Abstract
Therapeutic drug monitoring (TDM) is gaining importance for improving the success of antiretroviral treatment in human immunodeficiency virus-infected patients. However, enfuvirtide (ENF) concentrations are not regularly determined. The objective of this work was to study the pharmacokinetics (PK) of ENF in patients treated in routine clinical settings, to develop a population PK model describing the concentration-time profile, and to establish PK reference values. A liquid chromatography-tandem mass spectrometry method was developed and applied to serum samples submitted for TDM. A two-compartment model with linear absorption and elimination was fitted to 329 concentrations from 131 patients. The PK model was used for simulations resulting in percentile curves for ENF levels for the full dosing interval. The model predicted that a median concentration of 1,968 ng/ml would be reached 12 h after administration of 90 mg of ENF, and 23% and 58% of patients are expected to have concentrations below 1,000 ng/ml and 2,200 ng/ml, respectively. Both values have been proposed as cutoffs for virological efficacy. The median maximum concentration of drug in serum (Cmax) of 3,943 ng/ml, predicted for 3 h after drug administration, is lower than the Cmax reported previously. We found an enormous interpatient variability at every time point, with concentration spectrums covering >1 log and 52% and 123% interindividual variabilities in the typical clearance and volume of distribution, respectively, in contrast to preexisting PK data. In summary, ENF levels are lower and more variable than expected. Many patients may achieve insufficient concentrations. Further covariate analysis in the population PK model might help to identify factors influencing the variability in ENF concentrations.
Therapeutic drug monitoring (TDM) of protease inhibitors (PI) and nonnucleoside inhibitors of the reverse transcriptase (NNRTI) is slowly becoming established as an important tool for improving the success of antiretroviral treatment of human immunodeficiency virus (HIV)-infected patients. The ATHENA trial has shown that nelfinavir concentrations below a certain threshold are associated with high rates of virological failure (3, 4). Patients taking efavirenz also have lower chances of sustained viral suppressions when drug concentrations are below 1,000 ng/ml than when they are above this concentration (17). At the other end of the concentration spectrum, TDM may be beneficial for patients with excessive toxic drug effects. Concentration-controlled dose reductions in patients on ritonavir-boosted indinavir who suffer from renal toxicity may alleviate side effects without increasing the risk of therapeutic failure (3). With monitoring of drug levels, individual patients with extremely low or high concentrations can be identified, and a search for the underlying cause can be started, preventing precocious viral failure or unnecessary toxicity. The growing recognition of these advantages has led to the generation of an enormous body of data on the pharmacokinetics (PK) of members of these two drug classes.
Unlike PI and NNRTI, enfuvirtide (ENF), the first HIV fusion inhibitor, is administered by subcutaneous injection. It is not metabolized via the cytochrome system (11). Because of these characteristics, drug exposure is assumed to be less variable and drug-drug interactions are thought to be less important with ENF than with PI or NNRTI. Therefore, less attention has been paid to the investigation of factors influencing the PK of ENF in HIV-infected patients.
Our objectives were to develop an assay for the quantification of ENF in human serum and to characterize the PK of ENF by population PK modeling using PK data generated in the normal patient population.
MATERIALS AND METHODS
Patient samples.
From July 2003 through June 2005, our therapeutic drug monitoring service received 782 samples for the quantification of ENF in serum from unselected patients treated in different clinical centers. ENF was prescribed at a dose of 90 mg, injected every 12 h twice a day (BID). Samples had been collected in the course of standard treatment monitoring during routine visits. Patients had been told that drug levels were going to be measured, and they had been asked to report the time of the last ENF administration. We have performed a retrospective analysis on these requests. Samples were considered acceptable for statistical analysis only if the TDM request form contained all of the following information: patient characteristics (age, weight, sex, CD4 count, and virus load), time and date of blood collection, time and date of last ENF administration, and dose of ENF. Three hundred twenty-nine samples from 131 patients taking ENF in combination with different antiretroviral background regimens met these criteria and were included in the analysis.
Drug standards, reagents, and special tubes.
Analytical reference standards of ENF and deuterium-labeled ENF were generously provided by Roche Bioscience, Pharma Division, Palo Alto, CA. Ammonium hydroxide solution, trifluoroacetic acid, and glacial acetic acid were purchased from Sigma-Aldrich, Munich, Germany. Acetonitrile (ACN) and H2O for chromatography were purchased from VWR International, Berlin, Germany. Prototypes of “protein LoBind tubes” were generously provided by Eppendorf AG, Hamburg, Germany.
Sample preparation.
After blood collection, samples were centrifuged and the serum was shipped at ambient temperature to the laboratory. Upon arrival in the laboratory, samples were immediately frozen and stored at −25°C until analysis. The sample preparation method presented here is based upon previous work by Chang et al. (5). In summary, a 100-μl aliquot of serum was spiked into a low-binding polypropylene microreaction vial. Twenty microliters of an internal standard working solution (deuterium-labeled ENF [2,500 ng/ml] in stock solution buffer [H2O-ACN-NH3 {80:20:0.05, vol/vol/vol}]) was added. After the mixture was vortexed, 200 μl of acetonitrile was added, and the sample was vortexed again and allowed to equilibrate at room temperature for 30 min. After extraction, the sample was spun at 13,000 × g for 6 min. An aliquot of 200 μl of the supernatant was transferred into a clean low-binding microreaction vial and evaporated to dryness in a vacuum centrifuge (Bachofer, Reutlingen, Germany). The dry extract was then reconstituted in reconstitution solution (mobile phase A-ACN [70:30, vol/vol]), vortexed gently for 30 min, and centrifuged at 13,000 × g for 6 min.
HPLC conditions.
A 50-μl volume of the reconstituted sample was injected onto a Eurogel 100 polymery reversed phase column (8 μm, 100 by 2 mm) with an integrated guard column (Knauer, Berlin, Germany). Mobile phase A was H2O containing acetic acid (0.002%) and trifluoroacetic acid (0.0002%). Mobile phase B was acetonitrile containing acetic acid (0.08%). The high-pressure liquid chromatography (HPLC) system consisted of the following components: a mobile-phase delivery pump (Rheos 2000; Flux Instruments, Basel, Switzerland), a mobile-phase degasser (Degasys DG 1210; Uniflows, Tokyo, Japan), and an autosampler (Micro 215 liquid handler; Gilson S.A., Villiers-le-Bel, France). HPLC separation was achieved with mobile-phase-gradient elution (flow, 0.25 ml/min) using the following sequence: 0 min, 65% A; −1.0 min, 65% A; −1.5 min, 20% A; −3.0 min, 20% A; −3.5 min, 10% A; and −6.0 min, 10% A. The total run time was 15 min. After every injection, the HPLC components with direct contact with the sample (injection needle, injection port, and loop) were intensely rinsed with ACN-H2O (60:40, vol/vol). The total effluent entered the interface of the mass spectrometer.
MS-MS conditions.
An API 3000 (Applied Biosystems, Ontario, Canada) tandem mass spectrometer (MS-MS) equipped with an electrospray ionization ion source and run with Analyst software (version 1.2, service pack 1) was used for detection. ENF and the internal standard were monitored in the positive ion mode with the following transitions of precursor to product ions: m/z 1,123.7 to 1,343.0 (ENF) and 1,126.4 to 1,346.6 (deuterium-labeled ENF). This apparent mass gain after fragmentation represents the transition of quadruply charged parent ions into triply charged fragment ions.
The ion source temperature was set to 400°C. The mass spectrometric parameters for ENF and the internal standard (IS) were optimized manually and are shown in Table 1.
TABLE 1.
MS-MS parameters
| Parameter | Value for:
|
|
|---|---|---|
| ENF | IS | |
| Declustering potential (V) | 65 | 74 |
| Focusing potential (V) | 260 | 295 |
| Entrance potential (V) | 10 | 10 |
| Collision energy (V) | 27 | 26 |
| Cell exit potential (V) | 32 | 10 |
| Dwell time (ms) | 300 | 300 |
| Nebulizer gasa | 8 | 8 |
| Curtain gasa | 8 | 8 |
| Collision gasa | 10 | 10 |
| Ionspray voltage (V) | 5,500 | 5,500 |
Values for nebulizer, curtain, and collision gases are dimensionless and apply to the API 3000 only.
Calibration and QC.
Standards and quality control (QC) samples were prepared in blank pool serum from inpatients treated in our clinic for reasons other than HIV infection. With each batch containing a maximum of 36 unknown samples, an eight-point standard calibration curve run in duplicate was analyzed with samples containing ENF in concentrations ranging from 110 ng/ml to 14,024 ng/ml. A weighted (1/x, with x being the analyte concentration) linear regression was used to generate the regression formula. QC samples analyzed during the validation process were prepared from a different stock solution (ENF in stock solution buffer) at concentrations of 110 ng/ml, 297 ng/ml, 2,971 ng/ml, and 14,857 ng/ml. Assay performance during analytical runs was controlled for by analyzing samples at concentrations of 297 ng/ml, 2,971 ng/ml, and 14,024 ng/ml.
Population PK analysis.
PK model building for describing the typical concentration-time profile and characterizing the variability in the population was performed using the nonlinear mixed-effects modeling approach implemented in NONMEM, version V 1.1. First-order conditional estimation with interaction was used as the estimation method. In a stepwise approach, first a structural model was developed by investigating different numbers of compartments as well as mono- and bidirectional transport processes. Afterwards, interindividual and residual variability were characterized. The model was parameterized in terms of clearance (CL) and distribution volumes with PREDPP subroutines ADVAN 4 and TRANS 4. The steady-state routine SS 4 was used to account for the mere steady-state concentrations in the data set.
Interindividual variability in, e.g., PK parameter clearance was modeled using an exponential error term according to the equation 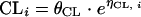 , where CLi represents the clearance estimate of the individual i, θCL the typical population clearance estimate, and η the (proportional) difference between both. Residual variability, that is, the discrepancy between the individual observed (Cobs,i) and the individual model-predicted (Cpred,i) serum concentrations, was expressed as a combined-error model with a proportional part, ɛ1, and an additive one, ɛ2, as follows: Cobs,i = Cpred,i · (1 + ɛ1,i) + ɛ2,i. The random-effect parameters η and ɛ were assumed to be symmetrically distributed with zero means and variances of ω2 and σ2, respectively.
, where CLi represents the clearance estimate of the individual i, θCL the typical population clearance estimate, and η the (proportional) difference between both. Residual variability, that is, the discrepancy between the individual observed (Cobs,i) and the individual model-predicted (Cpred,i) serum concentrations, was expressed as a combined-error model with a proportional part, ɛ1, and an additive one, ɛ2, as follows: Cobs,i = Cpred,i · (1 + ɛ1,i) + ɛ2,i. The random-effect parameters η and ɛ were assumed to be symmetrically distributed with zero means and variances of ω2 and σ2, respectively.
The model-building process was guided by analyzing the goodness-of-fit plots created with Xpose, version 3.104 (12), precision-of-parameter estimates and the objective function value provided by NONMEM. The latter was used for discrimination between hierarchical models in the likelihood ratio test. The addition of a parameter was considered significant if the decrease in objective function value was >3.84, corresponding to a P value of 0.05 (df = 1).
Simulations and statistical analysis.
The parameters estimated by the population PK model were used to simulate concentration-time profiles of 1,000 individuals by the software NONMEM, version V 1.1. Statistical analysis was carried out using the software S-PLUS 6.0 Professional. For the simulated individuals, the median concentration-time profile and the 5, 10, 25, 75, 90, and 95% quantile profiles were determined.
RESULTS
Assay performance and adhesion problem.
Like other peptides, ENF tends to adsorb to a variety of materials. The impact of this effect on ENF concentrations in stock solutions, in patient and spiked samples, and on extracts was investigated. Polypropylene containers were suitable for the storage of stock solutions. We used low-binding microreaction vials designed for protein research for sample preparation and extraction because these vials caused less adhesion of ENF to the vial surface, which permitted constant rates of extraction.
Sensitivity and selectivity.
ENF was spiked into blank sera from eight HIV-infected patients on different antiretroviral treatment regimens and into pool serum from 50 HIV-negative patients at a concentration of 112 ng/ml, which is close to the lower limit of quantification (LLOQ) of the assay. The signals and calculated concentrations were evaluated against the corresponding values from unspiked samples. There was no relevant signal at the retention time of ENF in any of the unspiked samples. The typical signal-to-noise ratio at the LLOQ was 31. The intra-assay LLOQ accuracy and precision generated from these samples were as follows: the relative error (RE) was −3.7%, and the coefficient of variation (CV) was 8.2%. These data are comparable to the intra-assay accuracy and precision achieved with QC samples from single HIV-negative donors, indicating that there are no matrix effects specific to the donor of the blank serum and the donor's concomitant HIV medication. Since the analyte peak areas were comparable in spiked samples from HIV-infected and -uninfected donors, it seems unlikely that ENF cross-reactive antibodies which are present in the majority of HIV-infected patients (21) influence the extraction and recovery of ENF.
Precision and accuracy.
Multiple validation batches were run to generate data on assay precision and accuracy. A linear regression with a 1/x weighting was fitted best to the calibration standards. The goodness of the fit was expressed by the low percentage of REs of all standards (<5%) and a mean correlation coefficient (r2) of 0.9996 (n = 9).
QC samples at four concentrations were prepared from a stock solution different from that used for the standards. One series of QC samples was prepared at a concentration above the highest standard. Multiple replicas of QC samples at each concentration were analyzed in at least five validation batches. The interassay accuracy and precision at these four concentrations are shown in Table 2.
TABLE 2.
Assay precision and accuracy
| Parameter | Value | |||
|---|---|---|---|---|
| Nominal concn (ng/ml) | 297 | 1,490 | 2,970 | 14,900 |
| Mean concn (ng/ml) | 298 | 1,417 | 2,824 | 14,611 |
| SD | 15 | 48 | 135 | 436 |
| CV (%) | 5.1 | 3.4 | 4.8 | 3.0 |
| RE (%) | 0.2 | −4.9 | −4.9 | −1.9 |
Extraction.
The levels of recovery of ENF and the IS were comparable at all concentration levels and at three different temperatures during sample preparation (4°C, 21°C, and 50°C).
Sample storage stability.
Patient samples were stored at room temperature for 24, 36, and 72 h. ENF concentrations were determined and compared to an aliquot which had been stored at −25°. After 72 h, ENF concentrations were reduced by 15% (95% confidence interval, −19 to −11%).
Serum versus plasma.
The concentrations of ENF in serum were compared to those measured in EDTA-plasma in paired samples from seven different patients. ENF concentrations in serum were slightly but significantly lower than those in plasma (−11% [95% confidence interval, −8 to −15%]).
Ruggedness.
The method described herein has been validated only with a single mass spectrometer. However, the performance of the method did not vary with the use of HPLC columns from different batches or after changes in other components of the HPLC system.
Patient samples and the population PK model.
Three hundred twenty-nine samples from 131 patients treated with ENF (90 mg BID) in routine clinical settings were analyzed. Seventy-two patients had a single blood sample collected. The numbers of patients who had two, three, four, five, six, seven, and eight planned serial blood samples collected were 16, 9, 8, 2, 13, 10, and 1, respectively. Blood had been collected 1 h (±15 min) (n = 49), 2 h (±15 min) (n = 41), 3 h (±15 min) (n = 21), 4 h (±15 min) (n = 33), 6 h (±15 min) (n = 29), 9 h (±15 min) (n = 25), 12 h (±15 min) (n = 65), 24 h (±15 min) (n = 8), and at other time points (n = 58) after drug administration. Patient characteristics for the model-building population are shown in Table 3.
TABLE 3.
Patient characteristics of the model-building program
| Characteristic | Value |
|---|---|
| No. of patients | 131 |
| % Male | 82 |
| Median age (range) | 45 (24-68) |
| Median wt (kg) (range) | 67 (41-104) |
| Median no. of CD4 cells/μl (range) | 157 (3-724) |
| Median log10 VL (copies/ml) (range) | 3.5 (1.3-6.6) |
VL,viralload.
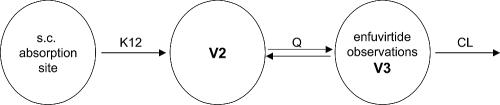
In the population PK approach, all serum concentration data were fitted simultaneously. Several structural PK models, comprised of one, two, and three compartmental structures, were investigated. A two-compartment model (with the absorption constant [K12], volumes of distribution V2 and V3, intercompartmental clearance [Q], and clearance [CL]) exhibited the best fit to the data. Due to the sparseness of the data, the bioavailability fraction was fixed. The structure of the final model is shown in Fig. 1, with population parameter estimates and their relative standard errors listed in Table 4. All parameters were estimated with acceptable precision (5 to 50% relative standard error). The volume of the observation compartment approximated the serum volume. The comparatively higher value of the peripheral volume of distribution indicated a good distribution into the tissue.
FIG. 1.
Structure of the final population PK model after subcutaneous (s.c.) ENF administration.
TABLE 4.
Final parameters of the population PK modela
| Model parameter | Population estimate | RSE (%) |
|---|---|---|
| CL (liters/h) | 2.15b | 5.5 |
| V2 (liters) | 15.2b | 14.1 |
| Q (liters/h) | 1.72b | 41.3 |
| V3 (liters) | 5.43b | 42.2 |
| K12 (liters/h) | 2.12 | 49.5 |
| Interindividual variability (ω) | ||
| CL, CV (%) | 52.4 | 15.8c |
| V3, CV (%) | 122.5 | 38.5c |
| Residual errors (σ) | ||
| Proportional, CV (%) | 22.8 | 17.7c |
| Additive (ng/ml) | 0.0001 | NAd |
| Calculated parameters | ||
| AUC0-12 (mg · h/liters) | 35.29 | |
| t1/2 (h) | 1.75 |
RSE, relative standard error (standard error divided by population estimate × 100); AUC0-12, area under the concentration-time curve from 0 to 12 h; t1/2, plasma half-life.
Assuming a bioavailability fraction of 0.843.
Given on the variance scale.
No standard error could be calculated, as the additive error was fixed.
Interindividual variability to characterize the variation among the patients could be quantified for the two primary PK parameters, CL and V3, revealing substantial CV values of 52% and 122%, respectively. The residual variability estimated with a combination of the additive- and proportional-error models was quite low. The additive error, fixed at 0.0001 ng/ml, was negligible and included only for reasons of model stability; the proportional error was 23%.
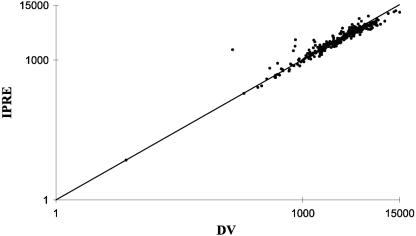
In Fig. 2, the observed ENF concentrations in serum are plotted against the individual predicted concentrations based on the final model. The symmetrically distributed data points around the line of identity indicate the adequacy of the data description of the model.
FIG. 2.
Diagnostic plot for the final PK model showing observed (dependent variable, DV) (ng/ml) versus individual prediction (IPRE) (ng/ml) ENF concentrations and the line of unity.
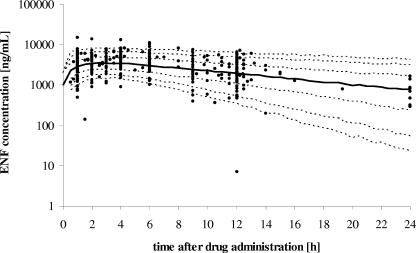
Based on the typical and variability estimates of the final population model, 1,000 concentration-time profiles were generated. Table 5 shows drug levels expected to be exceeded by 5%, 10%, 25%, 50%, 75%, 90%, and 95% of the normal population for a given time point within the dosing interval. Figure 3 summarizes these data for the complete dosing interval. The concentration range found in this population was enormous, with log coefficients of variation ranging from 5.8% to 9.2%. Although the highest concentration was observed in a sample taken 1 h after the injection of ENF into one individual, the model predicted a maximum concentration of drug in serum (Cmax) after 3 to 4 h of administration in all percentile courses.
TABLE 5.
ENF concentrations expected to be exceeded by 5%, 10%, 25%, 75%, 90%, and 95% of the population at a given time point of the dosing intervala
| Cumulative probability (%) | Concn (ng/ml) at indicated time (h) after ENF administration
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 12 | 24 | |
| 5 | 7,303 | 8,086 | 7,906 | 8,155 | 7,735 | 6,137 | 4,219 |
| 10 | 5,847 | 6,558 | 6,587 | 6,661 | 6,126 | 4,856 | 3,074 |
| 25 | 4,099 | 4,726 | 4,949 | 4,714 | 4,357 | 3,115 | 1,690 |
| 50 | 2,839 | 3,366 | 3,471 | 3,353 | 3,008 | 1,968 | 771 |
| 75 | 1,937 | 2,378 | 2,459 | 2,392 | 2,030 | 1,071 | 244 |
| 90 | 1,363 | 1,689 | 1,790 | 1,748 | 1,378 | 538 | 53 |
| 95 | 1,040 | 1,414 | 1,432 | 1,353 | 1,081 | 349 | 23 |
All values were generated by simulation of 1,000 individuals based on the developed population pharmacokinetic model.
FIG. 3.
Concentration-time profiles generated from the population PK model by simulating 1,000 individuals. The dashed lines from bottom to top represent the 5%, 10%, 25%, 75%, 90%, and 95% quantiles of the population. The thick line corresponds to the predicted median concentration-time profile. Observed concentrations are shown as filled circles.
DISCUSSION
Assay development.
Unlike the previously developed method, whose calibration range was 5 to 2,000 ng/ml (5), the assay that we have developed covers the complete spectrum of ENF concentrations found in clinical serum samples. The performance of the method has been assessed through a vigorous validation procedure, and the results are comparable with those published by other groups (5; A. D'Avolio, M. Sciandra, S. Bonora, A. Ibanez, D. Aguilar Marucco, and G. Di Perri, Abstr. 5th Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 8.3, 2004). Concentrations of all QC samples and calibration standards fell within the acceptance range of ±15% as proposed by the FDA (8).
Application to clinical samples and definition of reference values.
In our population, the observed mean trough concentration of drug in serum (Ctrough) 12 h after ENF injection was comparable to that seen in PK trials (16) (observed mean, 2,768 ng/ml versus 2,670 ng/ml). Cmaxs for ENF were measured in samples which had been collected 3 to 4 h after drug injection. This finding is consistent with the time to Cmax of 3.3 h reported in the product monograph. The observed peak concentrations in our population were lower than the official PK data (observed mean, 3,830 ng/ml versus 5,350 ng/ml), and the interindividual concentration variability within our population was considerably higher than that of the data from Roche (65.6 versus 31.9%).
Based on this data set, we developed a two-compartment PK model that proved superior to a one-compartment model which has been described in the literature previously (18). Using this model, percentile curves of ENF concentrations were generated for the complete dosing interval. The model predicts that 90% of patients will achieve Ctroughs between 349 ng/ml and 6,137 ng/ml. This enormous degree of variability, which is found at every time point throughout the dosing interval, is typical for orally administered protease inhibitors and NNRTI (H. Stocker, G. Kruse, C. Moecklinghoff, A. Hill, W. Sawyr, and M. Kurowski, Abstr. 4th Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 5.4, 2004). However, it is a rather surprising result for a subcutaneously administered drug. A number of reasons may account for the differences between the data generated in our population and those found in other studies.
Technical problems.
ENF is not stable in serum samples. After 3 days at room temperature, ENF concentrations are reduced by 15%. In our population, the median time from blood collection to storage at −25° was 4 days. In addition, we have found serum levels to be 10% lower than plasma levels. However, we consider this discrepancy too small to explain the low maximum concentrations. Our validation data and results from replicate analyses of clinical samples underline the good performance of the method. Even without a formal cross validation, we are confident about the accuracy of our assay because the ENF PK data presented by S. Bonora et al. are very similar to our results, despite the methodological difference of the assay used by this group (S. Bonora, D. Castagna, D. Aguilar Marucco, H. Hasson, F. Canta, M. Sciandra, A. D'Avolio, L. Veronese, D. Gonzalez de Requena, M. Boffito, A. Lazzarin, and G. Di Perri, Abstr. 5th Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 6.3, 2004).
Inadequate storage or handling of ENF.
After mixing of the sterile water with the powder, ENF takes half an hour or longer to dissolve completely. When dissolved, it should be stored at 4°C and used within 24 h. Some patients may have problems complying with these complex requirements and may not aspirate the total amount of the injection solution. Others may deliberately inject less than the total dose, hoping to reduce the severity of injection site reactions.
Influence of injection site.
Injecting ENF into the thigh results in a lower Cmax (4.71 μg/ml) than injecting ENF into the abdomen (5.35 μg/ml) (16). Our data set does not include information on the injection site. It is likely, though, that patients rotate injection sites, which could have contributed to the higher variability and the lower Cmax values found here.
Drug-drug interactions.
So far, three formal interaction trials have been conducted to investigate the impact of ritonavir (200 mg BID) (20), saquinavir-ritonavir (1,000 and 100 mg BID, respectively) (20), and rifampin (600 mg once a day) (2) on the PK of ENF. The most-pronounced changes in ENF PK were seen with saquinavir-ritonavir coadministration, causing a rise in Ctrough of 26%. The CYP3A4 inhibitor ritonavir alone and the inducer rifampin had only minor influence on ENF Ctroughs (+14% and −15%, respectively). However, the metabolism of ENF is not mediated by cytochromes because the peptide does not penetrate into the intracellular compartment. In addition, ENF has been shown not to affect the activity of a variety of cytochromes in vivo (24) and in vitro (11). ENF is rather deamidated in the first step at its C-terminal phenylalanine residue in the blood. The following steps also seem to take place in the blood, where unspecific proteolytic enzymes further degrade ENF into its constituent amino acids, which either appear in the excreta or remain in the body pool of amino acids (11).
Concomitant medical conditions.
The catabolism of ENF is possibly subject to regulation by the endocrine system and inflammatory cytokines. Candidate hormones with effects on protein catabolism are growth hormone, thyroid hormones, steroids, insulin, and others. Studies have shown that there is a profound dysregulation in some of these systems in patients with advanced HIV disease and especially with AIDS wasting (1, 6, 7, 9, 10, 19, 23). In these patients, lean body mass (proteins and carbohydrates) is being converted into fat, a process which may also impact the degradation of ENF. The PK data presented in the ENF product monograph were generated from a trial which included 12 HIV-infected individuals (16). The patients in that study, which was designed to compare ENF PK on the basis of injection site, were similar in age but had a higher body weight (mean, 79 kg) than our patients (mean, 69 kg). This may indicate that our group included a higher percentage of patients with advanced HIV disease and AIDS wasting. The lower area under the concentration-time curve (35.29 mg · h/liter for our group versus 43.3 mg · h/liter for the PK group) and the shorter plasma half-time (1.75 h for our group versus 3.80 h for the PK group) (16) predicted by our model support this interpretation.
Protein loss in HIV-related nephropathy may also lead to low ENF concentrations. However, this condition is seen predominantly in black patients, who represent only a small minority of our population.
Compliance is likely to have influenced the results of this study. Patients knew that their drug levels were going to be measured, and they all reported the time of the last dose, so the degree of noncompliance was likely that of a group of typical human subjects.
A number of studies have demonstrated a good relationship between the ENF dose and its antiviral effect (13, 14). Based on data from clinical trials, Lalezari et al. and Gonzalez de Requena et al. suggested concentrations of 1,000 ng/ml and 2,200 ng/ml, respectively (15; D. Gonzalez de Requena, D. Castagna, D. Aguilar Marucco, H. Hasson, L. Veronese, M. Sciandra, A. D'Avolio, A. Ibanez, A. Sinicco, A. Lazzarin, and G. Di Perri, Abstr. 12th Conf. Retrovir. Opportun. Infect., abstr. 643, 2005), as cutoffs for antiviral activity. According to our data, we expect 23% and 58% of patients to achieve Ctrough levels below 1,000 ng/ml and 2,200 ng/ml, respectively. Despite this high number of patients with low drug levels, we did not find any correlation between drug concentration and virus load (data not shown). Yet, in the clinical setting, ENF is still being used mainly as a last resort for patients carrying viruses with little or no sensitivity to antiviral drugs. Therefore, the relationship between the ENF concentration and its antiviral effect may be less visible, as the drug is often being used as functional monotherapy, and resistance may have developed when the samples for TDM were taken. This interpretation is also supported by the finding that the virological responses in triple class-experienced patients differed only slightly between patient groups receiving 67.5-mg ENF injections and those receiving 90-mg ENF injections (22). With an increasing number of patients starting ENF treatment in combination with a greater number of active background drugs, sufficient ENF levels may become more and more important, and the recognition of conditions affecting ENF PK will be crucial.
In conclusion, our study was not designed to assess the PK of ENF in study patients treated in an ideal setting. Rather, it provides a picture of real life and may serve as an explanation for why patients do so much better in clinical trials than in the clinical routine. The reference values obtained by simulations from the PK model will serve as a valuable tool for the clinician to interpret ENF drug levels and to identify those patients with concentrations in the lower spectrum of the concentration range. We suggest that in cases of low drug concentrations, physicians should start an investigation for the underlying cause.
In future investigations, our PK model could also be used for further covariate analysis. By investigating patient-specific characteristics as covariates, it might be possible to distinguish patients with low drug levels from those with higher ones, i.e., to predict concentrations for each individual patient. Overall, the typical PK parameters along with their obtained variability parameters are a prerequisite for performing a Bayesian TDM analysis of individual HIV-infected patients. This might add to the individualization of therapy and improve therapeutic success.
Acknowledgments
This work was carried out within the Network of Competence HIV/AIDS Germany and was supported by a corresponding grant (01 KI 0211) from the German Ministry of Education and Research (BMBF). The population PK analysis was supported by the Berliner Programm zur Foerderung der Chancengleichheit fuer Frauen in Forschung und Lehre.
Hoffmann-La Roche GmbH generously provided the reference substances of T-20 and deuterium-labeled T-20, which was used as an internal standard. Eppendorf Hamburg donated prototypes of low-bind microreaction vials, which were not commercially available at the time this work was carried out. We also thank Steffi Lehmann and Renate Rogall, who helped a lot with the laboratory work.
REFERENCES
- 1.Belec, L., D. Meillet, G. Gresenguet, and R. K. Gherardi. 1995. Increased tumor necrosis factor-alpha serum levels in patients with HIV wasting syndrome and euthyroid sick syndrome. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:212-214. [DOI] [PubMed] [Google Scholar]
- 2.Boyd, M. A., X. Zhang, A. Dorr, K. Ruxrungtham, S. Kolis, K. Nieforth, T. Kinchelow, N. Buss, and I. H. Patel. 2003. Lack of enzyme-inducing effect of rifampicin on the pharmacokinetics of enfuvirtide. J. Clin. Pharmacol. 43:1382-1391. [DOI] [PubMed] [Google Scholar]
- 3.Burger, D., P. Hugen, P. Reiss, I. Gyssens, M. Schneider, F. Kroon, G. Schreij, K. Brinkman, C. Richter, J. Prins, R. Aarnoutse, and J. Lange. 2003. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naive HIV-1-infected individuals. AIDS 17:1157-1165. [DOI] [PubMed] [Google Scholar]
- 4.Burger, D. M., P. W. Hugen, R. E. Aarnoutse, R. M. Hoetelmans, M. Jambroes, P. T. Nieuwkerk, G. Schreij, M. M. Schneider, M. E. van der Ende, and J. M. Lange. 2003. Treatment failure of nelfinavir-containing triple therapy can largely be explained by low nelfinavir plasma concentrations. Ther. Drug Monit. 25:73-80. [DOI] [PubMed] [Google Scholar]
- 5.Chang, D., S. J. Kolis, T. F. Julian, K. H. Linderholm, R. Nachi, E. Linder, P. Lin, J. W. Lee, and S. K. Bansal. 2001. LC/MS/MS assay validation of T-20 in human EDTA plasma. Abstract. AAPS PharmSci 3(S1). [Online.] http://www.aapspharmsci.org/.
- 6.Coodley, G. O., M. O. Loveless, H. D. Nelson, and M. K. Coodley. 1994. Endocrine function in the HIV wasting syndrome. J. Acquir. Immune. Defic. Syndr. 7:46-51. [PubMed] [Google Scholar]
- 7.Darko, D. F., M. M. Mitler, and J. C. Miller. 1998. Growth hormone, fatigue, poor sleep, and disability in HIV infection. Neuroendocrinology 67:317-324. [DOI] [PubMed] [Google Scholar]
- 8.FDA. 2001. Guidance for industry: bioanalytical method validation. FDA, Washington, D.C.
- 9.Frost, R. A., J. Fuhrer, R. Steigbigel, P. Mariuz, C. H. Lang, and M. C. Gelato. 1996. Wasting in the acquired immune deficiency syndrome is associated with multiple defects in the serum insulin-like growth factor system. Clin. Endocrinol. 44:501-514. [DOI] [PubMed] [Google Scholar]
- 10.Hellerstein, M. K. 2001. Pathophysiology of body composition and metabolic abnormalities in HIV-infection: therapeutic implications. Int. J. Sport Nutr. Exerc. Metab. 11(Suppl.):S105-S110. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann-La Roche. 2004. Fuzeon product monograph including updated 48 week TORO data. Hoffmann-La Roche, Switzerland.
- 12.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 13.Kilby, J. M., J. P. Lalezari, J. J. Eron, M. Carlson, C. Cohen, R. C. Arduino, J. C. Goodgame, J. E. Gallant, P. Volberding, R. L. Murphy, F. Valentine, M. S. Saag, E. L. Nelson, P. R. Sista, and A. Dusek. 2002. The safety, plasma pharmacokinetics, and antiviral activity of subcutaneous enfuvirtide (T-20), a peptide inhibitor of gp41-mediated virus fusion, in HIV-infected adults. AIDS Res. Hum. Retrovir. 18:685-693. [DOI] [PubMed] [Google Scholar]
- 14.Lalezari, J. P., E. DeJesus, D. W. Northfelt, G. Richmond, P. Wolfe, R. Haubrich, D. Henry, W. Powderly, S. Becker, M. Thompson, F. Valentine, D. Wright, M. Carlson, S. Riddler, F. F. Haas, R. DeMasi, P. R. Sista, M. Salgo, and J. Delehanty. 2003. A controlled phase II trial assessing three doses of enfuvirtide (T-20) in combination with abacavir, amprenavir, ritonavir and efavirenz in non-nucleoside reverse transcriptase inhibitor-naive HIV-infected adults. Antivir. Ther. 8:279-287. [PubMed] [Google Scholar]
- 15.Lalezari, J. P., J. J. Eron, M. Carlson, C. Cohen, E. DeJesus, R. C. Arduino, J. E. Gallant, P. Volberding, R. L. Murphy, F. Valentine, E. L. Nelson, P. R. Sista, A. Dusek, and J. M. Kilby. 2003. A phase II clinical study of the long-term safety and antiviral activity of enfuvirtide-based antiretroviral therapy. AIDS 17:691-698. [DOI] [PubMed] [Google Scholar]
- 16.Lalezari, J. P., I. H. Patel, X. Zhang, A. Dorr, N. Hawker, Z. Siddique, S. J. Kolis, and T. Kinchelow. 2003. Influence of subcutaneous injection site on the steady-state pharmacokinetics of enfuvirtide (T-20) in HIV-1-infected patients. J. Clin. Virol. 28:217-222. [DOI] [PubMed] [Google Scholar]
- 17.Marzolini, C., A. Telenti, L. A. Decosterd, G. Greub, J. Biollaz, and T. Buclin. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71-75. [DOI] [PubMed] [Google Scholar]
- 18.Mould, D. R., X. Zhang, K. Nieforth, M. Salgo, N. Buss, and I. H. Patel. 2005. Population pharmacokinetics and exposure-response relationship of enfuvirtide in treatment-experienced human immunodeficiency virus type 1-infected patients. Clin. Pharmacol. Ther. 77:515-528. [DOI] [PubMed] [Google Scholar]
- 19.Rondanelli, M., S. B. Solerte, M. Fioravanti, D. Scevola, M. Locatelli, L. Minoli, and E. Ferrari. 1997. Circadian secretory pattern of growth hormone, insulin-like growth factor type I, cortisol, adrenocorticotropic hormone, thyroid-stimulating hormone, and prolactin during HIV infection. AIDS Res. Hum. Retrovir. 13:1243-1249. [DOI] [PubMed] [Google Scholar]
- 20.Ruxrungtham, K., M. Boyd, S. E. Bellibas, X. Zhang, A. Dorr, S. Kolis, T. Kinchelow, N. Buss, and I. H. Patel. 2004. Lack of interaction between enfuvirtide and ritonavir or ritonavir-boosted saquinavir in HIV-1-infected patients. J. Clin. Pharmacol. 44:793-803. [DOI] [PubMed] [Google Scholar]
- 21.Walmsley, S., K. Henry, C. Katlama, M. Nelson, A. Castagna, J. Reynes, B. Clotet, J. Hui, M. Salgo, R. DeMasi, and J. Delehanty. 2003. Enfuvirtide (T-20) cross-reactive glycoprotein 41 antibody does not impair the efficacy or safety of enfuvirtide. J. Infect. Dis. 188:1827-1833. [DOI] [PubMed] [Google Scholar]
- 22.Wheat, J., J. P. Lalezari, J. M. Kilby, D. A. Wheeler, M. Salgo, R. DeMasi, and J. Delehanty. 2002. A week-48 assessment of high strength T-20 formulations in multi-class experienced patients, poster 417-W. Presented at the 9th Conference on Retroviruses and Opportunistic Infections, Seattle, Wash., 24-28 February 2002.
- 23.Yarasheski, K. E., J. J. Zachwieja, J. Gischler, J. Crowley, M. M. Horgan, and W. G. Powderly. 1998. Increased plasma gln and Leu Ra and inappropriately low muscle protein synthesis rate in AIDS wasting. Am. J. Physiol. 275:E577-E583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, X., J. P. Lalezari, A. D. Badley, A. Dorr, S. J. Kolis, T. Kinchelow, and I. H. Patel. 2004. Assessment of drug-drug interaction potential of enfuvirtide in human immunodeficiency virus type 1-infected patients. Clin. Pharmacol. Ther. 75:558-568. [DOI] [PubMed] [Google Scholar]