Abstract
The effect of ergosterol on the electrochemical features of the phosphatidylcholine bilayer membrane was investigated by impedance spectroscopy. The experimental impedance values obtained in the presence of different amounts of ergosterol showed evidence of domain structures within the bilayer containing <0.06 molar fraction of ergosterol. Based on derived mathematical equations, the surface area of phospholipid/ergosterol domain was calculated; it amounts to 262 Å2. This value is consistent, taking into consideration the presented measurements as well as ordering and condensation effects of ergosterol, with a stoichiometry of such a domain equal to 3:1. The result of the investigation is the proposal of a new and simple method for the determination of the surface area and description stoichiometry of domains formed in any two-component system.
INTRODUCTION
Lipid lateral organization is an important issue in membrane biology. There is increasing evidence that functional lipid domains of micrometer sizes exist in biological membranes under physiological conditions (1,2), but relatively less is known about lipid organization at the molecular level inside and outside those domains. Most of the techniques used to characterize domains are based on fluorescence microscopy. A limitation of fluorescence-based measurements is the inability to provide information at the molecular structure level and interactions of domain constituents. Furthermore, the area of the domains detected is limited to >∼106 Å2. Infrared and Raman spectroscopies provide a means to overcome these limitations. Direct molecular structure information is inherent in vibrational spectroscopy. Certain infrared spectral parameters are sensitive to domain formation in a size regime much smaller than fluorescence-determined domains (3).
Because cell membranes are extremely complex, a molecular understanding of membrane lipid lateral organization must first come from simple model systems such as two-component lipid bilayers. In such systems, the membrane components can be domain-segregated (4), or randomly distributed (5), or regularly distributed (6). The distribution depends on experimental conditions and may change with temperature, pressure, lipid compositions, and mixing ratio, and with the type of lipids (7).
Sterols are membrane components and as such regulate membrane fluidity and permeability. The paradigmatic example from this group of compounds is cholesterol. It is often found distributed nonrandomly in domains in biological and model membranes (8–12). These domains are believed to be important for the maintenance of membrane structure and function. Recent observations suggest that cholesterol exerts many of its actions by maintaining a specialized type of membrane domain, termed a lipid raft, in a functional state (8,13,14). Although cholesterol is the major sterol present in plasma membranes of higher eukaryotes, ergosterol is the major component present in lower eukaryotes such as certain protozoa, yeast, and other fungi, and in insects such as Drosophila (15). Although detailed biophysical characterization of the effect of cholesterol on membranes is well documented, the effect of ergosterol on the physical properties of membranes has not been studied in detail and has never been studied by means of electrochemical impedance spectroscopy. The effect of ergosterol on membrane organization and dynamics assumes significance in view of the recent reports about isolation of lipids rafts from organisms such as yeast (16) and Drosophila (17).
In this article, we model biomembranes using a two-component bilayer system. The lipids chosen are phosphatidylcholine from egg yolk and ergosterol. Egg phosphatidylcholine was selected mainly due to its acyl chain composition, which resembles many biological membranes. Ergosterol was used because its effect on the organization of membranes is not very clear (18). We have monitored the effect of ergosterol on the capacitance and resistance of the phosphatidylcholine membranes utilizing electrochemical impedance spectroscopy. Our results show the formation of the phosphatidylcholine/ergosterol domains in lipid bilayers containing <0.06 molar fraction of ergosterol. The determination of the area occupied by one phosphatidylcholine/ergosterol domain is the final research result. Equations presented in this article can be used, e.g., for determination of area and describe stoichiometry of domains formed in any two-component system.
THEORY
Any two-component system, regardless whether it is a monolayer or a bilayer, can be described in terms of the presented equations here describing additivity of electric capacity and electric conductance,
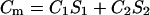 |
(1a) |
and
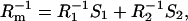 |
(1b) |
here
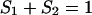 |
(2) |
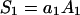 |
(3) |
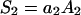 |
(4) |
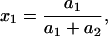 |
(5) |
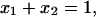 |
(6) |
where Cm [μF cm−2] is capacitance of the membrane; C1, C2 [μF cm−2] are capacitances of the membrane built of components 1 and 2, respectively; 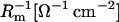 is conductance of the membrane;
is conductance of the membrane; 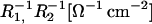 are conductances of the membrane built of components 1 and 2, respectively; S1, S2 are surface fractions of the membrane covered by components 1 and 2, respectively; a1, a2 [mol m−2] are surface concentrations of components 1 and 2, respectively, in the membrane; A1, A2 [m2 mol−1] are surface areas of one mole of the membrane formed from components 1 and 2, respectively; and x1, x2 are molar fractions of components 1 and 2, respectively.
are conductances of the membrane built of components 1 and 2, respectively; S1, S2 are surface fractions of the membrane covered by components 1 and 2, respectively; a1, a2 [mol m−2] are surface concentrations of components 1 and 2, respectively, in the membrane; A1, A2 [m2 mol−1] are surface areas of one mole of the membrane formed from components 1 and 2, respectively; and x1, x2 are molar fractions of components 1 and 2, respectively.
After solution of the equations system above (Eqs. 1–6), the linear dependencies are derived as
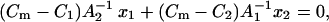 |
(7a) |
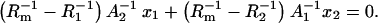 |
(7b) |
Spatial regionalization of components occurs in biological membranes. It is due to specific interactions between membrane components resulting in the appearance of membrane regions of diverse chemical character, structure, and function. Such specialized structures of various sizes have been called domains (19). The equilibrium of domain formation can be described in terms of such physicochemical parameters as electric capacity and electric conductance. Let us assume that, in the domain (denoted by subscript 3) formation process in a two-component lipid membrane, every molecule of component 2 is surrounded by a certain, possible to determine, quantity of component 1. The equilibrium state of the discussed system is described by
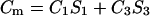 |
(8a) |
and
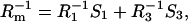 |
(8b) |
in which
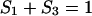 |
(9) |
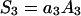 |
(10) |
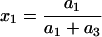 |
(11) |
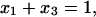 |
(12) |
where C3 [μF cm−2] is the capacitance of the membrane built of a domain; 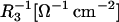 is the conductance of the membrane built of a domain; S3 is the surface fraction of the membrane covered by a domain; a3 [mol m−2] is the surface concentration of a domain in the membrane; A3 [m2 mol−1] is the surface area of one mole of the membrane formed from a domain; and x3 is the molar fraction of a domain.
is the conductance of the membrane built of a domain; S3 is the surface fraction of the membrane covered by a domain; a3 [mol m−2] is the surface concentration of a domain in the membrane; A3 [m2 mol−1] is the surface area of one mole of the membrane formed from a domain; and x3 is the molar fraction of a domain.
Elimination of S1, S3, a1, and a3 yields the equations
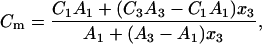 |
(13a) |
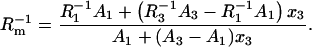 |
(13b) |
Equations 13a and 13b are quotients of polynomials. Dividing the numerator of each quotient by its denominator yields a series of increasing exponents of the power of molar fraction, x3. Further, taking into account the two first terms of each series results in linear expressions that are correct at low molar fractions (for x3 → 0):
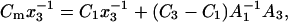 |
(14a) |
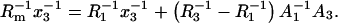 |
(14b) |
MATERIALS AND EXPERIMENTAL DETAILS
Reagents and preparation of the forming solutions
Ninety-nine percent egg phosphatidylcholine was purchased from Fluka (Neu-Ulm, Germany) and it had the following fatty acid composition: 16:0 ≤ 33%, 18:0 ∼ 4%, 18:1 ∼ 30%, 18:2 ∼ 14%, and 20:4 ∼ 4%. Ninety-seven percent ergosterol was also obtained from Fluka. The lipids were dissolved in chloroform to prevent oxidizing and mixed in appropriate proportions to achieve the desired molar fractions. The solvent was evaporated under a stream of argon. Dried residues were dissolved in a hexadecane-butanol mixture (10:1 by volume). The resultant solution used to form the model membrane contained 20 mg ml−1 of lipids in solution. During membrane formation, the solvent mixture was removed and the membrane created has the same proportion as in the resultant solution. The samples were stored for at least five days at 4°C before examination.
The solvents were of chromatographic standard grade: chloroform and butanol were from Aldrich (Milwaukee, WI); hexadecane was from Fluka.
Potassium chloride solution of 0.1 mol dm−3 was used as the electrolyte for experiments. KCl from POCh (Gliwice, Poland) was analytical grade and was roasted before use at 400°C for 4 h to remove traces of organic material. Water purified by Milli-Qll (18.2 M, Millipore, Billerica, MA) was used to make the electrolyte, and in all cleaning procedures.
Preparation of the bilayer membranes
Bilayer membranes were obtained as bubbles at the Teflon cap constituting a measuring vessel component. The use of hexadecane as the solvent allows one to obtain membranes of thickness and capacity values similar to those of membranes formed of monolayers (20,21); there is almost no solvent retained in the bilayer. A small quantity of butanol added has a negligible effect on the impedance parameters of the bilayers created; however, it considerably accelerates the formation of the membranes. The formation of the bilayers was monitored visually and electrically by measuring the membrane capacitance at low frequency (1 Hz). Capacity of the membranes increased with time after bilayers formation until a steady-state value was reached some 10–20 min later. The measurements were begun only after the low frequency capacitance was stable, increasing by <1%/h. When the capacitance had stabilized, it was assumed that diffusion of solvent out of the bilayer was complete, although some hexadecane molecules would remain dissolved in the membrane interior. The bilayers area were determined with a microscope with a micrometer scale built into the lens, and were between 4 × 10−2 − 8 × 10−2 cm2 (the values were given for the bilayers area with subtracted margin).
Impedance analysis
Electrochemical impedance spectroscopy was performed with an AC impedance system (Model 388; EG&G, Princeton Applied Research, Princeton, NJ) that included a personal computer, a two-phase lock-in amplifier (Model 5208) and a potentiostat/galvanostat (Model 273), in which a four-electrode input was applied within the preamplifier. The electrochemical cell contained two identical reversible silver-silver chloride electrodes and two identical current platinum electrodes (described exactly in (22–24)). The use of the four-electrode system in the studies of electric phenomena occurring in membranes makes it possible to reduce, considerably, the errors caused by electrode and electrolyte impedance (25,26). A 4-mV amplitude sine-wave signal perturbation was applied in the 0.1–10,000 Hz frequency range. Impedance data were analyzed by using the nonlinear least-squares (NLLQ) fitting to a model represented by an equivalent electrical circuit. The NLLQ program used in this work was EQUIVCRT.PAS (27). All experiments were carried out at room temperature (20° ± 1°C).
RESULTS
The effect of ergosterol on capacitance and resistance (reciprocal of conductance) of the phosphatidylcholine bilayer was examined in the presence of different amounts of ergosterol using electrochemical impedance spectroscopy. The ergosterol content was varied up to a 0.11 molar fraction; above this, sterol induced disorder of the acyl chains of phosphatidylcholine, and we were not able to form a bilayer sufficiently stable upon which to carry out measurements. It has been reported before (28) that the solubility limit of ergosterol found in egg phosphatidylcholine bilayers amounts to 0.25 molar fraction. The impedance technique was used in our study to characterize the membrane features, since this method has been shown to be able to measure the membrane capacitance and resistance on bilayer lipid membranes accurately. The mean values of the measured parameters were obtained from six independent measurements of the lipid bilayer. The experimental impedance values presented here refer to the bilayer surface-area unit.
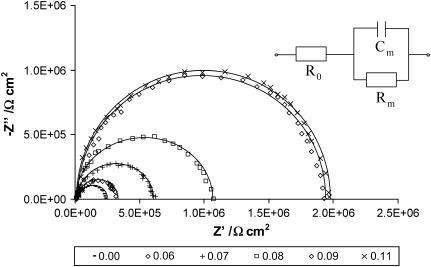
Fig. 1 shows the results of impedance measurements conducted on the phosphatidylcholine bilayers, pure and containing different amounts of ergosterol. For the sake of clarity, spectra for some molar fractions have been omitted (otherwise the figure would be illegible by superimposed spectra caused by too few differences in the impedance parameters values). Very simple impedance diagrams were obtained for all examined membranes; they had the form of impedance semicircles in the entire analyzed frequency range. The centers of the semicircles lie on the real axis, provided that the lipid bilayer is considered as a dielectric layer with leakage. The spectra of phosphatidylcholine/ergosterol bilayers were higher than that for pure phosphatidylcholine membranes, confirming that ergosterol has been successfully incorporated into the phosphatidylcholine bilayer, and have an effect on the membrane capacitance and resistance; it caused capacitance of the membrane Cm to decrease and resistance of the membrane Rm to increase. The equivalent circuit used for data analysis (inset in Fig. 1) consists of a parallel arrangement of the capacitor Cm and resistor Rm, attributed to the electrical properties of the bilayer, completed by a serial resistor R0 for the conductivity of the bulk. The possibility of misinterpretation of the recorded data is reduced by simplicity of the circuit. This electric circuit is characteristic for an artificial lipid membrane only when ionophore systems, specific channels, pores, and adsorption are absent (29). Based on this equivalent circuit, the nonlinear least-squares analysis was used to simulate the impedance plots; then the values of Rm and Cm were extracted from the fit. The NLLQ fits are represented by the solid lines in Fig. 1 and are in good agreement with the data obtained.
FIGURE 1.
Dependence of an imaginary part (−Z″) on the real part (Z′) over a frequency range of 0.1 Hz to 10 kHz for a phosphatidylcholine membrane modified with ergosterol. A different content of ergosterol (expressed as a molar fraction) is illustrated by the different point's shapes of the impedance spectra. The solid lines represent the results of the fitting procedure. The equivalent circuit used for impedance data analysis is shown in the inset: R0 represents the resistance of the electrolyte, Rm the resistance of the membrane, and Cm the capacitance of the membrane.
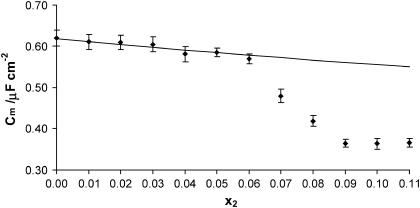
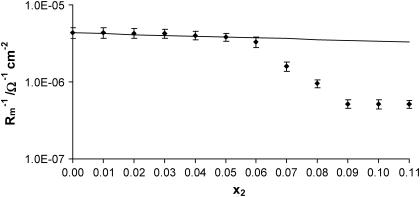
Dependences of the capacitance and the conductance of the phosphatidylcholine/ergosterol membrane on the molar fraction of ergosterol are presented in Figs. 2 and 3, respectively. The resulting curves deviate from linearity, indicating that specific interactions between membrane components are presented in the membrane. In these figures, points denote experimental values, and the solid lines are calculated on the basis of Eqs. 13a and 13b (describing the domain formation process) using values whose determination will be presented in further parts of this article. That the theoretical values agree well with the experimental data in the 0.00–0.06 molar-fraction range of ergosterol suggests the existence of phosphatidylcholine/ergosterol domains with a defined stoichiometry and a constant area in this region. Ergosterol had significant effect on the capacitance and the conductance membranes up to 0.09 molar fraction; Cm and 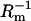 reached a plateau value when the ergosterol molar fraction was over 0.09. At a molar fraction of ergosterol in the range 0.06–0.09, the lipid composition and physico-chemical properties of phosphatidylcholine/ergosterol domains change in comparison with the domains formed in the range 0.00–0.06. Our results are consistent with the studies indicating that the effect of ergosterol on the rotational mobility of phosphatidylcholine membranes stabilizes after a certain concentration (∼0.10 molar fraction) of ergosterol in the membrane (18). The C1 and
reached a plateau value when the ergosterol molar fraction was over 0.09. At a molar fraction of ergosterol in the range 0.06–0.09, the lipid composition and physico-chemical properties of phosphatidylcholine/ergosterol domains change in comparison with the domains formed in the range 0.00–0.06. Our results are consistent with the studies indicating that the effect of ergosterol on the rotational mobility of phosphatidylcholine membranes stabilizes after a certain concentration (∼0.10 molar fraction) of ergosterol in the membrane (18). The C1 and 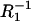 values obtained for a pure bilayer of phosphatidylcholine are equal to 0.62 μF cm−2 and 4.35 × 10−6 Ω−1 cm−2, respectively. The C2 and
values obtained for a pure bilayer of phosphatidylcholine are equal to 0.62 μF cm−2 and 4.35 × 10−6 Ω−1 cm−2, respectively. The C2 and 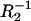 values for a pure bilayer of ergosterol (evaluated from plateau values) are equal to 0.37 μF cm−2 and 5.12 × 10−7 Ω−1 cm−2, respectively. It is clear that inclusion of sterol molecules into membrane results in the increase of the membrane thickness (as evident by examining the distance from the bilayer center to phosphorus atoms (30)). The increase in the membrane thickness results in decrease in its electrical capacity. Such an increase represents a main manifestation of the sterol condensing effect on the membranes (31,32).
values for a pure bilayer of ergosterol (evaluated from plateau values) are equal to 0.37 μF cm−2 and 5.12 × 10−7 Ω−1 cm−2, respectively. It is clear that inclusion of sterol molecules into membrane results in the increase of the membrane thickness (as evident by examining the distance from the bilayer center to phosphorus atoms (30)). The increase in the membrane thickness results in decrease in its electrical capacity. Such an increase represents a main manifestation of the sterol condensing effect on the membranes (31,32).
FIGURE 2.
Dependence of capacitance Cm of the phosphatidylcholine/ergosterol membrane on the molar fraction of ergosterol x2. Experimental points represent the mean ± SE obtained from six membranes. The solid line represents the theoretical values calculated according to Eq. 13a.
FIGURE 3.
Dependence of conductance 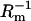 of the phosphatidylcholine/ergosterol membrane on the molar fraction of ergosterol x2. Experimental points represent the mean ± SE obtained from six membranes. The solid line represents the theoretical values calculated according to Eq. 13b.
of the phosphatidylcholine/ergosterol membrane on the molar fraction of ergosterol x2. Experimental points represent the mean ± SE obtained from six membranes. The solid line represents the theoretical values calculated according to Eq. 13b.
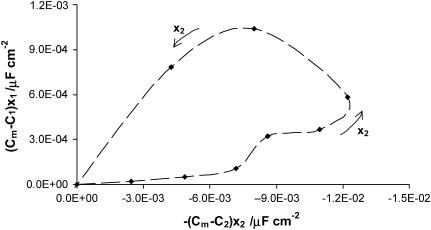
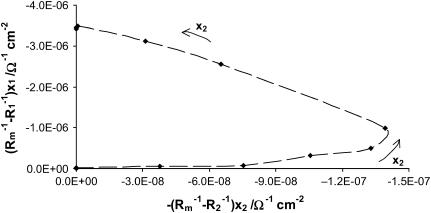
Figs. 4 and 5 present the dependences resulting from Eqs. 7a and 7b, respectively, expressed in the coordinate systems in which the plots should be straight lines in the case when they are lacking specific interactions between membrane components. Their actual shapes prove that they do not correspond to Eqs. 7a and 7b, suggesting that there are specific interactions in the phosphatidylcholine/ergosterol bilayer. Since Eqs. 7a and 7b do not describe the system under the study sufficiently, we assume, on the basis of the literature (7,18,33), that the creation of domains within the phospholipids bilayer are enriched with respect to ergosterol, and that all ergosterol is present in the phosphatidylcholine/ergosterol domains. Consequently, Eqs. 8a and 8b, describing a domain formed in the bilayer lipid membrane, complete the theoretical description. After simple modifications of Eqs. 8a and 8b, one can obtain information of great interest presented by Eqs. 14a and 14b.
FIGURE 4.
The dependence of (Cm−C1)x1 versus (Cm−C2)x2: Cm-capacitance of the membrane, C1-capacitance of the phosphatidylcholine membrane, C2-capacitance of the ergosterol membrane, x1-molar fraction of the phosphatidylcholine, and the x2-molar fraction of ergosterol. The arrows denote the direction of the increasing x2 values and the dashed line indicates the order of points.
FIGURE 5.
The dependence of 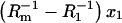 versus
versus 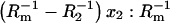 -conductance of the membrane,
-conductance of the membrane, 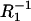 -conductance of the phosphatidylcholine membrane,
-conductance of the phosphatidylcholine membrane, 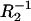 -conductance of the ergosterol membrane, x1-molar fraction of the phosphatidylcholine, and the x2-molar fraction of ergosterol. The arrows denote the direction of the increasing x2 values and the dashed line indicates the order of points.
-conductance of the ergosterol membrane, x1-molar fraction of the phosphatidylcholine, and the x2-molar fraction of ergosterol. The arrows denote the direction of the increasing x2 values and the dashed line indicates the order of points.
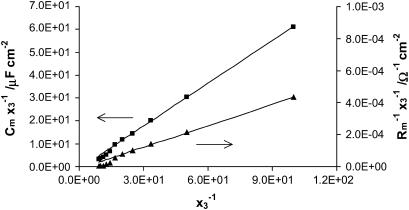
Fig. 6 presents the dependences illustrating Eqs. 14a and 14b in the entire analyzed molar fraction of the ergosterol range. Provided that a domain is formed, the plots of Eqs. 14a and 14b show straight lines. The six points obtained for the lowest concentrations of ergosterol lie on ideal straight lines. These points correspond to the ergosterol content up to 0.06 molar fraction, and confirm that in this molar fraction range there are created domains with a defined stoichiometry and a relatively constant area.
FIGURE 6.
A plot illustrating Eqs. 14a and 14b, from which the surface area of phosphatidylcholine/ergosterol domain can be determined. The value Cm represents capacitance of the membrane, 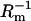 conductance of the membrane, and x3 molar fraction of the domain. Solid lines represent the theoretical lines calculated according to Eqs. 14a and 14b.
conductance of the membrane, and x3 molar fraction of the domain. Solid lines represent the theoretical lines calculated according to Eqs. 14a and 14b.
The Eq. 14a and 14b can be written in the form of y = ax + b. The a and b parameters were determined for six of the least molar fractions of ergosterol using a linear regression according to numerical recipes worked out by Press et al. (34). These are equal to 0.619 and −0.715, respectively, from capacitance measurements. We calculated additional numbers to characterize the property of the probable uncertainty for the parameters' estimation: the variances in the estimates of a and b amount to 5.56 × 10−8 and 1.00 × 10−4, respectively; the covariance of a and b is equal to −1.88 × 10−6; and the coefficient of the correlation between the uncertainty in a and the uncertainty in b is tantamount to −0.80. The a and b parameters are equal to 4.49 × 10−6 and −1.27 × 10−5 from conductance measurements (the variances in the estimates of a and b amount to 7.91 × 10−17 and 1.34 × 10−13, respectively; the covariance of a and b is equal to −2.69 × 10−15, and the coefficient of the correlation between the uncertainty in a and the uncertainty in b is tantamount to −0.83).
The parameters determined by the regression were applied to present the agreement of the Eqs. 14 data (solid lines) with the experimental data (points) in Fig. 6. The slope values of the straight lines are equal to C1 and 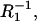 respectively, and are in agreement (in deviation limits) with experimental values obtained for a pure phosphatidylcholine membrane. The intersections of the straight lines with y axes yield the (
respectively, and are in agreement (in deviation limits) with experimental values obtained for a pure phosphatidylcholine membrane. The intersections of the straight lines with y axes yield the (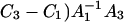 and
and 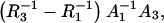 respectively, which allow one to determine an area occupied by one phosphatidylcholine/ergosterol domain (denoted by A3). The surface area occupied by one phosphatidylcholine molecule (A1) is also necessary for calculation of the A3 value. This surface depends on the way the phospholipid is prepared, because this affects the length, conformation, and degree of unsaturation of the fatty acids chains. Therefore, the values of the literature range between 54 Å2 and 99 Å2 (35–37). We chose the A1 value, determined in our laboratory (38), equaling 85 Å2. Knowing the surface area per phosphatidylcholine molecule, the capacitance of the membrane built of phosphatidylcholine C1 (0.62 μF cm−2) and the capacitance of the membrane built of domain C3 (0.37 μF cm−2), as well as the conductance of the membrane built of phosphatidylcholine
respectively, which allow one to determine an area occupied by one phosphatidylcholine/ergosterol domain (denoted by A3). The surface area occupied by one phosphatidylcholine molecule (A1) is also necessary for calculation of the A3 value. This surface depends on the way the phospholipid is prepared, because this affects the length, conformation, and degree of unsaturation of the fatty acids chains. Therefore, the values of the literature range between 54 Å2 and 99 Å2 (35–37). We chose the A1 value, determined in our laboratory (38), equaling 85 Å2. Knowing the surface area per phosphatidylcholine molecule, the capacitance of the membrane built of phosphatidylcholine C1 (0.62 μF cm−2) and the capacitance of the membrane built of domain C3 (0.37 μF cm−2), as well as the conductance of the membrane built of phosphatidylcholine 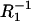 (4.35 × 10−6 Ω−1 cm−2) and the conductance of the membrane built of domain
(4.35 × 10−6 Ω−1 cm−2) and the conductance of the membrane built of domain 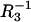 (5.12 × 10−7 Ω−1 cm−2), the area occupied by one phosphatidylcholine/ergosterol domain could be determined. The resulting A3 values were 243 Å2 from capacitance measurements and 281 Å2 from conductance measurements, which gave the mean value amounting to 262 Å2.
(5.12 × 10−7 Ω−1 cm−2), the area occupied by one phosphatidylcholine/ergosterol domain could be determined. The resulting A3 values were 243 Å2 from capacitance measurements and 281 Å2 from conductance measurements, which gave the mean value amounting to 262 Å2.
DISCUSSION
The molecular interaction between ergosterol and phosphatidylcholine is specific, and as a consequence of this, ergosterol-rich domains are formed in mixed bilayers having a low concentration of ergosterol (18). In this study, the effect of ergosterol on capacitance (Fig. 2) and conductance (Fig. 3) of the phosphatidylcholine bilayer has been examined with respect to the formation of domains in bilayers.
The newly bilayer formed by our technique reaches stable conductance and capacitance values within 30–40 min. The reason that it takes 30–40 min until the new formed bilayer displays stable 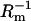 and Cm values can be attributed to the fact that the hydrophobic interior has not yet reached its completely ordered configuration. Residues of organic solvents (mostly hexadecane) must redistribute between the membrane core and the lipid deposit on the Teflon rim (pretreatment procedure) forming the Gibbs-Plateau border. Drainage of solvent residues to that border is accompanied by an increasing order of the hydrocarbon chains forming the hydrophobic interior of the bilayer.
and Cm values can be attributed to the fact that the hydrophobic interior has not yet reached its completely ordered configuration. Residues of organic solvents (mostly hexadecane) must redistribute between the membrane core and the lipid deposit on the Teflon rim (pretreatment procedure) forming the Gibbs-Plateau border. Drainage of solvent residues to that border is accompanied by an increasing order of the hydrocarbon chains forming the hydrophobic interior of the bilayer.
The high capacitance values obtained for phosphatidylcholine membranes (Fig. 2) leave no doubts that the membrane is really a bilayer. In our earlier article (23) we inferred that gramicidin D causes the formation of trans-membrane ion channels in the membranes created by us, which is an ultimate proof for the bilayer status of the lipid membrane (the gramicidin dimmer is too short to penetrate membranes thicker than bimolecular ones (39,40). Based on our experimental results and numerous literature data (20,30,41), we assume that our membranes do not stay solvent. If some of these quantities, which are not large in number, are contained in the membranes, then one should treat them as traces of impurities. Since it is impossible to determine the quantity of these impurities, it is impossible to make a thorough qualitative determination of their nature and so one cannot take them into account in quantitative considerations (except as a possible qualitative indication). If quantitative analysis were possible, we would take into account the possibility of solvent's presence in the derived equations.
We were able to form stable phosphatidylcholine bilayers with the ergosterol content in the range 0.00–0.11 molar fraction. Our results are similar to the studies indicating that ergosterol only ordered acyl chains up to 0.15–0.20 molar fraction; above this ergosterol induced disorder of the acyl chains and disrupt the phosphatidylcholine bilayers instead (42,43). Relatively recent data (44–48) indicate that in Trypanosoma cruzi, a protozoan human parasite that requires ergosterol and other 24-alkyled sterols for growth, the sterol content of the plasma membranes, which are rich in unsaturated fatty acids, is 0.23 molar fraction (of which three-quarters are ergosterol or its 24-ethylated analogs). The ergosterol content of the plasma membrane of ergosterol-containing organisms such as Drosophila has also been shown to be within this range (17).
From the experiments on model systems and natural membranes it has been concluded that the interactions between phospholipids and sterols are complex, and depend on the details of sterol structure and the types of acyl chains present in the phospholipid molecules (e.g., 15,28,49–51). Sterols such as cholesterol with a planar sterol nucleus, an intact side chain, and a 3β-hydroxy group reveal strong effects on the lipid bilayer membrane. These effects are shown by reduction in the membrane permeability, which is strongly influenced by a reduction in motional freedom of the hydrocarbon region (ordering effect) and by decrease in the surface area occupied by the phospholipid (a condensing effect). Compounds sharing structural features with cholesterol—e.g., cholestanol, lanosterol, 7-dehydrocholesterol, and β-norcholesterol—induce a strong condensation and ordering effect. Sterols with a different side-chain structure, e.g., stigmasterol and ergosterol, cause smaller effects. Compounds with no side chain (androstan-3β-ol) with a nonplanar sterol nucleus (coprostanol) or a 3α-hydroxy group (epicholesterol) show no effect or increase the permeability (28,50,51).
The types of acyl chains present in the phospholipid molecules are very important in the phospholipid-sterol interactions. Studies using 2H-, 13C-, and 31P-NMR spectroscopy show that ergosterol is less effective at increasing order and restricting the mobility of 1-palmitoyl-2-oleoyl-phosphatidylcholine than is cholesterol, whereas the opposite is true with dimyristoylphosphatidylcholine bilayers. The lanosterol is less effective than ergosterol and cholesterol, with both saturated and unsaturated lipids. A possible reason for these complex effects is that the rigid and bulky ergosterol molecule (or the methylated α of lanosterol) is unable to interact effectively with the phospholipid molecules when unsaturated acyl chains are present (43).
The condensing and ordering effects are not limited to specific phospholipids; they are also shown by phosphatidylcholines and phosphatidylethanolamines, as well as phosphatidic acid, sphingomyelin, phosphatidylglycerol, or other derivatives of phospholipids (50).
The model of intermolecular interactions resulting from complex formation or molecular realignment assumes that, at a constant surface pressure, the area per sterol molecule is also constant. The condensation effect of sterol is related to the decrease of the area per phospholipid molecule (52). The surface area per ergosterol molecule, reported in the literature, varies from 32 Å2 (53) to 39.3 Å2 (51). Taking into account the surface area occupied by one ergosterol molecule equaling 38.5 Å2 (49), the surface area occupied by one phosphatidylcholine molecule (85 Å2), the mean experimental surface area occupied by one phosphatidylcholine/ergosterol domain determined by us (262 Å2) and the possibility of existence of condensation and ordering effects, we suggest that the stoichiometry of phosphatidylcholine/ergosterol domain is equal to 3:1. The mean experimental A3 value is higher than the sum of areas per two phosphatidylcholines and one ergosterol molecule (208.5 Å2) but lower than the sum of areas per three phosphatidylcholines and one ergosterol molecule (293.5 Å2). Deviation of the surface area calculated on the basis of experiments from the theoretical value shows a negative value, which means that a reduction in the surface area occurs as a result of component mixing. This is thus a condensation effect observed between the phosphatidylcholine and ergosterol, indicating that ergosterol can work as a reinforcer for phosphatidylcholine bilayers. The condensation can be attributed to area changes within the phosphatidylcholine when this lipid is mixed with ergosterol, since ergosterol is a rigid molecule whose area does not significantly change when a phosphatidylcholine/ergosterol bilayer is formed. This is in agreement with the model of intermolecular interactions. The surface area occupied by one phosphatidylcholine molecule within the phosphatidylcholine/ergosterol domain amounts to ∼75 Å2, whereas the presence of cholesterol in the phosphatidylcholine bilayer can reduce the molecular area of the phosphatidylcholine to 56 Å2 (36).
During the last few years, many research groups have intensively studied the function of lipid rafts and microdomains in biological membranes and in models of biomembranes. The concept of rafts or glycosphingolipid/cholesterol domains, which are involved in protein and lipid transport and in several signaling cascades, was first presented in 1997 (1). The raft hypothesis suggests that lipids can form domains or aggregates in the plane of the membrane and that these domains form due to interactions between the lipid molecules. The existence of such rafts has been disputed, but more and more evidence has been gathered showing the important functions of such lipid domains in the cellular membranes (54).
The lipid rafts are enriched in cholesterol and saturated fatty acids, and therefore are highly ordered as compared to the surrounding lipid bilayer (55). In addition, sterol-rich domains exist within the plasma membrane as structures referred to as caveoli (56). Cholesterol is typically associated with separate kinetic domains, and is thus considered to be distributed nonrandomly within the plasma membrane (57). Regulation of the size and physico-chemical properties of these kinetic domains may influence extra- and intracellular cholesterol transport (58). Investigators have proposed that cholesterol domains may modulate the activity of membrane proteins that localize specifically to cholesterol-rich and cholesterol-poor domains (59). It has also been hypothesized that sterol-rich regions play a crucial role in cellular function that induces signal transduction, cell adhesion, motility, and the sorting and trafficking of membrane components (1,9). In the membrane, cholesterol tends to aggregate into clusters at cholesterol/phospholipid mole ratios in excess of 0.3 (60) and forms separate domains at ratios in excess of 1 (i.e., 0.5 molar fraction (61)). Numerous theoretical and model monolayer and bilayer studies have demonstrated that the systematic addition of cholesterol to biological membranes can eventually yield to lateral phase separation and the formation of membrane-restricted sterol domains (60–62). In well-defined lipid monolayer systems, the addition of cholesterol produces lateral sterol domains (62). The formation of these domains appeared to correlate with the growth-promoting capacity of the sterols in a Mycoplasma capricolum system. Although cholesterol is the sterol preferred by most organisms, it has been established that sterol analogs having blocked 3 β-OH functions (e.g., cholesteryl methyl ether and cholesteryl acetate) can support the growth of M. capricolum nearly as well as cholesterol can (63,64). Similarly, ergosterol methyl ether can support the growth of Saccharomyces cerevisiae nearly as efficiently as the native yeast sterol ergosterol (64).
Although conventional techniques to purify rafts, e.g., detergent extraction of cell membranes, were first employed to study raft lipid composition, they might be affected by artifacts (2,65). Optical microscopy allows for a straightforward visualization of lipid domains in a noninvasive way (66,67). By detecting fluorescence from lipid probes expected to partition preferentially either into rafts or into nonraft phase, raftlike domains could be visualized in monolayers and supported bilayers (68–70). A systematic investigation of the morphology of raftlike domains as a function of cholesterol concentration has been also attempted (71,72). Sizes of artificial rafts are consistently on the micrometer scale (1–50 μm), in striking contrast to the putative raft size in cells (∼20–500 nm). This often raises the question whether what we observe in bilayer or monolayer membranes actually corresponds to a good model for rafts in vivo (72). Our impedance data clearly show that domains in bilayers composed of phosphatidylcholine and ergosterol exist, and have size close to the size of rafts in living cells. These data, together with the theoretical model proposed by us, indicate that phosphatidylcholine/ergosterol domains represent an accurate model for rafts and allow for investigation of raftlike properties under controllable conditions.
CONCLUSION
Application of impedance spectroscopy to the study of electrochemical behavior of lipid bilayers allows one to provide a quantitative description of equilibria in a two-component membrane. Based on derived mathematical equations, a new method for calculation of the surface area of the domain between phosphatidylcholine and ergosterol was proposed. The domain formation is the main reason for which deviation from rectilinearity of the parameters of the system described by the additivity rule is observed.
Data presented in this work, obtained from the mathematical derivation and confirmed experimentally are of great importance for the interpretation of phenomena occurring in lipid monolayers and bilayers. In our opinion, these results can help in a better understanding of biological membranes and with biophysical studies. A new, simple, and very interesting method proposed by us can be used with success for the quantitative determination of area and describe the stoichiometry of domains formed in any two-component system.
References
- 1.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- 2.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. [DOI] [PubMed] [Google Scholar]
- 3.Mendelssohn, R., and D. J. Moore. 1998. Vibrational spectroscopic studies of lipid domains in biomembranes and model systems. Chem. Phys. Lipids. 96:141–157. [DOI] [PubMed] [Google Scholar]
- 4.Lee, K. Y. C., J. F. Klingler, and H. M. McConnell. 1994. Electric field-induced concentration gradients in lipid monolayers. Science. 263:655–658. [DOI] [PubMed] [Google Scholar]
- 5.Mabrey, S., and J. M. Sturtevant. 1976. Investigation of phase transitions of lipid mixtures by high sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA. 73:3862–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somerharju, P. J., J. A. Virtanen, K. K. Eklund, P. Vainio, and P. K. J. Kinnunen. 1985. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 24:2773–2781. [DOI] [PubMed] [Google Scholar]
- 7.Chong, P. L. G., and I. P. Sugar. 2002. Fluorescence studies of lipid regular distribution in membranes. Chem. Phys. Lipids. 116:153–175. [DOI] [PubMed] [Google Scholar]
- 8.Yeagle, P. L. 1985. Cholesterol and the cell membrane. Biochim. Biophys. Acta. 822:267–287. [DOI] [PubMed] [Google Scholar]
- 9.Simons, K., and E. Ikonen. 2000. How cells handle cholesterol. Science. 290:1721–1725. [DOI] [PubMed] [Google Scholar]
- 10.Xu, X., and E. London. 2000. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 39:843–849. [DOI] [PubMed] [Google Scholar]
- 11.Rukmini, R., S. S. Rawat, S. C. Biswas, and A. Chattopadhyay. 2001. Cholesterol organization in membranes at low concentrations: effects of curvature stress and membrane thickness. Biophys. J. 81:2122–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder, F., J. K. Woodford, J. Kavexansky, W. G. Wood, and C. Joiner. 1995. Cholesterol domains in biological membranes. Mol. Membr. Biol. 12:113–119. [DOI] [PubMed] [Google Scholar]
- 13.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- 14.Edidin, M. 2003. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32:257–283. [DOI] [PubMed] [Google Scholar]
- 15.Bloch, K. E. 1983. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 14:47–92. [DOI] [PubMed] [Google Scholar]
- 16.Bagnat, M., S. Keranen, A. Shevchenko, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA. 97:3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rietveld, A., S. Neutz, K. Simons, and S. Eaton. 1999. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J. Biol. Chem. 274:12049–12054. [DOI] [PubMed] [Google Scholar]
- 18.Arora, A., H. Raghuraman, and A. Chattopadhyay. 2004. Influence of cholesterol and ergosterol on membrane dynamics: a fluorescence approach. Biochem. Biophys. Res. Commun. 318:920–926. [DOI] [PubMed] [Google Scholar]
- 19.Dołowy, K., A. Szewczyk, and S. Pikuła. 2003. Biological Membranes. Science Publishing “Silesia”. Katowice, Poland.
- 20.Benz, R., O. Frohlich, O. Lauger, and M. Montal. 1975. Electrical capacity of black films and of lipid bilayers made from monolayers. Biochim. Biophys. Acta. 374:323–334. [DOI] [PubMed] [Google Scholar]
- 21.Karolins, C., H. G. L. Coster, T. C. Chilcott, and K. D. Barrow. 1998. Differential effects of cholesterol and oxidized-cholesterol in egg lecithin bilayers. Biochim. Biophys. Acta. 1368:247–255. [DOI] [PubMed] [Google Scholar]
- 22.Naumowicz, M., A. D. Petelska, and Z. A. Figaszewski. 2003. Capacitance and resistance of the bilayer lipid membrane formed of phosphatidylcholine and cholesterol. Cell. Mol. Biol. Lett. 8:5–18. [PubMed] [Google Scholar]
- 23.Naumowicz, M., and Z. A. Figaszewski. 2003. Impedance analysis of phosphatidylcholine membranes modified with gramicidin D. Bioelectrochemistry. 61:21–27. [DOI] [PubMed] [Google Scholar]
- 24.Naumowicz, M., A. D. Petelska, and Z. A. Figaszewski. 2005. Impedance analysis of phosphatidylcholine-cholesterol system in bilayer lipid membranes. Electrochim. Acta. 50:2155–2161. [Google Scholar]
- 25.Kalinowski, S., and Z. A. Figaszewski. 1995. A four-electrode system for measurement of bilayer lipid membranes. Meas. Sci. Technol. 6:1043–1049. [Google Scholar]
- 26.Kalinowski, S., and Z. A. Figaszewski. 1995. A four-electrode potentiostat-galvanostat for studies of bilayer lipid membranes. Meas. Sci. Technol. 6:1050–1055. [Google Scholar]
- 27.Boukamp, B. A. 1989. Equivalent Circuit Users Manual, 2nd Ed. University of Twente, The Netherlands.
- 28.Demel, R. A., K. R. Bruckdorfer, and L. L. M. van Deenen. 1972. The effect of sterol structure on the permeability of liposomes to glucose, glycerol and Rb+. Biochim. Biophys. Acta. 255:321–330. [DOI] [PubMed] [Google Scholar]
- 29.Krysiński, P. 1982. Applications of pulse techniques in the investigations of artificial lipid membranes. Post. Biochim. 28:227–249. [PubMed] [Google Scholar]
- 30.Smondyrev, A. M., and M. L. Berkowitz. 2001. Molecular dynamics simulation of the structure of dimyristoylphosphatidylcholine bilayers with cholesterol, ergosterol, and lanosterol. Biophys. J. 80:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lecuyer, H., and D. G. Dervichian. 1969. Structure of aqueous mixtures of lecithin and cholesterol. J. Mol. Biol. 45:39–57. [DOI] [PubMed] [Google Scholar]
- 32.Levine, Y. K., and M. H. F. Wilkins. 1971. Structure of oriented lipid bilayers. Nature New Biol. 230:69–72. [DOI] [PubMed] [Google Scholar]
- 33.Liu, F., I. P. Sugar, and P. L. G. Chong. 1997. Cholesterol and ergosterol superlattices in three-component liquid crystalline lipid bilayers as revealed by dehydroergosterol fluorescence. Biophys. J. 72:2243–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Press, W. H., B. P. Flannery, S. A. Teukolsky, and W. T. Vetterling. 1986. Numerical Recipes: The Art of Scientific Computing. Cambridge University Press, New York. Chapt. 14.
- 35.Joos, P., and R. A. Demel. 1969. The interaction energies of cholesterol and phosphatidylcholine in spread mixed monolayers at the air-water interface. Biochim. Biophys. Acta. 183:447–457. [DOI] [PubMed] [Google Scholar]
- 36.Tien, H. T. 1974. Bilayer Lipid Membrane: Theory and Practice. Marcel Dekker, New York.
- 37.Jain, M. K. 1972. The Bimolecular Lipid Membrane. Litton Educational Publishing, New York. Chapt. 9.
- 38.Petelska, A., and Z. A. Figaszewski. 2000. Effect of pH on the interfacial tension of lipid bilayer membrane. Biophys. J. 78:812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen, O. S. 1984. Gramicidin channels. Annu. Rev. Physiol. 46:531–548. [DOI] [PubMed] [Google Scholar]
- 40.Urry, D. W., M. C. Goodall, J. D. Glickson, and D. F. Mayers. 1971. The gramicidin A transmembrane channel. Characteristics of head-to-head dimerized p(L, D) helices. Proc. Natl. Acad. Sci. USA. 68:1907–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler, W., J. Gaburjakova, M. Gaburjakova, B. Sivak, V. Rehacek, V. Tvarozek, and T. Hianik. 1998. Agar-supported lipid bilayers—basic structures for biosensor design. Electrical and mechanical properties. Colloids Surf. A Physicochem. Eng. Aspects. 140:357–367. [Google Scholar]
- 42.Semer, R., and E. Gelerinter. 1979. A spin label study of the effects of sterols on egg phosphatidylcholine bilayers. Chem. Phys. Lipids. 23:201–211. [Google Scholar]
- 43.Urbina, J. A., S. Pekerar, H. Le, J. Patterson, B. Montez, and E. Oldfield. 1995. Molecular order and dynamics of phosphatidylcholine bilayer membranes in the presence of cholesterol, ergosterol and lanosterol: a comparative study using 2H-, 13C- and 31P-NMR spectroscopy. Biochim. Biophys. Acta. 1238:163–176. [DOI] [PubMed] [Google Scholar]
- 44.Beach, D. H., L. J. Goad, and G. G. Holz, Jr. 1986. Effects of ketoconazole on sterol biosynthesis by Trypanosoma cruzi epimastigotes. Biochem. Biophys. Res. Commun. 136:851–856. [DOI] [PubMed] [Google Scholar]
- 45.Larralde, G., J. Vivas, and J. A. Urbina. 1988. Concentration and time dependence of the effects of ketoconazole on growth and sterol synthesis by Trypanosoma (Schizotrypanum) cruzi epimastigotes. Acta Cient. Venez. 39:140–146. [PubMed] [Google Scholar]
- 46.Urbina, J., K. Lazardi, T. Aguirre, M. M. Piras, and R. Piras. 1988. Antiproliferative synergism of the allylamine SF-86327 and ketoconazole on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 32:1237–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urbina, J., K. Lazardi, T. Aguirre, M. M. Piras, and R. Piras. 1991. Antiproliferative effects and mechanism of action of ICI 195,739, a novel bis-triazole derivative, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 35:730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urbina, J., K. Lazardi, E. Marchan, G. Visbal, T. Aguirre, M. M. Piras, R. Piras, R. A. Maldonado, and W. De Souza. 1993. Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotrypanum) cruzi: in vivo and in vitro studies. Antimicrob. Agents Chemother. 37:580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demel, R. A., K. R. Bruckdorfer, and L. L. M. van Deenen. 1972. Structural requirements of sterols for the interaction with lecithin at the air-water interface. Biochim. Biophys. Acta. 255:311–320. [DOI] [PubMed] [Google Scholar]
- 50.Demel, R. A., and B. De Kruyff. 1976. The function of sterols in membranes. Biochim. Biophys. Acta. 457:109–132. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh, D., and J. Tinaco. 1972. Monolayer interactions of individual lecithins with natural sterols. Biochim. Biophys. Acta. 266:41–49. [DOI] [PubMed] [Google Scholar]
- 52.Brzozowska, I., and Z. A. Figaszewski. 2002. The equilibrium of phosphatidylcholine-cholesterol in monolayers at the air/water interface. Colloids Surf. B Biointerf. 23:51–58. [Google Scholar]
- 53.Seoane, R., J. Minones, O. Conde, M. Casas, and E. Iribarnegaray. 1998. Molecular organisation of amphotericin B at the air-water interface in the presence of sterols: a monolayer study. Biochim. Biophys. Acta. 1375:73–83. [DOI] [PubMed] [Google Scholar]
- 54.Ohvo-Rekilä, H., B. Ramstedt, P. Leppimäki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41:66–97. [DOI] [PubMed] [Google Scholar]
- 55.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- 56.Edidin, M. 1997. Lipid microdomains in cell surface membranes. Curr. Opin. Struct. Biol. 7:528–532. [DOI] [PubMed] [Google Scholar]
- 57.Mason, R. P., T. N. Tulenko, and R. F. Jacob. 2003. Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathobiology. Biochim. Biophys. Acta. 1610:198–207. [DOI] [PubMed] [Google Scholar]
- 58.Bretscher, M. S., and S. Munro. 1993. Cholesterol and the Golgi apparatus. Science. 261:1280–1281. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee, S., and A. Chattopadhyay. 1996. Membrane organization at low cholesterol concentrations: a study using 7-nitrobenz-2-oxa-1,3-diazol-4-y-labled cholesterol. Biochemistry. 35:1311–1322. [DOI] [PubMed] [Google Scholar]
- 60.Engelman, D. M., and J. E. Rothman. 1972. The planar organization of lecithin-cholesterol bilayers. J. Biol. Chem. 247:3694–3697. [PubMed] [Google Scholar]
- 61.Houslay, M. D., and K. K. Stanley. 1982. Dynamics of Biological Membranes: Influence and Synthesis, Structure and Function. Wiley, New York.
- 62.Slotte, J. P. 1995. Lateral domain formation in mixed monolayers containing cholesterol and dipalmitoylphosphatidylcholine or N-palmitoylsphingomyelin. Biochim. Biophys. Acta. 1237:127–134. [DOI] [PubMed] [Google Scholar]
- 63.Odrizola, J. M., E. Waitzkin, T. I. Smith, and K. Bloch. 1978. Sterol requirement of Mycoplasma capricolum. Proc. Natl. Acad. Sci. USA. 75:4107–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lala, A. K., T. M. Buttke, and K. Bloch. 1979. On the role of the sterol hydroxyl group in membranes. J. Biol. Chem. 254:10582–10585. [PubMed] [Google Scholar]
- 65.Brown, D. A. 2001. Seeing is believing: visualization of rafts in model membranes. Proc. Natl. Acad. Sci. USA. 98:10517–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bagatolli, L. A., and E. Gratton. 1999. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys. J. 77:2090–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bagatolli, L. A., and E. Gratton. 2000. A correlation between lipid domain shape and binary phospholipid mixture composition in freestanding bilayers: a two-photon fluorescence microscopy study. Biophys. J. 79:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dietrich, C., L. A. Badatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Gratton. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dietrich, C., Z. N. Volovyk, M. Levi, N. L. Thompson, and K. Jacobson. 2001. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc. Natl. Acad. Sci. USA. 98:10642–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan, C., J. Furlong, P. Burgos, and L. J. Johnston. 2002. The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes. Biophys. J. 82:2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahya, N., D. Scherfeld, K. Bacia, B. Poolman, and P. Schwille. 2003. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 278:28109–28115. [DOI] [PubMed] [Google Scholar]
- 72.Kahya, N., D. Scherfeld, K. Bacia, and P. Schwille. 2004. Lipid domain formation and dynamics in giant unilamellar vesicles explored by fluorescence correlation spectroscopy. J. Struct. Biol. 147:77–89. [DOI] [PubMed] [Google Scholar]