Abstract
The mechanism by which rotavirus and other nonenveloped viruses enter the cell is still not clear. We have proposed an endocytosis model where the critical step for virus uncoating and membrane permeabilization is the decrease in Ca2+ concentration in the endosome. In this paper, we monitored rotavirus entry by measuring α-sarcin-rotavirus coentry and infectivity in MA104 cells. The participation of endocytosis, acidification, and endosomal Ca2+ concentration on virus entry was studied by inhibiting the endosomal H+-ATPase with bafilomycin A1 and/or increasing the extracellular calcium reservoir by addition of 10 mM CaEGTA. Rotavirus-α-sarcin coentry was inhibited by bafilomycin A1 and by addition of 10 mM CaEGTA. These effects were additive. These substances induced a significant inhibition of infectivity without affecting virus binding and postentry steps. These results are compatible with the interpretation that bafilomycin A1 and CaEGTA block rotavirus penetration from the endosome into the cytoplasm and support our hypothesis of a Ca2+-dependent endocytosis model.
The mechanism by which rotavirus and other nonenveloped viruses enter the cell is still not clear. By contrast, it is known that viruses with a lipid envelope can penetrate the cell by fusion of the viral membrane with either the plasma membrane or endosomal membrane. In the latter case, fusion usually requires an acidic environment provided by the operation of the vesicular H+ pump (26).
Rotaviruses belong to the Reoviridae family presenting an icosahedral capsid formed by six proteins organized in three concentric layers containing a genome of 11 genomic segments of double-stranded RNA. The outer shell consists of two proteins, glycoproteins VP7 and VP4, which are organized in dimers to form 60 spikes. VP4 is involved in the early interaction with the host cell (7, 22), and its proteolytic cleavage (VP5* and VP8*) is required for infectivity (6, 12).
Endocytosis and direct penetration through the plasma membrane have been proposed as pathways for rotavirus entry. Ultrastructural images of rotavirus particles within coated pits, coated vesicles, and endosomes during entry have been observed (23, 27, 28), as well as images suggestive of direct entry (32). The efflux of intracellular space markers from preloaded MA104 cells during rotavirus entry supports the direct pathway hypothesis (19). Lysosomotropic drugs, endosomal H+-ATPase inhibitors, or agents that block the intracellular traffic of endosomes do not appear to affect rotavirus infection, arguing against the endosomal pathway (1, 8, 14, 19, 21, 23).
Both routes would entail the transient permeabilization of a lipid barrier, the endosomal membrane or the plasma membrane. Solubilized and trypsinized outer proteins (VP4 and VP7), but not the intact triple-layer particle (TLP), induce membrane permeabilization (2, 25, 28, 29). Solubilization of these proteins occurred by lowering Ca2+ concentration of the medium to the nanomolar level (28, 30). The permeabilizing activity of outer proteins has been ascribed to VP5 (10, 11) and to solubilized and trypsinized VP7 (2) and may play a role in rotavirus entry. If this were the case, solubilization of the external proteins and permeabilization should occur in a compartment having a low Ca2+ concentration and not in the extracellular medium that contains millimolar concentrations of calcium. In this context, we have proposed an alternative endocytosis model where Ca2+ concentration in the endosome decreases by dissipation of the gradient. This would induce the solubilization of the outer layer proteins, in turn provoking the permeabilization of the endosomal membrane (28, 31).
Entry of viruses into the cell has been found to induce the coentry of other macromolecules, such as α-sarcin, which inhibits translation in cell-free systems and to which cells are normally impermeable (13). Entry of this toxin correlates with productive rotavirus penetration (8, 21). In these studies, rotavirus-mediated coentry of toxins was not affected either by bafilomycin A1 or calcium ionophores (8, 21). This suggested that an acidic pH in the endosome and a low intracellular concentration of Ca2+ were not required for toxin entry (8). However, the ionic fluxes across the plasma or endosomal membrane that might be involved in these processes are likely to occur faster than the time resolution of rotavirus-α-sarcin coentry and l-[35S]methionine incorporation used by these workers.
Searching for evidence supporting our model, we have manipulated endosomal pH and Ca2+ concentrations and studied its effects on viral synthesis and on the entry of α-sarcin mediated by rotavirus for short time periods. The role of acidification was studied by inhibiting the endosomal H+-ATPase with bafilomycin A1, whereas that of endosomal Ca2+ concentration was investigated by increasing the endosomal Ca2+ reservoir by the addition of 10 mM CaEGTA to the extracellular medium.
Virus entry was measured as an inhibition of protein synthesis due to the coentry of α-sarcin with the sialic acid-dependent rotavirus strains OSU (porcine, serotype P9[7]G5 [5], kindly supplied by F. Liprandi [Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela]) or SA11 (simian, serotype P[2]G3 [9], kindly supplied by J. Cohen [Institut National de la Recherche Agronomique, Paris, France]) into MA104 cells (embryonic rhesus monkey kidney cells, kindly supplied at passage 7 by J. Cohen). The entry of OSU and SA11 virus strains into cells depends on the presence of sialic acid residues on the cell surface. Cells were seeded at a density of 4,000 cells/well in 96-well plates (Costar), grown in minimal essential medium (MEM) supplemented with 10% fetal calf serum and used at confluency (50,000 cells/well) 3 to 4 days later. At this stage, cells were not differentiated (3). Rotaviruses used in the experiments were grown in MA104 cells in the presence of trypsin (0.1 μg/ml). Viral suspensions were treated with 10 μg of trypsin per ml for 30 min at 37°C to cleave VP4 (Trypsin Type IX; Sigma Chemical Co). The titer of the rotavirus preparations was determined by titration in microplates using an anti-VP6 monoclonal antibody (4B2D2) for immunocytochemical staining after methanol fixation (4) and expressed as focus-forming units (FFU).
To measure α-sarcin-rotavirus coentry, confluent monolayers were washed three times with phosphate-buffered saline (PBS) and incubated with methionine-free MEM for 1 h at 37°C. During the last 30 min, cells were preincubated with or without bafilomycin A1 (500 nM) and then incubated for 15 min at 37°C with the virus at a multiplicity of infection of 500 FFU/cell and with 100 μg of α-sarcin (5.95 μM) per ml in a final volume of 50 μl/well with or without bafilomycin A1 and/or 10 mM CaEGTA. After infection, the medium was replaced by 100 μl of MEM containing 0.1 μCi of l-[35S]methionine in the continuous presence of bafilomycin A1 and/or 10 mM CaEGTA. These conditions were used in all experiments unless specified otherwise. After 15 min of incubation, cells were fixed and extracted as previously described (21). Results are expressed as percent inhibition of protein synthesis induced by the coentry of TLP and α-sarcin. The incorporation values obtained with virus and α-sarcin in the presence of bafilomycin A1 and/or 10 mM CaEGTA were related to label incorporation of infected cells in the absence of α-sarcin but with bafilomycin A1 and/or 10 mM CaEGTA. Mock infection was performed with MEM without virus or trypsin but with bafilomycin A1 and/or CaEGTA.
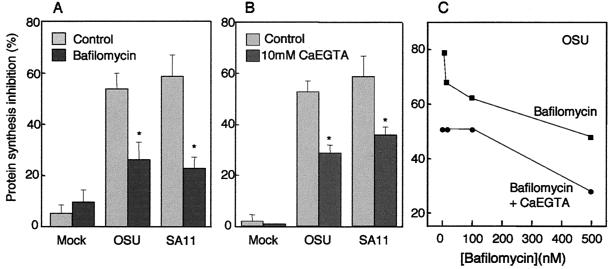
Effects of bafilomycin A1 and CaEGTA on rotavirus-mediated entry of α-sarcin.
In pilot experiments, we compared α-sarcin incorporation and protein synthesis inhibition using nonpurified or partially purified virus (Freon extraction and concentration by ultracentrifugation on a 40% sucrose cushion). We found that toxin coentry induced by both rotavirus preparations was rather similar (results not shown). Furthermore, to assess that α-sarcin incorporation was due to TLP, we pretreated the virus suspension (purified or not) with 5 mM EGTA for 15 min to induce the solubilization of the external protein layer. Under these conditions, there was no infectivity and no α-sarcin incorporation, even when a normal Ca2+ concentration (1.8 mM) was reestablished (results not shown). This indicates that the incorporation of α-sarcin was due to infectious virus entry and that cellular components in the nonpurified virus suspension had no effect on toxin entry or protein synthesis inhibition. Therefore, for practical reasons, nonpurified virus was used in the following experiments.
The effect of bafilomycin A1 on rotavirus entry is presented in Fig. 1A. Exposure of the monolayer to α-sarcin induced a small inhibition of protein synthesis in mock-infected cells (5%), whereas in the presence of OSU and SA11 virus strains, α-sarcin inhibited l-[35S]methionine incorporation by more than 50%. Bafilomycin A1 significantly reduced the inhibition of protein synthesis to about half in the presence of either virus, with no effect on mock-infected cells. These results indicate that rotavirus induced the coentry of α-sarcin, which in turn inhibited protein synthesis, confirming the results of previous reports (8, 21). As the H+/ATPase is associated with the endocytic compartment, the inhibition of the α-sarcin effect by bafilomycin A1 represents the first evidence for the involvement of the endocytic pathway in rotavirus penetration. In the presence of bafilomycin A1, endocytosed rotavirus would not be subjected to low pHs. However, previous results suggest that the acidification component may not participate in the mechanism of rotavirus entry. Lysosomotropic drugs failed to inhibit rotavirus infectivity (1, 14, 19, 23). Furthermore, pretreatment of rotavirus at low pHs does not induce the expression of a permeabilizing activity (25, 29), and recombinant VP5 has an optimal permeabilizing effect at neutral pH (10).
FIG. 1.
Effect of bafilomycin A1 or 10 mM CaEGTA in the extracellular medium on rotavirus-mediated entry of α-sarcin. (A) MA104 cells were preincubated in a methionine-free MEM for 1 h. During the last 30 min, bafilomycin A1 (500 nM) was added to a group of wells, while another group of wells were left untreated for controls. Then, monolayers were infected with OSU or SA11 rotavirus at a multiplicity of infection of 500 FFU/cell in the presence or absence of α-sarcin (100 mg/ml) and/or bafilomycin A1. After 15 min, inoculum was removed and replaced by methionine-free MEM supplemented with l-[35S]methionine (0.1 μCi/well) without α-sarcin but in the continuous presence or absence of bafilomycin A1. (B) Preincubation, infection, and protein labeling were performed as described above for panel A, except that 10 mM CaEGTA was added only during infection and protein labeling periods. Each value is the mean ± standard error (error bar) (In panel A, n = 21 values from seven experiments with the OSU strain or n = 6 values from two experiments with the SA11 strain. In panel B, n = 9 values from three experiments with the OSU strain or n =6 values from two experiments with SA11 strain. The OSU and SA11 values were compared, and statistically significant different values are indicated by an asterisk [P < 0.001 in paired Student's t test]). (C) Different concentrations of bafilomycin A1 (0, 10, 100, and 500 nM) were used during preincubation, infection, and protein labeling periods with or without 10 mM CaEGTA during infection and labeling periods. Data are plotted as the means of four measurements from one experiment.
The acidification component is required for the entry of numerous enveloped and nonenveloped viruses (17, 20, 24, 26). A low pH is required to elicit a fusion-inducing or permeabilizing conformational change of one or more viral capsid proteins, usually by exposure of a hydrophobic domain. Even in some viruses requiring a low pH, acidic conditions per se are not sufficient to promote virus entry into cells; rather, this step of virus infection requires the electrochemical gradient generated by the bafilomycin A1-sensitive H+ pump (16, 18, 26). The activity of this pump would generate an electrochemical gradient, resulting in an acidification of the endosomal medium and an electrical potential difference. In this sense, the electrical component may be required for the generation of other ion gradients necessary for the uncoating of rotavirus in the endosome. During endocytosis, extracellular fluid containing millimolar concentrations of Ca2+ is taken up into the vesicle. It has been shown that Ca2+ is extruded within a few minutes from the endosomes into the cytoplasm over the same period as endosomal acidification takes place (15). Both processes are coupled, since Ca2+ loss from the endosomes was blocked by bafilomycin A1 and acidification was inhibited by reducing the extracellular Ca2+ concentration (15). Rapid loss of Ca2+ from early endosomes would be driven by the electrical gradient, positive inside, generated by the H+ pump. In the case of rotavirus taken up into the endosome, inhibition of the pump by bafilomycin A1 would leave a high-Ca2+ environment where virus uncoating, membrane permeabilization, rotavirus entry, and α-sarcin coentry into the cytoplasm would be delayed.
Next, we investigated whether an increase in the concentration of endosomal Ca2+ would also affect rotavirus-α-sarcin coentry. To do this, we increased total extracellular calcium by adding 10 mM CaEGTA during rotavirus entry to increase the calcium content of the endosomal compartment and delay the dissipation of the Ca2+ gradient. It needs to be pointed out that under these conditions, EGTA is saturated with Ca2+ and the total free Ca2+ concentration in the extracellular medium does not significantly increase. As expected, this medium did not compromise the stability of the TLP outer layer and did not modify viral infectivity (data not shown). The presence of 10 mM CaEGTA reduced α-sarcin incorporation coupled to OSU or SA11 rotavirus entry, whereas no effect was observed in mock-infected cells (Fig. 1B).
We also studied the effect of the combined addition of bafilomycin A1 and 10 mM CaEGTA on rotavirus-α-sarcin coentry. Bafilomycin A1 reduced α-sarcin inhibition of protein synthesis in a concentration-dependent manner (Fig. 1C). The addition of 10 mM CaEGTA to the extracellular medium in the presence of the inhibitor induced an additive effect at all bafilomycin A1 concentrations used. This supports the interactive role of endosomal Ca2+ extrusion and the activity of the H+ pump in rotavirus entry. In this case, Ca2+ should stay longer in the endosome due to a larger reservoir and the lack of the active driving force for Ca2+ extrusion provided by the H+ pump.
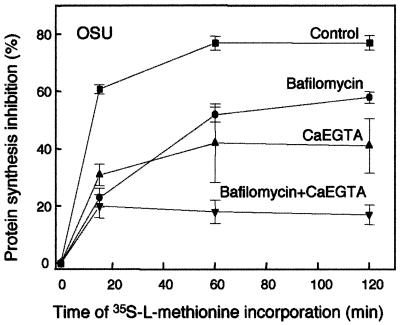
Previous studies using the same experimental model did not show an inhibitory effect of bafilomycin A1 on α-sarcin entry (8, 21). This may be due to differences in their experimental protocols where drugs were removed after infection and longer times of rotavirus-α-sarcin coentry and l-[35S]methionine incorporation were used. To evaluate this possibility using the protocol described above, we studied the inhibition of protein synthesis in the presence or absence of CaEGTA and/or bafilomycin A1 using a 15-min infection period but different times of l-[35S]methionine incorporation (Fig. 2). In the presence of virus alone, inhibition of protein synthesis by α-sarcin reached its maximum (80%) after 60 min of addition of the label. Therefore, there is a lag between the removal of α-sarcin from the extracellular medium and the maximal effect of the toxin. This may be due to the time needed for the toxin to reach the cytoplasm and/or to exert its action on the ribosome. A reduction of the inhibition of protein synthesis was clearly observed at 15 min of labeling in the presence of bafilomycin A1 and/or CaEGTA, as described above for Fig. 1. With longer labeling times, the effects of bafilomycin A1 and CaEGTA tended to fade. However, after 2 h, it had not yet reached the value obtained with the virus. The combination of CaEGTA and bafilomycin A1 reduced the inhibition of protein synthesis with no reversal after 2 h of labeling. Removal of the drug and the longer sampling times, when a reversal of the effect had already occurred, can explain the discrepancy between this and previous studies (8, 21). The fact that in our experiments, the blockade by bafilomycin A1 or CaEGTA was not complete and was somewhat reversible with time may be due to slow diffusion of Ca2+ driven by the large concentration gradient, which only delays virus uncoating and the entry process.
FIG. 2.
Inhibition of rotavirus-mediated entry of α-sarcin induced by bafilomycin A1 and/or CaEGTA as a function of the incorporation time of l-[35S]methionine. Preincubation and infection with OSU strain of MA104 cell monolayers were performed as detailed in the legend to Fig. 1. The concentration of bafilomycin was constant (500 nM), and protein labeling periods were 15, 60, and 120 min. Data are plotted as the means plus standard errors (error bars) of nine measurements from three experiments.
Effects of bafilomycin A1 and 10 mM CaEGTA on viral synthesis.
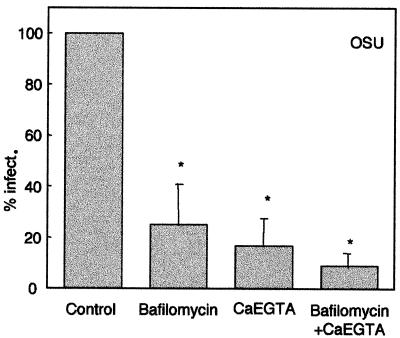
Since we can partially block α-sarcin entry coupled to rotavirus infection with bafilomycin A1 and CaEGTA, we studied the effects of these substances on infectivity as a more direct expression of virus entry (Fig. 3). Rotavirus infectivity was determined in the presence or absence of 500 nM bafilomycin A1 (Sigma-Aldrich, St. Louis, Mo.) and/or 10 mM CaEGTA in cells grown in 96-well plates. When bafilomycin A1 was used in the experiments, cells were pretreated with the drug (500 nM) for 30 min at 37°C. Then, monolayers were infected with serial dilutions of rotavirus (from 107 FFU/ml) in the continuous presence of bafilomycin A1 or other substances. After 15 min, the medium was removed, and monolayers were washed twice with PBS containing 5 mM EGTA (without Ca2+) to solubilize the capsid external layer and remove bound virus. Then, MEM containing 1.8 mM Ca2+ and bafilomycin A1, 10 mM CaEGTA, or both was added, and infection continued up to 18 h postinfection (hpi). The number of foci was determined by immunofluorescence (4).
FIG. 3.
Effects of 10 mM CaEGTA and bafilomycin A1 on viral synthesis. MA104 cells were preincubated in a medium containing bafilomycin A1 (500 nM) for 30 min. Then, they were infected by serial dilutions of OSU rotavirus in a medium supplemented or not supplemented with 10 mM CaEGTA and/or bafilomycin A1. After 15 min, the virus was removed and washed twice with a medium containing 5 mM EGTA without Ca2+ to remove bound virus. Infection was allowed to proceed for 18 h in the presence of CaEGTA or bafilomycin A1, and infectivity (infect.) was determined. Values are means plus the standard errors of the means (error bars) of six independent experiments performed in triplicate. Values that were statistically significantly different from the control value are indicated by an asterisk (P < 0.01 by paired Student's t test).
Bafilomycin A1, 10 mM CaEGTA, or their combination induced a significant inhibition of infectivity determined at 18 hpi, indicating that a smaller number of infectious viral particles reached the cytoplasm under these conditions. As these substances can affect different points of virus replication, we performed control experiments to rule out effects on virus binding and the postentry steps of virus replication. First, we determined whether bafilomycin A1 or CaEGTA inhibited infectivity when added after virus entry. In these experiments, cells were inoculated with serial dilutions of OSU rotavirus (15 min at 37°C) and washed with PBS containing 5 mM EGTA (without Ca2+) to remove bound virus. At this point, bafilomycin A1 and/or CaEGTA were added and maintained up to 18 hpi. With these conditions, we did not detect any effect of these substances on infectivity (results not shown). In a second series of experiments, we studied the possible effects of bafilomycin A1 or 10 mM CaEGTA on rotavirus binding to the cell. Monolayers were preincubated with or without bafilomycin A1 (500 nM) for 30 min at 37°C. Then, monolayers were cooled down to 4°C for 1 h and incubated with serial dilutions of OSU rotavirus containing the different substances (bafilomycin A1 or CaEGTA). At this temperature, only virus attachment to the cells without internalization should occur. Monolayers were then washed three times with ice-cold PBS (with normal Ca2+ levels) and rewarmed with fresh medium without bafilomycin A1 and CaEGTA at 37°C to induce internalization of the virus into the cell. Infection was allowed to proceed up to 18 hpi, when infectivity was determined by immunofluorescence. In parallel experiments, monolayers were frozen after being washed with cold PBS, and then the bound virus was titrated in fresh MA104 cell monolayers. In either case, no reduction of infectivity was observed (results not shown). These control experiments indicate that bafilomycin A1 and CaEGTA do not affect virus binding or the post entry steps of replication. Our results are compatible with the interpretation that bafilomycin A1 and CaEGTA block rotavirus penetration from the endosome into the cytoplasm.
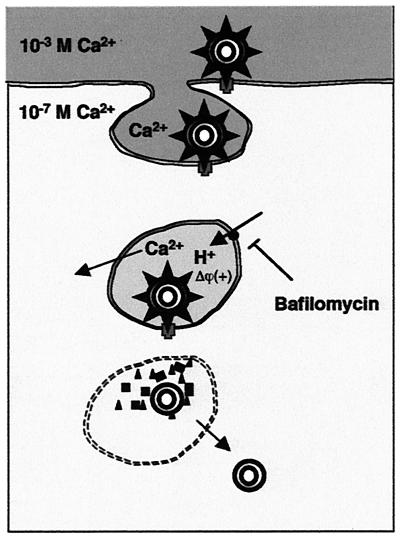
The results presented in this paper support our hypothesis of a Ca2+-dependent endocytosis model (Fig. 4) where the critical step for virus uncoating and membrane permeabilization is the decrease in Ca2+ concentration in the endosome (28, 31). After rotavirus particles bind to receptors on the cell surface, they are endocytosed into clathrin-coated vesicles together with extracellular fluid containing Ca2+ in the millimolar range. Once inside the endocytic vesicle, Ca2+ is transported into the cytoplasm driven by the large concentration gradient (from 1 mM to 100 nM). In addition, we now postulate that the electrical gradient (positive inside) generated by the V-type H+ pump provides an additional force for Ca2+ extrusion out of the endosome. Once Ca2+ has dropped to a critical concentration, the virus uncoats, and the external solubilized proteins, VP5* and perhaps VP7, permeabilize and lyse the endosomal membrane. In this way, the double-layer particle gains access to the cytoplasm and replication is started. Acidification per se would not required to uncoat the virus particle and trigger membrane permeabilization.
FIG. 4.
Hypothetical model of rotavirus entry by endocytosis. See text for details.
Acknowledgments
This work was supported in part by CONICIT/FONACIT (Venezuela) grants 95000520 and 2001000329.
REFERENCES
- 1.Bass, D. M., M. Baylor, C. Chen, and U. Upadhyayula. 1995. Dansylcadaverine and cytochalasin d enhance rotavirus infection of murine l cells. Virology 212:429-437. [DOI] [PubMed] [Google Scholar]
- 2.Charpilienne, A., M. J. Abad, F. Michelangeli, F. Alvarado, M. Vasseur, J. Cohen, and M. C. Ruiz. 1997. Solubilized and cleaved VP7, the outer glycoprotein of rotavirus, induces permeabilization of cell membrane vesicles. J. Gen. Virol. 78:1367-1371. [DOI] [PubMed] [Google Scholar]
- 3.Ciarlet, M., S. E. Crawford, and M. K. Estes. 2001. Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains. J. Virol. 75:11834-11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciarlet, M., M. Hidalgo, M. Gorziglia, and F. Liprandi. 1994. Characterization of neutralization epitopes on the VP7 surface protein of serotype G11 porcine rotaviruses. J. Gen. Virol. 75:1867-1873. [DOI] [PubMed] [Google Scholar]
- 5.Ciarlet, M., J. E. Ludert, M. Iturriza-Gomara, F. Liprandi, J. J. Gray, U. Desselberger, and M. K. Estes. 2002. Initial interaction of rotavirus strains with N-acetylneuraminic (sialic) acid residues on the cell surface correlates with VP4 genotype, not species of origin. J. Virol. 76:4087-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, S. M., J. R. Roth, L. Clark, B. B. Barnett, and R. S. Spendlove. 1981. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J. Virol. 39:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford, S. E., M. Labbe, J. Cohen, M. H. Burroughs, Y. J. Zhou, and M. K. Estes. 1994. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 68:5945-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuadras, M. A., C. F. Arias, and S. Lopez. 1997. Rotaviruses induce an early membrane permeabilization of MA104 cells and do not require a low intracellular Ca2+ concentration to initiate their replication cycle. J. Virol. 71:9065-9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunliffe, N. A., P. A. Woods, J. P. Leite, B. K. Das, M. Ramachandran, M. K. Bhan, C. A. Hart, R. I. Glass, and J. R. Gentsch. 1997. Sequence analysis of NSP4 gene of human rotavirus allows classification into two main genetic groups. J. Med. Virol. 53:41-50. [PubMed] [Google Scholar]
- 10.Denisova, E., W. Dowling, R. LaMonica, R. Shaw, S. Scarlata, F. Ruggeri, and E. R. Mackow. 1999. Rotavirus capsid protein VP5* permeabilizes membranes. J. Virol. 73:3147-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowling, W., E. Denisova, R. LaMonica, and E. R. Mackow. 2000. Selective membrane permeabilization by the rotavirus VP5* protein is abrogated by mutations in an internal hydrophobic domain. J. Virol. 74:6368-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes, M. K., D. Y. Graham, and R. B. Mason. 1981. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J. Virol. 39:879-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Puentes, C., and L. Carrasco. 1980. Viral infection permeabilizes mammalian cells to protein toxins. Cell 20:769-775. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara, N., O. Yoshie, S. Kitaoka, T. Konno, and N. Ishida. 1987. Evidence for endocytosis-independent infection by human rotavirus. Arch. Virol. 97:93-99. [DOI] [PubMed] [Google Scholar]
- 15.Gerasimenko, J. V., A. V. Tepikin, O. H. Petersen, and O. V. Gerasimenko. 1998. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr. Biol. 8:1335-1338. [DOI] [PubMed] [Google Scholar]
- 16.Guinea, R., and L. Carrasco. 1994. Concanamycin A blocks influenza virus entry into cells under acidic conditions. FEBS Lett. 349:327-330. [DOI] [PubMed] [Google Scholar]
- 17.Guinea, R., and L. Carrasco. 1995. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J. Virol. 69:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irurzun, A., J. L. Nieva, and L. Carrasco. 1997. Entry of Semliki forest virus into cells: effects of concanamycin A and nigericin on viral membrane fusion and infection. Virology 227:488-492. [DOI] [PubMed] [Google Scholar]
- 19.Kaljot, K. T., R. D. Shaw, D. H. Rubin, and H. B. Greenberg. 1988. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J. Virol. 62:1136-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasermann, F., and C. Kempf. 1996. Low pH-induced pore formation by spike proteins of enveloped viruses. J. Gen. Virol. 77:3025-3032. [DOI] [PubMed] [Google Scholar]
- 21.Liprandi, F., Z. Moros, M. Gerder, J. E. Ludert, F. H. Pujol, M. C. Ruiz, F. Michelangeli, A. Charpilienne, and J. Cohen. 1997. Productive penetration of rotavirus in cultured cells induces coentry of the translation inhibitor alpha-sarcin. Virology 237:430-438. [DOI] [PubMed] [Google Scholar]
- 22.Ludert, J. E., N. G. Feng, J. H. Yu, R. L. Broome, Y. Hoshino, and H. B. Greenberg. 1996. Genetic mapping indicates that VP4 is the rotavirus cell attachment protein in vitro and in vivo. J. Virol. 70:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludert, J. E., F. Michelangeli, F. Gil, F. Liprandi, and J. Esparza. 1987. Penetration and uncoating of rotaviruses in cultured cells. Intervirology 27:95-101. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, C. G., R. Guinea, J. Benavente, and L. Carrasco. 1996. The entry of reovirus into l cells is dependent on vacuolar proton-ATPase activity. J. Virol. 70:576-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandi, P., A. Charpilienne, and J. Cohen. 1992. Interaction of rotavirus particles with liposomes. J. Virol. 66:3363-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez, L., and L. Carrasco. 1994. Involvement of the vacuolar H+-ATPase in animal virus entry. J. Gen. Virol. 75:2595-2606. [DOI] [PubMed] [Google Scholar]
- 27.Quan, C. M., and F. W. Doane. 1983. Ultrastructural evidence for the cellular uptake of rotavirus by endocytosis. Intervirology 20:223-231. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz, M. C., M. J. Abad, A. Charpilienne, J. Cohen, and F. Michelangeli. 1997. Cell lines susceptible to infection are permeabilized by cleaved and solubilized outer layer proteins of rotavirus. J. Gen. Virol. 78:2883-2893. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz, M. C., S. R. Alonso Torre, A. Charpilienne, M. Vasseur, F. Michelangeli, J. Cohen, and F. Alvarado. 1994. Rotavirus interaction with isolated membrane vesicles. J. Virol. 68:4009-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz, M. C., A. Charpilienne, F. Liprandi, R. Gajardo, F. Michelangeli, and J. Cohen. 1996. The concentration of Ca2+ that solubilizes outer capsid proteins from rotavirus particles is dependent on the strain. J. Virol. 70:4877-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz, M. C., J. Cohen, and F. Michelangeli. 2000. Role of Ca2+ in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium 28:137-149. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, H., S. Kitaoka, T. Konno, T. Sato, and N. Ishida. 1985. Two modes of human rotavirus entry into MA 104 cells. J. Virol. 85:25-34. [DOI] [PubMed] [Google Scholar]