Abstract
A practical preclinical model for the hyperbilirubinemia produced by human immunodeficiency virus protease inhibitors has been developed. Indinavir and atazanavir produced significant hyperbilirubinemia, whereas amprenavir, the negative control, was indistinguishable from the ritonavir booster dose. This model was used to disqualify an exploratory protease inhibitor from development.
Human immunodeficiency virus (HIV) protease inhibitors (PIs) are one of the drug classes used in combination to provide highly active antiretroviral therapy, which lowers HIV RNA in plasma to unquantifiable levels and substantially extends the lives of people with HIV infection. However, PIs are associated with side effects as well. For example, two PIs, indinavir and atazanavir, produce significant elevations of unconjugated serum bilirubin. Bilirubin is conjugated as its glucuronide in the liver by the enzyme UDP-glucuronosyltransferase 1A1 (UGT1A1) and is subsequently secreted by hepatocytes into the bile canaliculi for excretion. The appearance of increased unconjugated bilirubin in serum suggests inhibition of conjugation by the PIs rather than overt hepatotoxicity. PIs inhibit UGT1A1 in vitro (4, 7); however, the rank order of inhibitory potency does not correlate with clinical observations unless protein binding is taken into account (6). PIs also have been reported to inhibit the human organic anion transporting protein 1B1, which transports unconjugated bilirubin to the liver, at lower micromolar concentrations (1). Consequently, the mechanism of hyperbilirubinemia induced by these two PIs remains to be unequivocally established.
Recently, Zucker et al. reported modest increases in plasma bilirubin in Gunn rats upon treatment with indinavir (7). Gunn rats are heterozygous for an inherited deficiency in hepatic bilirubin-conjugating activity caused by a −1 frameshift mutation in the UGT1A1 gene (2) and are more susceptible to bilirubin elevations than normal rats. We have investigated the utility of this observation for the preclinical evaluation of new PIs to assess the potential to induce this side effect.
Initially, we investigated the effects of indinavir and atazanavir in Gunn rats under conditions similar to those employed by Zucker et al. (7). Indinavir was administered in three doses of 360 mg/kg of body weight twice a day (BID), and blood samples were drawn 4 hours after the final dose for serum bilirubin evaluation (Zucker et al. used four doses of 240 mg/kg three times a day). Unlike the previous report, in which a small (0.042 mg/dl) but significant increase from baseline was observed, bilirubin levels following the third dose of indinavir were not statistically significantly different than baseline (mean change, 0.02 ± 0.04 mg/dl). We also administered atazanavir under similar conditions (100 mg/kg BID) and observed no change in bilirubin between baseline and day 2 (4 h after the third dose). However, in an additional modification to the reported procedure, we also collected blood 4 hours following the initial dose of each of the above PIs. In contrast to the very small effects observed on day 2, five of six animals treated with indinavir experienced a bilirubin increase of 0.1 mg/dl or greater, with one animal displaying an increase of 0.5 mg/dl.
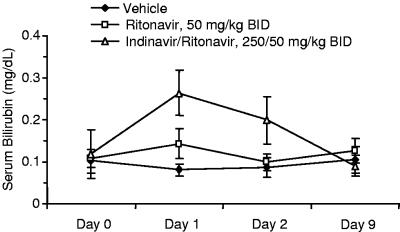
Based on the above preliminary results, we repeated the above procedure (three doses in a BID format), assessing serum bilirubin on day 0, prior to the initial dosing day, and 4 hours after both the first and third doses (day 1 and day 2). In an attempt to optimize the plasma exposure of indinavir, which has a relatively short half-life in rats, we also boosted the indinavir pharmacokinetics by codosing with ritonavir (3). All PIs were dosed in 5% ethanol:95% propylene glycol with appropriate equivalents of p-toluene sulfonic acid. Two separate groups of Gunn rats were treated with either vehicle or ritonavir only. The ritonavir boosting dose (50 mg/kg) produced a small but significant (P < 0.001, analysis of variance) bilirubin elevation after a single dose, which declined back to near-vehicle levels by day 2. As anticipated, the indinavir-ritonavir combination (250 and 50 mg/kg, respectively [250-50 mg/kg]) produced a substantial elevation, and serum bilirubin levels remained significantly higher than levels for either vehicle- or ritonavir-treated animals, despite a partial decline on day 2 (Fig. 1) (P < 0.001). A third sample taken from indinavir-ritonavir-treated rats on day 9 (7 days after the final dose) indicated that the hyperbilirubinemia was reversible, and the same set of rats was randomized and reused for subsequent experiments.
FIG. 1.
Bilirubin changes in Gunn rats treated with indinavir-ritonavir.
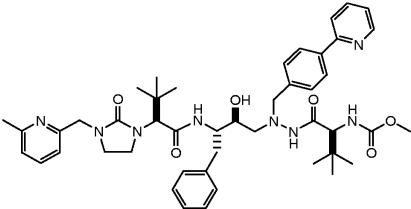
Several HIV PIs were examined with this model (n = 8 to 10 rats/arm/experiment). Lopinavir-ritonavir and amprenavir-ritonavir were chosen as negative controls because these drugs do not produce clinically significant hyperbilirubinemia in humans. Amprenavir-ritonavir (250-50 mg/kg) produced elevations that were indistinguishable from the ritonavir boosting dose (Table 1). Lopinavir-ritonavir produced an incremental increase that was slight but nonetheless statistically significantly different from results with ritonavir alone. In contrast, treatment of the Gunn rats with atazanavir-ritonavir produced marked hyperbilirubinemia. Plasma levels of all of the PIs (determined from the same blood sample 4 h after the first and third doses) were similar to those observed with efficacious doses in humans (Table 1). Finally, we evaluated the effect of an exploratory PI, A-681799 (5), part of the structure of which is the same as atazanavir (Fig. 2). Elevations in serum bilirubin significantly greater than those produced by atazanavir-ritonavir (P < 0.001) were observed. Interestingly, in contrast to the other PIs examined, serum bilirubin continued to increase following multiple doses of A-681799-ritonavir.
TABLE 1.
Mean changes in bilirubin and mean serum concentrations of PIs in Gunn rats
| Treatment (n) | BID dose (mg/kg) | Mean (SD) change in bilirubin (mg/dl)a
|
Mean (SD) serum PI concn (μg/ml)a
|
||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 1 | Day 2 | ||
| Vehicle (26) | −0.012 (0.026) | 0.013 (0.060) | NA | NA | |
| Ritonavir (27) | 50 | 0.056 (0.034) | 0.017 (0.045) | 5.66 (3.07) | 3.64 (2.53) |
| Amprenavir-ritonavir (10) | 250-50 | 0.069 (0.031) | NA | 12.84 (1.65) | NA |
| Lopinavir-ritonavir (8) | 250-50 | 0.1b | 0.093 (0.053) | 4.21 (0.53) | 3.37 (0.62) |
| Indinavir-ritonavir (10) | 250-50 | 0.146 (0.059) | 0.074 (0.073) | 5.74 (2.21) | 5.36 (2.41) |
| Atazanavir-ritonavir (8) | 250-50 | 0.355 (0.139) | 0.199 (0.157) | 16.62 (4.42) | 9.23 (4.01) |
| A-681799-ritonavir (9) | 100-50 | 0.536 (0.216) | 0.685 (0.415) | 8.87 (6.86) | 8.88 (5.35) |
Day 1 and day 2 determinations were performed with samples obtained from the jugular vein 4 hours after the first and third BID doses, respectively. NA, not available.
Serum bilirubin was measured to the nearest 0.01 mg/ml, except for lopinavir/ritonavir (day 1).
FIG. 2.
Structure of A-681799.
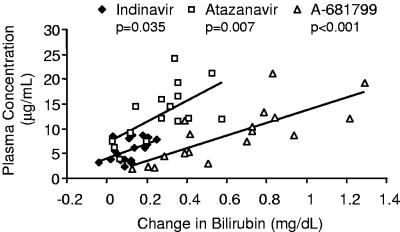
The pharmacokinetic/pharmacodynamic relationships in this model were explored with the three PIs producing substantial bilirubin elevations (indinavir, atazanavir, and A-681799). As shown in Fig. 3, the change in bilirubin in individual rats was positively correlated to the serum drug concentration at the time of blood sampling. This relationship was statistically significant for both atazanavir-ritonavir (P = 0.007, linear regression) and A-681799-ritonavir (P < 0.001) and marginally significant for indinavir-ritonavir (P = 0.035), which displayed a lower range of both drug concentrations and bilirubin elevations.
FIG. 3.
Association of serum bilirubin elevations and drug concentrations in Gunn rats treated with ritonavir-boosted indinavir, atazanavir, or A-681799.
In summary, we have developed a practical preclinical model for assessing the potential for unconjugated hyperbilirubinemia by HIV PIs. This convenient in vivo model, which appears to be predictive with only a single dose of a PI boosted by ritonavir, recapitulates the hyperbilirubinemia produced clinically with indinavir and atazanavir therapy and may be useful in the identification of new PIs with low potential for this undesired side effect.
REFERENCES
- 1.Campbell, S. D., S. M. de Morais, and J. J. Xu. 2004. Inhibition of the human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chem.-Biol. Interact. 150:179-187. [DOI] [PubMed] [Google Scholar]
- 2.Iyanagi, T., T. Watanabe, and Y. Uchiyama. 1989. The 3-methylcholanthrene-inducible UDP-glucuronosyltransferase deficiency in the hyperbilirubinemic rat (Gunn rat) is caused by a −1 frameshift mutation. J. Biol. Chem. 264:21302-21307. [PubMed] [Google Scholar]
- 3.Kempf, D. J., K. C. Marsh, G. Kumar, A. D. Rodrigues, J. F. Denissen, E. McDonald, M. J. Kukulka, A. Hsu, G. R. Granneman, P. A. Baroldi, E. Sun, D. Pizzuti, J. J. Plattner, D. W. Norbeck, and J. M. Leonard. 1997. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob. Agents Chemother. 41:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Mara, E., V. Mummaneni, B. Burchell, D. Randall, N. Sagali, T. Tanner, A. Schuster, M. Geraldes, R. Raymond, and S. Kaul. 2000. Relationship between uridine diphosphate glucuronosyl transferase (UDP-GT) 1A1 genotype and total bilirubin elevations in healthy subjects receiving BMS-232632 and saquinavir. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1645. American Society for Microbiology, Washington, D.C.
- 5.Randolph, J. T., P. Huang, C. Flentge, D. A. DeGoey, W. Flosi, D. Grampovnik, C. Yeung, H. J. Chen, L. L. Klein, T. Dekhtyar, H. Mo, L. Colletti, W. Kati, J. M. Schmidt, T. Turner, K. C. Marsh, A. Molla, and D. J. Kempf. 2004. A-681799, a novel HIV protease inhibitor. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstract F-485. American Society for Microbiology, Washington, D.C.
- 6.Zhang, D., T. J. Chando, D. W. Everett, C. J. Patten, S. S. Dehal, and W. G. Humphreys. 2005. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab. Dispos. 33:1729-1739. [DOI] [PubMed] [Google Scholar]
- 7.Zucker, S. D., X. Qin, S. D. Rouster, F. Yu, R. M. Green, P. Keshavan, J. Feinberg, and K. E. Sherman. 2001. Mechanism of indinavir-induced hyperbilirubinemia. Proc. Natl. Acad. Sci. USA 98:12671-12676. [DOI] [PMC free article] [PubMed] [Google Scholar]