Abstract
Sensory rhodopsin II, a repellent phototaxis receptor from Natronomonas (Natronobacterium) pharaonis (NpSRII), forms a complex with its cognate transducer (NpHtrII). In micelles the two proteins form a 1:1 heterodimer, whereas in membranes they assemble to a 2:2 complex. Similarly to other retinal proteins, sensory rhodopsin II undergoes a bleaching reaction with hydroxylamine in the dark which is markedly catalyzed by light. The reaction involves cleavage of the protonated Schiff base bond which covalently connects the retinal chromophore to the protein. The light acceleration reflects protein conformation alterations, at least in the retinal binding site, and thus allows for detection of these changes in various conditions. In this work we have followed the hydroxylamine reaction at different temperatures with and without the cognate transducer. We have found that light irradiation reduces the activation energy of the hydroxylamine reaction as well as the frequency factor. A similar effect was found previously for bacteriorhodopsin. The interaction with the transducer altered the light effect both in detergent and membranes. The transducer interaction decreased the apparent light effect on the energy of activation and the frequency factor in detergent but increased it in membranes. In addition, we have employed an artificial pigment derived from a retinal analog in which the critical C13=C14 double bond is locked by a rigid ring structure preventing its isomerization. We have observed light enhancement of the reaction rate and reduction of the energy of activation as well as the frequency factor, despite the fact that this pigment does not experience C13=C14 double bond isomerization. It is suggested that retinal excited state polarization caused by light absorption of the “locked” pigment polarizes the protein and triggers relatively long-lived protein conformational alterations.
INTRODUCTION
Archaeal phototaxis in Halobacterium salinarum is mediated by two sensory rhodopsins, SRI and SRII (called also phoborhodopsin), which are closely related to the two ion pumps bacteriorhodopsin (bR) and halorhodopsin (HR). This family of membrane proteins consists of seven transmembrane helices which covalently bind a retinal chromophore via a protonated Schiff bond with a protein lysine residue (for recent reviews see (1–4)). SRI serves primarily as a receptor of positive phototaxis responsible for obtaining suitable light conditions for the bacteria ion pumps function. SRII is a photoreceptor for negative phototaxis, and its physiological role, which was not entirely established, may allow the bacteria to avoid harmful high oxygen conditions in the presence of light (5). Both receptors are bound to specific membrane proteins (halobacterial transducers of rhodopsins, HtrI and HtrII) consisting of a two-helical transmembrane and a coiled/coil cytoplasmic domain. Light excitation of the receptors activates the cytoplasmic region of the transducer which triggers a cytoplasmic signaling cascade. Light absorption by SRII isolated from Natronomonas pharaonis (NpSRII), initiates a photocycle characterized by several photochemically induced intermediates which are similar to bR, however, the regeneration of the pigment is two orders of magnitude slower (6). The photocycle of SRII contains, like that of bR, an M intermediate which is characterized by a retinal Schiff base (rather than a protonated Schiff base).
It is widely assumed that all light-induced protein conformational alterations in retinylidene proteins are initiated by isomerization of the retinal chromophore. However, alternative approaches have been suggested in which isomerization is not the only trigger for biological activity or protein structural changes (7). One proposal attributed protein conformational alterations to a large charge redistribution in the retinal chromophore developed after light absorption (8–10). We have recently shown, using atomic force sensing (AFS), that protein conformational alterations are indeed induced in bR after light absorption, even when the crucial C13=C14 double bond isomerization is prevented by a rigid ring structure (“locked” pigment) (11). In addition, we have used chemical reactions with spin-labeled artificial bR pigments derived from “locked” retinal to probe light-induced conformational changes. It was concluded that certain domains of the protein experience conformational alterations even though retinal isomerization is prevented (12). Thus, the data questions (providing direct experimental results) the hypothesis that all primary events in retinal proteins can occur only due to an initial trans-cis isomerization. Furthermore, from examination of the light-catalyzed cleavage of the retinal-protein covalent bond by hydroxylamine (HA) (13) it was concluded that this reaction was caused by light-induced conformational alterations extending into the μs-ms timescale. These protein changes are not correlated to an optically detectable photocycle, which is associated with C13=C14 isomerization.
In this work, we have applied the hydroxylamine reaction approach, which offers a possibility to identify protein conformational alterations in the retinal binding site after light absorption by the NpSRII system. This reaction was checked previously for the NpSRII native system at room temperature (14). We have revealed that light absorption decreases the activation energy of the reaction as well as the frequency factor. A decrease of activation energy and frequency factor is detected also in an artificial pigment derived from a retinal analog in which isomerization around the critical C13=C14 double bond is prevented. It is concluded that after light absorption the artificial pigment experiences conformational alterations which are not associated with double bond isomerization.
MATERIALS AND METHODS
Solubilized WT NpSRII (in 0.1% n-dodecyl-β-d-maltopyranoside (DDM)) was prepared as described elsewhere (15), as well as samples reconstituted in membranes without NpHtrII (16). Briefly, purple membrane lipids (PML) were extracted with a 1:1 chloroform:methanol mixture. The chloroform layer was evaporated, and the residue was mixed with the solubilized protein sample and a few Bio-Beads SM-2 adsorbent (Bio-Rad, Hercules, CA) for 16 h at room temperature. The beads were subsequently discarded and the sample was centrifuged and resuspended in Tris buffer at pH 7. Samples reconstituted in PML containing also NpHtrII and artificial pigments of NpSRII derived from locked-trans retinal chromophore 2 (Scheme 1) were prepared in a similar manner.
SCHEME 1.
Retinal (1) and “locked-trans” retinal (2).
Apoprotein was prepared by incubating the WT protein with 1M hydroxylamine at pH 7, and irradiation with a long-pass (λ > 475 nm) cutoff filter (Schott, Mainz, Germany). The sample was passed through a PD-10 sepharose column (Amersham Pharmacia Biotech AG, Uppsala, Sweden) and the eluted sample was incubated with 1.2 equivalents of locked-trans retinal for 1 week at 25°C. Formation of the pigment was monitored by following the appearance of a characteristic absorption band at 524 nm. During this period no detectable formation of wild-type NpSRII was observed due to retinal oxime hydrolysis and retinal rebinding with the apoprotein. In addition, incubation of apomembrane at 25°C for one week did not produce detectable amount of wild-type NpSRII.
Experiments with hydroxylamine were carried out by stabilizing the temperature of the sample and the hydroxylamine for 10 min before measurement in an Agilent 4583 diode-array spectrophotometer (Agilent Technologies, Palo Alto, CA) equipped with an Agilent 89090A thermostated cuvette holder (Agilent Technologies). Since hydroxylamine is not completely thermally stable, a fresh hydroxylamine sample was used for each experiment. Irradiation was carried out with a Schott 250W cold light source (Carl Zeiss Microscopy, Jena, Germany) equipped with a heat absorbing filter and an optic fiber. The sample temperature was monitored, and the deviation in temperature did not exceed ±0.1°C, even during irradiation. Light was filtered through a long-pass cutoff filter, λ > 475 nm for wild-type NpSRII, and λ > 505 nm for the “locked” pigment.
The reactions with hydroxylamine were followed at various temperatures in the dark and under constant illumination by monitoring the disappearance of the main absorption band of the pigment (500 nm for the WT and 524 nm for the locked-trans pigment). Reactions in the dark showed only one component, and were fitted to the equation 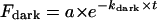 ; where Fdark is the fraction that did not undergo the reaction, kdark is the reaction rate in the dark, and a is a coefficient related to the total amount of pigment, which in this case should equal 1. Reactions under constant illumination were fitted to a double exponential equation, where one of the terms was the contribution of the dark reaction. Thus,
; where Fdark is the fraction that did not undergo the reaction, kdark is the reaction rate in the dark, and a is a coefficient related to the total amount of pigment, which in this case should equal 1. Reactions under constant illumination were fitted to a double exponential equation, where one of the terms was the contribution of the dark reaction. Thus, 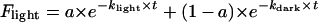 ; where Flight is the fraction of remaining pigment, klight and kdark are the reaction rates of the light and dark reactions, respectively, and a is a coefficient related to the relative amount of each component. kdark was taken from the experiments in the dark, or by extrapolating the data from the Arrhenius plot for the reaction in the dark. It was necessary to conduct the experiments in the dark at higher temperatures than those required for the light reaction, since the rate of these reactions is very slow.
; where Flight is the fraction of remaining pigment, klight and kdark are the reaction rates of the light and dark reactions, respectively, and a is a coefficient related to the relative amount of each component. kdark was taken from the experiments in the dark, or by extrapolating the data from the Arrhenius plot for the reaction in the dark. It was necessary to conduct the experiments in the dark at higher temperatures than those required for the light reaction, since the rate of these reactions is very slow.
The reaction rates in the dark and in the light at various temperatures were fitted to the Arrhenius equation: 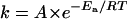 ; where k is the reaction rate, A is the frequency factor which is a constant indicating how many collisions have the correct orientation to lead to products, Ea is the activation energy in kcal/mol, R is the gas constant and T is the temperature. A plot of ln(k) versus 1/T should be linear. From this plot it is possible to calculate the A coefficient as well as the activation energy.
; where k is the reaction rate, A is the frequency factor which is a constant indicating how many collisions have the correct orientation to lead to products, Ea is the activation energy in kcal/mol, R is the gas constant and T is the temperature. A plot of ln(k) versus 1/T should be linear. From this plot it is possible to calculate the A coefficient as well as the activation energy.
RESULTS
Addition of a hydroxylamine solution to a pigment eliminates the covalent bond between the retinal and the protein, and forms apoprotein and a retinal oxime molecule (17). Apart from the reaction usefulness in preparing apoprotein for incorporation of synthetic retinal analogs, it can serve as a probe for monitoring protein conformational alterations (13). The reaction of hydroxylamine with bR in the dark proceeds very slowly. It is well established that the reaction rate is substantially increased by irradiation. Arrhenius plots of the reaction in the dark and under irradiation have shown a dramatic reduction in the activation energy of the reaction after irradiation (13). To evaluate the light effect on the Arrhenius parameters of the hydroxylamine reaction in NpSRII, we have examined the reaction of NpSRII in DDM detergent in the dark and under illumination. Fig. 1 shows the spectral changes associated with the reaction of hydroxylamine and NpSRII. The hydroxylamine concentration was much higher than that of the pigment, thus, it may be assumed that the dark and light reactions are both first-order. The reaction in the dark was considerably slower than that under irradiation (Fig. 2). Therefore, the contribution of the dark reaction to the overall rate of the light reaction is negligible. In fact, in WT NpSRII the rate of the light reaction fits well to a first-order reaction, as was previously observed in WT bR (13).
FIGURE 1.
Spectral changes associated with the hydroxylamine reaction of (A and C) WT NpSRII and (B and D) locked-trans NpSRII, in detergent. Panels A and B show the spectra, and panels C and D show the respective difference spectra after and before the reaction. Reactions were carried out in the presence of 50 mM Tris buffer, 300 mM NaCl, 0.1% DDM, pH 7.
FIGURE 2.
Comparison of reaction rates with hydroxylamine of WT NpSRII in the dark (solid line) and under irradiation (dashed line), and of locked-trans NpSRII in the dark (dotted line) and under irradiation (dotted-dashed line).
An Arrhenius plot of the rate constants at various temperatures in the dark (Figs. 3, 5 and Table 1) indicates that upon irradiation the activation energy for the reaction decreases considerably from 16.9 kcal/mol to ∼0 kcal/mol. The latter change was accompanied by a significant decrease of the frequency factor by ∼9 orders of magnitude (Table 1). The decrease in the frequency factor indicates that after light irradiation the probability for correct collisions between the reactive molecules leading to reaction is reduced. This light effect is stronger than that detected in bR membranes (13). We note that by increasing the temperatures the reaction rates are affected but the rate of the photocycle and the lifetime of various intermediates may be affected as well. Therefore, the measured reaction rates and calculated energies of activation and frequencies factors are actually apparent values.
FIGURE 3.
Arrhenius plots of dark reactions (1M hydroxylamine) of WT NpSRII in detergent (DDM) and in membranes (purple membrane lipids, PML), with and without transducer (HtrII).
FIGURE 5.
Arrhenius plot of dark and light reactions of WT NpSRII and locked-trans NpSRII in detergent (DDM), with 1M hydroxylamine.
TABLE 1.
Apparent reaction rates and Arrhenius parameters of NpSRII at various temperatures, in detergent (0.1% DDM) or in membranes, and in the absence or presence of transducer
| WT NpSRII
|
WT NpSRII + NpHtrII
|
LT NpSRII
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detergent
|
PML
|
Detergent
|
PML
|
Detergent
|
||||||
| Dark | Light | Dark | Light | Dark | Light | Dark | Light | Dark | Light | |
| °C | Reaction rates (×10−3/s)* | |||||||||
| 20 | 2.2 | 58 | 48 | 2.3 | 70 | |||||
| 25 | 3.5 | 58 | 3.6 | 49 | 3.3 | 49 | 4.0 | 70 | 0.23 | 0.82 |
| 30 | 6.4 | 60 | 4.9 | 72 | 4.6 | 61 | 5.6 | 69 | 0.45 | 0.92 |
| 35 | 8.5 | 57 | 8.0 | 74 | 7.2 | 72 | 9.1 | 65 | 1.2 | 1.4§ |
| 40 | 13 | 78 | 14.6 | 1.5 | 1.9 | |||||
| Arrhenius parameters | ||||||||||
| A† | 8.0×109 | 5.7×10−2 | 1.8×109 | 1.5×103 | 2.7×107 | 8.2×102 | 2.2×109 | 2.0×10−2 | 2.9×1014 | 3.6×104 |
| Ea‡ | 16.9 | 0 | 16.0 | 6.0 | 13.5 | 5.7 | 16.0 | 0 | 24.6 | 10.5 |
Error in reaction rates is in the last significant digit.
Frequency factor (s−1). Error is ∼10%.
Activation energy (kcal/mol). Error is ∼10%.
Since the rate of the light reaction was very similar to that of the dark reaction, a monophasic scheme was used to calculate the rate, whereas in all other cases, the light reaction rate was calculated using a biphasic scheme (see Materials and Methods).
Next we have examined the effect of the membrane matrix on the rate of the hydroxylamine reaction and repeated the above experiments in NpSRII reconstituted in purple membrane lipids. The reaction activation energy, both in the dark (Fig. 3 and Table 1) and under irradiation (Fig. 4 and Table 1), was comparable to that obtained for bR (13). Comparison of membranes and detergent indicates that light affects the NpSRII system more significantly in the detergent preparation than in membranes (Table 1). Since NpSRII binds with a cognate transducer, we proceeded to examine the effect of the transducer, NpHtrII, on the rate of the hydroxylamine reaction, both in the dark and light, and in detergent or membrane preparations. Addition of transducer to the pigment forms a very strong complex with a dissociation constant in the nM range (16,18).
FIGURE 4.
Arrhenius plot of light reactions (1M hydroxylamine) of WT NpSRII in detergent (DDM) and in membranes (purple membrane lipids, PML), with (A) and without (B) transducer (HtrII).
The transducer clearly had an impact on both the activation energy and frequency factor in detergent and lowered both of them in the dark. The compensation of both these factors causes the actual rate of the reaction in the dark to remain largely unchanged. In addition, the apparent light effect was weakened due to the transducer interaction and both the energy of activation and the frequency factor were lowered by ∼8 kcal/mole and five orders of magnitude relative to 17 kcal/mole and 9 orders of magnitude without transducer (Fig.4 and Table 1). In contrast, in membrane preparation the transducer increased the apparent light effect and actually the effect was similar to that observed in detergent preparation without transducer (Table 1).
It was proposed that the observed light rate acceleration of the pigment reaction with hydroxylamine is due to a greater reactivity of the reagent in the M photochemically induced intermediate of NpSRII (14). This hypothesis was suggested for bR as well, but later it was proposed that the L intermediate (the precursor of M) is the species that is reactive to hydroxylamine (19). Namely, the reaction is a direct consequence of a protein conformational change occurring after light-induced retinal all-trans → 13-cis isomerization, but before Schiff base deprotonation. However, more recently it was shown for bR that the hydroxylamine reaction is light-catalyzed even when retinal double bond isomerization is prevented by a rigid ring structure in the chromophore (13). This observation suggests that long-lived conformational changes occur in the protein, which are not reflected in spectroscopic changes, and enhance the reactivity of the chromophore toward hydroxylamine. Therefore, we have measured the rate of the hydroxylamine reaction both in the dark and under irradiation of an NpSRII artificial pigment derived from chromophore 2 in which isomerization around the critical C13=C14 double bond was prevented (Scheme 1). Similarly to bR, also in NpSRII artificial pigment an appreciable acceleration of the reaction rate occurred under irradiation (almost fourfold at 25°C; Table 1), which cannot be attributed to a photocycle intermediate. We note that we could not detect any photointermediate after flash-irradiation of the artificial pigment derived from chromophore 2, whereas similar irradiation of native pigment produced the well known photochemically induced intermediates. To exclude the possibility that irradiation caused rate acceleration due to temperature increase, rather than just sample light excitation, we have irradiated the sample with a cutoff filter of λ > 600 nm which is not absorbed by the retinal chromophore. In this case the reaction rate was identical to the dark reaction excluding the possibility of sample warming due to irradiation. The hydroxylamine reactions were slow relative to native NpSRII (Figs. 2 and 5), due to a higher activation energy. The frequency factor was significantly higher (five orders of magnitude in the dark and two orders of magnitude under illumination), than that detected in WT, and partially compensated for the higher activation energy (Table 1). The decrease in activation energy due to irradiation was lower (∼14 kcal/mol) than that detected in WT in detergent (17 kcal/mol). In the “locked” artificial pigment, it was not possible to neglect the contribution of the dark reaction under light, since the dark and light reaction rates were of comparable magnitude. Therefore, the rates of the reaction were fitted to a scheme composed of two phases. In cases where the rates of the dark and light reactions were very similar, a monophasic scheme was used instead.
DISCUSSION
The biological functions of retinal proteins are based on changes in the protein structure induced via light absorption by the retinal chromophore. It is generally assumed that protein changes follow retinal double bond isomerization. Thus, light provides the necessary energy for double bond isomerization which in turn leads to sustainable changes in the protein structure that drive the biological activity. Despite the general consensus as to the fundamental role of C=C double bond isomerization, theoretical considerations have suggested (7,8,10) that light-induced charge redistribution may develop in the retinal chromophore which in turn polarizes the protein and may induce protein conformational alterations. Indeed, a large light-induced dipole was measured in bR in its FC transition (20–22), and was suggested to originate from excited state positive charge stabilization by tryptophan residues located in the vicinity of the retinal ring (23). Recently, it was proposed that in bR protein polarization (triggered by the retinal excited state charge redistribution) is required to obtain retinal isomerization and initiation of a normal photocycle (24). Studies of artificial bR pigments in which isomerization of the critical C13=C14 double bond was prevented demonstrated that several chemical reactions in the protein are light catalyzed despite lacking double bond isomerization and detection of a normal photocycle (12,25,26). We note that the possibility of isomerization around another double bond, which can cause protein conformational alterations, was excluded since in the “locked” pigment a detectable photocycle was not observed by flash photolysis studies (9,27–29) as well as by time resolved Raman measurements (30). Moreover, light-induced reaction acceleration was observed in pigments in which the retinal chromophore was substituted by dyes lacking double bonds free to isomerize (25). Therefore, it was suggested that protein conformational alterations in bR can occur as a result of protein polarization and do not require a normal photocycle. This conclusion was based also on studies of the hydroxylamine reaction in bR which demonstrated that the reaction was light catalyzed even in artificial pigments in which double bond isomerization was prevented (13). The reaction acceleration as well as alterations in activation energy do not necessary imply significant changes in the protein conformation. Relatively small changes in the retinal binding site may affect the reaction. These studies indicate that the hydroxylamine reaction rate in NpSRII is light catalyzed also in an artificial pigment in which isomerization around the C13=C14 double bond is prevented. The activation energy of the reaction of the artificial pigment in the dark is higher than that of the native system (24.6 vs.16.9 kcal/mol (Table 1)) probably since the additional ring spanning the retinal carbons C12–C14 alters the binding site and modifies the reaction pathway. This is further reflected in the frequency factor which is significantly increased and partially compensates for the higher activation energy. It is striking that the light reaction activation energy of the “locked” pigment is markedly reduced relative to the dark reaction by 14.1 kcal/mole. The frequency factor is reduced in the “locked” pigment light reaction by 10 orders of magnitude similar to that detected in the native pigment (nine orders), and reduces the light-induced rate acceleration in the “locked” pigment. Since the “locked” NpSRII pigment lacks detectable photocycle intermediates, we suggest that similarly to bR the chemical reactivity of the protein to hydroxylamine reaction is significantly affected by light in NpSRII even when no C13=C14 isomerization takes place. As stated above, in bR “locked” artificial pigment isomerization around another double bond after light absorption was excluded. Similarly, it is very unlikely that isomerization around another double bond occurs in NpSRII “locked” pigment since no detectable photocycle was observed. Thus, we suggest that in the “locked” molecule the reaction is due to protein conformational alterations which are light-induced by charge redistribution in the retinal excited state. It is plausible that the hydroxylamine chemical reaction does not occur during the short excited state lifetime, but rather on ns to μs time. Therefore, the protein conformational alterations in the “locked” pigment are not accompanied by retinal configurational changes and should persist on a timescale which is longer than ns. It appears that these protein conformational alterations occur not only in bR (as was previously demonstrated), but in NpSRII as well. In bR and in NpSRII the lifetime of the retinal excited state is ∼500 fs (31–33), but in the “locked” bR pigment the excited state is characterized by a life time of ∼20 ps (34,35). A similar lifetime was not measured for NpSRII artificial “locked” pigment but it is likely that similarly to bR it is longer than the in native system. Therefore, these results indicate that at least in the “locked” pigment it is possible to significantly affect the hydroxylamine reaction and thus to induce protein conformational alterations without retinal isomerization. It was suggested that the hydroxylamine reaction is accelerated in NpSRII due to the formation of M intermediate (14). Our results do not exclude this possibility but rather demonstrate that an additional route is possible. The question arises as to the possibility that the effect detected in the “locked” pigment is valid also for the native system whose excited lifetime is shorter (∼500 fs). This short lifetime probably prevents atom movement but rather induces protein polarization including possible polarization of tryptophans in the retinal vicinity, bound water, or peptide dipoles. The possibility that protein polarization induces protein conformational changes on a longer time scale even in the native pigment should be the subject of future studies. In this respect it is interesting to note that bR artificial pigments derived from “locked” retinal analogs induce, after light absorption, protein conformational alterations in the same protein domains that experience changes in the native pigment, which does undergo retinal isomerization (12).
The results obtained for WT NpSRII indicate that the hydroxylamine reaction in lipid reconstituted samples is not significantly different from that in detergent. In both cases light reduces the activation energy accompanied by a decrease in frequency factor. A similar light effect was detected for bR (in membranes) which may indicate that both proteins experience similar light-induced structural alterations. As detected by x-ray studies (36–39), both proteins are characterized by a similar retinal binding structure which may lead to a similar protein response after retinal light absorption. In addition, both proteins experience similar changes in the protein cytoplasmic domain in the M intermediate. NpSRII association with its transducer did not considerably affect the rate of the reaction in the dark both in detergent and membrane preparations (in agreement with previous results (40)), even though it reduced the energy of activation in detergent (16.9 vs. 13.5 kcal/mol). The smaller activation energy in the complex was compensated by a lower frequency factor. The small effect of the transducer binding on the hydroxylamine dark reaction is in keeping with the crystal structure of NpSRII which indicates close similarity to the structure of the transducer complex (41). Binding of transducer to detergent preparation reduced, however, the light effect. Similarly to the free pigment, the complex experienced a significant reduction in activation energy and in frequency factor but the effect was smaller. In membranes the apparent light effect on the complex was stronger than in detergent. The effect of light on the activation energy and the frequency factor probably reflects light-induced alterations in the retinal binding site. The transducer forms in detergent a 1:1 heterodimer with the pigment whereas a 2:2 complex is detected in membrane preparations. The transducer binds to the cytoplasmic side of the protein but since the complex is different in detergent and membranes the light effect may be different. Interestingly, recent FTIR studies (42) indicated similar secondary structure of M intermediate after transducer binding in membrane preparations. Although the light effect can originate also from other intermediates besides M, it is plausible that the light effect originates from relatively mild changes in the retinal binding site. The binding of transducer can affect differently these changes in membranes and detergent preparations. We note (as stated in the results section) that the measured rates are apparent rates since temperature change affects the intermediates' lifetime. This phenomenon may induce differences between preparations with and without transducer as well.
Acknowledgments
The work was supported by the Fund for Basic Research (administered by The Israel Academy of Sciences and Humanities), by the Human Frontier Science Program, and by a grant from The Estate of Klara Seidman. M.S. holds the Katzir-Makineni professorial chair in chemistry.
References
- 1.Sasaki, J., and J. L. Spudich. 2000. Proton transport by sensory rhodopsins and its modulation by transducer-binding. Biochim. Biophys. Acta. 1460:230–239. [DOI] [PubMed] [Google Scholar]
- 2.Haupts, U., J. Tittor, and D. Oesterhelt. 1999. Closing in on bacteriorhodopsin: progress in understanding the molecule. Annu. Rev. Biophys. Biomol. Struct. 28:367–399. [DOI] [PubMed] [Google Scholar]
- 3.Lanyi, J. K. 2000. Molecular mechanism of ion transport in bacteriorhodopsin: insights from crystallographic, spectroscopic, kinetic, and mutational studies. J. Phys. Chem. B. 104:11441–11448. [Google Scholar]
- 4.Klare, J., V. Gordeliy, J. Labahn, G. Büldt, H. J. Steinhoff, and M. Engelhard. 2004. The archaeal sensory rhodopsin II/transducer complex: a model for transmembrane signal transfer. FEBS Lett. 564:219–224. [DOI] [PubMed] [Google Scholar]
- 5.Spudich, J. L. 1998. Variations on a molecular switch: transport and sensory signalling by archaeal rhodopsins. Mol. Microbiol. 28:1051–1058. [DOI] [PubMed] [Google Scholar]
- 6.Chizhov, I., G. Schmies, R. Seidel, J. R. Sydor, B. Lüttenberg, and M. Engelhard. 1998. The photophobic receptor from Natronobacterium pharaonis: temperature and pH dependencies of the photocycle of sensory rhodopsin II. Biophys. J. 75:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salem, L., and P. Bruckmann. 1975. Conversion of a photon to an electrical signal by sudden polarisation in the N-retinylidene visual chromophore. Nature. 258:526–528. [DOI] [PubMed] [Google Scholar]
- 8.Lewis, A. 1978. The molecular mechanism of excitation in visual transduction and bacteriorhodopsin. Proc. Natl. Acad. Sci. USA. 75:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaney, J. K., T. L. Brack, G. H. Atkinson, M. Ottolenghi, G. Steinberg, and M. Sheves. 1995. Primary picosecond molecular events in the photoreaction of the BR5.12 artificial bacteriorhodopsin pigment. Proc. Natl. Acad. Sci. USA. 92:2101–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu, D., C. Martin, and K. Schulten. 1996. Molecular dynamics study of early picosecond events in the bacteriorhodopsin photocycle: dielectric response, vibrational cooling, and the J, K intermediates. Biophys. J. 70:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rousso, I., E. Khachatouriants, Y. Gat, I. Brodsky, M. Ottolenghi, M. Sheves, and A. Lewis. 1997. Microsecond atomic force sensing of protein conformational dynamics: implications for the primary light-induced events in bacteriorhodopsin. Proc. Natl. Acad. Sci. USA. 94:7937–7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aharoni, A., L. Weiner, M. Ottolenghi, and M. Sheves. 2000. Bacteriorhodopsin experiences light-induced conformational alterations in nonisomerizable C13=C14 pigments. J. Biol. Chem. 275:21010–21016. [DOI] [PubMed] [Google Scholar]
- 13.Rousso, I., Y. Gat, A. Lewis, M. Sheves, and M. Ottolenghi. 1998. Effective light-induced hydroxylamine reactions occurs with C13=C14 nonisomerizable bacteriorhodopsin pigments. Biophys. J. 75:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwamoto, M., Y. Sudo, K. Shimono, and N. Kamo. 2001. Selective reaction of hydroxylamine with chromophore during the photocycle of pharaonis phoborhodopsin. Biochim. Biophys. Acta. 1514:152–158. [DOI] [PubMed] [Google Scholar]
- 15.Hohenfeld, I. P., A. A. Wegener, and M. Engelhard. 1999. Purification of histidine tagged bacteriorhodopsin, pharaonis halorhodopsin and pharaonis sensory rhodopsin II functionally expressed in Escherichia coli. FEBS Lett. 442:198–202. [DOI] [PubMed] [Google Scholar]
- 16.Wegener, A. A., J. Klare, M. Engelhard, and H. J. Steinhoff. 2001. Structural insights into the early steps of receptor-transducer signal transfer in archaeal phototaxis. EMBO J. 20:5312–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oesterhelt, D., L. Schumann, and H. Gruber. 1974. Light-dependent reaction of bacteriorhodopsin with hydroxylamine in cell suspensions of Halobacterium halobium: demonstration of an apo-membrane. FEBS Lett. 44:257–261. [DOI] [PubMed] [Google Scholar]
- 18.Sudo, Y., M. Iwamoto, K. Shimono, and N. Kamo. 2001. Pharaonis phoborhodopsin binds to its cognate truncated transducer even in the presence of a detergent with a 1:1 stoichiometry. Photochem. Photobiol. 74:489–494. [DOI] [PubMed] [Google Scholar]
- 19.Subramaniam, S., T. Marti, S. J. Rosselet, K. J. Rothschild, and H. G. Khorana. 1991. The reaction of hydroxylamine with bacteriorhodopsin studied with mutants that have altered photocycles: Selective reactivity of different photointermediates. Proc. Natl. Acad. Sci. USA. 88:2583–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, J., Z. Chen, and A. Lewis. 1989. Second harmonic generation in purple membrane-poly (vinyl alcohol) films: probing the dipolar characteristics of the bacteriorhodopsin chromophore in bR570 and M412. J. Phys. Chem. 93:3314–3319. [Google Scholar]
-
21.Birge, R., and C. Zhang. 1990. Two photon double resonance spectroscopy of bacteriorhodopsin. Assignment of the electronic and dipolar properties of the low-lying
 -like and
-like and 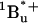 -like π,π* states. J. Chem. Phys. 92:7178–7195. [Google Scholar]
-like π,π* states. J. Chem. Phys. 92:7178–7195. [Google Scholar] - 22.Clays, K., E. Hendrickx, M. Triest, T. Verbiest, A. Persoons, C. Dehu, and J.-L. Bredas. 1993. Nonlinear optical properties of proteins measured by Hyper-Rayleigh scattering in solution. Science. 262:1419–1422. [DOI] [PubMed] [Google Scholar]
- 23.Aharoni, A., A. Khatchatouriants, A. Manevitch, A. Lewis, and M. Sheves. 2003. Protein-β-ionone interactions enhance the light induced dipole of the chromophore in bacteriorhodopsin. J. Phys. Chem. B. 107:6221–6225. [Google Scholar]
- 24.Zadok, U., A. Khatchatouriants, A. Lewis, M. Ottolenghi, and M. Sheves. 2002. Light-induced charge redistribution in the retinal chromophore is required for initiating the bacteriorhodopsin photocycle. J. Am. Chem. Soc. 124:11844–11845. [DOI] [PubMed] [Google Scholar]
- 25.Aharoni, A., L. Weiner, A. Lewis, M. Ottolenghi, and M. Sheves. 2001. Nonisomerizable non-retinal chromophores initiate light-induced conformational alterations in bacterioopsin. J. Am. Chem. Soc. 123:6612–6616. [DOI] [PubMed] [Google Scholar]
- 26.Aharoni, A., L. Weiner, A. Lewis, M. Ottolenghi, and M. Sheves. 2001. Light induced hydrolysis in Non-isomerizable C13=C14 bacteriorhodopsin pigment. Biophys. J. 82:2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheves, M., N. Friedman, and A. Albeck. 1985. Primary photochemical event in bacteriorhodopsin: study with artificial pigments. Biochemistry. 24:1260–1265. [Google Scholar]
- 28.Chang, C., R. Govindjee, T. Ebrey, K. Bagley, G. Dollinger, L. Eisenstein, J. Marque, H. Rodex, J. Vittow, J. Fang, and K. Nakanishi. 1985. Trans/13-cis isomerization is essential for both the photocycle and proton pumping of bacteriorhodopsin. Biophys. J. 47:509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharya, S., T. Marti, H. Otto, M. Heyn, and H. Khorana. 1992. A bacteriorhodopsin analog reconstituted with a nonisomerizable 13-trans retinal derivative displays light insensitivity. J. Biol. Chem. 267:6757–6762. [PubMed] [Google Scholar]
- 30.Uji, L., Y. Zhou, M. Sheves, M. Ottolenghi, S. Ruhman, and G. Atkinson. 2000. Vibrational spectrum of a picosecond intermediate in the artificial bR5.12 photoreaction: picosecond time resolved CARS of T-5.12. J. Am. Chem. Soc. 122:96–106. [Google Scholar]
- 31.Sharkov, S., A. Pakulev, S. Chekalin, and Y. Matveetz. 1985. Primary events in bacteriorhodopsin probed by subpicosecond spectroscopy. Biophys. Biochim. Acta. 808:94–102. [Google Scholar]
- 32.Mathies, R. A., C. H. Brito Cruz, W. T. Pollard, and C. V. Shank. 1988. Direct observation of the femtosecond excited-state cis-trans isomerization in bacteriorhodopsin. Science. 240:777–779. [DOI] [PubMed] [Google Scholar]
- 33.Lutz, I., A. Sieg, A. A. Wegener, M. Engelhard, I. Boche, M. Otsuka, D. Oesterhelt, J. Wachtveitl, and W. Zinth. 2001. Primary reactions of sensory rhodopsins. Proc. Natl. Acad. Sci. USA. 98:962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong, Q., S. Ruhman, M. Ottolenghi, M. Sheves, N. Friedman, G. H. Atkinson, and J. K. Delaney. 1996. Reexamining the primary light-induced events in bacteriorhodopsin using a synthetic C13=C14-locked chromophore. J. Am. Chem. Soc. 118:12828–12829. [Google Scholar]
- 35.Ye, T., N. Friedman, Y. Gat, G. Atkinson, M. Sheves, M. Ottolenghi, and S. Ruhman. 1999. On the nature of the primary light-induced events in bacteriorhodopsin: ultrafast spectroscopy of native and C13=C14 locked pigments. J. Phys. Chem. B. 103:5122–5130. [Google Scholar]
- 36.Luecke, H., B. Schobert, H. T. Richter, J. P. Cartailler, and J. K. Lanyi. 1999. Structure of bacteriorhodopsin at 1.55 angstroms resolution. J. Mol. Biol. 291:899–911. [DOI] [PubMed] [Google Scholar]
- 37.Berhali, H., P. Nollert, A. Royant, C. Menzel, J. P. Rosenbusch, E. M. Landau, and E. Pebay-Peyroula. 1999. Protein, lipid and water organization in bacteriorhodopsin crystals: a molecular view of the purple membrane at 1.9 angstroms resolution. Structure. 7:909–917. [DOI] [PubMed] [Google Scholar]
- 38.Royant, A., P. Nollert, K. Edman, R. Neutze, E. M. Landau, E. Pebay-Peyroula, and J. Navarro. 2001. X-ray struture of sensory rhodopsin II at 2.1 angstroms resolution. Proc. Natl. Acad. Sci. USA. [DOI] [PMC free article] [PubMed]
- 39.Luecke, H., B. Schobert, J. K. Lanyi, E. N. Spudich, and J. L. Spudich. 2001. Crystal structure of sensory rhodopsin II at 2.4 angstroms: insight into color tuning and transducer interaction. Science. 293:1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sudo, Y., M. Iwamoto, K. Shimono, and N. Kamo. 2002. Association of pharaonis phoborhodopsin with its cognate tranducer decreases the photo-dependence reactivity by water-soluble reagents of azide and hydroxylamine. Biochim. Biophys. Acta. 1558:63–69. [DOI] [PubMed] [Google Scholar]
- 41.Gordeliy, V., J. Labahn, R. Moukhametzianov, R. Efremov, J. Granzin, R. Schlesinger, G. Buldt, T. Savopol, A. Scheidig, J. Klare, and M. Engelhard. 2002. Molecular basis of transmembrane signaling by sensory rhodopsin II-transducer complex. Nature. 419:484–487. [DOI] [PubMed] [Google Scholar]
- 42.Furutani, Y., K. Kamada, Y. Sudo, K. Shimono, N. Kamo, and H. Kandori. 2005. Structural changes of the complex between pharaonis phoborhodopsin and its cognate transducer upon formation of the M photointermediate. Biochemistry. 44:2909–2915. [DOI] [PubMed] [Google Scholar]








