Abstract
The multidrug-resistant mutant Streptococcus pneumoniae M22 constitutively overexpresses two genes (patA and patB) that encode proteins homologous to known efflux proteins belonging to the ABC transporter family. It is shown here that PatA and PatB were strongly induced by quinolone antibiotics and distamycin in fluoroquinolone-sensitive strains. PatA was very important for growth of S. pneumoniae, and it could not be disrupted in strain M22. PatB appeared to control metabolic activity, particularly in amino acid biosynthesis, and it may have a pivotal role in coordination of the response to quinolone antibiotics. The induction of PatA and PatB by antibiotics showed a pattern similar to that exhibited by SP1861, a homologue of ABC-type transporters of choline and other osmoprotectants. A second group of quinolone-induced transporter genes comprising SP1587 and SP0287, which are homologues of, respectively, oxalate/formate antiporters and xanthine or uracil permeases belonging to the major facilitator family, showed a different pattern of induction by other antibiotics. There was no evidence for the involvement of PmrA, the putative proton-dependent multidrug transporter that has been implicated in norfloxacin resistance, in the response to quinolone antibiotics in either the resistant mutant or the fluoroquinolone-sensitive strains.
Large epidemiological studies of Streptococcus pneumoniae in clinical infections have associated mutations in the genes encoding gyrase and topoisomerase IV with fluoroquinolone resistance (13, 40). However, resistance to fluoroquinolones can also be mediated by active efflux (5, 9-11, 17, 18, 22, 34, 44, 53). Until recently, the only efflux pump implicated in pneumococcal fluoroquinolone resistance was PmrA (22), but it now appears that there must be other efflux pumps involved in this resistance phenotype (11, 45). Multidrug-resistant strain M22, a mutant selected after exposure of S. pneumoniae NCTC 7465 (strain M4) to ciprofloxacin, appeared to have such an efflux-mediated resistance mechanism (46). The mutation frequency of 6.9 × 10−8 and stable resistance without antibiotic pressure suggested a mutation in a single gene (46). However, no mutations in the fluoroquinolone resistance-determining regions of the A subunits of DNA gyrase or topoisomerase IV have been detected (36). Strain M22 was at least fourfold more resistant than strain M4 to ciprofloxacin, norfloxacin, acriflavine, ethidium bromide, doxorubicin, tetracycline, erythromycin, and cetrimide. The MICs of clinafloxacin, gatifloxacin, grepafloxacin, levofloxacin, and sitafloxacin were reproducibly twofold higher for strain M22 than for strain M4, but those of moxifloxacin, ofloxacin, sparfloxacin, and chloramphenicol were identical for the two strains. The accumulation of ciprofloxacin, gatifloxacin, and ofloxacin by strain M22 was significantly less than that observed in strain M4, whereas the accumulation of norfloxacin and ethidium was consistently higher than in strain M4. Addition of reserpine increased the uptake of norfloxacin but had no effect on the uptake of ciprofloxacin by either strain. The uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) increased the uptake of norfloxacin, ciprofloxacin, and ofloxacin by strain M4 but had no effect on their uptake by strain M22 (46). Thus, while strain M22 exhibits some of the features associated with the participation of an energy-dependent efflux system in resistance, it has not been possible to identify its phenotype with a single system that is the major determinant of multidrug resistance by inhibitor sensitivity studies (cf. references 22 and 29). In earlier work (36), it was shown, by using DNA microarray analysis to compare the gene expression profiles of strain M22 with those of strain M4 under various conditions, that strain M22 constitutively expressed 22 genes at levels higher than those observed in strain M4 under all of the conditions studied. Among the up-regulated genes were two (patA and patB) with sequences homologous to ABC transporter proteins. Expression of the patA and patB genes was induced by ciprofloxacin in both strains, but in strain M4 it only reached the levels observed in strain M22 after exposure to high concentrations of ciprofloxacin or long incubation with lower concentrations. There were at least six other transport proteins whose expression was significantly altered in strain M22, but none of these belong to transporter families that have been associated with drug efflux. However, the genome sequences of two strains of S. pneumoniae now available show that this species possesses at least 25 genes that might encode components of efflux pump systems (27, 52). The involvement of the potential efflux proteins PatA and PatB and of the other putative efflux transporters in the response of S. pneumoniae to fluoroquinolones was investigated in more detail.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pneumoniae NCTC 7465 (strain M4), ATCC 49619, R6, and TIGR4, previously KNR0.7/87 (52), were used as control strains throughout. Strains M4 and M22 were maintained at −80°C on Protect beads (Protect Bacterial Preservers; Technical Service Consultants, Ltd., Heywood, United Kingdom) without antibiotic and, except for microarray analysis (where Todd-Hewitt broth was used), were grown overnight in brain heart infusion broth at 37°C in 5% CO2. The solid medium was Iso-Sensitest agar supplemented with 5% defibrinated horse blood. Gram staining and optochin sensitivity were used to confirm identity.
Antibiotics and susceptibility determination.
The MIC of each substance for each strain was determined as recommended by the British Society for Antimicrobial Chemoptherapy (3). p-Aminobenzoic acid, acriflavine, actinonin, actinomycin, azithromycin, bacitracin, blasticidin, cerulenin, cethromycin, chloramphenicol, ciprofloxacin, clarithromycin, clindamycin, daunomycin, distamycin, doxorubicin, erythromycin, ethidium bromide, fosfomycin, fusidic acid, gramicidin, lincomycin, mitomycin C, monensin, mupirocin, nalidixic acid, nigericin, norfloxacin, novobiocin, oleandomycin, penicillin G, polymyxin B, puromycin, reserpine, sodium orthovanadate, sulfamethoxazole, thiamphenicol, trimethoprim, tunicamycin, tylosin, valinomycin, and verapamil were purchased from Sigma-Aldrich.
Expression of pmrA.
Northern blotting was performed with RNA (20 μg) extracted with Trizol (Gibco BRL) and as described in the Amersham Gene Images kits. Sample-to-sample RNA uniformity was determined by examining 16S rRNA expression in parallel. The PCR was used to generate a 558-bp fragment of the structural gene for PmrA (GenBank accession number AJ007367; nucleotides 431 to 988), which was used as the probe. Quantitative competitive reverse transcriptase PCR was performed on the RNA template to generate cDNA of pmrA as described previously (46).
Microarray analysis of gene expression in S. pneumoniae R6.
The antisense ROEZ06a array custom fabricated by Affymetrix (Santa Clara, Calif.) to cover the genomes of both S. pneumoniae and Haemophilus influenzae was used. Probe selection, open reading frame (ORF) coverage and array design are described by Hakenbeck et al. (25) and de Saizieu et al. (20). The array area covering S. pneumoniae has over 130,000 oligonucleotide probes that are complementary to the S. pneumoniae KNR0.7/87 genome (20) sequence, now published as TIGR4 (52). In total, 1,968 putative genes, predicted by GeneMark software, and 323 intergenic regions longer than 200 bp from S. pneumoniae are represented. Each gene is represented by at least 20 (for short genes) and, in general, by 25 probe pairs. The probe pairs (25-residue oligonucleotides) comprise a perfect-match probe (PM) and a mismatch probe (MM) that differs by a single base change at the central position. Bacteria were grown on at least two separate occasions to an optical density at 600 nm (OD600) of 0.3 in Todd-Hewitt medium, and the cells were harvested by centrifugation and frozen in liquid nitrogen. The effects of antibiotics on gene expression were examined by harvesting cultures after 15 min of exposure to the antibiotics at concentrations equal to 0.1 times the MIC, the MIC, and 10 times the MIC, where the MIC was determined in liquid culture in Todd-Hewitt medium after overnight incubation. Antibiotic-free cultures were analyzed in parallel. RNA extraction and cDNA labeling were performed as described by de Saizieu et al. (20). Fragmented, biotin-labeled cDNA was hybridized to the chips and stained as previously described (20), with minor modifications. The hybridization mixtures contained 5 μg of biotin-labeled cDNA, and TOP-BLOCK (Juro) was used instead of acetylated bovine serum albumin (Sigma) at 1.5 g/liter in the hybridization solutions and 2 g/liter in the staining solution. The microarrays were scanned at 570 nm (3-μm resolution) with an Affymetrix gene chip scanner and analyzed as previously described (32). Assessment of reproducibility and validation of genomic hybridization on the microarray were done as previously described (19). The signal for each gene was calculated as the average intensity difference (AID) represented by the equation Σ(intensity PM − intensity MM)/number of probe pairs. All experiments were performed twice and the AID value averaged. The intensity ratios were defined as the value of the AID under the conditions where the gene was expressed at the highest level divided by the AID under the condition where the gene was expressed at the lowest level. Change factors (CHFs) were computed as previously described (20, 25) with the boundary condition of CHF = ±1.6 chosen for a significant effect because >98% of the variance between repeated samples is encompassed within the limits −1.6 < CHF < 1.6 in these experiments (36).
Construction of gene disruption mutants of S. pneumoniae R6 and M22.
Mutants of strain R6 were obtained by site-directed insertional inactivation as described by Stieger et al. (49). Modifications of this method to adapt it to working with strain M22 were as follows. Genomic DNA was isolated from M22 with the QIAGEN Genomic DNA Purification Kit according to the instructions of the manufacturer. Plasmid DNA was prepared with a QIAprep Miniprep Kit (QIAGEN). pRKO plasmids were constructed exactly as described by Stieger et al. (49) with pJDC9 (15). pEM1 was constructed with pRKO2 as the starting vector and contains a portion of pRKO2 (Ery-R, tetR, Psyn, to, P57/P57opt, and the cloning cassette) combined with an 1,811-bp AatII-AflIII fragment from pUC19 (38) containing a β-lactamase gene and ColE1. pRKO2 carries an erythromycin resistance cassette and does not replicate autonomously in S. pneumoniae. An internal fragment of patB (388 nucleotides) was generated by PCR and cloned into pEM1. The resulting plasmid was pEM2. Recombinants were propagated in Escherichia coli M15. Strain M22 proved difficult to transform, even by electroporation, presumably due to its capsule. It was assumed that removal of part of the capsular polymers would enhance the uptake of DNA and a search for compounds that would remove sufficient polysaccharide without killing the bacteria revealed that the cationic agent cetyltrimethylammonium bromide (CTAB) acted in this way. The main application of this detergent to date is the removal and precipitation of polysaccharides, e.g., for the pneumococcal vaccine (14). Low concentrations of CTAB had to be used due to its antibacterial activity. To transform strain M22, the bacteria were grown to an OD600 of 0.2 to 0.3 in Todd-Hewitt medium, and CTAB (0.0001% in growth medium) was added and the cultures were incubated for 15 min at 37°C in 10% CO2. The cultures were centrifuged (4,000 × g for 2 min at room temperature), the supernatant was discarded, and 500 μl of prewarmed Streptococcus medium (30) containing 200 ng/ml competence-stimulating peptide (26) was added. After 20 min of incubation at 37°C in a 10% CO2 atmosphere, 1 μg of DNA (pEM2) was added to the mixture, which was incubated for a further 3 h (37°C, 10% CO2). Finally, 0.1 ml of cell suspension was plated on sheep blood agar plates containing 1 μg/ml erythromycin. Correct chromosomal integration of pEM2 was confirmed by PCR with an internal reverse primer for the plasmid M13 region and a forward primer annealing to the 5′ end of the inactivated gene. Liquid cultures of strains with gene disruptions were prepared for experimentation by growing an overnight inoculum in Todd-Hewitt medium containing 1 μg/ml erythromycin. An aliquot of this culture was used as the inoculum for fresh Todd-Hewitt medium free of erythromycin. The carryover of erythromycin resulted in medium concentrations of less than 1 ng/ml (<0.01 times the MIC), at which concentration erythromycin has no significant effect on gene expression.
Measurement of the accumulation of quinolones by S. pneumoniae.
The modified fluorescence method was used for fluoroquinolones essentially as described by Mortimer and Piddock (41), but with slight changes to allow for the growth characteristics of S. pneumoniae, for measuring the concentration accumulated by each mutant and parent strain after 5 min of exposure to a 10-μg/ml concentration of each agent (46). To a parallel set of tubes, reserpine (20 mg/liter) or CCCP (100 μM) was added at time zero or after 10 min of exposure to a fluoroquinolone. The accumulation data were converted and expressed as nanograms of quinolone per milligram (dry weight) of cells. All experiments were performed in duplicate on at least three separate occasions and the mean values and standard deviations determined. To determine whether any differences in data were statistically significant, the values obtained after 5 min of exposure to each agent were compared by using the Student two-tailed t test. A P value of less than 0.05 was considered significant.
RESULTS
Absence of a functional PmrA efflux system in S. pneumoniae M4 and M22.
It was not possible to detect pmrA expression in either strain by Northern blotting or reverse transcriptase PCR (data not shown). DNA sequencing revealed that both strains contain a large deletion in pmrA. Upstream of the coding region for pmrA (extending 350 bp upstream from the start codon), M4 and M22 were identical. Compared with strain R6, there were only two nucleotide changes; an A-to-G change at −140 and a T-to-G change at −342. The latter change is also present in a clinical isolate (L. Weigel, personal communication). Except for two nucleotide changes between positions 1 and 473, the DNA sequence was identical to that of strain R6. Following this point, there was a complete disruption of homology with strain R6. From position 558 of R6 (position 474 of pmrA of M4 and M22), the DNA sequences were identical and remained in frame thereafter, a deletion of 84 nucleotides. This deletion of 28 amino acids, compared with the model of Gill et al. (22), removes most of putative transmembrane segment 6 of PmrA.
Effects of antibiotics on transporter gene expression in S. pneumoniae R6.
Seventeen transporter genes were induced by quinolone antibiotics, of which only five genes were consistently induced by quinolones (Tables 1 and 2). The latter genes formed two clusters according to their response to exposure to antibiotics.
TABLE 1.
Effects of antibiotics on expression of putative transporter genes in S. pneumoniae R6a
| TIGR4 IDb | Description | Inducer(s) in R6 | Repressor(s) in R6 |
|---|---|---|---|
| SP0522 | ABC transporter, ATP-binding protein | CHL, CIP, DAU, DIS, ERY | |
| SP0523 | ABC transporter, permease protein, putative | ACM, CIP, CLR, DAU, DIS, ERY, FUS, NOR | |
| SP2101 | Cation-transporting ATPase, E1-E2 family | ACM, CIP, DIS, | |
| SP1717 | ABC transporter, ATP-binding protein | NAL | ACM, CIP, DAU, DIS, NOR, NOV, POL |
| SP0636 | ABC transporter, ATP-binding protein | ACM, CIP, DAU, DIS, NOR | |
| SP0770 | ABC transporter, ATP-binding protein | NOR | ACM, CIP, DIS, MUP |
| SP2221 | ABC transporter, ATP-binding protein | NOR, NOV | ACM, CIP, DIS, MUP |
| SP2219 | ABC transporter, ATP-binding protein | NOV, PEN | ACM, DIS, MUP, POL |
| SP1398 | Phosphate ABC transporter, permease protein, putative | PEN | ACM, CIP, DAU, DIS, POL |
| SP1400 | ABC transporter, ATP-binding protein | PEN | ACM, CIP, DAU, DIS |
| SP1387 | Spermidine/putrescine ABC transporter, permease protein (PotC) | DIS | ACM, CIP, MUP, POL, SMX |
| SP2169 | Zinc ABC transporter | THI, LCN, NOR | ACM, CIP, NOV, TMP |
| SP1939 | MATE efflux family protein DinF | ABA | ACM, MUP |
| SP0483 | ABC transporter, ATP-binding protein | NOR, VAL | ACM, DAU, PEN |
| SP1553 | ABC transporter, ATP-binding protein | NOR | ACM |
| SP1114 | ABC transporter, ATP-binding protein | PEN | ACM, DIS, CIP, MUP, NOV |
| SP0756 | Cell division ABC transporter, ATP-binding protein FtsE | DIS | ACM, CIP, MUP |
| SP0656 | Hypothetical protein, Na+/H+ antiporter | ACM, CIP, CLR, MUP | |
| SP1357 | ABC transporter, ATP-binding/permease protein | ACM, CIP, CLR, DIS, MUP, NOV, OLE | |
| SP1648 | Manganese ABC transporter, ATP-binding protein (PsaB) | ACM | CIP, DAU, NOV |
| SP1116 | Transporter, putative | DXR, NAL, PEN | ACM, CIP, DIS, NOR, NOV, POL, TUN |
| SP0787 | Permease, putative | BAC, CET, CHL, FUS | ACM, CIP, NOR |
| SP0786 | ABC transporter, ATP-binding protein | BAC, BLA, CET, CHL, CLI, ERY, FUS, LCN, OLE, NAL, TUN | ACM, CIP, DIS |
| SP1957 | ABC transporter, ATP-binding protein | CHL, LCN, MUP, NOR, OLE | MON, NOV |
| SP2075 | ABC transporter, ATP-binding protein | BOT, CHL, CET, MUP, DIS, NOV, CIP, NOR, NAL, VAL | ACM |
| SP2073 | ABC transporter, ATP-binding protein | CHL, CIP, CLI, DIS, LCN, MUP, NAL, NOR, NOV, TEL | ACM, DAU |
| SP1861 | Choline transporter (ProV) | ABA, CIP, DIS, FUS, MUP, NAL, NOR, SMX, VAL | ACM, NOV |
| SP1341 | ABC transporter, ATP-binding protein | ABA, CHL, GRA, NAL, THI, VAL | ACM, CIP, DAU, DXR, DIS, ERY, MIT, NOR, NOV, POL |
| SP1625 | Cadmium resistance transporter, putative | CLI, CLR | CIP, DAU, DIS, DXR, GRA, THI, MON, MUP, NIG, NOV, VAL |
| SP0707 | ABC transporter, ATP-binding protein | ACM, BLA, CLI, CLR, GRA, LCN, TYL, VAL | ACM, BAC, CER, DAU, DIS, DXR, MUP, NIG, NOV, PEN, POL |
| SP1715 | ABC transporter, ATP-binding protein | BAC, DAU, DIS, GRA, MON, NIG, POL, PUR, RES, TUN, VAL | ACM, CHL, CIP, PEN, THI |
| SP0957 | ABC transporter, ATP-binding protein | ABA, DIS, LCN, PEN, TYL, VAL | BAC, CIP, MUP, NOR, PEN, TUN |
| SP1342 | Toxin secretion ABC transporter, ATP-binding/permease protein | GRA, NAL, MON, PEN, THI, VAL | ACM, CIP, DAU, DIS, DXR, ERY, MUP, NOR, NOV, POL |
| SP1389 | Spermidine/putrescine ABC transporter, ATP-binding protein (PotA) | DIS | ACM, CIP, DAU, MUP, SMX |
| SP1035 | Iron compound ABC transporter, ATP-binding protein | CHL, CLR, ERY, OLE, PEN | ACM, BAC, CIP, DIS, MUP, NOV, POL, RES, THI, TUN |
| Sp0972 | PmrA | ACM, CIP, DIS, MUP, THI | |
| SP1682 | Sugar ABC transporter, permease protein | AZM, BLA, CHL, ERY, MON, NIG, PUR, RES, THI, TYL | ACM, BOT, CER, DAU, DXR, FOF, MIT, NOV, OLE, POL |
| SP2129 | PTSc IIC component, putative | BLA, CHL, CLR, FUS, MON, TUN, TYL | ABA, ACM, BAC, BLA, CER, CET, CIP, DAU, DIS, ERY, MIT, NAL, NIG, NOR, |
| NOV, OLE, POL, PUR, SMX | |||
| SP1826 | ABC transporter, substrate-binding protein | BLA, MON, PUR, THI, TYL | ACM, CER, CIP, DAU, DIS, DXR, MIT, NOR, NOV, OLE, PEN, POL, TMP |
| SP1824 | ABC transporter, permease protein | AZM, BLA, CHL, FUS, NAL, PEN, PUR, THI, TYL | ACM, CER, CIP, DAU, DIS, DXR, MIT, MON, NIG, NOR, NOV, OLE, PEN, POL, TMP |
| SP1587 | Oxalate:formate antiporter | CET, CIP, CLI, CLR, DAU, DIS, DXR, ERY, FUS, LCN, MUP, NAL, NOR, OLE, PEN, SMX, TEL | CHL, CLR, ERY, TUN, PEN |
| SP0287 | Xanthine/uracil permease family protein | CER, CIP, CLI, DAU, DIS, FUS, LCN, MUP, NAL, NOR, PEN, SMX | ABA, BAC, CHL, CIP, DAU, ERY, MON, NOV, PEN, POL, PUR, RES, THI, VAL |
| SP1282 | ABC transporter, ATP-binding protein | AZM, BLA, CET, CHL, CIP, CLI, CLR, ERY, FUS, GRA, LCN, OLE, TMP, TYL, VAL | DIS, MUP |
| SP0042 | Competence factor-transporting ATP-binding/permease protein | ABA, ACN, MUP, NIG, PEN, TMP, VAL | ABA, CER, CHL, CIP, CLR, DAU, DXR, ERY, FUS, NAL, NIG, NOR, POL, RES, SMX, THI, TUN |
The putative transporter genes that showed CHFs of >1.6 (induced in column 3) or <−1.6 (repressors in column 4) after exposure to antibiotics at either 0.1 or 1 times the MIC are listed. Genes with a clear indication of function in amino acid and carbohydrate transport (49) have been excluded from the list. Abbreviations used for the antibiotics are as follows: ABA, p-aminobenzoic acid; ACN, actinonin; ACM, actinomycin; AZM, azithromycin; BAC, bacitracin; BLA, blasticidin; CER, cerulenin; CHL, chloramphenicol; CIP, ciprofloxacin; CLR, clarithromycin; CLI, clindamycin; DAU, daunomycin; DIS, distamycin; DXR, doxorubicin; ERY, erythromycin; FOF, fosfomycin; FUS, fusidic acid; GRA, gramicidin; LCN, lincomycin; MIT, mitomycin C; MON, monensin; MUP, mupirocin; NAL, nalidixic acid; NIG, nigericin; NOR, norfloxacin; NOV, novobiocin; OLE, oleandomycin; PEN, penicillin G; POl, polymyxin B; PUR, puromycin; RES, reserpine; SMX, sulfamethoxazole; THI, thiamphenicol; TMP, trimethoprim; TUN, tunicamycin; TYL, tylosin; VAL, valinomycin.
ID, identification.
PTS, phosphotransferase system.
TABLE 2.
Effect of ciprofloxacin on the expression levels of putative transporter genes of strains M4 and M22a
| TIGR4 IDb | Inducer(s) in R6 | Largest CHF
|
||||
|---|---|---|---|---|---|---|
| Controls (M22 vs M4) | Strain M4
|
Strain M22
|
||||
| +CIP | +NOR | +CIP | +NOR | |||
| SP1282 | CIP | −1.4 | 0.4 | 0.02 | −1.1 | 0.1 |
| SP0770 | NOR | 1.3 | −0.38 | −.09 | 1.7 | 0.12 |
| SP2169 | NOR | 7.2 | −8.8 | 0.28 | 1.8 | 0.3 |
| SP0483 | NOR | −1.6 | 0.35 | 0.06 | 3.0 | 0.05 |
| SP1553 | NOR | 0.3 | −0.6 | 0.13 | 1.2 | 0.0 |
| SP1957 | NOR | −5.4 | −1.2 | −0.03 | −2.8 | −0.47 |
| SP1717 | NAL | −0.8 | −12 | 0.18 | −0.5 | −0.12 |
| SP0786 | NAL | 1.9 | −5.0 | 0.08 | −2.6 | 0.16 |
| SP1341 | NAL | −1.4 | −3.6 | −0.09 | −0.34 | −0.02 |
| SP0111 | CIP, NAL | −3.1 | −0.6 | 0.23 | −14 | 0.0 |
| SP2073 | CIP, NOR, NAL | 3.9 | 9.0 | 0.26 | 0.8 | 0.15 |
| SP2075 | CIP, NOR, NAL | 3.1 | 5.1 | 0.62 | 3.7 | 0.28 |
| SP1861 | CIP, NOR, NAL | −1.0 | −20 | 0.2 | 0.35 | −0.1 |
| SP1587 | CIP, NOR, NAL | 1.9 | 5.1 | 0.72 | 1.4 | 0.59 |
| SP0287 | CIP, NOR, NAL | 1.9 | −5.7 | 0.92 | 0.9 | 0.37 |
The genes in boldface are those identified by Marrer et al. (36) as being expressed at a higher level in strain M22 than in strain M4 after exposure to ciprofloxacin. Induction (column 2) was taken to mean that the CHF after exposure to the indicated antibiotic at 0.1 or 1 × MIC was greater than 1.6. The antibiotics are ciprofloxacin (CIP), norfloxacin (NOR), and nalidixic acid (NAL). For the experiments with strains M4 and M22, the concentrations of CIP were 2 μg/liter (MIC of strain M4), 12 μg/liter (MIC of strain M22), and 80 μg/liter and the concentrations of NOR were 4 μg/liter (MIC of strain M4) and 32 μg/liter (MIC of strain M22).
ID, identification.
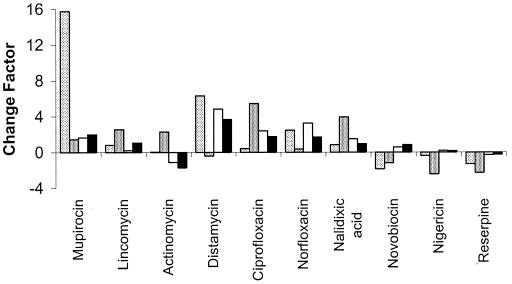
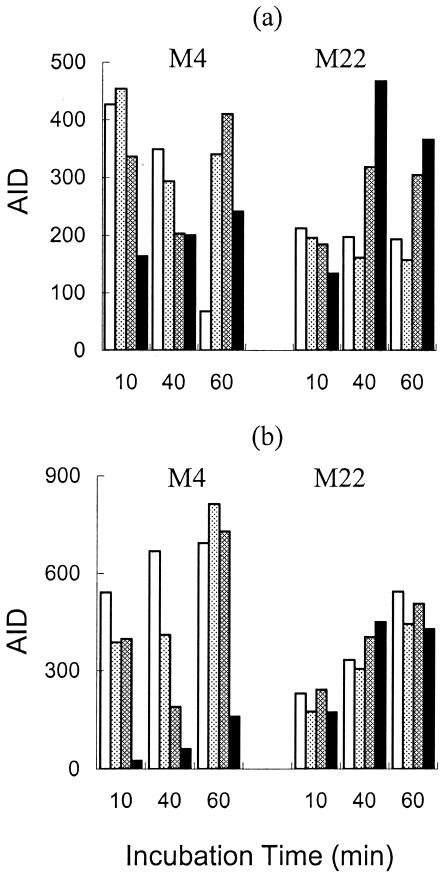
One cluster, comprising three putative ABC-type transporters, SP1861 (homologous to proteins involved in choline and other osmoprotectant transport; 52), SP2075 (patA), and SP2073 (patB), was strongly induced in strain R6 by the quinolones distamycin and mupirocin (Fig. 1). SP1861 had a regulation different from that of PatA and PatB in response to exposure of strains M4 and M22 to ciprofloxacin and norfloxacin. In strain M4, the expression of SP1861 increased with the time of incubation but was repressed by exposure to high concentrations of ciprofloxacin (Fig. 2). In strain M22, the expression of SP1861 was repressed compared to that in strain M4, still increased with time, but was unaffected by exposure to ciprofloxacin. The net effect was that strain M22 expressed higher levels of SP1861 in the presence of high concentrations of ciprofloxacin, and thus, the gene product could contribute to resistance.
FIG. 1.
Effects of antibiotics on expression in S. pneumoniae R6 of SP0287 (light gray), SP1587(medium gray), SP2073 (patB; white), and SP2075 (patA; black). The antibiotic was present at 0.1, 1, or 10 times the MIC for 15 min of exposure. The mean CHF was computed relative to an antibiotic-free culture that had been exposed to the solvent used for the antibiotic.
FIG. 2.
Effect of ciprofloxacin on expression of SP1587 (a) and SP1861 (b) in S. pneumoniae M4 and M22. In each case, the bars represent unexposed controls (white), samples exposed to 2 μg/ml ciprofloxacin (light gray), samples exposed to 12 μg/ml ciprofloxacin (medium gray), and samples exposed to 80 μg/ml ciprofloxacin (black).
The second cluster comprised SP0287 (a putative purine/pyrimidine transporter) and SP1587 (a putative oxalate/formate antiporter), which were strongly induced in strain R6 by the three quinolones, as well as daunorubicin, doxorubicin, and mupirocin (Fig. 1). The two genes in the second cluster induced by quinolones, SP0287 and SP1587, had similar expression profiles. In strain M4, the expression of both genes decreased with time and showed a bimodal response to ciprofloxacin in the 60-min incubation samples, where both genes were induced at a low ciprofloxacin concentration but repressed at a high concentration (Fig. 2). In strain M22, both genes were expressed at about the same level at all three incubation times but showed diverse responses to ciprofloxacin (Fig. 2). At the highest concentration of ciprofloxacin, both genes were expressed at a higher level in strain M22 than in strain M4 and therefore their products might contribute to resistance.
Gene disruption mutants of S. pneumoniae R6 and M22.
patA and patB gene disruption mutants of strain R6 were readily obtained. The growth rate of the R6 derivative with a disruption in patA (R6 patA disruptant) was significantly decreased (doubling time, 130 min), whereas that of the strain with a patB disruption (R6 patB disruptant; doubling time, 90 min) was within the range observed for constructs in which nonessential genes had been disrupted (doubling times, 80 to 100 min). Mutants with the pmrA gene disrupted were also readily obtained, and the disruption had no effect on the growth of the bacteria (doubling times, 80 to 90 min). Transformation of strain M22 with pEM2(patB) yielded nine putative transformants that had a disruption of the chromosomal patB gene that was verified by PCR. Only very few colonies were obtained after transformation of M22 with pEM3(patA) despite numerous attempts. PCR analysis revealed that the disruption plasmid was not correctly integrated and that the patA gene was still intact in these isolates. It appears that PatA is important for growth of S. pneumoniae and probably essential for strain M22.
Effect of gene disruption mutants on gene expression in S. pneumoniae R6 and M22.
Microarray analysis of the expression profiles of the three gene disruption mutants, the R6 patA disruptant, the R6 patB disruptant, and the M22 patB disruptant, revealed that the expression of neither patA nor patB was significantly affected by the disruption of either partner gene in R6 or by patB disruption in M22 (Table 3). In contrast to its effects on strain M4 or M22, ciprofloxacin did not significantly induce expression of patA or patB in the M22 patB disruptant. Of the 17 putative transporters that were induced by exposure to quinolones in R6, only the expression of SP1587 was greatly affected and this only in the knockouts of patB (CHF < −1.6).
TABLE 3.
Effect of patB disruption on the transcription of genes constitutively expressed at higher levels in strain M22 under all conditionsa
| TIGR4 IDb | Description | CHF
|
||||
|---|---|---|---|---|---|---|
| M22 vs M4 | M22 patB vs M22 | M22 patB vs M4 | R6 patA vs R6 | R6 patB vs R6 | ||
| SP0141 | Transcriptional regulator | 2.8 | −2.0 | 0.27 | −0.08 | −0.37 |
| SP0159 | Mn(II) and Fe(II) symporter | 2.7 | 0.4 | 4.9 | 0.09 | −0.95 |
| SP0445 | Acetolactate synthase large subunit | 3.8 | −2.0 | 0.45 | −0.12 | −0.08 |
| SP0446 | Acetolactate synthase small subunit | 4.9 | −3.1 | 0.42 | 0.2 | −0.16 |
| SP0447 | Ketol acid reductoisomerase | 3.7 | −2.1 | 0.46 | 0.14 | −0.73 |
| SP0448 | DNA helicase II | 6.4 | −2.5 | 1.5 | −0.13 | −0.1 |
| SP0449 | Hypothetical protein | 3.7 | −3.2 | 0.12 | −0.03 | 0.16 |
| SP0450 | Threonine dehydratase | 2.0 | −1.8 | 0.42 | 0.02 | −0.08 |
| SP0757 | ABC transporter | 2.3 | −1.2 | 0.45 | 0.09 | 0.02 |
| SP0789 | Transcriptional regulator | 3.1 | −1.8 | 0.47 | 0.14 | −0.1 |
| SP0790 | Hypothetical protein | 2.6 | −0.97 | 0.83 | 0.28 | 0.6 |
| SP0823 | Glutamine ABC transporter, | 2.8 | 1.1 | 6.9 | −0.47 | −1.6 |
| SP0824 | Glutamine ABC transporter, ATP-binding protein | 2.4 | −1.3 | 0.48 | 0.15 | 0.07 |
| SP0856 | Branched-chain amino acid aminotransferase | 2.4 | −1.2 | 0.47 | −0.07 | −0.1 |
| SP1394 | Glutamine-binding periplasmic protein | 2.6 | 2.2 | 10.7 | 0.05 | −0.05 |
| SP1429 | Peptidase of U32 family | 1.7 | −0.8 | 0.15 | 0.03 | 0.09 |
| SP1460 | Amino acid ABC transporter ATP-binding protein | 2.8 | 0.4 | 4.5 | −0.09 | −0.31 |
| SP1461 | Amino acid ABC transporter permease | 2.4 | 0.5 | 4.1 | −0.01 | −0.38 |
| SP1624 | 1-Acyl-sn-glycerol-3-phosphate acyltransferase | 6.4 | −2.6 | 1.6 | −0.15 | −0.56 |
| SP2073 | PatB | 2.5 | 0.29 | 3.5 | 0.4 | −0.4 |
| SP2075 | PatA | 3.8 | 0.92 | 8.1 | 0.5 | 0.2 |
| SP2126 | Dihydroxyacid dehydratase | 2.4 | −1.3 | 0.48 | 0.1 | −0.42 |
The mean CHFs computed for comparison of antibiotic-free cultures at the mid- and late log phases of the growth curve are shown. The comparisons are between strains M22 and M4 (column 3), the patB disruption mutant of strain M22 (M22 patB) and M22 (column 4), the patB disruption mutant of strain M22 (M22 patB) and M4 (column 5), the patA disruption mutant of strain R6 (R6 patA) and R6 (column 6), and the patB disruption mutant of strain R6 (R6 patB) and R6 (column 7).
ID, identification.
Of the 22 genes that were constitutively induced in strain M22 (36), seven (including patA and patB) retained or increased their level of expression in the M22 patB disruptant strain (Table 3). The patB disruption in strain M22 reversed the constitutive overexpression of DNA helicase II and the genes involved in branched-chain amino acid biosynthesis (Table 3). Neither the patA nor the patB disruption had a significant effect on the expression of these genes in strain R6, again showing that this is a specific effect on the regulatory network in strain M22.
Uptake of fluoroquinolones by S. pneumoniae M4 and M22 and effects of inhibitors of efflux.
Ciprofloxacin and norfloxacin were accumulated to about the same level by respiring cells of S. pneumoniae M4 (41 and 39 ng mg of cells−1, respectively). The fluoroquinolone-resistant mutant M22 accumulated about half as much ciprofloxacin as did strain M4 (22 ng mg of cells−1), whereas the accumulation of norfloxacin was slightly higher (48 ng mg of cells−1). The M22 patB disruptant strain accumulated about as much ciprofloxacin as did strain M4 (40 ng mg of cells−1) and nearly twice as much norfloxacin as did strain M4 (76 ng mg of cells−1). Reserpine and CCCP did not have a significant effect on the accumulation levels in any strain.
Susceptibility of S. pneumoniae M4 and M22 to antibiotics.
The resistance of the M22 patB disruptant strain to ciprofloxacin, norfloxacin, and ethidium bromide was lower than that of strain M22, but the susceptibility did not return to the level of strain M4 (Table 4). The patA and patB disruption mutants of strain R6 were both reproducibly more sensitive to ciprofloxacin than strain R6. The R6 patA disruptant mutant was more susceptible to norfloxacin but not ciprofloxacin (Table 4). Reserpine lowered the MICs of tetracycline, ciprofloxacin, norfloxacin, acriflavine, and ethidium bromide for both M4 and M22 when present at 20 μg/ml (cf. MIC of 600 to 700 μg/ml). The reserpine effect was greater for strain M22 than for strain M4 and resulted in MICs that were two- to fourfold higher than those observed with M4.
TABLE 4.
Antibiotic susceptibilities of strains M4 and R6 and derivatives
| Substance(s) | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| M4 | M22 | M22 patB disruptant | R6 | R6 patA disruptant | R6 patB disruptant | |
| Ciprofloxacin | 2 | 12 | 4 | 2 | 0.5 | 0.5 |
| Ciprofloxacin + reserpinea | 0.5 | 1 | 1 | NDb | ND | ND |
| Norfloxacin | 4 | 32 | 20 | 2 | 1 | 2 |
| Norfloxacin + reserpinea | 2 | 16 | 16 | ND | ND | ND |
| Ethidium | 2 | 32 | 32 | 1 | 1 | 1 |
| Ethidium + reserpinea | 0.5 | 4 | 4 | ND | ND | ND |
| Acriflavine | 4 | 16 | 16 | 16 | 16 | 16 |
| Acriflavine + reserpinea | 1 | 2 | 1 | ND | ND | ND |
| Chloramphenicol | 4 | 4 | 16 | 2 | 2 | 2 |
| Tetracycline | 0.25 | 1 | 1 | 2 | 2 | 2 |
Present at a fixed concentration of 20 μg/ml.
ND, not done.
Effect of reserpine on gene expression.
Of the 23 genes that were induced by ciprofloxacin under all of the conditions tested, 17 (74%) were repressed by reserpine and 8 of these (34% of the total) were strongly repressed with CHF > −1.6 (Table 5). Further, of the 60 genes that were strongly repressed by reserpine (CHF < −1.6), 34 were induced (CHF > 1.6) by at least one of the quinolones (data not shown). Thus, it appears that reserpine antagonizes the gene expression changes that occur during adaptation to exposure to quinolone antibiotics.
TABLE 5.
Effects of reserpine on genes induced in strain R6 in response to ciprofloxacina
| Affy IDb | Description | CHF
|
|||
|---|---|---|---|---|---|
| +RES | +CIP | +NOR | +NAL | ||
| SP0047_at | Hypothetical protein | −1.8 | 4.1 | 1.1 | 0.67 |
| SP0082_at | para-Aminobenzoate synthetase | −0.3 | 28 | 60 | 2.4 |
| SP0136_at | Attenuation regulatory protein | −2.5 | 2.8 | 0.76 | 0.11 |
| SP0192_at | Phosphoribosylaminoimidazole-succinocarboxamide synthase | 0.5 | 14 | 2.7 | 5.4 |
| SP0194_at | Transport ATP-binding protein coma | 0.0 | 8.6 | 3.0 | 4.9 |
| SP0293_at | Xanthine phosphoribosyltransferase | −0.15 | 5.4 | 1.9 | 2.0 |
| SP0609_at | Hypothetical ABC transporter | −0.4 | 4.2 | 3.3 | 2.7 |
| SP0190_at | PRPPc amidotransferase | −0.3 | 5.1 | 9.3 | 5.7 |
| SP0954_at | Arginine hydroximate resistance protein | −0.4 | 14 | 3.7 | 1.5 |
| SP1650_at | Orotate phosphoribosyltransferase | −1.9 | 2.6 | 0.76 | 0.15 |
| SP2176_at | Hypothetical | −3.5 | 8.8 | 0.36 | 11.1 |
| SP2261_at | PTS permease for mannose | 1.5 | 2.4 | 0.96 | 2.2 |
| SP2821_at | Hypothetical | 2.2 | 3.8 | 0.05 | 0.98 |
| SP3091_at | Dihydroorotate dehydrogenase b | −1.3 | 3.0 | 2.2 | 0.37 |
| SP3151_at | Xaa-Pro dipeptidyl-peptidase | −0.4 | 16 | 12 | 3.5 |
| SP3331_at | Sialidase a | 1.1 | 5.3 | 5.7 | 0.61 |
| SP3533_at | ABC transporter homolog | −0.3 | 3.1 | 1.7 | 1.6 |
| SP3586_at | Hypothetical 18.7-kDa protein | −0.4 | 3.7 | 30 | 3.2 |
| SP3644_g_at | Autolysin response regulator | −1.7 | 2.2 | 2.6 | 3.7 |
| SP1570_at | Hypothetical | 1.9 | 4.3 | 0 | 1.5 |
| SP1187_at | Transmembrane protein Tmp7 | −4.6 | 2.2 | 16 | 23 |
| SP2818_at | Bacteriocin 513 immunity protein | −1.8 | 2.1 | 4.1 | 4.0 |
| SP0138_at | Glutaminase of carbamoyl-phosphate synthase | −1.8 | 3.2 | 0.37 | 0.09 |
Mean CHFs computed for comparison between antibiotic-exposed and antibiotic-free cultures after 10 and 40 min of exposure are shown. The antibiotics were reserpine (RES) at 20 μg/ml, ciprofloxacin (CIP) at 2 μg/ml, norfloxacin (NOR) at 2 μg/ml, and nalidixic acid (NAL) at 128 μg/ml.
ID, identification.
PRPP, S-phosphoribosyl-1-pyrophosphate.
DISCUSSION
Overexpression of drug efflux systems that confer resistance to particular agents has been observed for the NorA (29) and Mde (28) systems of Staphylococcus aureus, the PmrA and Mef systems of S. pneumoniae (16, 22), the Lde pump of Listeria monocytogenes (23), the Bmr (2) and EbrAB (37) systems of Bacillus subtilis, and systems in a number of gram-negative organisms (24, 40, 42, 47, 50, 51). We have found six transporter genes that were consistently expressed at a higher level in the presence of ciprofloxacin by the multidrug-resistant mutant M22 than by its fluoroquinolone-susceptible parent M4. Of these, five were also consistently induced by quinolones in strain R6, suggesting a general role in the fluoroquinolone response. Three of the transporter genes, SP0287 (purine/pyrimidine transporter), SP1587 (oxalate/formate antiporter), and SP1861 (ABC transporter involved in choline and other osmoprotectant transport), encode proteins that do not have an association with antibiotic efflux. They might be involved in adaptation of strain M22 to fluoroquinolone resistance and not directly in efflux-mediated resistance. Only two of the genes, patA and patB, are homologues of proteins that have been directly implicated in antibiotic efflux and could be directly involved in mediating fluoroquinolone resistance by active efflux.
In favor of a direct role for at least PatB is the observation that the M22 patB disruptant strain with patB disrupted was more susceptible to both norfloxacin and ciprofloxacin than strain M22 (1.6- and 3-fold more susceptible, respectively) and accumulated larger amounts of both agents than did M22 (1.5- and 2.2-fold higher accumulation, respectively). The increases in susceptibility to, and accumulation of, ciprofloxacin were larger than those for norfloxacin in the knockout, suggesting that if indeed it is a substrate, ciprofloxacin is a better substrate for PatB than is norfloxacin. The strain M22 patB disruptant was as resistant as, or even more resistant than, strain M22 to acriflavine, ethidium bromide, cetrimide, and chloramphenicol. It is possible that these agents are transported preferentially by PatA and that the increased expression of this gene in the patB deletion mutant can lead to their increased efflux. The lesser effect of the patB knockout on norfloxacin could also be due to some transport of this compound by PatA, such that the decreased efflux of norfloxacin due to the knockout is partially compensated for by increased transport through increased expression of PatA. The observation that reserpine reverses the resistance phenotype of strain M22 fits the hypothesis that there is an active efflux system, or systems, involved in the fluoroquinolone resistance of strain M22. However, we could not demonstrate an effect of reserpine on accumulation, which should be observed if reserpine were directly inhibiting the pumps, and we found that reserpine antagonizes many of the gene expression changes that appear to contribute to adaptation to fluoroquinolone-induced stress in strains M4 and M22. Thus, in the growth inhibition studies it is quite likely that reserpine is acting by blocking the expression of the key resistance determinants and not by inhibiting the function of an efflux system. This observation indicates that it is necessary to exercise care when interpreting the effect of reserpine in susceptibility studies, which have been used to indicate the role of efflux in resistance (5, 6, 9, 10, 21, 35, 39, 43, 48). In principle, even the selection of reserpine-resistant mutants in transporter genes (1), which might be taken as evidence of a specific interaction, needs careful interpretation since mutations that improve the efficiency of transport or level of production of the transporter would generate the same phenotype.
The evidence for energy-dependent efflux of fluoroquinolones mediated by the PatA and PatB proteins is not unequivocal. Examination of the concentration dependence of the induction or repression of genes in the two strains showed that a significant number of genes had similar dependencies in the two strains, which suggests that the effect of the internal concentration is not very strong. We were unable to demonstrate a direct effect of the uncoupler CCCP, which suggests that, for those agents where the accumulation was lowered in strain M22, the accumulation was not driven by a proton motive force-dependent mechanism.
The strong effect of the patA disruption on the growth of strain R6 and the lack of success in creating such a disruption in strain M22 point to an important role for PatA in the normal growth of the cell. The lesser impact of patB disruption suggests that this partner is not vital to cell growth or the physiology of the normal cell. The consequences of patB disruption on gene expression in strain M22 suggest that the activity of PatB impacts on branched-chain amino acid biosynthesis. PatA might impact in a similar way on glutamine biosynthesis, as the expression of several genes involved in glutamine metabolism and uptake follows the regulation pattern of PatA in the patB disruption mutant.
PatA and PatB have close homologues that always occur as linked pairs in gram-positive bacteria belonging to Mollicutes (e.g., Mycoplasma), Firmicutes (e.g., Clostridium, Thermoanaerobobacter, Bacillus, Listeria, Lactobacillus, Enterococcus, and Streptococcus), Actinobacteria (e.g., Corynebacterium, Mycobacterium, Nocardia, and Streptomyces), and Spirochaetes (Treponema). Among the homologues identified are two systems that have been implicated in efflux-mediated multidrug resistance in Enterococcus faecalis (EfrA and EfrB; 31) and Lactococcus lactis (YdaG and YdbA; 33) and three systems that are part of biosynthetic pathway operons for the polyene macrolactone antibiotics nystatin (NysG and NysH; 8), pimaricin (PimA and PimB; 4), and amphotericin (AmphG and AmphH; 12). Many bacterial ABC transporters implicated in drug efflux are thought to function as homodimers or as pairs where both gene products work together as a two-component multidrug efflux pump (7). The observations that the coding sequences for the homologues in streptomycetes overlap (4, 8, 12), which suggests that the two components of the transport system may be translationally coupled, and that the homologues in Lactobacillus are interdependent for their activity in drug resistance (33) are consistent with this model. However, such an overlap is not observed for the coding sequences of PatA and PatB in S. pneumoniae, the genes are not coordinately regulated in response to growth phase or induction by antibiotics, and PatA still exerts some effect in the absence of PatB. The lack of coordinate translation of the two genes and the clear differences in the effects of the patA and patB knockouts indicate that PatA and PatB are not components of a single functional unit that mediates the resistance phenotype of strain M22. Rather, it seems likely that PatA and PatB represent two independent systems that interact to contribute to the resistance phenotype of M22.
Acknowledgments
We are grateful to Linda Weigel (Centers for Disease Control and Prevention, Atlanta, Ga.) for bringing to our attention the deletion of part of pmrA of M4 and M22. We also thank Antoine de Saizieu for sharing his microarray expertise and for helpful discussions.
REFERENCES
- 1.Ahmed, M., C. M. Borsch, A. A. Neyfakh, and S. Schuldiner. 1993. Mutants of the Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensive alkaloid reserpine. J. Biol. Chem. 268:11086-11089. [PubMed] [Google Scholar]
- 2.Ahmed, M., L. Lyass, P. N. Markham, S. S. Taylor, N. Vazquez-Laslop, and A. A. Neyfakh. 1995. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J. Bacteriol. 177:3904-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, J. M. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48(Suppl. 1):5-16. [DOI] [PubMed] [Google Scholar]
- 4.Aparicio, J. F., R. Fouces, M. V. Mendes, N. Olivera, and J. F. Martin. 2000. A complex multienzyme system encoded by five polyketide synthase genes is involved in the biosynthesis of the 26-membered polyene macrolide pimaricin in Streptomyces natalensis. Chem. Biol. 7:895-905. [DOI] [PubMed] [Google Scholar]
- 5.Baranova, N. N., and A. A. Neyfakh. 1997. Apparent involvement of a multidrug transporter in the fluoroquinolone resistance of Streptococcus pneumoniae. Antimicrob. Chemother. 41:1396-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer, R., E. Pestova, J. J. Millichap, V. Stosor, G. A. Noskin, and L. R. Peterson. 2000. A convenient assay for estimating the possible involvement of efflux of fluoroquinolones by Streptococcus pneumoniae and Staphylococcus aureus: evidence for diminished moxifloxacin, sparfloxacin, and trovafloxacin efflux. Antimicrob. Agents Chemother. 44:798-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolhuis, H., H. W. Van Veen, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1997. Mechanisms of multidrug transporters. FEMS Microbiol. Rev. 21:55-84. [DOI] [PubMed] [Google Scholar]
- 8.Brautaset, T., O. N. Sekurova, H. Sletta, T. E. Ellingsen, A. R. Strom, S. Valla, and S. B. Zotchev. 2000. Biosynthesis of the polyene antifungal antibiotic nystatin in Streptomyces noursei ATCC 1145: analysis of the gene cluster and deduction of the biosynthetic pathway. Chem. Biol. 7:395-403. [DOI] [PubMed] [Google Scholar]
- 9.Brenwald, N. P., M. J. Gill, and R. Wise. 1997. The effect of reserpine, an inhibitor of multi-drug efflux pumps, on the in vitro susceptibilities of fluoroquinolone-resistant strains of Streptococcus pneumoniae to norfloxacin. J. Antimicrob. Chemother. 40:458-460. [DOI] [PubMed] [Google Scholar]
- 10.Brenwald, N. P., M. J. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenwald, N. P., P. Appelbaum, T. Davies, and M. J. Gill. 2003. Evidence for efflux pumps other than PmrA associated with fluoroquinolone resistance in Streptococcus pneumoniae. Clin. Microbiol. Infect. 9:140-143. [DOI] [PubMed] [Google Scholar]
- 12.Caffrey, P., S. Lynch, E. Flood, S. Finnan, and M. Oliynyk. 2001. Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes. Chem. Biol. 8:713-723. [DOI] [PubMed] [Google Scholar]
- 13.Canton, R., M. Morosini, M. C. Enright, and I. Morrissey. 2003. Worldwide incidence, molecular epidemiology and mutations implicated in fluoroquinolone-resistant Streptococcus pneumoniae: data from the PROTEKT surveillance programme. J. Antimicrob. Chemother. 52:944-952. [DOI] [PubMed] [Google Scholar]
- 14.Carlo, D. J., J. Y. Zeltner, T. H. Stoudt, K. H. Nollstadt, and R. B. Walton. 1980. South African patent SFXXABZA 7806664 1980730.
- 15.Chen, J. D., and D. A. Morrison. 1988. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene 64:155-164. [DOI] [PubMed] [Google Scholar]
- 16.Daly, M. M., S. Doktor, R. Flamm, and D. Shortridge. 2004. Characterization and prevalence of MefA, MefE, and the associated msr(D) gene in Streptococcus pneumoniae clinical isolates. J. Clin. Microbiol. 42:3570-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daporta, M. T., J. L. Munoz Bellido, G. Y. Guirao, M. S. Hernandez, and J. A. Garcia-Rodriguez. 2004. In vitro activity of older and newer fluoroquinolones against efflux-mediated high-level ciprofloxacin-resistant Streptococcus pneumoniae. Int. J. Antimicrob. Agents 24:185-187. [DOI] [PubMed] [Google Scholar]
- 18.Davies, T. A., G. A. Pankuch, B. E. Dewasses, M. R Jacobs, and P. C. Appelbaum. 1999. In vitro development of resistance to five quinolones and amoxicillin-clavulanate in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:1177-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Saizieu, A., U. Certa, J. Warrington, C. Gray, W. Keck, and J. Mous. 1998. Bacterial transcript imaging by hybridization of total RNA to oligonucleotide arrays. Nat. Biotechnol. 16:45-48. [DOI] [PubMed] [Google Scholar]
- 20.de Saizieu, A., C. Gardès, N. Flint, C. Wagner, M. Kamber, T. J. Mitchell, W. Keck, K. E. Amrein, and R. Lange. 2000. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J. Bacteriol. 182:4696-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbons, S., and E. E. Udo. 2000. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother. Res. 14:139-140. [DOI] [PubMed] [Google Scholar]
- 22.Gill, M. J., N. P. Brenwald, and R. Wise. 1999. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godreuil, S., M. Galimand, G. Gerbaud, C. Jacquet, and P. Courvalin. 2003. Efflux pump Lde is associated with fluoroquinolone resistance in Listeria monocytogenes. Antimicrob. Agents Chemother. 47:704-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grkovic, S., M. H. Brown, and R. A. Skurray. 2001. Transcriptional regulation of multidrug efflux pumps in bacteria. Semin. Cell Dev. Biol. 12:225-237. [DOI] [PubMed] [Google Scholar]
- 25.Hakenbeck, R., N. Balmelle, B. Weber, C. Gardès, W. Keck, and A. de Saizieu. 2001. Mosaic genes and mosaic chromosomes: intra- and interspecies genomic variation of Streptococcus pneumoniae. Infect. Immun. 69:2477-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havarstein, L. S., G. Coomaraswamy, and D. A. Morrison. 1995. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 92:11140-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. De Hoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. Le Blanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, J., P. W. O'Toole, W. Shen, H. Amrine-Madsen, X. Jiang, N. Lobo, L. M. Palmer, L. Voelker, F. Fan, M. N. Gwynn, and D. McDevitt. 2004. Novel chromosomally encoded multidrug efflux transporter MdeA in Staphylococcus aureus. Antimicrob. Agents Chemother. 48:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaatz, G. W., and S. M. Seo. 1995. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 39:2650-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim. Biophys. Acta 39:508-517. [DOI] [PubMed] [Google Scholar]
- 31.Lee, E.-W., M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimcrob. Agents Chemother. 47:3733-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lockhart, D. J., H. Dong, M. C. Byrne, M. T. Follettie, M. V. Gallo, M. S. Chee, M. Mittmann, C. Wang, M. Kobayashi, H. Horton, and E. L. Brown. 1996. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat. Biotechnol. 14:1675-1680. [DOI] [PubMed] [Google Scholar]
- 33.Lubelski, J., R. Van Merkerk, W. N. Konings, and A. J. M. Driessen. 2004. ydaG and ydbA of Lactococcus lactis encode a heterodimeric ABC-type multidrug transporter, abstr. C1-1190, p. 79. In Abstracts 44th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C.
- 34.Madaras-Kelly, K. J., C. Daniels, M. Hegbloom, and M. Thompson. 2002. Pharmacodynamic characterization of efflux and topoisomerase IV-mediated fluoroquinolone resistance in Streptococcus pneumoniae. J. Antimicrob. Chemother. 50:211-218. [DOI] [PubMed] [Google Scholar]
- 35.Markham, P. N. 1999. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob. Agents Chemother. 43:988-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrer, E., A. T. Satoh, M. M. Johnson, L. J. V. Piddock, and M. G. P. Page. 2005. Global transcriptome analysis of the responses of a fluoroquinolone-resistant Streptococcus pneumoniae mutant and its parent to ciprofloxacin. Antimicrob. Agents Chemother. 50:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masaoka, Y., Y. Ueno, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2000. A two-component multidrug efflux pump, EbrAB, in Bacillus subtilis. J. Bacteriol. 182:2307-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messing, J., C. Yanisch-Perron, and J. Vieira. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 39.Montanari, M. P., E. Tili, I. Cochetti, M. Mingoia, A. Manzin, and P. E. Varaldo. 2004. Molecular characterization of clinical Streptococcus pneumoniae isolates with reduced susceptibility to fluoroquinolones emerging in Italy. Microb. Drug Resist. 10:209-217. [DOI] [PubMed] [Google Scholar]
- 40.Morita, Y., T. Murata, T. Mima, S. Shiota, T. Kuroda, T. Mizushima, N. Gotoh, T. Nishino, and T. Tsuchiya. 2003. Induction of mexCD-oprJ operon for a multidrug efflux pump by disinfectants in wild-type Pseudomonas aeruginosa PAO1. J. Antimicrob. Chemother. 51:991-994. [DOI] [PubMed] [Google Scholar]
- 41.Mortimer, P. G. S., and L. J. V. Piddock. 1991. Comparison for the methods used for measuring the accumulation of quinolones into Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J. Antimicrob. Chemother. 28:639-653. [DOI] [PubMed] [Google Scholar]
- 42.Nair, B. M., K.-J. Cheung, Jr., A. Griffith, and J. L. Burns. 2004. Salicylate induces an antibiotic efflux pump in Burkholderia cepacia complex genomovar III (B. cenocepacia). J. Clin. Investig. 113:464-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pestova, E., J. J. Millichap, F. Siddiqui, G. A. Noskin, and L. R. Peterson. 2002. Non-PmrA-mediated multidrug resistance in Streptococcus pneumoniae. J. Antimicrob. Chemother. 49:553-556. [DOI] [PubMed] [Google Scholar]
- 44.Piddock, L. J. V., M. Johnson, V. Ricci, and S. L. Hill. 1998. Activities of new fluoroquinolones against fluoroquinolone-resistant pathogens of the lower respiratory tract. Antimicrob. Agents Chemother. 42:2956-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piddock, L. J. V., M. M. Johnson, S. Simjee, and L. Pumbwe. 2002. Expression of the efflux pump gene pmrA in fluoroquinolone-resistant and -susceptible clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piddock, L. J. V., and M. M. Johnson. 2002. Accumulation of 10 fluoroquinolones by wild-type or efflux mutant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouquette-Loughlin, C., I. Stojiljkovic, T. Hrobowski, J. T. Balthazar, and W. M. Shafer. 2002. Inducible, but not constitutive, resistance of gonococci to hydrophobic agents due to the MtrC-MtrD-MtrE efflux pump requires TonB-ExbB-ExbD proteins. Antimicrob. Agents Chemother. 46:561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz, F.-J., A. C. Fluit, M. Luckefahr, B. Engler, B. Hofmann, J. Verhoef, H.-P. Heinz, U. Hadding, and M. E. Jones. 1998. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in-vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 42:807-810. [DOI] [PubMed] [Google Scholar]
- 49.Stieger, M., B. Wohlgensinger, M. Kamber, R. Lutz, and W. Keck. 1999. Integrational plasmids for the tetracycline-regulated expression of genes in Streptococcus pneumoniae. Gene 226:243-251. [DOI] [PubMed] [Google Scholar]
- 50.Tanabe, H., K. Yamasaki, M. Furue, K. Yamamoto, A. Katoh, M. Yamamoto, S. Yoshioka, H. Tagami, H. Aiba, and R. Utsumi. 1997. Growth phase-dependent transcription of emrKY, a homolog of multidrug efflux emrAB genes of Escherichia coli, is induced by tetracycline. J. Gen. Appl. Microbiol. 43:257-263. [DOI] [PubMed] [Google Scholar]
- 51.Teran, W., A. Felipe, A. Segura, A. Rojas, J.-L. Ramos, and M.-T. Gallegos. 2003. Antibiotic-dependent induction of Pseudomonas putida DOT-T1E TtgABC efflux pump is mediated by the drug binding repressor TtgR. Antimicrob. Agents Chemother. 47:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 53.Zeller, V., C. Janoir, M.-D. Kitzis, L. Gutmann, and N. J. Moreau. 1997. Active efflux as a mechanism of resistance to ciprofloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:1973-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]