Abstract
The avian leukosis virus (ALV) entry mechanism is controversial, with evidence for and against a low-pH requirement for viral fusion. To further address this question, we tested the entry of human immunodeficiency virus type 1 (HIV-1) pseudotyped with the envelope protein of subgroup B ALV (ALV-B) in the presence of three different lysosomotropic agents. These lysosomotropic agents were able to block the entry of wild-type and pseudotyped ALV-B in two different cell lines, strongly suggesting that ALV-B requires a low-pH step for entry. ALV-B and pH-dependent Semliki Forest virus (SFV) entered cells with slower uptake kinetics than HIV-1, which is pH independent. These slow uptake rates support the theory that ALV-B utilizes endocytic pathways to enter cells. Using immunofluorescence and electron microscopy analysis, we visualized the colocalization of virus particles with the endosomal marker transferrin and demonstrated virus particles in clathrin-coated vesicles and endosome-like structures. Surprisingly, a low-pH treatment did not overcome the inhibition of ALV-B entry by lysosomotropic agents. This indicates that, in contrast to SFV, ALV-B is unable to fuse at the cellular surface, even at a low pH. Taken together, our findings suggest that endocytosis and a subsequent low-pH step are critical for successful ALV-B infection.
Viruses exploit different cellular processes to infect and replicate in target cells. Endocytosis, an essential cell function, is used by several viruses to gain entry into target cells (41). Transport of virus particles into acidic compartments is a prerequisite for the fusion of enveloped pH-dependent viruses, e.g., alphaviruses (Semliki Forest virus [SFV] and Sindbis virus [25, 26, 35, 37, 39, 54]), flaviviruses (West Nile virus), Retroviridae (mouse mammary tumor virus [48]), Orthomyxoviridae (influenza A [50]), and Rhabdoviridae (vesicular stomatitis virus [21]). Low pH triggers a conformational change of the viral envelope protein, from a fusion-inactive to a fusion-active state, which leads to the exposure of the hydrophobic fusion peptide and allows lipid mixing between the viral and cellular membranes (19, 22, 55). Viral fusion in endosomes results in the release of the viral nucleocapsid into the cytosolic compartment.
Viral endocytosis is required in some instances for reasons other than pH dependence (40). For example, forced fusion of SFV with the plasma membrane by acid treatment renders a productive infection in baby hamster kidney (BHK-21) cells but not in Chinese hamster ovary (CHO) cells. This indicates the presence of a postentry barrier in CHO cells that is overcome by endocytosis of virus particles (36). Similarly, the entry mechanism of ecotropic murine leukemia virus (e-MLV) is cell type specific. e-MLV fuses directly at the cell surface in rat XC sarcoma cells but requires endocytosis in NIH 3T3 cells (29). In addition, human immunodeficiency virus type 1 (HIV-1) macropinocytosis plays an important role in macrophage infection, demonstrating the use of an endocytic pathway of a pH-independent virus (34). This indicates that the endocytic pathway might overcome cell-specific restrictions in viral entry for reasons other than pH dependence.
In the present study, we have examined the mechanism of retroviral entry by using subgroup B avian leukosis virus (ALV-B) as a model system. ALV is divided into seven major subgroups based on receptor usage (subgroups A through F and J). Subgroup specificity of ALV is determined by the viral envelope glycoprotein (Env). Env-receptor interactions play a critical role in the entry of ALV and are thought to trigger conformational changes that drive virus-cell fusion (1, 7, 11, 17, 34). Entry of ALV-B into target cells is mediated by the primary binding receptor, TVBS3, which belongs to the tumor necrosis factor receptor superfamily (11). TVBS3 contains three extracellular cysteine-rich domains, a single transmembrane region, and a cytoplasmic “death domain.” Activation of TVBS3 is able to trigger apoptotic pathways as well as an NF-κB protective pathway (10, 13). Determinants for cell killing have been mapped to the receptor-binding domain of ALV-B Env (17). However, direct involvement of TVBS3 in cytopathic effects remains to be shown. Two homologues of TVBS3 have been identified, TVBS1 and TVBT. TVBS1 mediates entry of ALV subgroups B, D, and E, while the turkey homologue, TVBT, confers susceptibility to ALV-E only (1, 2).
The determinants for ALV-B entry have been mapped to a 15-amino-acid peptide (TVB32-46) in the N-terminal region of TVBS3. This peptide functions as a binding receptor and mediates ALV-B entry into TVBS3-negative cells (30). The mechanism of ALV entry is controversial, and there is evidence for and against a low-pH requirement for viral fusion. Gilbert and colleagues demonstrated that lysosomotropic agents, which neutralize endocytic compartments, do not inhibit ALV-A and ALV-C entry by using the infectivity and fluorescence fusion-dequenching assays (23). Hernandez and colleagues demonstrated that ALV-A Env interaction with liposomes is pH independent by using a liposome-binding assay (27). In contrast, Mothes and colleagues demonstrated that entry of ALV-A and ALV-B is blocked by lysosomotropic agents by using a PCR-based assay to monitor viral entry (42). The studies of Mothes and colleagues suggested a role of endocytosis in ALV entry that has not been directly addressed.
To further evaluate the mechanism of ALV entry, we generated HIV-1 virus particles pseudotyped with the envelope protein of ALV-B. We found that three different lysosomotropic agents were able to block the entry of pseudotyped and wild-type ALV-B. Viral uptake kinetics showed that ALV-B internalization was comparable to SFV uptake, which requires endocytosis. Immunofluorescence and ultrastructural analysis of chicken embryo fibroblasts infected with ALV-B identified virus particles in clathrin-coated vesicles and endosome-like structures. We also demonstrated the inability of ALV-B to fuse at the cell surface when provided with a low external pH, which, in contrast, forces the fusion of SFV with the cell surface. Taken together, our findings provide evidence that endocytosis is required for successful ALV-B entry.
MATERIALS AND METHODS
Cell lines, plasmids, and ALV-B.
293T cells and DF-1 chicken embryonic fibroblasts were obtained from the American Type Culture Collection. HEK 293 cells have been described elsewhere (1). U-251 human astrocytoma cells were obtained from David Weinstein (52). Cells were grown in complete Dulbecco's modified Eagle medium (DMEM medium; Cellgro, Herndon, Va.) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. HEK 293 cells, seeded on 100-mm-diameter tissue culture plates, were transfected with 10 μg of pBK-TVBS3 (11) and 1 μg of pPur vector (Clontech, Palo Alto, Calif.) with 20 μl of lipofectamine (Gibco/BRL, Rockville, Md.). Single-cell clones were selected in 1 μg of puromycin/ml (Clontech) and isolated by using cloning cylinders. U-251 cells, seeded on 100-mm-diameter tissue culture plates, were transfected with 10 μg of pBK-TVBS3 with 20 μl of lipofectamine. Single-cell clones were selected in 1 mg of Geneticin (Gibco/BRL)/ml and isolated by the same procedure. Wild-type ALV-B was produced by transfecting DF-1 cells with pRCASBP(B)-eGFP (42). Supernatant was collected and stored at −80°C 48 h after transfection. Viral titers were expressed as endpoint dilutions determined by infecting DF-1 cells.
Pseudotyped virus production.
A total of 3 × 106 293T cells seeded on 100-mm-diameter tissue culture plates was cotransfected with 5 μg of NLluc+env− and 15 μg of envelope-expressing plasmids by following the calcium phosphate procedure described previously (14). Pseudotyped particles were collected and stored at −80°C 48 h after transfection. Human T-cell leukemia virus type 1 (HTLV-1), SFV, and amphotropic murine leukemia virus (a-MLV) envelope-expressing plasmids were obtained from Tanya Dragic (31, 45). p24 levels of pseudotyped viruses were measured by using the HIV-1 p24 enzyme-linked immunosorbent assay kit (NEN, Boston, Mass.).
Viral entry and luciferase assay.
Pseudotyped viruses were prebound to 105 cells on 24-well tissue culture plates at 4°C for 1 h. Supernatant was removed, and fresh medium with or without lysosomotropic agents was incubated at 37°C for 4 h. Subsequently, medium was replaced with complete DMEM and cellular extracts were prepared 48 h after infection to assay luciferase activity. Extracts were made with 250 μl of luciferase lysis buffer (Promega, Madison, Wis.), and after one freeze-thaw cycle, samples were centrifuged at 12,000 × g for 5 min. Ninety microliters of the supernatant was mixed with 30 μl of luciferase substrate (Promega) and incubated for 3 min at room temperature. Luciferase activity was measured with a luminometer (PerkinElmer Wallac Inc., Boston, Mass.).
Lysosomotropic agents and inhibitors.
Ammonium chloride, chloroquine, bafilomycin A1, and chlorpromazine were obtained from Sigma (St. Louis, Mo.). The following concentrations were used in the inhibition experiments: 50 mM ammonium chloride, 100 μg of chloroquine/ml, 50 nM bafilomycin A1, and 50 μM chlorpromazine. None of these concentrations affected the pH of the cell culture medium.
Immunoblotting.
Cell lines were grown on 100-mm-diameter tissue culture plates to a confluency of 90%. Extracts were prepared with a homogenization buffer (10 mM Tris [pH 7.5], 10 mM NaCl, 1 mM EDTA) containing a cocktail of protease inhibitors (Roche, Indianapolis, Ind.). Cells were homogenized in a Dounce-fitted homogenizer and centrifuged at 2,500 × g to obtain postnuclear supernatants. Protein concentration was determined using a bicinchoninic acid protein assay reagent kit (Pierce, Rockford, Ill.). Ten micrograms of protein was applied to a 10% polyacrylamide-sodium dodecyl sulfate gel under reducing conditions and transferred to nitrocellulose membranes. The membranes were probed with soluble ALV-B Env fusion proteins (SUB-immunoglobulin G [11]) to detect TVBS3 and subsequently incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin (Amersham, Piscataway, N.J.). The TVBS3 signal was detected by using an enhanced chemiluminescence kit (Amersham).
Immunofluorescence of pseudotyped viruses and human transferrin internalization.
Cells grown overnight on 12-mm-diameter coverslips were incubated with pseudotyped virus particles and human transferrin-Alexa 488 (Molecular Probes, Eugene, Oreg.) at a concentration of 50 μg/ml at 4°C for 1 h. Infection and transferrin uptake were initiated by incubating cells at 37°C for 1 h. Samples were fixed in 3.9% paraformaldehyde (Sigma) in phosphate-buffered saline (PBS; Cellgro) for 30 min. Cells were washed in PBS and incubated in 0.1 M glycine (Sigma) for 10 min, followed by washing in PBS and permeabilization with 0.05% saponin (Sigma) for 30 min. Samples were blocked with 10% donkey serum (Dako, Carpinteria, Calif.) for 30 min, incubated with anti-p24 antibody AG3.0 (National Institutes of Health AIDS Research and Reference Reagent Program) for 1 h, washed with PBS, and incubated with anti-mouse Cy3-labeled antibody (Jackson Immunoresearch, West Grove, Pa.) for 45 min. Samples were mounted for fluorescence microscopy by using the ProLong antifade kit (Molecular Probes).
Forced fusion at the cell surface.
Pseudotyped viruses were prebound to 105 cells. Cells were incubated with DMEM, pH 7.3 (complete medium without sodium bicarbonate and with 20 mM HEPES, adjusted to pH 7.3), or DMEM, pH 5.3 (complete medium without sodium bicarbonate and with 10 mM MES [morpholineethanesulfonic acid], adjusted to pH 5.3), at 37°C for 2 min. Subsequently, cells were incubated in complete DMEM in the presence or absence of 50 mM ammonium chloride for 4 h. Fresh medium was added to the cells, and the luciferase activity of cellular extracts was measured 48 h postinfection.
Electron microscopy analysis.
DF-1 cells were grown to confluency and incubated with 2 ml of ALV-B (108 infectious units/ml) at 4°C for 1 h. Cells were incubated at 37°C for 1 h to allow infection. Samples were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, postfixed with 1% osmium tetroxide followed by 1% uranyl acetate, dehydrated through a graded series of ethanol washes, and embedded in LX112 resin (LADD Research Industries, Burlington, Vt.). Ultrathin sections were cut on a Reichert Ultracut E apparatus, stained with uranyl acetate followed by lead citrate, and viewed on a JEOL 1200EX transmission electron microscope at 80 kV.
Exhaustive photon reassignment deconvolution and 3-D recontruction.
Z sections were acquired by using an Olympus (Melville, N.Y.) AX70 wide-field fluorescense microscope with a CH-350 15-bit, cooled charge-coupled camera with a PlanApo 60×, 1.4 NA objective (Olympus). Iplab software (Scanalytics, Fairfax, Va.) was used to prepare images for iterative deconvolution. A tridimensional (3-D) data set composed of 30 images separated by 200 nm in the axial direction was deconvolved with an acquired point spread function (PSF) by using exhaustive photon reassignment (Scanalytics). The 3-D recontruction of deconvolved images was performed by using IMARIS version 3.0 software (Bitplane AG, Zurich, Switzerland) generously provided by Robert H. Singer. The PSF data set was made by acquiring 60 images, each separated by 200 nm, in the axial direction of a fluorescent microsphere that was 200 nm in diameter (Molecular Probes).
RESULTS
HIV-1 pseudotyped with the envelope protein of ALV-B.
To study the entry mechanism of ALV-B, we used an HIV-1 vector pseudotyped with the envelope glycoprotein of ALV-B. The NLluc+env− HIV-1 vector contains the luciferase gene inserted into the nef open reading frame (14), which provides luciferase activity as a reporter of infection. This replication-deficient system contains the HIV-1 proteins Tat and Rev and requires infection of human cells for viral gene expression (8). However, mammalian cells are not susceptible to ALV-B infection unless they express the cognate receptor (11). Therefore, TVBS3, the primary binding receptor for ALV-B, was stably expressed in human HEK 293 and U-251 astrocytoma cells (Fig. 1A). Single-cell clones were screened by their susceptibility to infection by ALV-B-eGFP, a virus that expresses the enhanced green fluorescent protein (eGFP) upon infection (42). Three single-cell clones were highly permissive to ALV-B-eGFP infection as measured by flow cytometry. These clones displayed receptor expression levels comparable to those for chicken embryonic fibroblasts (DF-1), which endogenously express TVBS3 (Fig. 1A).
FIG. 1.
Entry assay of an HIV-1-based vector pseudotyped with the envelope protein of ALV-B. (A) Astrocytoma cells (U-251) and HEK 293 cells were stably transfected with TVBS3-expressing vector. Extracts from chicken embryonic fibroblasts (DF-1) and human cell lines transfected with or without TVBS3 were prepared, and 10 μg of protein was analyzed by Western blot analysis to detect TVBS3 expression. (B) HIV-1 vector (NLluc+env−) was pseudotyped with the envelope protein of ALV-B. Parental and HEK 293-TVBS3 cells were infected for 3 h with pseudotyped ALV-B viruses (10 ng of p24). Supernatant was removed and replaced with fresh medium, and luciferase activity was measured 48 h postinfection. (C) Parental and U-251-TVBS3 were infected with pseudotyped ALV-B (10 ng of p24) as described above for panel B. Experiments were performed in triplicates, and standard deviations are indicated for panels B and C.
HEK 293 and U-251 cells expressing TVBS3 were tested for susceptibility to infection by HIV-1 pseudotyped with the envelope protein of ALV-B. These cells were prebound with pseudotyped particles at 4°C for 1 h and incubated at 37°C for 3 h. Luciferase activity was measured in extracts prepared from infected cells 48 h postinfection. These experiments demonstrated that HEK 293-TVBS3 and U-251-TVBS3, in contrast to parental cell lines, were highly susceptible to infection by pseudotyped ALV-B particles (Fig. 1B and C).
Lysosomotropic agents block the entry of ALV-B.
To evaluate the requirement of a low-pH compartment in ALV-B entry, we used lysosomotropic agents that block the acidification of endocytic vesicles (49). As positive controls for pH-dependent viruses, we used HIV-1 vectors pseudotyped with the envelope protein of SFV. As controls for pH-independent viruses, we used HIV-1 viruses pseudotyped with the envelope protein of a-MLV and HTLV-1 (3, 41). The amount of virus used in the infection experiments was standardized by the amount of p24 (Table 1).
TABLE 1.
Luciferase activity and p24 levels of HIV-1-vector-pseudotyped particles with different envelope proteins
| Envelope | Luciferase activity (RLU/ml)a | p24 level (ng/ml)b |
|---|---|---|
| Env− | 865 ± 208 | 0.17 ± 0.03 |
| ALV-B | 431,053 ± 26,549 | 6.93 ± 0.29 |
| SFV | 3,520,743 ± 195,251 | 81.87 ± 7.38 |
| a-MLV | 1,627,197 ± 44,996 | 55.04 ± 5.00 |
| HTLV-1 | 21,792 ± 5,052 | 7.00 ± 0.70 |
Activity in relative light units per milliliter of pseudotyped viral supernatant was determined by infecting HEK 293-TVBS3 cells with pseudotyped viruses and measuring luciferase activity in cell extracts 48 h postinfection.
Level in nanograms of p24 protein per milliliter of supernatant containing pseudotyped viruses was determined by measuring p24 levels in viral supernatants as described in Materials and Methods.
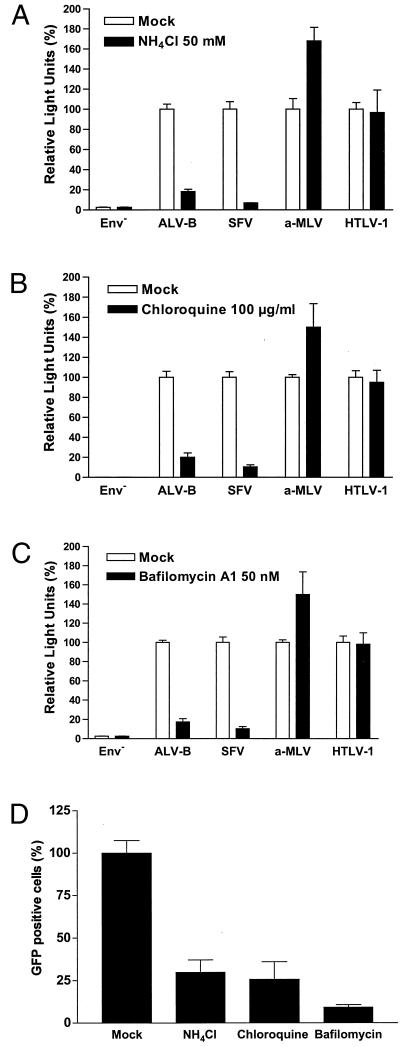
The lysosomotropic agent ammonium chloride, a weak base that selectively accumulates in endocytic compartments, was tested in an entry assay with several pseudotyped viruses. Ammonium chloride, at a concentration of 50 mM, reduced the entry of pseudotyped ALV-B and SFV by 91.7 and 93.1%, respectively (Fig. 2A). We further tested the effect of an additional inhibitor, chloroquine, a weak base that also accumulates in endocytic compartments and increases the endosomal pH. Chloroquine, at a concentration of 100 μg/ml, reduced the entry of pseudotyped ALV-B and SFV by 79.8 and 89.5%, respectively (Fig. 2B). To prevent the acidification of endocytic compartments by a different mechanism, we used bafilomycin A1 (44), a specific blocker of v-type H+-ATPase (9). This drug blocked the entry of pseudotyped ALV-B and SFV by 82.5 and 89.5%, respectively, at a concentration of 50 nM (Fig. 2C). As expected, these lysosomotropic drugs were unable to block the entry of pseudotyped a-MLV and HTLV-1, indicating that the inhibition of ALV-B and SFV was caused by blocking endosomal acidification and not by nonspecific cytotoxic effects. Similar results were obtained using the astrocytoma cell line U-251-TVBS3-3 (data not shown). Additionally, entry of wild-type ALV-B-eGFP into HEK 293-TVBS3 cells was also blocked by lysosomotropic agents as analyzed by flow cytometry (Fig. 2D). These results showed that ALV-B requires a low-pH compartment to develop a productive infection.
FIG. 2.
Entry of wild-type and pseudotyped ALV-B is inhibited by lysosomotropic agents. HEK 293-TVBS3 cells were incubated with pseudotyped ALV-B, SFV, a-MLV, or HTLV-1 for 4 h in the presence or absence of 50 mM NH4Cl (A), 100 μg of chloroquine/ml (B), or 50 nM bafilomycin A1 (C). Cells were challenged with pseudotyped ALV-B, SFV, a-MLV, and HTLV-1 with similar amounts of p24.Luciferase activity was measured from cellular extracts 48 h postinfection. (D) HEK 293-TVBS3 cells were incubated with wild-type ALV-B that expresses GFP (MOI of 10) in the presence or absence of 50 mM NH4Cl, 100 μg of chloroquine/ml, or 50 nM of bafilomycin A1 for 4 h. Infection was determined by flow cytometry analysis by counting GFP-positive cells 72 h postinfection. Experiments were performed in triplicate, and standard deviations are indicated.
Entry kinetics of ALV-B.
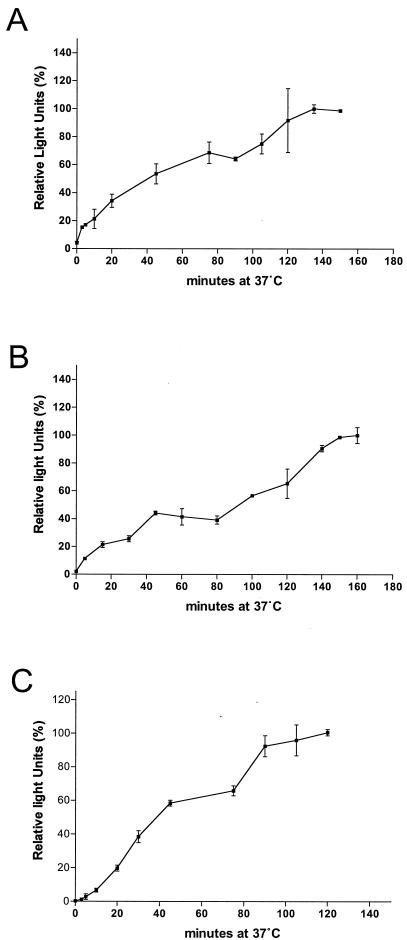
The low-pH requirement of wild-type and pseudotyped ALV-B strengthens the argument that endocytic pathways are involved in ALV-B entry. To evaluate the time required for pseudotyped ALV-B particles to travel from the cell surface to the fusion compartment and fuse with cellular membranes, we determined the kinetics of ALV-B entry. Pseudotyped ALV-B particles were prebound to cells at 4°C for 1 h. Infection was initiated by shifting the cells to 37°C, and 50 mM ammonium chloride was added at various times to prevent fusion (Fig. 3A and B). This experiment demonstrated that 45 to 50 min is required for the half-maximal internalization and fusion of pseudotyped ALV-B particles in two different cell types. Pseudotyped SFV, an additional pH-dependent virus, required 35 min to internalize and fuse half of the virus (Fig. 3C). This showed that ALV-B and SFV required a similar amount of time to travel from the cell surface to the fusion compartment.
FIG. 3.
Entry kinetics of pseudotyped ALV-B. Pseudotyped ALV-B (10 ng of p24) was prebound to HEK 293-TVBS3 (A) or U-251-TVBS3 (B) cells at 4°C for 1 h. Infection was initiated by shifting the temperature to 37°C. At the indicated time point, cells were incubated for 2 to 3 h with 50 mM NH4Cl to stop viral entry. Subsequently, supernatants were replaced with fresh medium and luciferase activity was determined 48 h postinfection. Pseudotyped SFV was prebound to HEK 293-TVBS3 cells, and entry kinetics were determined as described above (C). Experiments were performed in triplicate, and standard deviations are shown.
ALV-B particles colocalized with endosomal markers.
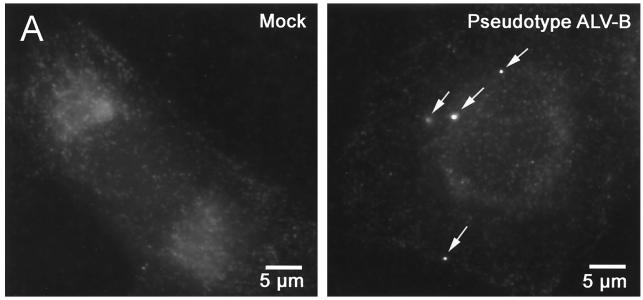
To visualize the fate and cellular localization of internalized virus particles, we used fluorescence microscopy. Pseudotyped virus particles were visualized by immunofluorescence with an anti-p24 (HIV-1 Gag protein) antibody (20, 33, 34). Experiments using pseudotyped SFV and ALV-B demonstrated discrete staining of virus particles with anti-p24 antibodies (Fig. 4A). The average numbers of virus particles per cell in ALV-B and SFV infections were 3.7 and 15.5, respectively. These numbers correlate with the ratio of luciferase activity produced by pseudotyped ALV-B and SFV (Table 1).
FIG. 4.
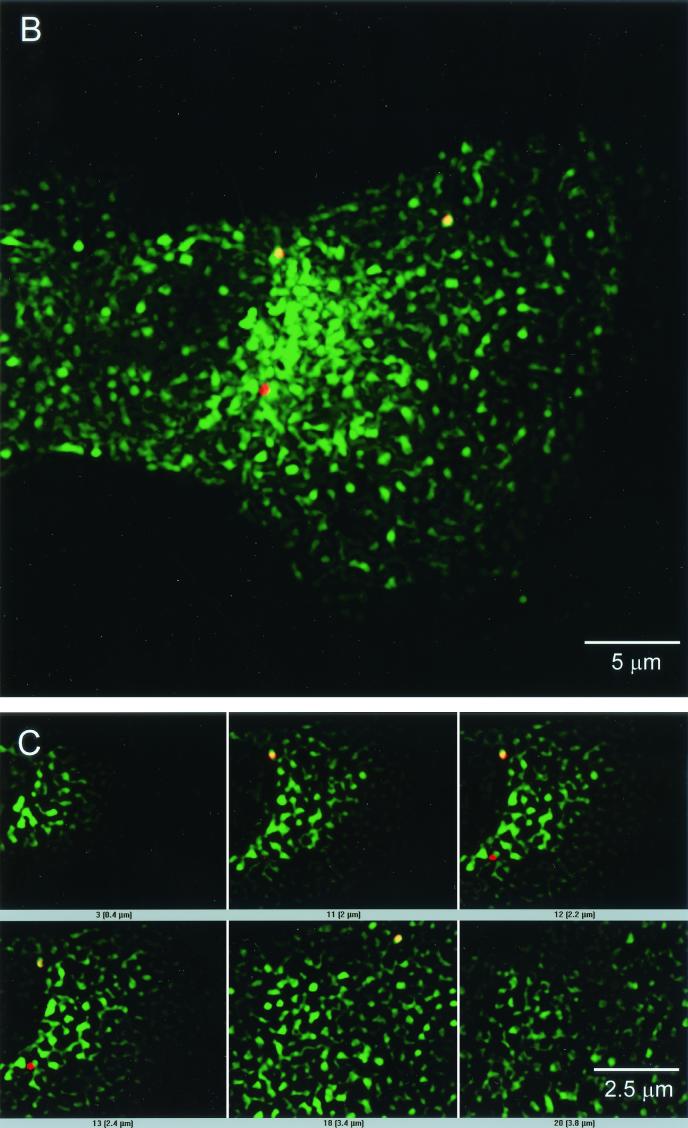
Pseudotyped ALV-B particles colocalize with an endosomal marker. (A) Pseudotyped ALV-B particles (10 ng of p24) were prebound to U-251-TVBS3-3 cells, and infection was started by shifting cells to 37°C. Cells were processed for immunofluorescence, stained using the anti-p24 antibodies, and developed with a Cy3-labeled antibody (arrows). (B) Pseudotyped ALV-B and human transferrin-Alexa 488 (green) were prebound to U-251-TVBS3-3 cells at 4°C for 1 h. Viral infection and transferrin uptake were initiated by shifting the temperature to 37°C. After 30 min, cells were processed for immunofluorescence with an anti-p24 antibody and developed with a Cy3-labeled antibody (red). Z sections were obtained in both filters (Alexa 488 and Cy3) of a wide-field fluorescence microscope. Images were deconvolved as described in Materials and Methods. The 3-D projection of an infected cell (30 sections) was done in Imaris version 3.0, showing pseudotyped ALV-B particles (red), endosomes (green), and colocalization (yellow). (C) Individual Z sections are shown, and the distance from the cell surface is indicated in micrometers.
To gain a better understanding of the cellular localization of virus particles, we labeled the endocytic pathway with human transferrin-Alexa 488. U-251-TVBS3-3 cells were challenged with pseudotyped ALV-B and SFV and incubated with labeled transferrin at 37°C for 30 min. Virus particles were identified by immunofluorescence with the anti-p24 antibody. Wide-field fluorescence and iterative image deconvolution with an acquired PSF were used to determine whether virus particles colocalized with endosomes. This procedure resolves the spatial separation of objects at a resolution of 100 nm (12). Thirty sections in the Cy3 (virus) and Alexa 488 (human transferrin) channels with a separation of 200 nm, used for deconvolution and reconstruction, showed that pseudotyped ALV-B particles colocalized with endosomal vesicles (Fig. 4B). From 15 independent experiments, we found that 73% of 200 pseudoyped ALV-B particles colocalized with endosomes. Similarly, we found that 64% of 200 pseudoyped SFV particles colocalized with endosomes (data not shown). Montage images of cells infected with pseudotyped ALV-B viruses identified several virus particles at different cellular planes (Fig. 4C), indicating the intracellular localization of pseudotyped ALV-B particles.
Ultrastructural analysis of ALV-B in chicken embryonyic fibroblasts.
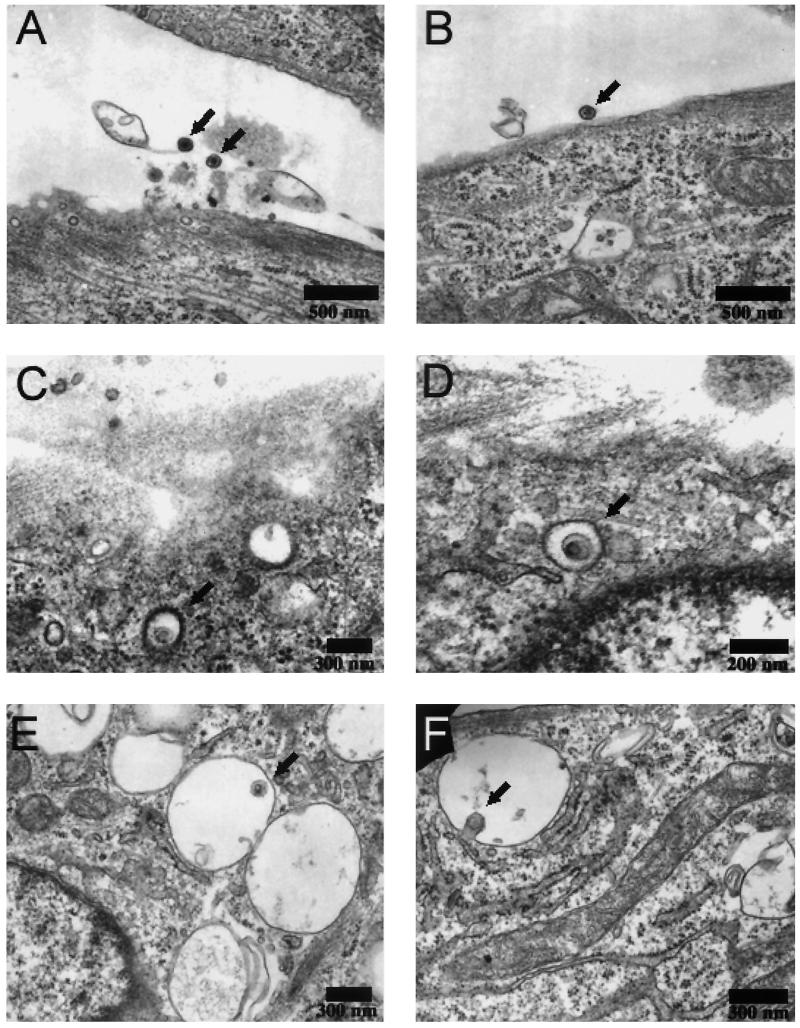
To further address the subcellular localization of ALV-B, electron microscopic studies were performed with the wild-type virus. Chicken embryonic fibroblasts were challenged with ALV-B at a multiplicity of infection (MOI) of 100 and processed for electron transmission microscopy. ALV-B particles were observed at the cell surface of fibroblasts with an average size of 100 nm (Fig. 5A and B). Virus particles were also observed in 200-nm vesicles with the characteristic morphology of clathrin-coated vesicles (Fig. 5B and C). We also observed virus particles in non-clathrin-coated cellular vesicles, which are several times bigger than virus particles (Fig. 5E). In addition, some virus particles appeared to be in the process of fusing with the vesicular membrane (Fig. 5F). Taken together, these data demonstrate, for the first time, the presence of ALV particles in endocytic and clathrin-coated vesicles.
FIG. 5.
Wild-type ALV-B is in clathrin-coated vesicles and endosome-like structures. Wild-type ALV-B viruses were prebound to DF-1 cells at 4°C for 1 h. Infection was initiated by shifting the temperature to 37°C. Thirty minutes after infection was initiated, samples were processed for ultrastructural analysis. Virus particles were observed (arrows) at a magnification of ×12,000 at the surface of DF-1 cells (A and B), in clathrin-coated vesicles (C and D), and in vesicles (E) while fusing to the vesicle membrane (F).
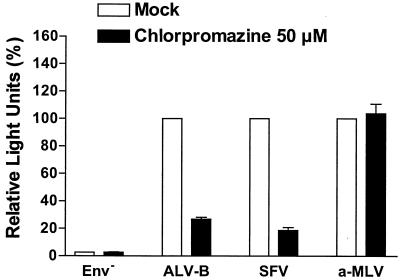
Since our ultrastructural analysis suggested the involvement of clathrin in the entry of ALV-B, we tested ALV-B entry in the presence of chlorpromazine, a specific inhibitor of clathrin-mediated endocytosis. Chlorpromazine, a cationic amphiphilic drug, disrupts clathrin-mediated uptake by relocating clathrin and adaptor protein 2 complexes from the cell surface (47, 51). Chlorpromazine, at a concentration of 50 μM, reduced the entry of pseudotyped ALV-B and SFV by 73.2 and 81.3%, respectively (Fig. 6). SFV was used as a positive control for viruses that require clathrin-mediated endocytosis (16, 18, 37). As expected, chlorpromazine did not block a-MLV entry, which does not require endocytosis (Fig. 6). These results provided further evidence that clathrin is involved in ALV-B entry.
FIG. 6.
Entry of pseudotyped ALV-B is inhibited by chlorpromazine. HEK 293-TVB S3 cells were incubated with pseudotyped ALV-B, SFV, or a-MLV for 3 h in the presence or absence of 50 μM chlorpromazine. Cells were challenged with pseudotyped ALV-B, SFV, or a-MLV by using similar amounts of p24. Luciferase activity was measured from cellular extracts 48 h postinfection. Experiments were performed in triplicate, and standard deviations are shown.
Low-extracellular-pH treatment does not overcome the ammonium chloride block of ALV-B entry.
pH-dependent viruses require the acidic environment of endosomes for viral fusion (35, 53, 54). However, in the case of SFV, the viral endocytosis requirement can be overcome by providing a transient acidic external pH, which forces viral fusion at the cell surface (36, 54). To test whether a transient low-pH treatment could also force fusion of ALV-B at the cellular surface, we treated cells prebound with wild-type and pseudotyped ALV-B with medium at either pH 7.3 or 5.3 at 37°C for 2 min. Subsequent to this treatment, the supernatant was replaced with medium containing or lacking 50 mM NH4Cl and cellular extracts were prepared 48 h postinfection to measure luciferase activity. Cells infected with wild-type ALV-B-eGFP were analyzed by flow cytometry. ALV-B-challenged cells yielded equivalent levels of infection in the presence or absence of a low-pH step, indicating that a low-pH treatment does not inactivate ALV-B (Fig. 7B and C). Strikingly, acid treatment of pseudotyped ALV-B did not overcome the entry inhibition caused by ammonium chloride in HEK 293-TVBS3 cells (Fig. 7B). We observed similar results in experiments using wild-type ALV-B (Fig. 7C). In contrast, a low-pH treatment overcame the ammonium chloride inhibition of SFV entry in HEK 293-TVBS3 cells (Fig. 7A), presumably by forcing viral fusion at the cell surface.
FIG.7.
Wild-type and pseudotyped ALV-B were unable to fuse at the cell surface upon low-pH treatment. Pseudotyped SFV (10 ng of p24) (A), pseudotyped ALV-B (10 ng of p24) (B), and wild-type ALV-B (MOI = 10) (C) were prebound to HEK 293-TVBS3 cells at 4°C for 1 h. Cells were treated with a medium, pH 5.3 or 7.0, for 2 min at 37°C. Subsequently, cells were incubated in fresh medium for 4 h in the presence or absence of 50 mM NH4Cl. Supernatants were replaced with fresh medium, and luciferase activity was measured 48 h postinfection (A and B). Flow cytometry analysis for GFP-positive cells was used to determine infection by wild-type ALV-B (C). Pseudotyped ALV-B (10 ng of p24) was prebound to HEK 293-TVBS3 cells at 4°C for 1 h. Cells were treated with a medium, pH 5.3, containing or lacking 50 mM NH4Cl for 5, 15, or 30 min at 37°C. Subsequently, cells were incubated with medium containing 50 mM NH4Cl for 4 h. Cells were grown in fresh medium, and extracts were prepared 48 h postinfection to measure luciferase activity (D). Experiments were performed in triplicate, and standard deviations are shown.
To test if longer exposure to an acidic pH could force the fusion of pseudotyped ALV-B with the plasma membrane, we treated cells prebound with pseudotyped ALV-B at pH 5.3 at 37°C for 5, 15, and 30 min in the presence or absence of 50 mM NH4Cl (Fig. 7D). Subsequently, cells were incubated with 50 mM NH4Cl for 4 h and luciferase activity was determined from cellular extracts 48 h postinfection. Extended incubation at pH 5.3 in the presence of NH4Cl did not promote fusion of pseudotyped ALV-B with the plasma membrane. These results indicated that the inability of pseudotyped ALV-B to fuse with the cell surface does not depend on time. In the absence of NH4Cl, infection proceeds normally even in the presence of a low-pH medium (Fig. 7D). Taken together, our results show that forced fusion of ALV-B at the cell surface does not occur, suggesting that additional factors besides low pH are required for ALV-B entry.
DISCUSSION
To study the role of endocytosis in ALV-B entry, we have used several approaches. We initially established an entry assay for ALV-B that depends on luciferase activity. The HIV-1 luciferase reporter system pseudotyped with ALV-B Env allows for the easy quantification of productive infection and is more sensitive than reporter systems using the β-galactosidase or GFP (14, 32).
Human 293 and astrocytoma cells are nonsusceptible to ALV-B infections. We demonstrated that expression of the ALV-B receptor TVBS3 renders these cell lines highly permissive to ALV-B infections.
pH-dependent viruses require endocytosis to reach acidic cellular compartments in order to mediate viral fusion with cellular membranes. We demonstrated that three independent lysosomotropic agents are able to block ALV-B entry, supporting the theory that ALV-B entry is pH dependent (42). As expected, these inhibitors were able to block entry by pH-dependent SFV but not by pH-independent a-MLV or HTLV-1 (3, 41). Surprisingly, the use of these lysosomotropic drugs enhanced a-MLV infection, suggesting that inhibition of degradation pathways by lysosomotropic agents increases the apparent titer of this virus. It is therefore possible that pH-independent viruses also use endocytic pathways to enter cells, as has been observed with HIV-1 (34). Our data suggest that endocytosis allows ALV-B to reach acidic compartments where viral fusion occurs.
Pseudotyped virus particles can acquire different proteins and lipids from the host producer cell that might affect the viral fusion properties of the virus, as shown for HIV-1 (24, 28, 43). However, we have observed that HIV particles pseudotyped with the envelope protein of SFV, a-MLV, or HTLV-1 preserve their corresponding fusion phenotype in our entry assay. Furthermore, we have shown that wild-type and pseudotyped ALV-B do not differ in their susceptibility to lysosomotropic agents.
Viruses that fuse at the cell surface are expected to enter cells with different kinetics than viruses that require endocytosis. Analysis of ALV-B entry kinetics with ammonium chloride to block fusion showed that ALV-B and SFV require 45 and 35 min, respectively, to reach the fusion compartment and fuse. In contrast, pH-independent viruses that have been described to fuse at the cellular surface, such as HIV-1, require only 10 min for half-maximal internalization of virus particles (46). Our data suggest that ALV-B entry is a relatively slow process and may involve endocytosis.
Furthermore, we established an immunofluorescence assay that allows for the visualization of virus particles by using an antibody against p24, a structural HIV-1 core protein. Antibodies against p24 have been previously used to visualize HIV-1 particles in cells (20, 33, 34). Colocalization of pseudotyped ALV-B particles with transferrin, a marker for endocytic compartments, indicates the presence of virus particles in endosomes, which has also been shown biochemically for SFV (35). A fraction of the intracellular pseudotyped viruses did not colocalize with endosomes, suggesting that these particles represent cytoplasmic postfusion viral cores. A 3-D reconstruction, using immunofluorescence, showed that virus particles were in separate section planes, indicating the intracellular localization of these particles.
Additional support for the presence of ALV-B in endocytic compartments came from ulltrastructural analysis. Electron microscopy analysis of chicken embryonic fibroblasts infected with ALV-B showed virus particles on the cellular surface, in clathrin-coated pits, and in endosomes. We challenged cells at low MOIs to study ALV-B entry under more physiological conditions (in the case of SFV, an MOI of 105 was used [25, 38]). In contrast, infections at high MOIs could promote viral uptake through alternative internalization pathways. While it is impossible to distinguish between replication-competent and replication-deficient viruses by immunofluorescence and electron microscopy, these studies further support the notion that ALV-B uses endocytic pathways to enter cells. In addition, we were able to block pseudotype ALV-B entry with chlorpromazine, a drug that specifically disrupts clathrin-mediated endocytosis (51). Interestingly, this drug was also able to block SFV entry, which has been shown through different lines of experimental evidence to be clathrin dependent (16, 25, 26).
The fusion of some enveloped pH-dependent viruses, such as vesicular stomatitis virus and SFV, can be forced at the cellular surface by a low external pH. We observed that pseudotyped SFV was able to fuse at the cell surface by a low-pH treatment, which leads to a productive infection. Surprisingly, wild-type and pseudotyped ALV-B were unable to fuse at the cellular surface at low pH, even though ALV-B, like SFV, is a pH-dependent virus. This result supports previous data shown by Mothes and colleagues (42), where a low-pH step is unable to overcome the lysosomotropic inhibition of ALV entry. However, Mothes and colleagues were able to overcome this inhibition when the low-pH treatment was preceded by an endocytosis step of 15 min at a neutral pH. These results suggest that ALV-B is unable to fuse at the cellular surface even under low-pH conditions. Therefore, it seems that there are factors in addition to Env-receptor interactions and to the appropriate pH that determine the ability of ALV-B to fuse with the cellular membrane. For example, entry by e-MLV requires the involvement of a cellular protease to activate the fusion process (4-6). The cellular localization of these potential factors might determine the mechanism by which specific viruses enter cells.
The inability of ALV-B to fuse at the cell surface is unexpected because ALV-B-infected cells are able to form syncytia with TVBS3-expressing cells at a low pH (42). However, the capacity of some viruses, such as HIV-1 and a-MLV, to form syncytia is virus strain and cell type specific (41). Therefore, parameters and conditions that drive syncytium formation appear to be distinct from those that are required for viral fusion with cellular membranes (15, 56).
Taken together, our data suggest that endocytosis is essential for ALV-B entry and supports the growing idea that endocytosis plays an important role in retroviral entry. Future studies will determine the specific endocytosis pathways involved in ALV entry.
Acknowledgments
We thank Shailesh Shenoy for support with the image deconvolution software and Elliot Schwarzenberger for technical assistance. We especially thank Volker Briken for helpful discussions and technical assistance. We are grateful to Steve Porcelli, Ganjam Kalpana, and Peter Arvan for critical reading of the manuscript.
REFERENCES
- 1.Adkins, H. B., J. Brojatsch, J. Naughton, M. M. Rolls, J. M. Pesola, and J. A. Young. 1997. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc. Natl. Acad. Sci. USA 94:11617-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins, H. B., J. Brojatsch, and J. A. Young. 2000. Identification and characterization of a shared TNFR-related receptor for subgroup B, D, and E avian leukosis viruses reveal cysteine residues required specifically for subgroup E viral entry. J. Virol. 74:3572-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, K. B. 1985. The fate of the surface protein gp70 during entry of retrovirus into mouse fibroblasts. Virology 142:112-120. [DOI] [PubMed] [Google Scholar]
- 5.Andersen, K. B., and B. A. Nexo. 1983. Entry of murine retrovirus into mouse fibroblasts. Virology 125:85-98. [DOI] [PubMed] [Google Scholar]
- 6.Andersen, K. B., and H. Skov. 1989. Retrovirus-induced cell fusion is enhanced by protease treatment. J. Gen. Virol. 70:1921-1927. [DOI] [PubMed] [Google Scholar]
- 7.Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. [DOI] [PubMed] [Google Scholar]
- 8.Berkhout, B., A. Gatignol, A. B. Rabson, and K. T. Jeang. 1990. TAR-independent activation of the HIV-1 LTR: evidence that tat requires specific regions of the promoter. Cell 62:757-767. [DOI] [PubMed] [Google Scholar]
- 9.Bowman, E. J., A. Siebers, and K. Altendorf. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 85:7972-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brojatsch, J., J. Naughton, H. B. Adkins, and J. A. Young. 2000. TVB receptors for cytopathic and noncytopathic subgroups of avian leukosis viruses are functional death receptors. J. Virol. 74:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. [DOI] [PubMed] [Google Scholar]
- 12.Carrington, W. A., R. M. Lynch, E. D. Moore, G. Isenberg, K. E. Fogarty, and F. S. Fay. 1995. Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science 268:1483-1487. [DOI] [PubMed] [Google Scholar]
- 13.Chi, Y., F. Diaz-Griffero, C. Wang, J. A. T. Young, and J. Brojatsch. 2002. An NF-κB-dependent survival pathway protects against cell death induced by TVB receptors for avian leukosis viruses. J. Virol. 76:5581-5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 15.Corbeau, P., M. Benkirane, R. Weil, C. David, S. Emiliani, D. Olive, C. Mawas, A. Serre, and C. Devaux. 1993. Ig CDR3-like region of the CD4 molecule is involved in HIV-induced syncytia formation but not in viral entry. J. Immunol. 150:290-301. [PubMed] [Google Scholar]
- 16.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorner, A. J., and J. M. Coffin. 1986. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell 45:365-374. [DOI] [PubMed] [Google Scholar]
- 18.Doxsey, S. J., F. M. Brodsky, G. S. Blank, and A. Helenius. 1987. Inhibition of endocytosis by anti-clathrin antibodies. Cell 50:453-463. [DOI] [PubMed] [Google Scholar]
- 19.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 20.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaudin, Y., R. W. Ruigrok, M. Knossow, and A. Flamand. 1993. Low-pH conformational changes of rabies virus glycoprotein and their role in membrane fusion. J. Virol. 67:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert, J. M., D. Mason, and J. M. White. 1990. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J. Virol. 64:5106-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez, M. B., and J. E. Hildreth. 1995. Antibody to adhesion molecule LFA-1 enhances plasma neutralization of human immunodeficiency virus type 1. J. Virol. 69:4628-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helenius, A., J. Kartenbeck, K. Simons, and E. Fries. 1980. On the entry of Semliki Forest virus into BHK-21 cells. J. Cell Biol. 84:404-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helenius, A., and M. Marsh. 1982. Endocytosis of enveloped animal viruses. Ciba Found. Symp. 1982:59-76. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez, L. D., R. J. Peters, S. E. Delos, J. A. Young, D. A. Agard, and J. M. White. 1997. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J. Cell Biol. 139:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildreth, J. E., and R. J. Orentas. 1989. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science 244:1075-1078. [DOI] [PubMed] [Google Scholar]
- 29.Kizhatil, K., and L. M. Albritton. 1997. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J. Virol. 71:7145-7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knauss, D. J., and J. A. T. Young. 2002. A fifteen-amino-acid TVB peptide serves as a minimal soluble receptor for subgroup B avian leukosis and sarcoma viruses. J. Virol. 76:5404-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landau, N. R., K. A. Page, and D. R. Littman. 1991. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J. Virol. 65:162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis, B. C., N. Chinnasamy, R. A. Morgan, and H. E. Varmus. 2001. Development of an avian leukosis-sarcoma virus subgroup A pseudotyped lentiviral vector. J. Virol. 75:9339-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marechal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh, M., E. Bolzau, and A. Helenius. 1983. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell 32:931-940. [DOI] [PubMed] [Google Scholar]
- 36.Marsh, M., and R. Bron. 1997. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 110:95-103. [DOI] [PubMed] [Google Scholar]
- 37.Marsh, M., and A. Helenius. 1980. Adsorptive endocytosis of Semliki Forest virus. J. Mol. Biol. 142:439-454. [DOI] [PubMed] [Google Scholar]
- 38.Marsh, M., A. Helenius, K. Matlin, and K. Simons. 1983. Binding, endocytosis, and degradation of enveloped animal viruses. Methods Enzymol. 98:260-266. [DOI] [PubMed] [Google Scholar]
- 39.Marsh, M., K. Matlin, K. Simons, H. Reggio, J. White, J. Kartenbeck, and A. Helenius. 1982. Are lysosomes a site of enveloped-virus penetration? Cold Spring Harbor Symp. Quant. Biol. 46:835-843. [DOI] [PubMed] [Google Scholar]
- 40.Marsh, M., and A. Pelchen-Matthews. 2000. Endocytosis in viral replication. Traffic 1:525-532. [DOI] [PubMed] [Google Scholar]
- 41.McClure, M. O., M. A. Sommerfelt, M. Marsh, and R. A. Weiss. 1990. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71:767-773. [DOI] [PubMed] [Google Scholar]
- 42.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishi, T., and M. Forgac. 2002. The vacuolar (H+)-ATPases—nature's most versatile proton pumps. Nat. Rev. Mol. Cell. Biol. 3:94-103. [DOI] [PubMed] [Google Scholar]
- 45.Page, K. A., N. R. Landau, and D. R. Littman. 1990. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J. Virol. 64:5270-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pauza, C. D., and T. M. Price. 1988. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J. Cell Biol. 107:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74:2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redmond, S., G. Peters, and C. Dickson. 1984. Mouse mammary tumor virus can mediate cell fusion at reduced pH. Virology 133:393-402. [DOI] [PubMed] [Google Scholar]
- 49.Seglen, P. O. 1983. Inhibitors of lysosomal function. Methods Enzymol. 96:737-764. [DOI] [PubMed] [Google Scholar]
- 50.Skehel, J. J., P. M. Bayley, E. B. Brown, S. R. Martin, M. D. Waterfield, J. M. White, I. A. Wilson, and D. C. Wiley. 1982. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc. Natl. Acad. Sci. USA 79:968-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinstein, D. E., M. L. Shelanski, and R. K. Liem. 1991. Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons. J. Cell Biol. 112:1205-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White, J., J. Kartenbeck, and A. Helenius. 1980. Fusion of Semliki Forest virus with the plasma membrane can be induced by low pH. J. Cell Biol. 87:264-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White, J. M. 1992. Membrane fusion. Science 258:917-924. [DOI] [PubMed] [Google Scholar]
- 56.Wilson, C. A., J. W. Marsh, and M. V. Eiden. 1992. The requirements for viral entry differ from those for virally induced syncytium formation in NIH 3T3/DTras cells exposed to Moloney murine leukemia virus. J. Virol. 66:7262-7269. [DOI] [PMC free article] [PubMed] [Google Scholar]