pathway, thus allowing them to single out initiation, signaling and maintenance phases (10, 33, 68, 70, 73). For example, the HCpro and 2b proteins of poty- and cucumoviruses, respectively, suppress the maintenance and signaling phases of RNA silencing, respectively (2, 9, 22, 29).
Viral RNA silencing suppressors are determinants of additional phenotypes such as viral vascular transport (9, 14, 38). They also determine symptom severity (virulence) as shown by the enhanced virulence of a chimeric Potato X potexvirus (PVX) that expresses heterologous RNA silencing suppressors derived from a wide range of virus genera, including Potyvirus, Cucumovirus, Tombusvirus, Sobemovirus, and Begomovirus (9, 26, 58, 72).
Viruses of the genus Hordeivirus contain a positive-stranded tripartite RNA genome. The three genomic RNAs (RNAα, RNAβ, and RNAγ) are capped at their 5′ termini and have a tRNA-like structure at their 3′ ends. RNAs α and γ are essential for the viral genome replication and encode three proteins, whereas RNAβ encodes CP and three proteins involved in virus movement (27, 32, 57, 61). RNAγ is bicistronic and encodes the RNA-dependent RNA polymerase (γa protein) and the γb protein that is expressed from a subgenomic RNA (sgRNA). γb is cysteine rich, has a putative zinc finger motif, possesses RNA-binding activity in vitro, and may influence hordeivirus gene expression (1, 15, 23, 41, 46, 48). It is dispensable for virus replication and affects infection phenotypes in the natural host barley (Hordeum vulgare, a monocot) and the experimental host Chenopodium amaranticolor (a dicot) (45, 47). Moreover, γb is implicated in seed transmissibility of hordeiviruses (19), and this protein also constitutes a virulence determinant (15, 48).
These findings suggest a role for γb in viral genome amplification and the counterdefensive viral mechanisms required for suppression of host antiviral mechanisms. Such functions could be revealed based on differences in viral genome replication and gene expression, an altered rate of virus movement in plant tissues, or modified symptom expression and/or interference with RNA silencing. In this study, the mentioned protein activities that are putatively attributable to γb were studied in the related members of the genus Hordeivirus, Barley stripe mosaic virus (BSMV) and Poa semilatent virus (PSLV). Furthermore, we tested whether the functions of the hordeiviral γb proteins are complementary or exchangeable with potyviral HCpro, a protein known to suppress RNA silencing and stimulate viral genome amplification and long-distance movement (30). Our data show that hordeivirus γb proteins are determinants for viral long-distance movement, genome amplification, and gene expression, as well as suppression of RNA silencing and modulation of symptom severity (i.e., virulence). Furthermore, our data show that HCpro prevents recovery of nontransgenic plants from viral infection, inhibits the formation of DGIs, and reduces the accumulation of siRNAs in both cases, thereby emphasizing these resistance phenotypes as manifestations of systemic and local RNA silencing, respectively.
MATERIALS AND METHODS
Plasmid constructs.
DNA manipulations and cloning were carried out using standard procedures (54). Primer sequences are available on request. To construct BγP, an NcoI restriction site was introduced into the full-length cDNA of BSMV strain ND18 (BSMVND18) RNAγ (44) at the start codon of the γb gene. The PSLV γb gene was amplified by PCR and ligated as an NcoI-BamHI fragment, together with a BamHI-MluI fragment that included the 3′ untranslated region sequence of BSMV RNAγ, into the NcoI-MluI-digested RNAγ plasmid. To obtain the BSMVdelγb construct (Fig. 1B), BγP was digested with NcoI and BamHI, end filled, and ligated. pPVX201 contained the complete copy of the PVX genome under control of the Cauliflower mosaic virus 35S promoter and carried a duplicate copy of the CP subgenomic promoter from the plasmid pTXS.P3C2 (8). The genes encoding the PSLVγb or BSMVγb protein were amplified by PCR and inserted between the NheI and SalI restriction sites of pPVX201. To obtain pPVX-GF, pPVX-PSγb-GF, and pPVX-BSγb-GF, the 5′-terminal 342 residues of the gfp (green fluorescent protein) gene flanked by SalI restriction sites were obtained by PCR from the template 30B-GFP-C3 (59). The fragment was ligated into the SalI site of pPVX201, and orientation of the insert was checked by restriction analysis. The TMV-GFP clone 30B-GFP-C3 (59) was kindly provided by William O. Dawson.
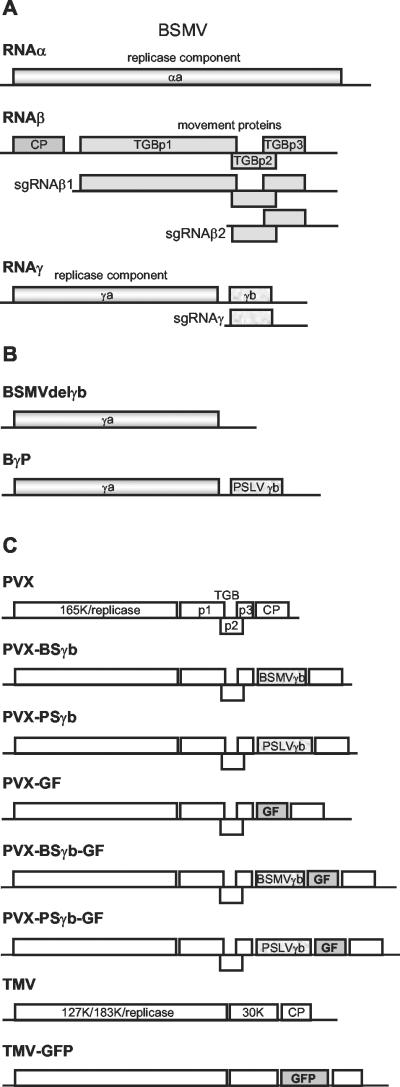
FIG. 1.
Viral genomes and chimeric derivatives. (A) Organization of the genome of BSMV. Three genomic RNAs are shown, and the protein coding regions are presented as boxes. The sgRNAs requiredfor expression of the 5′ distal genes are shown. (B) Mutants of the BSMV RNAγ: the γb gene deletion mutant (BSMVdelγb) and the chimera BγP of BSMV carrying the γb gene of PSLV. (C) Recombinant viral genomes used in the silencing suppression assays. PVX, the genome of PVX; PVX-BSγb and PVX-PSγb, modified genomes of PVX carrying the γb gene of BSMV and PSLV, respectively; PVX-GF, a modified genome of PVX carrying two-thirds of the gfp gene; PVX-BSγb-GF and PVX-PSγb-GF, modified genomes of PVX carrying two-thirds of the gfp gene (GF) and the γb gene of BSMV or PSLV, respectively; TMV, the TMV genome; TMV-GFP, the modified TMV genome expressing GFP.
In vitro transcription and plant inoculation.
BSMV cDNA constructs encoding full-length RNAα, RNAγ, BSMVdelγb, and BγP were linearized with MluI, and the full-length BSMV RNAβ cDNA was linearized with SpeI. All constructs were transcribed in vitro in the presence of a cap analog m7GpppG (New England Biolabs) using T7 RNA polymerase (Promega).
The T1 progeny of a transgenic N. benthamiana line (ab34) expressing the PVA HCpro have been described elsewhere (55). They were grown from seed in Fi-totron 600H growth chambers (17 to 19°C, 75% relative humidity, photoperiod, 16 h; Fisons Environmental Equipment, Loughborough, United Kingdom). The fully expanded leaves of the 4-week-old plants were mechanically inoculated with RNA in GKP buffer (50 mM glycine; 30 mM K2HPO4, pH 9.2; 1% bentonite; 1% celite) (45). pPVX201 and its derivatives were inoculated as plasmid DNA onto carborundum-dusted N. benthamiana leaves (7). TMV-GFP constructs were linearized with KpnI and transcribed as described above. The T7 RNA transcripts were mechanically coinoculated with cDNA clones pPVX-GF, pPVX-PSγb-GF, or pPVX-BSγb-GF.
Analysis of plants.
Total RNA was extracted from inoculated and systemically infected leaves at various time points after inoculation as described (69). For Northern blots, 5 μg RNA was separated by electrophoresis on a 1% (wt/vol) agarose formaldehyde gel, transferred to a Hybond-N membrane, and hybridized with [32P]cDNA probes specific to the respective viruses. For Western blot analysis, leaf samples (20 mg, fresh weight) were collected at various time points as indicated in the text, ground and homogenized in an ice-cold mortar in 20 μl of 250 mM Tris-HCl (pH 7.8), mixed with 20 μl of extraction buffer (75 mM Tris-HCl, pH 6.8; 9 M urea; 4.3% sodium dodecyl sulfate; 7.5% β-mercaptoethanol), heated 5 min at 100°C, and centrifuged (5 min at 10,000 × g) to remove all insoluble material. Aliquots of the supernatant (1 to 10 μl) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (31) on 12% gels. After electrophoresis, proteins were transferred to a Hybond-C membrane, and BSMV CP and TGBp1 were analyzed by Western blot analysis with specific antisera (kindly provided by V. K. Novikov and N. O. Kalinina) and the ECL Western blotting kit (Amersham Pharmacia Biotech). Quantification of the HCpro antigen (double antibody sandwich-enzyme-linked immunosorbent assay [DAS-ELISA]) was carried out as described previously (55).
Isolation and detection of siRNAs.
Low-molecular-weight RNAs were extracted from 0.2 to 0.3 g of plant tissues as described previously (28) using the RNA/DNA Midi Kit (QIAGEN), and 50 μg of RNA was analyzed on a 15% polyacrylamide TBE-7 M Urea Ready gel (Bio-Rad, Hercules, Calif.). The gel was run until the bromophenol blue dye had migrated to the bottom of the gel and then was soaked in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 15 min. A 22-mer oligonucleotide was electrophoresed along with the low-molecular-weight RNA and used as a size marker. The RNA was transferred from the gel to a HyBond-N membrane and fixed by UV cross-linking (1,200 μJ; UV Cross-linker; Amersham Pharmacia Biotech).
RNA blot hybridization was carried out as described (28). The CP gene and the tRNA-like structure were amplified from BSMV cDNA by PCR, and the purified PCR products were radiolabeled using the random prime labeling system Rediprime II (Amersham Pharmacia Biotech). Unincorporated nucleotides were removed using Micro Bio-Spin P-30 Tris chromatography columns (Bio-Rad). A mixture of the two probes was used for hybridization.
GFP imaging.
Detection of GFP fluorescence in whole plants was performed using a hand-held long-wave UV lamp UVL-56 (UV Products, Upland, Calif.). Plants were photographed with a digital camera (Nikon E990).
RESULTS
The long-distance movement function of hordeiviral γb is complemented by potyviral HCpro.
In previous studies, deletion of the BSMV gene encoding the cysteine-rich γb protein prevented systemic infection in barley (H. vulgare), a monocot host of the virus (46), and reduced the rate of systemic infection of BSMV in the dicot species N. benthamiana (47). We deleted the γb gene from the infectious cDNA of BSMV strain ND18 (BSMVND18) (45) and tested its infectivity on our strain of N. benthamiana. The resultant mutant virus (BSMVdelγb) (Fig. 1B) was unable to infect N. benthamiana systemically, but similar amounts of CP were detected in the leaves inoculated with the mutant and wt viruses (Fig. 2). Four weeks after inoculation, no symptoms were observed in the upper, noninoculated leaves of the 30 plants inoculated with BSMVdelγb, whereas control plants inoculated with BSMVND18 were systemically infected and showed mild mosaic symptoms at 10 to 11 days postinoculation (dpi). In plants inoculated with BSMVdelγb, Western blotting and RNA dot blot hybridization confirmed the absence of viral proteins and RNAs in the upper (noninoculated) leaves, and therefore the lack of systemic infection, at 6 weeks postinoculation (data not shown). These results differ from those reported previously (47) in which deletion of the γb gene delayed but did not fully abolish systemic infection. The different results could be explained by different genotypes of N. benthamiana used.
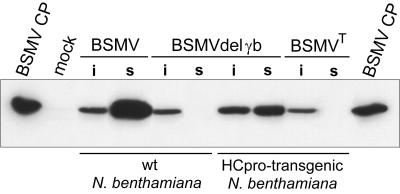
FIG. 2.
Western blot analysis of the BSMV CP accumulation in infected nontransgenic and HCpro-transgenic N. benthamiana plants. Lanes are as follows: BSMV CP, CP preparation loaded as a control; mock, healthy plants; BSMV, plants inoculated with BSMVND18; BSMVdelγb, plants inoculated with the γb gene deletion mutant of BSMVND18; BSMVT, plants inoculated with the type strain of BSMV; i, inoculated leaves; s, upper, noninoculated (systemically infected) leaves.
The ability of a potyviral long-distance movement protein (HCpro) (30) to complement defects in long-distance movement of BSMVdelγb was tested in transgenic N. benthamiana plants expressing the HCpro of PVA. Symptoms of mild yellow mosaic were observed in the second leaf above the inoculated leaf at 10 dpi, and BSMV CP was detected in the symptomatic leaves (Fig. 2). The HCpro-transgenic N. benthamiana plants were also inoculated with the type strain of BSMV (BSMVT), which is defective in long-distance movement in this host because of down-regulated expression of the γa gene (47). Virus accumulation was tested by Western analysis for CP and dot blot hybridization for viral RNA, but, in contrast to the inoculated leaves, no infection was observed in the upper, noninoculated leaves (Fig. 2, and data not shown). Results were similar for HCpro-transgenic plants and nontransgenic control plants.
Taken together, these results showed that the transgenically expressed PVA HCpro rescues defective long-distance movement of BSMVdelγb but not BSMVT. These data indicate that the long-distance movement functions of potyviral HCpro are specifically complementary with the hordeivirus γb protein and that the complementary functions can be provided in trans.
HCpro enhances the symptom severity of BSMV infection.
Potyviral HCpro can enhance infection of heterologous viruses in plants (50, 55, 65). It was therefore of particular interest to examine whether HCpro would have any effect on infection with BSMVND18. As before, BSMVND18 induced mild yellowing symptoms in wt N. benthamiana (Fig. 3A), but HCpro-transgenic plants showed a wider range of symptoms. Of the 26 plants tested, 10 displayed severe symptoms of yellowing, leaf malformation, and curling (Fig. 3B); 8 plants showed symptoms similar to those of BSMVND18 on wt N. benthamiana; and the symptoms of the remaining 8 plants were intermediate in their severity (data not shown).
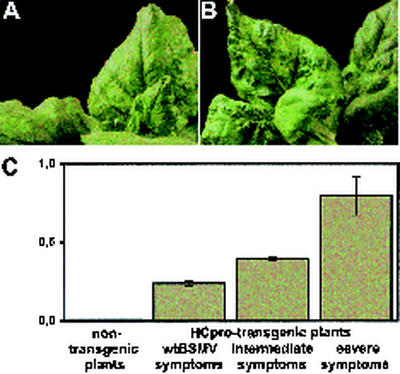
FIG. 3.
BSMV infection in HCpro-transgenic plants. (A) Symptoms of BSMV on systemically infected leaves of nontransgenic N. benthamiana. (B) Severe symptoms of the BSMV infection on systemically infected leaves of transgenic N. benthamiana expressing the PVA HCpro protein. (C) Correlation of symptom severity with levels of HCpro expression (detected by ELISA) in HCpro-transgenic N. benthamiana plants. The y axis indicates ELISA absorbance values. The bars indicate means and standard deviations for eight plants.
The T1 plants of the HCpro-transgenic N. benthamiana used in this study differed in their HCpro expression levels (55). The uppermost fully expanded leaves of the transgenic plants infected with BSMVND18 contained 760 to 4,000 ng HCpro per gram of leaf, as tested by DAS-ELISA, and the severity of symptoms correlated positively with the concentration of HCpro (Fig. 3C). Notably, the BSMV-infected plant with the highest HCpro concentration showed the most severe yellowing symptoms and was stunted in growth. Thus, potyviral HCpro promoted the BSMV infection-induced symptoms and contributed to the virulence of BSMV in N. benthamiana.
Substitution of the γb gene alters virulence and induces recovery.
The role of the hordeivirus γb proteins in long-distance movement was studied in further detail using a chimera of BSMVND18 (BγP; Fig. 1B), in which the γb gene was replaced with the γb gene of PSLV (57). Plants were inoculated with BSMVND18 (total 28 plants) and BγP (28 plants) in three experiments. The leaves inoculated with BSMVND18 showed mild chlorosis symptoms at 4 to 5 dpi, whereas the leaves challenged with BγP developed many bright yellow local lesions (∼3 mm in diameter) at 3 dpi. Both viruses infected the plants systemically, but symptoms were different and appeared at different times. In plants infected with BSMVND18, mild yellowing symptoms were observed in the upper, noninoculated leaves at 10 to 12 dpi. In the plants inoculated with BγP, however, symptoms were observed earlier (5 to 6 dpi), and they were more severe. Initially the leaves displayed severe mosaic symptoms and malformations (Fig. 4A) and later (9 to 12 dpi) developed necrosis. These results showed that replacement of the γb gene in BSMV with the corresponding gene of PSLV enhances symptom severity and infection rate in N. benthamiana, providing additional evidence for the involvement of hordeivirus γb in virus movement and virulence determination.
FIG. 4.
Induction of DGIs and recovery. (A) Symptoms of BγP infection in the systemically infected leaves in N. benthamiana. (B) A DGI on the chlorotic leaf systemically infected with BγP. (C) The upper systemically infected leaves on BγP-infected HCpro-transgenic plants showed no recovery. (D to G) DGIs in the systemic leaves of nontransgenic plants (D and E) and their absence on systemically infected leaves of the HCpro-transgenic plants (F), compared with a symptomless, healthy leaf (G). (H and I) Comparison of BγP infection in HCpro-transgenic (H) and nontransgenic (I) plants. Arrows indicate the recovered leaves.
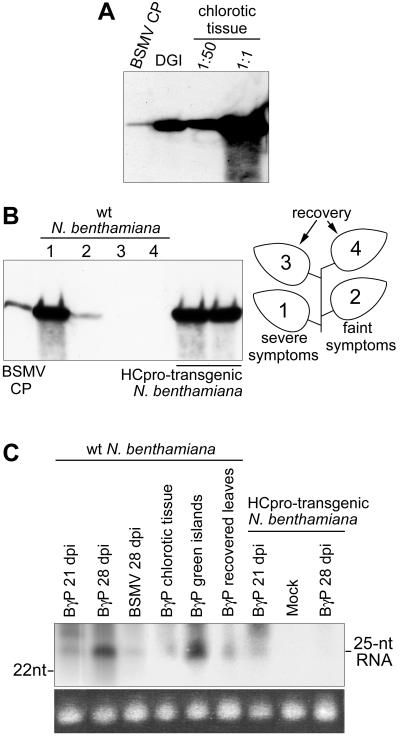
Leaves systemically infected with BγP and showing chlorosis developed discrete patterns of DGIs (Fig. 4B) at 12 to 40 dpi. DGI tissue was excised from the leaves and analyzed for viral CP, viral RNA, and siRNAs diagnostic of RNA silencing. Accumulation of viral CP (Fig. 5A) and RNA (data not shown) was reduced by ∼50-fold in DGIs compared with the surrounding chlorotic leaf tissue. The minute quantities of viral CP and RNA detected in DGI samples may have resulted from trace amounts of adjacent chlorotic tissue being included in the samples. This would be expected because the DGI margins are not always distributed homogeneously throughout the cell layers (40). DGIs did contain, however, high amounts of siRNAs derived from viral RNA (Fig. 5C). The surrounding chlorotic tissue also contained siRNAs but at ∼16-fold lower concentration than in the DGIs (Fig. 5C), as quantified by PhosphorImager. These data showed an accumulation of siRNAs in DGIs, which supports the idea that DGIs develop as a consequence of locally triggered RNA silencing.
FIG. 5.
Analysis of virus accumulation in N. benthamiana leaves showing DGIs and recovery. (A) Western blot of BSMV CP in the DGI tissue compared with the surrounding chlorotic tissue. The lanes labeled DGI and 1:1 contained equal amounts of plant material; the equivalent of a 1:50 dilution of the latter sample was loaded in the 1:50 lane. (B) BSMV CP accumulation in the systemically infected leaves of nontransgenic plants compared with transgenic plants expressing the PVA HCpro. The lanes with samples from nontransgenic plants are numbered according to the leaves shown to the right. (C) Detection of siRNAs in N. benthamiana leaves showing DGIs and recovery by Northern blot analysis with a probe specific to the virus CP gene and the 3′-terminal untranslated region. Positions of the 22-nucleotide marker DNA and the 25-nucleotide siRNAs are shown. Equal loading of the low-molecular-weight RNA was verified using ethidium bromide staining of tRNA (bottom panel).
At 21 to 40 dpi, most of the new leaves in plants infected with BγP were symptomless, indicating recovery from the viral infection (Fig. 4I), but some leaves still showed severe mosaic symptoms including DGIs. Thereafter, all new leaves were asymptomatic, whereas the new leaves in all plants infected with BSMVND18 continued to display symptoms. The symptomless leaves of BγP-infected plants contained only very low or no detectable amounts of viral CP (Fig. 5B) and viral RNA (data not shown), but they accumulated readily detectable amounts of siRNAs derived from the virus (Fig. 5 C). Apparently, DGI formation preceded recovery in BγP-infected plants, similar to the observations reported previously (40) on PVA CP-transgenic N. benthamiana plants infected with PVA. Our results showed that DGI formation (local recovery) and the exemption of the new developing leaves from virus infection are associated with the accumulation of siRNAs, which are diagnostic of RNA silencing.
BSMVND18 induced only mild symptoms in N. benthamiana, and no plant recovered from infection. In contrast, chimeric BSMV (BγP) carrying the γb gene of PSLV initially induced very severe symptoms, but the plants later recovered from infection. These data indicated the importance of γb for virulence (symptom severity). Recovery was preceded by development of DGIs in the upper leaves. DGIs and the recovered leaves contained greatly reduced amounts of viral RNA and CP, consistent with previous studies (3, 40), and accumulated high levels of virus-specific siRNAs that are diagnostic of RNA silencing targeted against the virus. We next studied whether γb directly affected the induction of host resistance (i.e., recovery from infection) or indirectly mediated its effects based on altered viral replication and/or gene expression.
The PSLV γb gene alters replication and gene expression in BSMV.
The BSMV γb protein regulates genome replication and expression (15), which could be the cause of the differences in virulence between BSMVND18 and BγP. To address this possibility, the accumulation of viral products such as CP, movement protein TGBp1, genomic and sgRNA, and negative-strand RNA was assessed in BSMV- and BγP-inoculated leaves from N. benthamiana during the course of infection.
Leaf tissue was collected at 5, 7, 11, and 15 dpi and was analyzed by Western blotting for CP and TGBp1. CP was detected at 5 dpi in plants inoculated with BSMV or BγP (Fig. 6A). CP levels increased through 15 dpi, and the amount of BγP CP consistently exceeded that of BSMV at all time points (Fig. 6A). A different pattern of accumulation was observed for TGBp1. In BSMV-inoculated leaves, a high level of TGBp1 accumulated at 5 dpi after which levels decreased until 11 dpi, and were undetectable at 15 dpi. However, higher levels of TGBp1 were evident in BγP-inoculated leaves, and the level was unchanged from 5 to 7 dpi, after which TGBp1 decreased but remained detectable through 15 dpi (Fig. 6B).
FIG. 6.
Accumulation of viral proteins and RNAs in leaves inoculated with BSMV and BγP. Samples were taken at 5, 7, 11, and 15 dpi. (A and B) Western blot detection of the BSMV CP and TGBp1 proteins, respectively. (C to F) Northern blot hybridization of representative samples with different probes. (C) BSMV RNAβ-specific probe detects accumulation of this genomic RNA and its sgRNAs. (D) Accumulation of RNAγ and RNAγ-derived sgRNA detected with a mixture of probes specific to the γb protein genes of BSMV andPSLV. (E) Accumulation of genomic RNAγ with a single probe specific to both wt RNA and the recombinant BγP. (F) Detection of negative-strand viral RNAs. (G) Ethidium bromide staining of rRNA as a loading control for panels C to F. Positions of proteins and virus-specific RNAs are indicated to the right.
For Northern hybridization, one probe was designed to detect the genomic RNAβ and its two sgRNAs, sgRNAβ1 and sgRNAβ2, which were used as templates for translation of TGBp1 and TGBp2/TGBp3, respectively (75). For detection of RNAγ, a mixture of two probes specific to the γb genes of BSMV and PSLV were used, permitting detection of the wt RNAγ in BSMV, the chimeric BγP RNAγ, and the sgRNAγ from which the γb protein is translated (44). Amount of genomic RNAγ in BSMV- and BγP infected leaves were also directly compared using a single probe detecting both these RNAs. In inoculated leaves, BSMV RNAs β and γ were detected from 5 to 15 dpi, reaching maximum concentrations at 11 dpi. In leaves inoculated with BγP, however, the highest concentrations of RNAs β and γ were observed at 5 and 7 dpi, after which they decreased (Fig. 6C to E). These results were reproducible in two experiments, in which 5 and 6 plants were tested.
Accumulation of negative-strand RNA was monitored by Northern blotting using a probe specific to the 3′-untranslated region of the BSMV genome. This probe could detect the negative-strand copies of all three genomic RNAs, and since they are of similar size the three bands appeared as a single band. In BSMV-infected leaves, the level of negative-strand RNA was constant from 5 to 7 dpi, increased at 11 dpi, and declined at 15 dpi (Fig. 6F). For BγP, the level of negative-strand RNA at 5 and 7 dpi was constant but higher than with BSMV. At 11 dpi, the level was much lower and was constant through 15 dpi (Fig. 6F).
Taken together, the data showed that the PSLV γb protein, when expressed in the genetic background of BSMV, altered both genome replication and gene expression patterns of the virus, which probably resulted in the altered virulence.
HCpro suppresses the recovery from infection with BSMV/PSLV chimera.
A total of nine HCpro-transgenic plants and nine nontransgenic (wt) N. benthamiana plants were inoculated with BγP in two experiments. The symptoms induced by BγP in the HCpro-transgenic plants were different from those observed in wt plants as the former group had fewer and smaller DGIs (Fig. 4E and F). As before, wt plants recovered from infection at 21 to 40 dpi (Fig. 4I), but the upper leaves of all HCpro-transgenic plants continued to be infected, displaying the typical yellow mosaic and leaf malformation symptoms (Fig. 4C and H). Viral CP and RNA were detected in the newly developing, symptomatic leaves of the HCpro-transgenic plants but not in the symptomless leaves of the wt N. benthamiana plants (Fig. 5B). The siRNAs were not detected in HCpro-transgenic plants at 28 dpi (Fig. 5C). Our studies on transgenically overexpressed potyviral HCpro uncovered two new functions for this protein: it is sufficient to suppress the formation of DGIs and prevents the recovery of plants from viral infection.
Hordeivirus γb proteins suppress RNA silencing.
As the γb protein of PSLV was found to have a profound effect on the severity of BSMV-induced disease, the effect of hordeivirus γb proteins on the virulence of an unrelated virus was also studied. The γb genes of PSLV and BSMV were inserted into an engineered cDNA of PVX (8), resulting in PVX chimeras designated as pPVX-PSγb and pPVX-BSγb, respectively (Fig. 1C), that each were used to inoculate a total of 12 N. benthamiana plants in two experiments. The wt PVX construct was used to inoculate a similar number of plants as a control. Systemic infection with PVX caused mild mosaic symptoms at 7 dpi (Fig. 7B). In contrast, necrosis and wilting of the upper, noninoculated leaves was observed at 9 to 10 dpi in plants inoculated with pPVX-PSγb (Fig. 7C) and pPVX-BSγb (Fig. 7D), and these plants died 2 to 3 days later. These severe symptoms resembled those reported for PVX chimeras expressing heterologous viral RNA silencing suppressors, such as HCpro of potyviruses or the 2b protein of cucumoviruses (9, 50).
FIG. 7.
Suppression of RNA silencing by the γb proteins of PSLV and BSMV. (A to D) Effect of the hordeivirus γb proteins on the symptoms of PVX infection. (A) Noninfected control plant of N. benthamiana. (B) Symptoms of PVX infection. (C and D) Necrosis in the systemically infected leaves in plants inoculated with PVX-PSγb and PVX-BSγb, respectively. (E to H) GFP expression from chimeric TMV (TMV-GFP) in the cross-protection assay. Pictures were taken under long-wave UV illumination. (E) GFP fluorescence in the inoculated and systemically infected leaves in a plant infected with TMV-GFP. (F) GFP fluorescence is restricted to inoculated leaves in the plant coinoculated with TMV-GFP and PVX-GF. (G and H) Coinoculation with TMV-GFP and either PVX-PSγb-GF or PVX-BSγb-GF, respectively, showed that hordeivirus γb expression allows systemic infection with TMV-GFP. Arrows indicate the systemically infected leaves showing GFP fluorescence.
Suppression of RNA silencing by the hordeivirus γb proteins was tested using a previously described cross-protection assay in N. benthamiana (52). The assay employed TMV-GFP (Fig. 1C), an engineered TMV cDNA expressing the bright mutant ′cycle3′ (59) of GFP. GFP fluorescence (indicating TMV-GFP replication) was readily detectable under long-wave UV illumination in both inoculated and systemically infected leaves at 10 dpi (Fig. 7E). For the cross-protection assay, a PVX derivative (PVX-GF) carrying a truncated gfp gene (half of the total gfp length; Fig. 1C) that was unable to produce functional GFP, was coinoculated with TMV-GFP. In coinoculated plants, GFP fluorescence was confined to the inoculated leaves, and was not observed in the upper, noninoculated leaves (Fig. 7F). These data were consistent with RNA silencing triggered by the two replicating virus chimeras carrying the homologous gfp sequence, as described (52). Detection of GFP-expressing lesions on inoculated leaves was expected because some expression may have taken place before silencing, and some initially infected cells may have been infected with only one virus. These data validated the assay, which was subsequently used to test the silencing suppression potential of the hordeivirus γb proteins.
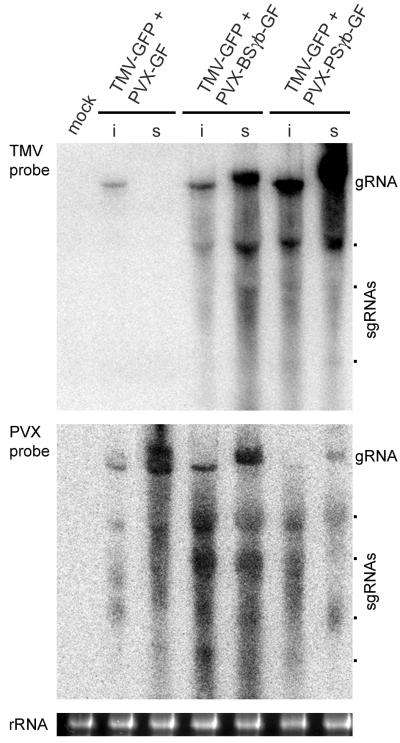
Derivatives of PVX-GF were made to express PSLV and BSMV γb proteins from the PVX genome (PVX-PSγb-GF and PVX-BSγb-GF, respectively; Fig. 1C), and they were used to coinoculate 15 plants with TMV-GFP in three experiments. The coinoculated plants developed apical necrosis at 9 to 10 dpi and died 2 to 3 days later. These symptoms were similar to those observed in the plants infected with pPVX-PSγb or pPVX-BSγb, as described above. Before necrotization, however, the newly emerging leaves exhibited bright GFP fluorescence under UV illumination (Fig. 7G, H). Northern blot analysis verified the presence of TMV-GFP RNA in the upper noninoculated leaves of these plants, whereas TMV was absent in the upper leaves of plants coinoculated with PVX-GF and TMV-GFP (Fig. 8). These data demonstrated that expression of the PSLV and BSMV γb proteins rescued ability of TMV-GFP to accumulate in the upper noninoculated leaves.
FIG. 8.
Accumulation of virus RNAs in cross-protection assay. Northern blot hybridization of samples from inoculated (i) and systemic (s) leaves with probes specific for the TMV and PVX CP genes. Inoculum components are indicated above the gels. Position of genomic RNAs (gRNA) and sgRNAs are shown. Mock, control buffer-inoculated plants. Loading of the gel was verified by ethidium bromide staining of rRNA (bottom panel).
Taken together, the data indicated that the γb proteins of PSLV or BSMV can suppress RNA silencing, permitting systemic movement of viral RNA from inoculated leaves. This conclusion is supported by previous studies showing that the silencing suppression achieved with a PVX chimera expressing HCpro or 2b proteins is not a consequence of the severe symptoms caused by these viruses (9).
DISCUSSION
In this study, we used two derivatives of BSMV and several other virus chimeras to analyze functions of the hordeivirus γb protein during plant infection, including a comparison with the functions of the unrelated, well-characterized potyvirus-encoded HCpro protein (28-30, 38). Our data showed that deletion of the γb gene from BSMV does not prevent virus accumulation in the inoculated leaves (consistent with its dispensability for viral replication and cell-to-cell movement) (46) but prevents viral long-distance movement. Replacement of the γb gene in the BSMV genome with that of PSLV, a hordeivirus closely related to BSMV (57), substantially enhanced viral long-distance movement as well as the severity of the symptoms. Similarly, when inserted into the genome of an unrelated virus (PVX), the γb proteins of BSMV and PSLV greatly enhanced the symptoms, converting the mosaic symptoms characteristic of PVX to necrosis and death of the systemically infected leaves. Our data showed that the potyviral HCpro complements the defective long-distance movement functions of the BSMV γb gene deletion mutation, and also enhances the symptoms induced by BSMV. Thus, the functions of the γb protein and HCpro in long-distance movement and virulence are analogous.
Many viral proteins previously considered to be long-distance movement factors and virulence determinants have lately been shown to be the suppressors of RNA silencing (9, 67, 72). It is hypothesized that coevolution of viruses and their plant hosts has resulted in mechanisms that allow the host to tolerate viral infections and the viruses to evade host defenses. RNA viruses replicate via dsRNA intermediates that are recognized and targeted by the host RNA surveillance system. Consequently, to replicate their genomes, viruses have developed mechanisms to counteract RNA silencing by producing proteins that suppress it (5, 11, 20, 35, 39, 70, 73). For example, potyviral HCpro enhances long-distance movement and virulence and acts as an RNA silencing suppressor, all of which are probably manifestations of the same underlying mechanism controlled by HCpro (30). Therefore, our data implicating the hordeivirus γb protein in viral long-distance movement and virulence and the direct evidence on the complementary functions of the γb protein and HCpro suggest that the γb protein could be a suppressor of RNA silencing. Furthermore, in a manner similar to several well-characterized RNA silencing suppressors (9, 50, 67), γb expressed from PVX converted mosaic symptoms of plants into lethal necrosis. Subsequently, the silencing suppression capabilities of γb were tested using a cross-protection assay (52). Plants were coinfected with chimeric TMV that expressed GFP and chimeric PVX carrying a truncated gfp gene. The truncated gfp was unable to produce functional GFP but was sufficient to trigger RNA silencing, which was observed as an inability of TMV-GFP to move systemically in coinfected plants. Our data showed that expression of γb from one of the virus chimeras allowed systemic movement of TMV-GFP. This result indicated that the γb protein interferes with RNA silencing.
Some recent data provide a basis for speculation on the relative importance of different domains of the BSMV γb protein in RNA silencing. The cysteine-rich proteins of Beet necrotic yellow vein benyvirus (BNYVV) and Peanut clump pecluvirus (PCV) suppress RNA silencing (18). These proteins, together with the analogous hordeivirus proteins examined in this study, represent a new group of viral silencing suppressors. The cysteine-rich protein of PCV is most closely related to γb proteins of hordeiviruses (57). The regions of conservation include a cluster of positively charged residues located at the N-proximal part of the protein that are responsible for RNA binding, a cysteine-rich motif positioned in the central part, a C-terminal region resembling a coiled-coil sequence, and the C-terminal tripeptide SKL (15, 18, 57). The antisilencing activity of the PCV cysteine-rich proteins maps to the coiled-coil sequence (18). This sequence may not, however, be the only determinant of silencing suppression in cysteine-rich proteins of peclu- and hordeiviruses, as point mutations in the cysteine-rich region of BSMV γb result in symptoms typical for a γb gene deletion mutant (15). This hypothesis is supported by a recent study showing that the cysteine-rich region of the C2 protein controls symptom induction and suppression of RNA silencing in Tomato yellow leaf curl begomovirus (67).
Replacement of the γb gene in BSMV with the corresponding gene of PSLV not only enhanced symptom severity but resulted in a novel phenotype characterized by the development of DGIs in the systemically infected leaves followed by recovery of the new developing leaves from viral infection. The recovered leaves were free of BSMV RNA but accumulated BSMV-derived siRNAs that are diagnostic of RNA silencing. Similarly, only a minute level of BSMV RNA, in contrast to the high levels of the BSMV-derived siRNAs, was detected in DGIs. Neither DGIs nor recovery was observed in plants infected with the wt BSMV.
It was intriguing that the chimeric BSMV exhibited enhanced virulence (due to the expression of a heterologous γb protein from PSLV) yet was sensitive to RNA silencing. We sought an explanation for this phenomenon by analyzing putative alterations in viral gene expression. The data indicated that the higher rate of infection with the BSMV chimera was attributable to enhanced production of the virus movement protein TGBp1. Alternatively, the stronger plant defense response to the BSMV chimera, expressed as a local and systemic induction of RNA silencing (recovery), may have resulted from altered viral replication. Northern blot analysis showed increased amounts of negative-strand RNAs at early stages of infection by the BSMV chimera compared with wt virus. These data are consistent with previous studies on BSMV (15) and the mutations in the cysteine-rich proteins of BNYVV and PCV that downregulate synthesis of negative strands of viral RNAs (17, 25). The observed enhanced synthesis of minus strand RNA in the chimeric virus BγP implicated several fold increased level of double-stranded, replicative-form viral RNA, which may have served as a strong inducer for RNA silencing and subsequent recovery of plants from infection. This possibility is suggested by previous studies, hypothesizing that dsRNA (e.g., the replicative forms of viral RNA genomes) constitutes a strong RNA inducer, compared to single-stranded RNA with a limited amount of secondary structure (28). The opposite forces of silencing induction by dsRNA and silencing suppression by the viral RNA suppressors may then determine, whether or not the plants recover from infection (56).
Analysis of the DGIs in leaves systemically infected with the BSMV chimera expressing the PSLV γb protein revealed 16-fold-higher concentrations of the BSMV-derived siRNAs in DGIs compared to the surrounding chlorotic tissues. Based on the greatly reduced amounts of viral RNA in DGIs, it has been suggested that DGIs could result from locally triggered RNA silencing (40). The data in this study showed that the siRNAs diagnostic of RNA silencing indeed accumulate in DGIs. Our data on the PVA HCpro-transgenic plants also showed that HCpro alone reduces DGI formation and is sufficient to prevent systemic recovery of plants from viral infection. Because the BSMV chimera that induced the DGIs and recovery in N. benthamiana has no sequence homology with the HCpro transgene, the data demonstrated the effect of HCpro on natural recovery (i.e., recovery not associated with resistance conferred by a transgene homologous to the infecting virus). These data confirm the previously proposed idea that the recovery of plants from viral infection may be a manifestation of RNA silencing triggered by the dsRNA of the replicating virus (12, 51).
Acknowledgments
We are grateful to S. Zvereva for assistance in preparation of figures and to A. O. Jackson for valuable materials.
This work was supported by the Swedish Royal Academy of Sciences (KVA) and INTAS-01-2379.
REFERENCES
- 1.Agranovsky, A. A., A. V. Karasev, V. K. Novikov, N. A. Lunina, S. Loginov, and L. G. Tyulkina. 1992. Poa semilatent virus, a hordeivirus having no internal polydisperse poly(A) in the 3′ non-coding region of the RNA genome. J. Gen. Virol. 73:2085-2092. [DOI] [PubMed] [Google Scholar]
- 2.Anandalakshmi, R., G. J. Pruss, X. Ge, R. Marethe, A. C. Mallory, T. H. Smith, and V. B. Vance. 1998. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 95:13079-13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson, P. H., and R. E. Matthews. 1970. On the origin of dark green tissue in tobacco leaves infected with tobacco mosaic virus. Virology 40:344-356. [DOI] [PubMed] [Google Scholar]
- 4.Beclin, C., R. Berthome, J. C. Palauqui, M. Tepfer, and H. Vaucheret. 1998. Infection of tobacco or Arabidopsis plants by CMV counteracts systemic post-transcriptional silencing of nonviral (trans)genes. Virology 252:313-317. [DOI] [PubMed] [Google Scholar]
- 5.Baulcombe, D. 2001. RNA silencing. Diced defence. Nature 409:295-296. [DOI] [PubMed] [Google Scholar]
- 6.Baulcombe, D. 2002. RNA silencing. Curr. Biol. 12:R82-R84. [DOI] [PubMed] [Google Scholar]
- 7.Baulcombe, D. C., S. Chapman, and S. Santa Cruz. 1995. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J. 7:1045-1053. [DOI] [PubMed] [Google Scholar]
- 8.Boevink, P., S. Santa Cruz, C. Hawes, N. Harris, and K. J. Oparka. 1996. Virus-mediated delivery of the green fluorescent protein to the endoplasmic reticulum of plant cells. Plant J. 10:935-941. [Google Scholar]
- 9.Brigneti, G., O. Voinnet, W. X. Li, L. H. Ji, S. W. Ding, and D. C. Baulcombe. 1998. Viral pathogenicity determinants are suppressors of transgene silencing in nicotiana benthamiana. EMBO J. 17:6739-6746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Carrington, J. C., K. D. Kasschau, and L. K. Johansen. 2001. Activation and suppression of RNA silencing by plant viruses. Virology 281:1-5. [DOI] [PubMed] [Google Scholar]
- 11.Chicas, A., and G. Macino. 2001. Characteristics of post-transcriptional gene silencing. EMBO Rep. 2:992-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covey, S. N., N. S. Al-Kaff, A. Langara, and D. S. Turner. 1997. Plants combat infection by gene silencing. Nature 385:781-782. [Google Scholar]
- 13.Dalmay, T., A. Hamilton, S. Rudd, S. Angell, and D. C. Baulcombe. 2000. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101:543-553. [DOI] [PubMed] [Google Scholar]
- 14.Ding, S. W., W. X. Li, and R. H. Symons. 1995. A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J. 14:5762-5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donald, R. G., and A. O. Jackson. 1994. The barley stripe mosaic virus γb gene encodes a multifunctional cysteine-rich protein that affects pathogenesis. Plant Cell 6:1593-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty, W. G., J. A. Lindbo, H. A. Smith, T. D. Parks, S. Swaney, and W. M. Proebsting. 1994. RNA-mediated virus resistance in transgenic plants: exploitation of a cellular pathway possibly involved in RNA degradation. Mol. Plant-Microbe Interact. 7:544-552. [PubMed] [Google Scholar]
- 17.Dunoyer, P., E. Herzog, O. Hemmer, C. Ritzenthaler, and C. Fritsch. 2001. Peanut clump virus RNA-1-encoded P15 regulates viral RNA accumulation but is not abundant at viral RNA replication sites. J. Virol. 75:1941-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunoyer, P., S. Pfeffer, C. Fritsch, O. Hemmer, O. Voinnet, and K. E. Richards. 2002. Identification, subcellular localization and some properties of a cysteine-rich suppressor of gene silencing encoded by peanut clump virus. Plant J. 29:555-567. [DOI] [PubMed] [Google Scholar]
- 19.Edwards, M., C. 1995. Mapping of the seed transmission determinants of barley stripe mosaic virus. Mol. Plant-Microbe Interact. 8:906-915. [DOI] [PubMed] [Google Scholar]
- 20.Fagard, M., and H. Vaucheret. 2000. Systemic silencing signal(s). Plant Mol. Biol. 43:285-293. [DOI] [PubMed] [Google Scholar]
- 21.Guo, H. S., and J. A. Garcia. 1997. Delayed resistance to plum pox potyvirus mediated by a mutated RNA replicase gene: involvement of a gene-silencing mechanism. Mol. Plant-Microbe Interact. 10:160-170. [Google Scholar]
- 22.Guo, H. S., and S. W. Ding. 2002. A viral protein inhibits the long range signaling activity of the gene silencing signal. EMBO J. 21:398-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafson, G., B. Hunter, R. Hanau, S. L. Armour, and A. O. Jackson. 1987. Nucleotide sequence and genetic organization of barley stripe mosaic virus RNA gamma. Virology 158:394-406. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton, A. J., and D. C. Baulcombe. 1999. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286:950-952. [DOI] [PubMed] [Google Scholar]
- 25.Hehn, A., S. Bouzoubaa, N. Bate, D. Twell, J. Marbach, K. Richards, H. Guilley, and G. Jonard. 1995. The small cysteine-rich protein P14 of beet necrotic yellow vein virus regulates accumulation of RNA 2 in cis and coat protein in trans. Virology 210:73-81. [DOI] [PubMed] [Google Scholar]
- 26.Hong, Y., K. Saunders, and J. Stanley. 1997. Transactivation of dianthin transgene expression by African cassava mosaic virus AC2. Virology 228:383-387. [DOI] [PubMed] [Google Scholar]
- 27.Jackson, A. O., B. G. Hunter, and G. D. Gustafson. 1989. Hordeivirus relationships and genome organization. Annu. Rev. Phytopathol. 27:95-121. [Google Scholar]
- 28.Johansen, L. K., and J. C. Carrington. 2001. Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol. 126:930-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasschau, K. D., and J. C. Carrington. 1998. A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell 95:461-470. [DOI] [PubMed] [Google Scholar]
- 30.Kasschau, K. D., and J. C. Carrington. 2001. Long-distance movement and replication maintenance functions correlate with silencing suppression activity of potyviral HC-Pro. Virology 285:71-81. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence, D. M., A. G. Solovyev, S. Y. Morozov, J. G. Atabekov, and A. O. Jackson. 2000. Hordeiviruses, p. 899-904. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom.
- 33.Li, W. X., and S. W. Ding. 2001. Viral suppressors of RNA silencing. Curr. Opin. Biotechnol. 12:150-154. [DOI] [PubMed] [Google Scholar]
- 34.Lindbo, J. A., and W. G. Dougherty. 1992. Untranslatable transcripts of tobacco etch virus coat protein gene sequence can interfere with tobacco etch virus replication in transgenic plants and protoplasts. Virology 189:725-733. [DOI] [PubMed] [Google Scholar]
- 35.Lindbo, J. A., W. P. Fitzmaurice, and G. Della-Cioppa. 2001. Virus-mediated reprogramming of gene expression in plants. Curr. Opin. Plant Biol. 4:181-185. [DOI] [PubMed] [Google Scholar]
- 36.Liu, H., B. Reavy, M. Swanson, and S. A. MacFarlane. 2002. Functional replacement of the tobacco rattle virus cysteine-rich protein by pathogenicity proteins from unrelated plant viruses. Virology 298:232-239. [DOI] [PubMed] [Google Scholar]
- 37.Llave, C., K. D. Kasschau, M. A. Rector, and J. C. Carringtom. 2002. Endogenous and silencing-associated small RNAs in plants. Plant Cell 14:1605-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maia, I. G., A. Haenni, and F. Bernardi. 1996. Potyviral HC-Pro: a multifunctional protein. J. Gen. Virol. 77:1335-1341. [DOI] [PubMed] [Google Scholar]
- 39.Matzke, M., A. J. Matzke, and J. M. Kooter. 2001. RNA: guiding gene silencing. Science 293:1080-1083. [DOI] [PubMed] [Google Scholar]
- 40.Moore, C. J., P. W. Sutherland, R. L. Forster, R. C. Gardner, and R. M. MacDiarmid. 2001. Dark green islands in plant virus infection are the result of posttranscriptional gene silencing. Mol. Plant-Microbe Interact. 14:939-946. [DOI] [PubMed] [Google Scholar]
- 41.Morozov, S. Y., V. V. Dolja, and J. G. Atabekov. 1989. Probable reassortment of genomic elements among elongated RNA-containing plant viruses. J. Mol. Evol. 29:52-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mourrain, P., C. Béclin, T. Elmayan, F. Feuerbach, C. Godon, J.-B. Morel, D. Jouette, A.-M. Lacombe, S. Nikic, N. Picault, K. Rémoué, M. Sanial, T.-A. Vo, and H. Vaucheret. 2000. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101:533-542. [DOI] [PubMed] [Google Scholar]
- 43.Napoli, C., C. Lemieux, and R. Jorgensen. 1990. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 7:599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petty, I. T., B. G. Hunter, and A. O. Jackson. 1988. A novel strategy for one-step cloning of full-length cDNA and its application to the genome of barley stripe mosaic virus. Gene 74:423-432. [DOI] [PubMed] [Google Scholar]
- 45.Petty, I. T., B. G. Hunter, N. Wei, and A. O. Jackson. 1989. Infectious barley stripe mosaic virus RNA transcribed in vitro from full-length genomic cDNA clones. Virology 171:342-349. [DOI] [PubMed] [Google Scholar]
- 46.Petty, I. T., R. French, R. W. Jones, and A. O. Jackson. 1990a. Identification of barley stripe mosaic virus genes involved in viral RNA replication and systemic movement. EMBO J. 9:3453-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petty, I. T., M. C. Edwards, and A. O. Jackson. 1990b. Systemic movement of an RNA plant virus determined by a point substitution in a 5′ leader sequence. Proc. Natl. Acad. Sci. USA 87:8894-8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petty, I. T., R. G. Donald, and A. O. Jackson. 1994. Multiple genetic determinants of barley stripe mosaic virus influence lesion phenotype on Chenopodium amaranticolor. Virology 198:218-226. [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer, S., P. Dunoyer, F. Heim, K. E. Richards, G. Jonard, and V. Ziegler-Graff. 2002. P0 of beet western yellows virus is a suppresssor of posttranscriptional gene silencing. J. Virol. 76:6815-6824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Pruss, G., X. Ge, X. M. Shi, J. C. Carrington, and V. B. Vance. 1997. Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 9:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ratcliff, F., B. D. Harrison, and D. C. Baulcombe. 1997. A similarity between viral defense and gene silencing in plants. Science 276:1558-1560. [DOI] [PubMed] [Google Scholar]
- 52.Ratcliff, F. G., S. A. MacFarlane, and D. C. Baulcombe. 1999. Gene silencing without DNA. RNA-mediated cross-protection between viruses. Plant Cell 11:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruiz-Medrano, R., B. Xoconostle-Cazares, and W. J. Lucas. 2001. The phloem as a conduit for inter-organ communication. Curr. Opin. Plant Biol. 4:202-209. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Savenkov, E. I., and J. P. Valkonen. 2001. Potyviral helper-component proteinase expressed in transgenic plants enhances titers of Potato leaf roll virus but does not alleviate its phloem limitation. Virology 283:285-293. [DOI] [PubMed] [Google Scholar]
- 56.Savenkov, E. I., and J. P. T. Valkonen. 2002. Silencing of a viral RNA silencing suppressor in transgenic plants. J. Gen. Virol. 83:2325-2335. [DOI] [PubMed] [Google Scholar]
- 57.Savenkov, E. I., A. G. Solovyev, and S. Y. Morozov. 1998. Genome sequences of poa semilatent and lychnis ringspot hordeiviruses. Arch. Virol. 143:1379-1393. [DOI] [PubMed] [Google Scholar]
- 58.Scholthof, H. B., K. B. Scholthof, and A. O. Jackson. 1995. Identification of tomato bushy stunt virus host-specific symptom determinants by expression of individual genes from a potato virus X vector. Plant Cell 7:1157-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shivprasad, S., G. P. Pogue, D. J. Lewandowski, J. Hidalgo, J. Donson, L. K. Grill, and W. P. Dawson. 1999. Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vectors. Virology 255:312-323. [DOI] [PubMed] [Google Scholar]
- 60.Smith, H. A., S. L. Swaney, T. D. Parks, E. A. Wernsman, and W. G. Dougherty. 1994. Transgenic plant virus resistance mediated by untranslatable sense RNAs: expression, regulation, and fate of nonessential RNAs. Plant Cell 6:1441-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solovyev, A. G., E. I. Savenkov, A. A. Agranovsky, and Morozov, S. Y. 1996. Comparisons of the genomic cis-elements and coding regions in RNA beta components of the hordeiviruses barley stripe mosaic virus, lychnis ringspot virus, and poa semilatent virus. Virology 219:9-18. [DOI] [PubMed] [Google Scholar]
- 62.Szittya, G., A. Molnar, D. Silhavy, C. Hornik, and J. Burgyan. 2002. Short defective interfering RNAs of tombusviruses are not targeted but trigger post-transcriptional gene silencing against their helper virus. Plant Cell 14:359-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tenllado, F., and J. R. Díaz-Ruíz. 2001. Double-stranded RNA-mediated interference with plant virus infection. J. Virol. 75:12288-12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueki, S., and V. Citovsky. 2001. Inhibition of systemic onset of post-transcriptional gene silencing by non-toxic concentrations of cadmium. Plant J. 28:283-291. [DOI] [PubMed] [Google Scholar]
- 65.Vance, V. B., P. H. Berger, J. C. Carrington, A. G. Hunt, and X. M. Shi. 1995. 5′-Proximal potyvirus sequences mediate potato virus X/potyviral synergistic disease in transgenic tobacco. Virology 206:583-590. [DOI] [PubMed] [Google Scholar]
- 66.Van der Krol, A. R., L. A. Mur, M. Beld, J. N. Mol, and A. R. Stuitje. 1990. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 2:291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Wezel, R., X. Dong, H. Liu, P. Tien, J. Stanley, and Y. Hong. 2002. Mutation of three cysteine residues in tomato yellow leaf curl virus-China C2 protein causes dysfunction in pathogenesis and posttranscriptional gene-silencing suppression. Mol. Plant-Microbe Interact. 15:203-208. [DOI] [PubMed] [Google Scholar]
- 68.Vaucheret, H., C. Beclin, and M. Fagard. 2002. Post-transcriptional gene silencing in plants. J. Cell Sci. 114:3083-3091. [DOI] [PubMed] [Google Scholar]
- 69.Verwoerd, T. C., B. M. M. Dekker, and A. Hoekema. 1989. A small scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 17:2362.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voinnet, O. 2001. RNA silencing as a plant immune system against viruses. Trends Genet. 17:449-459. [DOI] [PubMed] [Google Scholar]
- 71.Voinnet, O., C. Lederer, and D. C. Baulcombe. 2000. A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell 103:157-167. [DOI] [PubMed] [Google Scholar]
- 72.Voinnet, O., Y. M. Pinto, and D. C. Baulcombe. 1999. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA 96:14147-14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waterhouse, P. M., M. B. Wang, and T. Lough. 2001. Gene silencing as an adaptive defence against viruses. Nature 411:834-842. [DOI] [PubMed] [Google Scholar]
- 74.Xie, Z., B. Fan, C. Chen, and Z. Chen. 2001. An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc. Natl. Acad. Sci. USA 98:6516-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou, H., and A. O. Jackson. 1996. Expression of the barley stripe mosaic virus RNA beta “triple gene block.” Virology 216:367-379. [DOI] [PubMed] [Google Scholar]