Abstract
The pharmacokinetic profiles, safety, and efficacies of different dosing schedules of posaconazole oral suspension in patients with possible, probable, and proven refractory invasive fungal infection (rIFI) or febrile neutropenia (FN) were evaluated in a multicenter, open-label, parallel-group study. Sixty-six patients with FN and 32 patients with rIFI were randomly assigned to one of three posaconazole regimens: 200 mg four times a day (q.i.d.) for nine doses, followed by 400 mg twice a day (b.i.d.); 400 mg q.i.d. for nine doses, followed by 600 mg b.i.d.; or 800 mg b.i.d. for five doses, followed by 800 mg once a day (q.d.). Therapy was continued for up to 6 months in patients with rIFI or until neutrophil recovery occurred in patients with FN. The 400-mg-b.i.d. dose provided the highest overall mean exposure, with 135% (P = 0.0004) and 182% (P < 0.0001) greater exposure than the 600-mg-b.i.d. and 800-mg-q.d. doses, respectively. However, exposure in allogeneic bone marrow transplant (BMT) recipients (n = 12) was 52% lower than in non-BMT patients. Treatment-related adverse events (occurring in 24% of patients) were mostly gastrointestinal in nature. Twenty-four percent of patients had adverse events leading to premature discontinuation (none were treatment related). In efficacy-evaluable patients, successful clinical response was observed in 43% with rIFI (56% of patients receiving 400 mg b.i.d., 17% receiving 600 mg b.i.d., and 50% receiving 800 mg q.d.) and 77% with FN (74% receiving 400 mg b.i.d., 78% receiving 600 mg b.i.d., and 81% receiving 800 mg q.d.). Posaconazole is well tolerated and absorbed. Divided doses of 800 mg (400 mg b.i.d.) provide the greatest posaconazole exposure.
Invasive fungal infections (IFIs) are a significant cause of morbidity and mortality in immunocompromised patients. During the past few decades, a shift has been noted in the epidemiology of fungal infections, with fluconazole-resistant yeasts, such as Candida krusei and Candida glabrata, and echinocandin-resistant molds, such as Fusarium species and zygomycetes, becoming increasingly more common causes of IFI (1, 12, 14, 20, 29, 31). Although several therapeutic options exist for these life-threatening infections, mortality rates remain high, especially in certain high-risk patient populations. For example, in an analysis of 1,941 patients with invasive aspergillosis, the overall fatality rate was 58%, but among bone marrow transplant (BMT) recipients, the fatality rate was much higher (86.7%) (17).
The patients most vulnerable to opportunistic IFI include those with prolonged and profound neutropenia (8, 13, 15), solid-organ transplant recipients (14), and BMT recipients (3). Selecting appropriate antifungal therapy is complicated by drug-associated toxicities, development of resistance, and limited spectra of activity. Posaconazole is an investigational triazole with potent antifungal activity against a wide range of clinically relevant yeasts and molds, including Candida and Aspergillus species (10, 22, 25-27), but it also has clinically relevant activity against zygomycetes (7) and other filamentous fungi, including Fusarium species (19, 23, 27).
In healthy adult volunteers, oral posaconazole exhibits dose-proportional pharmacokinetics up to a dose of 800 mg/day (4). Posaconazole has a long half-life (35 h) and attains steady-state concentrations after 7 to 10 days of multiple-dose administration. Concomitant administration of food or a liquid nutritional supplement enhances posaconazole bioavailability (6). Posaconazole exposure (area under the plasma concentration-time curve [AUC]) can also be enhanced by dividing the daily dose (400 mg twice a day [b.i.d.] or 200 mg four times a day [q.i.d.]), regardless of food intake (11). In patients who are unable to tolerate oral medications, posaconazole oral suspension has been administered via nasogastric tube and resulted in successful treatment (J. Lewis, G. Anstead, and J. Graybill, Abstr. 15th Congr. Int. Soc. Hum. Anim. Mycol., abstr. 256, 2003). Although posaconazole pharmacokinetics are well defined in healthy subjects (4, 6, 11), the pharmacokinetics of posaconazole in patients at doses greater than 400 mg b.i.d. require further evaluation. To that end, we conducted a multicenter, randomized, open-label, parallel-group study to evaluate the pharmacokinetic profiles, safety, tolerabilities, and efficacies of different dosing schedules of posaconazole in patients with refractory IFI (rIFI) or febrile neutropenia (FN) that required empirical antifungal therapy.
(This paper was presented in part at the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., September 14 to 17, 2003.)
MATERIALS AND METHODS
Eligibility criteria.
The study participants were adults (≥18 years) of either sex and any race. Eligibility for enrollment required all patients to have either a proven, probable, or possible IFI according to the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Cooperative Group and the Mycoses Study Group (EORTC/MSG) criteria (2) that was refractory to other antifungal therapy (failure to respond to amphotericin B, fluconazole, itraconazole, ketoconazole, or flucytosine given for 7 to 90 days of treatment, depending on the mycosis) or FN, with an oral temperature greater than 38°C/100.4°F observed twice within 48 h despite systemic antibacterial therapy for longer than 72 h or fever recurrence during neutropenia (absolute neutrophil count [ANC], ≤500 cells/mm3) while undergoing broad-spectrum antibacterial therapy after previous resolution of fever with longer than 72 h of antibacterial therapy. All participants had to be willing and able to take the study medication orally or via nasogastric tube. Women could not be pregnant or lactating. Women with childbearing potential were required to use an adequate form of birth control and to have a negative pregnancy test result within 72 h of study initiation.
Patients were ineligible for study participation if they had FN with uncontrolled bacteremia; FN with a newly diagnosed, previously untreated, documented systemic IFI at the time of enrollment; a history of hypersensitivity or idiosyncratic reaction to azole antifungals; serum alanine aminotransferase or aspartate aminotransferase values elevated to >10 times the upper limit of normal; an electrocardiogram with a corrected QT (QTc) interval ≥20% above normal (>480 milliseconds); or a life expectancy of less than 14 days. Patients could not be on artificial ventilation. Patients taking concomitant medications known to lower the plasma concentrations and/or efficacies of azole antifungals (rifampin/rifabutin, phenytoin, and barbiturates) or known to potentially interact with azoles and cause life-threatening adverse events (terfenadine, cisapride, or ebastine 24 h before therapy or astemizole within 10 days before therapy) were also excluded.
Written informed consent was obtained from all study participants before the initiation of any study-related activities. The study protocol and informed-consent form were reviewed and approved by an independent ethics committee or institutional review board at each study site.
Treatments.
Eligible patients were assigned to one of the three following treatments based on a computer-generated two-step randomization schedule taking into account the two prognostic factors of mucositis (grades 0 to 2 versus grades 3 and 4) and rIFI versus FN. The mucositis grade was determined using Common Toxicity Criteria version 2.0 (revised 23 March 1998). Posaconazole was given as an oral suspension, and the treatment groups were as follows: (i) group 1, posaconazole 200 mg q.i.d. for nine doses, followed by 400 mg b.i.d.; (ii) group 2, posaconazole 400 mg q.i.d. for nine doses, followed by 600 mg b.i.d.; (iii) group 3, posaconazole 800 mg b.i.d. for five doses, followed by 800 mg q.d.
When possible, posaconazole was administered with food. Therapy was continued for up to 6 months in patients with rIFI or until neutrophil recovery (maximum of 42 days) occurred in patients with FN. The pharmacokinetic portion of the study was completed after 10 days of treatment regardless of the underlying disease.
Plasma posaconazole concentration determination.
Blood samples for posaconazole concentration determination were collected on day 3 and day 10 at 0 h (8:00 a.m.) and at 2, 3, 4, 6, 8, 10, and 12 h postdose for all treatment groups. To allow collection of plasma samples for 12 h after the first dose on day 3, the second dose was not given in groups 1 and 2. Thus, the second portion of the dosing regimen began at 8 p.m. on day 3 in groups 1 and 2. An additional blood sample was collected on day 10 at 24 h postdose. Plasma posaconazole concentrations were determined by means of a liquid chromatography tandem mass spectrometry method (PPD Development, Richmond, Va.). The lower limit of quantitation was 1.0 ng/ml (calibration range, 1 to 4,000 ng/ml) (5).
Posaconazole pharmacokinetic analysis.
Plasma posaconazole concentrations were used to determine the pharmacokinetic parameters using compartmental analyses and were conducted using the SAS system (version 8.2). Posaconazole concentrations were excluded from the analysis for patients who either did not follow the correct dosing regimen prior to sampling or terminated the study prior to sample collection. The maximum plasma concentration (Cmax) and time to Cmax (Tmax) were the observed values. The AUC from 0 to 12 h (AUC0-12) was calculated on day 3, and the AUC over the dosing interval (AUCτ) was calculated on day 10 (i.e., at 24 h for q.d. and 12 h for b.i.d.). With 400 mg as the reference dose (for group 1), AUC(0-τ) values were normalized by dividing by 1.5 for the 600-mg-b.i.d. dose (group 2) or by 2 for the 800-mg-q.d. dose (group 3). In previous studies, posaconazole demonstrated dose-proportional pharmacokinetics up to single doses of 800 mg (4). Under the assumption that plasma posaconazole concentrations attain steady state by day 10, it follows, according to linear pharmacokinetics, that the dose-normalized AUCτ (NAUC) values would be identical for the three different dosing regimens.
Although the parameters AUC0-12 and AUCτ are both reported, actual sample times were used in this analysis; therefore, the last sample times may not be exactly 12 or 24 h. Consequently, average concentration (Cav) values were calculated using the equation AUCτ/τ, where τ represents the actual time of the dosing interval. Cav values were considered the primary pharmacokinetic parameter. Additionally, the least-square means based on the log-transformed Cav were calculated to minimize the influence of extreme values. Total apparent body clearance (CL/F) was calculated as the ratio of the dose to the AUC. The apparent volume of distribution (V/F) was calculated as the ratio of CL/F to the terminal-phase rate constant (k).
Posaconazole statistical analyses.
Summary statistics were used to evaluate baseline and demographic values and pharmacokinetic data between treatment groups. All pharmacokinetic parameters were log transformed (natural base) before being statistically analyzed using a general linear model. Log-transformed Cav values (day 3 or day 10) and log-transformed NAUC values (day 10) were the response variables, whereas dose regimen (a factor variable on three levels) was the main effect. The covariates (history of BMT, type of transplant, primary diagnosis, sex, age, race, and body weight) were explored for statistical significance using the day 10 data. The “estimate” statement of the general linear model procedure was used to estimate the difference and to test statistical significance in exposure between any two levels of a factor variable (e.g., regimen, BMT, and sex). By taking the exponent of the estimated difference, an estimated ratio is obtained. Outliers, determined as having studentized residuals greater than 3, were excluded from the analysis.
Preliminary analysis of the results from a study of IFI patients that either had refractory disease or had developed intolerance to antifungal therapy suggested that the minimum effective dose exposure, AUC0-12, was approximately 4,000 ng · h/ml or an AUC0-24 of approximately 8,000 ng · h/ml. The results of the interim analysis of this study showed that the investigator-determined response rate (complete and partial response) for various fungal pathogens ranged from 50% to 85% (T. Walsh, T. Patterson, A. Langston, J. van Burik, A. Louie, R. Herbrecht, S. Hadley, J. Perfect, C. Hsu, J. Gogate, C. Hardalo, H. Patino, L. Pedicone, G. Corcoran, I. Raad, and A. Wayne, Abstr. 45th Am. Soc. Hematol. Meet., abstr. 682, 2003). Assuming a coefficient of variation equal to 0.55, an underlying relative difference of 0.56 or higher in AUC between the low- and high-dose groups could be detected with a power of at least 80% at the 0.05 (two-tailed) level of significance with a sample size of 25 patients per treatment group. Therefore, if the response rate for achieving a targeted AUC0-24 of 8,000 ng · h/ml was 50% for the low dose, a response rate at 85% or higher for the high dose (i.e., a difference of 35% or more) would be detectable with a power of at least 80% at the 0.05 (two-tailed) level of significance.
Safety analyses.
Safety evaluations included monitoring of adverse events and results of serum chemistry tests, hematology studies, electrocardiograms, and neurologic examinations. Investigators graded all reported adverse events for severity according to the Common Toxicity Criteria grading system and assessed their relationship to posaconazole treatment (unlikely, possible, or probable).
Efficacy analysis.
Efficacy was evaluated by using an assessment of clinical and microbiological responses. Clinical response was evaluated monthly and at the end of treatment in patients with rIFI, where clinical response was defined according to the following criteria: complete response (success), resolution of all attributable symptoms, signs, and radiographic or bronchoscopic abnormalities; partial response (success), clinically meaningful improvement in attributable symptoms, signs, and radiographic or bronchoscopic abnormalities; stable disease (nonsuccess), no improvement in attributable symptoms, signs, and radiographic or bronchoscopic abnormalities; and treatment failure (nonsuccess), deterioration in clinical or radiographic abnormalities necessitating alternative antifungal therapy or resulting in death. If for any reason the response could not be assessed, the patient was considered to have a nonsuccessful outcome.
In patients with FN, efficacy was evaluated at the end of treatment and at 7 days posttreatment. A patient was considered to have had a successful response if all of the following criteria were met: survival through 7 days after the last day of treatment, defervescence during the neutropenic period, microbiological eradication of a baseline IFI (if documented at study entry), no breakthrough IFIs during posaconazole therapy or within 7 days after the last day of dosing, and no discontinuation of posaconazole therapy due to toxicity or lack of efficacy. For patients who did not meet all of these criteria, therapy was considered to be nonsuccessful.
Efficacies were evaluated for the all-randomized (all patients who were randomized into the study) and the efficacy-evaluable (all randomized patients who took at least one dose of drug and had an outcome assessment at the end of treatment or 7 days posttreatment) subsets.
RESULTS
Patient demographics.
A total of 98 patients were randomly assigned to one of three posaconazole dosing groups at 12 different study sites (Table 1). The primary reason for enrollment was FN (n = 66); the remainder of the patients were enrolled because of an rIFI (n = 32). There were no notable differences between dose groups with respect to age, sex, race, weight, reason for enrollment, or underlying risk factors for fungal infection. Underlying hepatic or renal disease was noted in 20% and 24% of patients, respectively. The primary risk factors for development of a fungal infection included hematologic malignancy (n = 89) and bone marrow transplantation (n = 32). Of the 98 patients randomized, 93 received at least one dose of posaconazole (35 patients in group 1, 30 patients in group 2, and 28 patients in group 3). Most patients with rIFI had experienced treatment failure with caspofungin and/or amphotericin B. Sixty-nine patients (70%) were assessable for pharmacokinetic analysis on day 3 following the initial high doses, and 61 patients (62%) were assessable for pharmacokinetic analysis on day 10. For the majority of patients not included in the pharmacokinetic analysis, assessments were limited due to significant dosing regimens and/or sampling collection errors.
TABLE 1.
Summary of patient demographic data
| Characteristic | Value
|
|||
|---|---|---|---|---|
| Group 1 (200 mg q.i.d./ 400 mg b.i.d.) (n = 35) | Group 2 (400 mg q.i.d./ 600 mg b.i.d.) (n = 31) | Group 3 (800 mg b.i.d./ 800 mg q.d.) (n = 32) | Total (n = 98) | |
| Mean age [yr (range)] | 45.3 (18-73) | 49.2 (20-73) | 51.2 (20-74) | 48.4 (18-74) |
| Age (yr) | ||||
| ≥18 to <65 [n (%)] | 30 (86) | 27 (87) | 26 (81) | 83 (85) |
| ≥65 [n (%)] | 5 (14) | 4 (13) | 6 (19) | 15 (15) |
| Sex [n (%)] | ||||
| Female | 14 (40) | 14 (45) | 10 (31) | 38 (39) |
| Male | 21 (60) | 17 (55) | 22 (69) | 60 (61) |
| Race [n (%)] | ||||
| Caucasian | 32 (91) | 31 (100) | 27 (84) | 90 (92) |
| Other | 3 (9) | 0 | 5 (16) | 8 (8) |
| Mean wt [kg (range)] | 71.7 (49.8-111.0) | 74.6 (52.8-112.4) | 74.7 (49.3-101.0) | 73.6 (49.3-112.4) |
| Primary diagnosis | ||||
| FN | 23 (66) | 21 (68) | 22 (69) | 66 (67) |
| rIFI | 12 (34) | 10 (32) | 10 (31) | 32 (33) |
Pharmacokinetic analyses.
On day 3, no statistically significant differences in the Cav values were detected between the three dosing regimens (P = 0.8) (Table 2). Mean Cmax values were attained at median times of 2 to 6 h postdose. As expected for a drug with a mean half-life of 35 h at steady state, the mean Cav values were slightly lower than the Cmax values, indicating that steady state had not been achieved by 3 days postdose.
TABLE 2.
Posaconazole pharmacokinetic parameters on day 3
| Parameter (units) | Value [mean (% coefficient of variation)]
|
||
|---|---|---|---|
| Group 1 (200 mg q.i.d.) (n = 24) | Group 2 (400 mg q.i.d.) (n = 21) | Group 3 (800 mg b.i.d.) (n = 24) | |
| Cmax (ng/ml) | 539 (82) | 531 (71) | 417 (60) |
| Cav (ng/ml) | 447 (84) | 423 (70) | 340 (63) |
| Tmaxa (h) | 2.65 (0, 11.8) | 5.78 (2, 12.4) | 4.21 (0, 11.9) |
| AUC(0-12) (ng · h/ml) | 5,314 (83) | 5,075 (71) | 4,035 (63) |
Median (minimum, maximum).
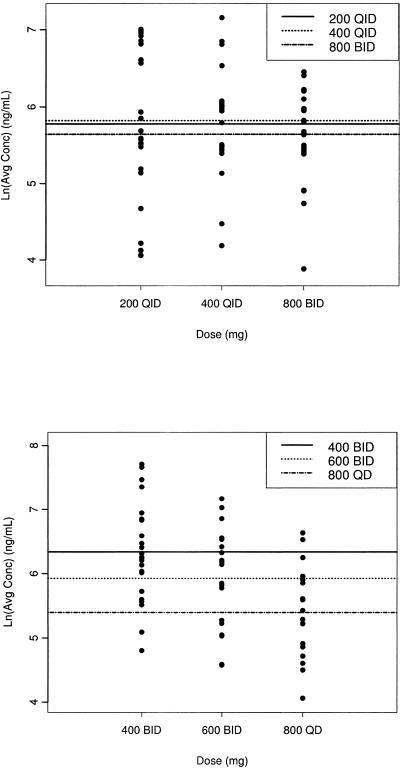
An analysis of variance using the day 10 Cav values showed a statistically significant difference between the three dose regimens (P = 0.01), with group 1 demonstrating the highest mean exposure (Table 3 and Fig. 1 and 2). Steady-state posaconazole plasma levels were achieved by day 10 for all three dose regimens, with Cmax attained at a median Tmax of 3 to 4 h postdose (Fig. 2). Analysis of variance showed that the NAUC for the 400-mg dose group was statistically different from those for the 600-mg and 800-mg dose groups. The NAUCs for the 600-mg and 800-mg dose groups were not statistically different (Table 3). These data indicate that no additional increase in AUC was attained with a dose exceeding 800 mg/day administered as 400 mg b.i.d. and that dividing the dose (800 mg q.d. versus 400 mg b.i.d.) results in significantly higher exposure to posaconazole in favor of twice-daily dosing (P = 0.01). NAUC values, which were used to compare the exposures of the three regimens, were 135% higher (P = 0.004) and 182% higher (P < 0.0001) in group 1 than in group 2 and group 3, respectively, indicating that patients who received posaconazole 400 mg b.i.d. had the greatest exposure.
TABLE 3.
Posaconazole pharmacokinetic parameters on day 10
| Parameter (unit)a | Value [mean (% coefficient of variation)]
|
||
|---|---|---|---|
| Group 1 (400 mg b.i.d.) (n = 24) | Group 2 (600 mg b.i.d.) (n = 19) | Group 3 (800 mg q.d.) (n = 18) | |
| Cmax (ng/ml) | 851 (82) | 579 (71) | 361 (74) |
| Cmin (ng/ml) | 640 (98) | 388 (64) | 252 (100) |
| Cav (ng/ml) | 723 (86) | 490 (71) | 259 (72) |
| Cav, least square (ng/ml) | 523 | 333 | 184 |
| Tmax (h)b | 3.0 (0, 12.5) | 3.83 (0, 10) | 4.04 (2.42, 12.5) |
| AUCτ (ng · h/ml) | 8,619 (86) | 5,823 (71) | 6,199 (71) |
| NAUC (ng · h/ml)c | 8,619 (86) | 3,882 (71) | 3,099 (71) |
| V/F (liters) | 2,447 (421) | 4,984 (919) | 5,061 (903) |
| CL/F (liters/h) | 283 (354) | 179 (82) | 215 (81) |
| T1/2 (h) | 11.9 (3) | 12.0 (3) | 24 (2) |
Cmin, Trough plasma concentration; T1/2, half-life.
Median (minimum, maximum).
P value for log-transformed NAUC: 400 mg b.i.d. versus 600 mg b.i.d., P = 0.0004; 400 mg b.i.d. versus 800 mg q.d., P < 0.0001; 600 mg b.i.d. versus 800 mg q.d., P = 0.447.
FIG. 1.
Individual average concentrations (natural log scale) of posaconazole at days 3 (top) and 10 (bottom) for each dosage regimen. The horizontal lines indicate mean values.
FIG. 2.
Mean posaconazole plasma concentration-time profile on day 10 in patients receiving 400 mg b.i.d., 600 mg b.i.d., or 800 mg q.d. posaconazole oral suspension.
Primary diagnosis (FN or rIFI), sex, age, and body weight were found to have no significant influence on posaconazole exposure on day 10 (P = 0.09 to 0.69; data not shown). However, exposure in allogeneic BMT recipients was 52% lower than that in patients with no history of BMT (191 versus 402 ng/ml, respectively; P = 0.003). Although this difference is statistically significant, there were only a small number (n = 12) of allogeneic BMT patients available for evaluation at day 10. A history of bone marrow transplantation was found to have an effect on the elimination rate constant. In allogeneic BMT patients, the terminal half-life was estimated to be shorter: 17 h compared to 29 h in patients with no history of BMT. The V/F was found to be influenced by the dosage regimen. Based upon the assumption that the mean estimates of V are similar in the three treatment groups, it follows that F in the 400-mg-b.i.d. group was double that of the 600-mg-b.i.d. or 800-mg-q.d. group. Additionally, though the randomization of patients was stratified by mucositis grade, there were too few patients with grade 3 or 4 mucositis in each group to perform an analysis.
Safety evaluation.
Most patients (84/98; 86%) received posaconazole therapy for less than 3 months. Only seven patients received posaconazole treatment for 6 months or longer. In the entire study population (n = 98), regardless of dosing regimen, the most frequent treatment-related adverse events were gastrointestinal events (Table 4). There was no clear trend or pattern suggesting that any of the other treatment-related adverse events occurred more frequently in one dosing group, with the exception of treatment-related nausea, which was reported only in group 1 (4/35; 11%). Severe or life-threatening adverse events were reported in 55% (54/98) of all randomized patients (49% of patients on 400 mg b.i.d.; 58% on 600 mg b.i.d.; 59% on 800 mg q.d.). One severe or life-threatening adverse event, abdominal pain, was considered by the investigator to be possibly or probably related to treatment (posaconazole 600 mg b.i.d. dose group). The patient remained in the study and received additional medication for the complaint. Twenty-four percent (24/98) of all randomized patients discontinued the study due to an adverse event (Table 5); none of the events leading to discontinuation were considered related to treatment. Laboratory evaluations (hemoglobin, hematocrit, serum alanine aminotransferase, aspartate aminotransferase, and total bilirubin levels; leukocyte, platelet, and absolute neutrophil counts; and creatinine values) revealed no trends or patterns that suggested an effect of posaconazole treatment. No neurologic abnormalities were noted. There were no clinically significant treatment-emergent changes in electrocardiograms, and prior to or during the trial, no patient had a QTc interval greater than 500 milliseconds.
TABLE 4.
Treatment-related adverse eventsa
| Event, body system, organ class | No. (%) of patients reporting
|
|||
|---|---|---|---|---|
| Group 1 (200 mg q.i.d./ 400 mg b.i.d.) (n = 35) | Group 2 (400 mg q.i.d./ 600 mg b.i.d.) (n = 31) | Group 3 (800 mg b.i.d./ 800 mg q.d.) (n = 32) | Total (n = 98) | |
| Total | 11 (31) | 6 (19) | 7 (22) | 24 (24) |
| General disorders (headache, edema, rigors) | 3 (9) | 2 (6) | 2 (6) | 7 (7) |
| Gastrointestinal system disorder | 7 (20) | 2 (6)b | 6 (19) | 15 (15) |
| Diarrhea | 3 (3) | 0 | 1 (3) | 4 (4) |
| Nausea | 4 (11) | 0 | 0 | 4 (4) |
| Vomiting | 3 (9) | 0 | 3 (9) | 6 (6) |
| Skin and subcutaneous tissue disorders (rash, pruritus, fissures) | 2 (6) | 1 (3) | 0 | 3 (3) |
All randomized patients reporting ≥2 events.
Only one of these adverse events (abdominal pain) was considered by the investigator to be severe and possibly related to treatment.
TABLE 5.
Treatment-emergent adverse events leading to study drug discontinuationa
| Event, body system | No. (%) of patients reporting
|
|||
|---|---|---|---|---|
| Group 1 (200 mg q.i.d./ 400 mg b.i.d.) (n = 35) | Group 2 (400 mg q.i.d./ 600 mg b.i.d.) (n = 31) | Group 3 (800 mg b.i.d./ 800 mg q.d.) (n = 32) | Total (n = 98) | |
| Total | 8 (23) | 9 (29) | 7 (22) | 24 (24) |
| General disorders (condition aggravated, fever, hypoxia, multiorgan failure) | 2 (6) | 2 (6) | 1 (3) | 5 (5) |
| Central and peripheral nervous system disorder | 2 (6) | 1 (3) | 3 (9) | 6 (6) |
| Gastrointestinal system disorder | 1 (3) | 2 (6) | 2 (6) | 5 (5) |
| Infection (pneumonia, sepsis, septic shock, aspergillosis) | 1 (3) | 2 (6) | 1 (3) | 4 (4) |
| Respiratory system disorders | 3 (9) | 2 (6) | 1 (3) | 6 (6) |
All randomized patients reporting ≥2 events.
Serious adverse events were reported by 46% of all randomized patients (49% of patients in group 1, 45% in group 2, and 44% in group 3). None of them were related to treatment with posaconazole. The overall mortality rate was 22% (22/98) for all randomized patients (9/35 patients in group 1, 7/31 in group 2, and 6/32 in group 3), with a total of 15/32 (47%) deaths reported for patients with rIFI and 7/66 (11%) for patients with febrile neutropenia; however, no deaths were considered to be related to posaconazole.
Clinical response in patients with rIFI.
Clinical responses were determined for the efficacy-evaluable (n = 21) and the all-randomized (n = 32) subsets (Table 6). Clinical responses were stratified by dosing regimen.
TABLE 6.
Clinical response at end of treatment in patients with rIFIs
| Clinical response | No. (%) of patients
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 (200 mg q.i.d./400 mg b.i.d.)
|
Group 2 (400 mg q.i.d./600 mg b.i.d.)
|
Group 3 (800 mg b.i.d./800 mg q.d.)
|
Total
|
|||||
| Efficacy evaluable (n = 9) | All randomized (n = 12) | Efficacy evaluable (n = 6) | All randomized (n = 10) | Efficacy evaluable (n = 6) | All randomized (n = 10) | Efficacy evaluable (n = 21) | All randomized (n = 32) | |
| Responder (complete + partial) | 5 (56) | 6 (50) | 1 (17) | 1 (10) | 3 (50) | 4 (40) | 9 (43) | 11 (34) |
| Complete | 0 | 0 | 0 | 0 | 2 (33) | 3 (30) | 2 (10) | 3 (9) |
| Partial | 5 (56) | 6 (50) | 1 (17) | 1 (10) | 1 (17) | 1 (10) | 7 (33) | 8 (25) |
| Failure | 4 (44) | 6 (50) | 5 (83) | 9 (90) | 3 (50) | 6 (60) | 12 (57) | 21 (66) |
| Stable disease | 1 (11) | 1 (8) | 1 (17) | 1 (10) | 1 (17) | 1 (10) | 3 (14) | 3 (9) |
| Clinical failure | 3 (33) | 3 (25) | 2 (33) | 2 (20) | 1 (17) | 1 (10) | 6 (29) | 6 (19) |
| Unable to determine | 0 | 0 | 1 (17) | 1 (10) | 1 (17) | 1 (10) | 2 (10) | 2 (6) |
| Missing | 0 | 2 (17) | 1 (17) | 5 (50) | 0 | 3 (30) | 1 (5) | 10 (31) |
The results for 15 patients (all in the efficacy-evaluable subset) who had a proven or probable IFI, according to the EORTC/MSG consensus criteria (2), that was considered refractory are listed in Table 6.
Clinical response in patients with FN.
Clinical responses were determined for the efficacy-evaluable (n = 53) and the all-randomized (n = 66) subsets. Overall, similar response rates were observed among the three dosing regimens (Table 7). The duration of neutropenia was shorter in group 1 (5.6 versus >10 days), but the sample size was too small for an evaluation of statistical significance. No patient with FN had a confirmed (proven or probable) invasive fungal infection during posaconazole treatment; however, one patient developed a Geotrichum infection 3 days after discontinuation of posaconazole therapy and three patients in the all-randomized population had possible pulmonary IFIs (Aspergillus, n = 1; unspecified IFI, n = 2) during treatment.
TABLE 7.
Clinical response at the end of treatment and 7 days after the end of treatment in patients with febrile neutropenia
| Efficacy outcome | No. (%) of patients
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 (200 mg q.i.d./ 400 mg b.i.d.)
|
Group 2 (400 mg q.i.d./ 600 mg b.i.d.)
|
Group 3 (800 mg b.i.d./ 800 mg q.d.)
|
Total
|
|||||
| Efficacy evaluable | All randomized | Efficacy evaluable | All randomized | Efficacy evaluable | All randomized | Efficacy evaluable | All randomized | |
| End of treatment | ||||||||
| Total | 19 | 23 | 18 | 21 | 16 | 22 | 53 | 66 |
| Success | 14 (74) | 14 (61) | 14 (78) | 14 (67) | 13 (81) | 14 (64) | 41 (77) | 42 (64) |
| Failure | 5 (26) | 9 (39) | 4 (23) | 7 (33) | 3 (19) | 8 (36) | 12 (23) | 24 (36) |
| Clinical failure | 5 (26) | 6 (26) | 3 (17) | 4 (19) | 2 (13) | 2 (9) | 10 (19) | 12 (18) |
| Missing | 0 | 3 (13) | 1 (6) | 3 (14) | 1 (6) | 6 (27) | 2 (4) | 12 (18) |
| Posttreatment plus 7 daysa | ||||||||
| Total | 17 | 17 | 13 | 47 | ||||
| Success | 13 (76) | 14 (82) | 11 (85) | 38 (81) | ||||
| Failure | 4 (24) | 3 (18) | 2 (15) | 9 (19) | ||||
| Clinical failure | 4 (24) | 2 (12) | 2 (15) | 8 (17) | ||||
| Missing | 0 | 1 (6) | 0 | 1 (2) | ||||
Data for clinical efficacy 7 days after end of treatment were not collected for all randomized patients.
Responses by underlying disease, infecting pathogen, site of infection, and neutropenic status for patients with rIFI or FN are shown in Table 8.
TABLE 8.
Overall efficacy based on underlying factors (all randomized patients)
| Underlying factor | Favorable outcome
|
|||
|---|---|---|---|---|
| Persistent febrile neutropenia (n/total)
|
Refractory disease (n[CR:PR]h/total)
|
|||
| Efficacy evaluable (n = 53) | All randomized (n = 66) | Efficacy evaluable (n = 21) | All randomized (n = 32) | |
| Underlying diseasea | ||||
| Leukemia | 32/39 | 33/50 | 9[2:7]/20 | 11[3:8]/32 |
| Non-Hodgkin's lymphoma | 6/7 | 6/8 | 0/1 | 0/1 |
| Myelodysplastic syndrome | 2/5 | 2/6 | 1[0:1]/3 | 1[0:1]/4 |
| Aplastic anemia | 2/3 | 2/4 | 0/0 | 0/0 |
| Other | 1/2b | 1/3c | 0/1d | 0/1d |
| Transplantation history | ||||
| Allogeneic BMT | 5/7 | 6/9 | 2[1:1]/5 | 4[2:2]/12 |
| Unrelated | 4/5 | 5/7 | 1[0:1]/1 | 2[0:2]/4 |
| Related | 1/2 | 1/2 | 1[1:0]/4 | 2[2:0]/8 |
| Matched | 5/6 | 6/8 | 2[1:1]/5 | 4[2:2]/11 |
| Mismatched | 0/1 | 0/1 | 0/0 | 0/1 |
| Solid-organ transplant | 0/0 | 0/0 | 0/0 | 0/0 |
| Pathogen | 0/0e | 2/3f | 9[2:7]/21 | 11[3:8]/32 |
| Aspergillus spp. | 0/0 | 1/1 | 2[1:1]/10 | 4[2:2]/17 |
| Fusarium spp. | 0/0 | 0/0 | 2[0:2]/2 | 2[0:2]/3 |
| Zygomycetes | 0/0 | 0/0 | 1[0:1]/3 | 1[0:1]/3 |
| Chromoblastomycosis | 0/0 | 0/0 | 1[1:0]/1 | 1[1:0]/1 |
| Othersg | 0/0 | 1/2 | 3[0:3]/5 | 3[0:3]/8 |
| Site of infection | 0/0e | 2/3f | 9[2:7]/21 | 11[3:8]/32 |
| Pulmonary | 0/0 | 2/3 | 8[1:7]/20 | 9[2:7]/28 |
| Proven | 0/0 | 0/0 | 2[1:1]/4 | 2[0:2]/8 |
| Probable | 0/0 | 0/0 | 1[0:1]/9 | 2[1:1]/10 |
| Possible | 0/0 | 2/3 | 5[0:5]/7 | 5[1:4]/10 |
| Nonpulmonary | 0/0 | 0/0 | 1[0:1]/1 | 2[1:1]/4 |
| Sinus | 0/0 | 0/0 | 1[0:1]/1 | 1[0:1]/1 |
| Skin | 0/0 | 0/0 | 0/0 | 1[1:0]/1 |
| Blood | 0/0 | 0/0 | 0/0 | 0/1 |
| Oral | 0/0 | 0/0 | 0/0 | 0/1 |
| Neutropenic status | 41/53 | 42/66 | 9[2:7]/21 | 11[3:8]/32 |
| ANC ≤500 cells/μl involvement | 38/49 | 39/60 | 1[0:1]/2 | 2[1:1]/3 |
| ANC >500 cells/μl | 2/3 | 2/3 | 8[2:6]/15 | 9[2:7]/21 |
| Missing | 1/1 | 1/3 | 0/4 | 0/8 |
Patients could have had more than one underlying condition.
Other underlying diseases were Hodgkin's disease (n = 1) and malignant testis neoplasm (n = 1).
Other underlying diseases were Hodgkin's disease (n = 1), malignant testis neoplasm (n = 1), and neoplasm not otherwise specified (n = 1).
Other underlying disease was malignant breast neoplasm (n = 1).
One efficacy-evaluable patient had nonpulmonary (perianal) Geotrichum infection 3 days after posaconazole discontinuation and was not included in the analysis.
Only three all-randomized patients had possible IFI during posaconazole treatment.
Other pathogens were not specified.
CR, complete response; PR, partial response.
DISCUSSION
Posaconazole was safe, well tolerated, orally absorbed, and efficacious in this study, an indication that posaconazole may play a role in the treatment of rIFI. The results of this study indicate that a variety of dosing regimens ranging from 800 mg/day to 1,600 mg/day were well tolerated. Initial doses that were relatively higher or given more frequently in divided doses (200 mg q.i.d., 400 mg q.i.d., and 800 mg b.i.d.) were included in the study to determine if greater exposure could be achieved rapidly with larger or more frequent short-term doses. The rational for a loading dose was the concern about unsatisfactory absorption in a sick population and achieving a steady state more rapidly. The rising single-dose and multiple-dose study demonstrated dose proportionality up to 800 mg and no increase in exposure above a dose of 800 mg (4, 11). Following a comparison of the three dosing regimens on day 3, similar posaconazole exposures were observed after administration of the 200-mg-q.i.d. and 400-mg-q.i.d. doses; these exposures were slightly higher than that following administration of 800 mg b.i.d., though the difference was not statistically significant (P = 0.8). Thus, there appears to be no pharmacokinetic benefit to initiating posaconazole therapy at daily doses greater than the clinically recommended dose of 800 mg/day in divided doses.
After patients received initial posaconazole doses for 3 days, the dosing regimen was changed to relatively lower posaconazole doses or the same dose in fewer divided doses for the remainder of the study. The 400-mg-b.i.d. dose provided the best overall mean exposure. Further, using log-transformed data, which creates a more robust analysis minimizing the influence of extreme values, the Cav least-square mean value was 57% higher in the 400-mg-b.i.d. group than in the 600-mg-b.i.d. group. The reason for lower mean exposure in group 2 than in group 1 remains unclear. A concentration-dependent first-pass effect may play a role but was not further investigated. The greater exposure observed in group 1 (200 mg q.i.d./400 mg b.i.d.) relative to group 3 (800 mg b.i.d./800 mg q.d.) is presumably due to the division of the daily 800-mg dose, which increases the soluble fraction of posaconazole, thereby enhancing overall absorption. In addition, the lack of an increase in exposure in group 2 (400 mg q.i.d./600 mg b.i.d.) relative to group 1 is presumably due to dose-limited absorption above 800 mg per day.
Posaconazole is a drug with a relatively long half-life (35 h), and its pharmacokinetics are characterized by a relatively flat plasma concentration profile after multiple dosing. Thus, given the allowed variability of the assay and interindividual absorption of the drug, Cmax can occur at any time point in the dosing interval (Tables 2 and 3).
Exposure of posaconazole in allogeneic BMT recipients was significantly lower than that in patients with no history of BMT. A reason for this could be that the numerous medications patients must take after BMT procedures may lower posaconazole exposure. Due to concerns that severe mucositis might hamper absorption, patients were stratified by mucositis grade. However, there were too few patients (total, n = 11) with grades 3 and 4 mucositis in each group to perform an adequate comparative pharmacokinetic analysis. However, these patients were included in the pharmacokinetic, safety, and efficacy analyses.
There were some limitations to this study. For example, there was no systematic analysis of how posaconazole exposure may have been affected by drug-drug interactions. In general, posaconazole does not have major circulating oxidative (cytochrome P450-mediated) metabolites, and posaconazole plasma concentrations therefore are not expected to change significantly when posaconazole is coadministered with other drugs that are cytochrome P450 inhibitors or inducers.
All three posaconazole dosage regimens were well tolerated in this population of severely ill patients, according to the assessment of the investigator. Most adverse events were consistent with the patient's underlying disease. The types and frequencies of reported adverse events were similar among the three dosage groups, with the exception of treatment-related nausea, which was observed only in the 400-mg-b.i.d. group (4/35; 11%). This may be attributed to higher posaconazole exposure in this treatment group. Most treatment-related adverse events were gastrointestinal in origin, with few leading to treatment discontinuation. The safety and tolerability findings in this study are in agreement with the results of earlier posaconazole studies (R. Hachem, I. Raad, C. Afif, R. Negroni, J. Graybill, S. Hadley, H. Kantarjian, S. Adams, and G. Mukwaya, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr.1109, 2000; L. Nieto, R. Northland, P. Pitisuttithum, C. Firnhaber, S. Jacobson, W. Greaves, R. Plott, and M. Taglietti, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1108, 2000).
Posaconazole treatment resulted in an overall success rate of 77% in efficacy-evaluable patients with FN, with no significant differences noted among the three dosing groups at the end of treatment. Neutropenia resolved in most patients during posaconazole therapy, so it is difficult to discern the extent to which each factor (i.e., posaconazole treatment and/or recovery from neutropenia) contributed to the success rate. Although there appeared to be a shorter duration of neutropenia in group 1 (5.6 versus >10 days), the sample size was too small to detect any statistically significant differences among the groups. No patient with FN developed a confirmed (proven or probable) fungal infection during posaconazole treatment.
This trial demonstrated a favorable outcome in FN patients compared with other antifungal trials with similar patient populations (30, 32, 33). However, the number of patients in the present study is too small to predict outcomes regarding the empirical treatment of FN with posaconazole. Furthermore, the use of a composite end point for evaluating efficacy in these trials (30, 32, 33) is controversial; varying results have been seen, indicating that it is likely that other, unknown additional variables influence antifungal efficacy (28).
In efficacy-evaluable patients with rIFI, the overall posaconazole response rate of 43% is similar to that observed in a large open-label clinical trial that evaluated the efficacy and safety of posaconazole in patients with IFI who had refractory disease or were intolerant of previous antifungal therapy (I. Raad, S. Chapman, R. Bradsher, V. Morrison, M. Goldman, J. Graybill, J. Perfect, T. Patterson, T. Walsh, G. Corcoran, and P. Pappas, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-669, 2004). The overall response rate in the present study is also similar to that observed with other recently approved antifungals when used as salvage therapy. For example, salvage therapy success rates range from 35% to 45% for caspofungin (16, 18), from 37.5% to 46.6% for voriconazole (9, 24), and from 28% to 45% for micafungin (A. J. Ullmann, J. van Burik, P. McSweeney, V. Ratanatharathorn, J. Raymond, V. de Morais, J. McGuirk, W. Lau, D. Facklam, S. Koblinger, M. Reusch, K. Marr, T. Patterson, and D. Denning, Abstr. 13th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. 400, 2003) and are 43% for liposomal amphotericin B (21).
The results of this study demonstrate that posaconazole is both well tolerated and well absorbed in patients with rIFI or FN, though a final conclusion as to its safety cannot be made based on a single study. Divided doses of 800 mg (400 mg b.i.d.) provide the greatest posaconazole exposure. As an extended-spectrum antifungal agent, posaconazole offers antifungal activity against a broad range of clinically important fungal pathogens, together with a potentially better tolerability profile. Additional studies are needed to investigate the role of posaconazole in the treatment of rIFI and the possibility of early empirical or preemptive treatment of patients with FN who have persistent fever despite adequate antibacterial therapy.
Acknowledgments
This work was supported by Schering-Plough Research Institute, Kenilworth, NJ.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Baddley, J. W., T. P. Stroud, D. Salzman, and P. G. Pappas. 2001. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 32:1319-1324. [DOI] [PubMed] [Google Scholar]
- 4.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtney, R., A. Sansone, W. Smith, T. Marbury, P. Statkevich, M. Martinho, M. Laughlin, and S. Swan. 2005. Posaconazole pharmacokinetics, safety, and tolerability in subjects with varying degrees of chronic renal disease. J. Clin. Pharmacol. 45:185-192. [DOI] [PubMed] [Google Scholar]
- 6.Courtney, R., D. Wexler, E. Radwanski, J. Lim, and M. Laughlin. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannaoui, E., J. Meletiadis, J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 51:45-52. [DOI] [PubMed] [Google Scholar]
- 8.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 9.Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 10.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzet, F., D. Wexler, R. Courtney, G. Krishna, J. Lim, and M. Laughlin. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg, R. N., L. J. Scott, H. H. Vaughn, and J. A. Ribes. 2004. Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Curr. Opin. Infect. Dis. 17:517-525. [DOI] [PubMed] [Google Scholar]
- 13.Herbrecht, R., S. Neuville, V. Letscher-Bru, S. Natarajan-Ame, and O. Lortholary. 2000. Fungal infections in patients with neutropenia: challenges in prophylaxis and treatment. Drugs Aging 17:339-351. [DOI] [PubMed] [Google Scholar]
- 14.Husain, S., B. D. Alexander, P. Munoz, R. K. Avery, S. Houston, T. Pruett, R. Jacobs, E. A. Dominguez, J. G. Tollemar, K. Baumgarten, C. M. Yu, M. M. Wagener, P. Linden, S. Kusne, and N. Singh. 2003. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin. Infect. Dis. 37:221-229. [DOI] [PubMed] [Google Scholar]
- 15.Kontoyiannis, D. P., G. P. Bodey, H. Hanna, R. Hachem, M. Boktour, E. Girgaway, M. Mardani, and I. I. Raad. 2004. Outcome determinants of fusariosis in a tertiary care cancer center: the impact of neutrophil recovery. Leukoc. Lymphoma 45:139-141. [DOI] [PubMed] [Google Scholar]
- 16.Kontoyiannis, D. P., R. Hachem, R. E. Lewis, G. A. Rivero, H. A. Torres, J. Thornby, R. Champlin, H. Kantarjian, G. P. Bodey, and I. I. Raad. 2003. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 98:292-299. [DOI] [PubMed] [Google Scholar]
- 17.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 18.Maertens, J., I. Raad, G. Petrikkos, M. Boogaerts, D. Selleslag, F. B. Petersen, C. A. Sable, N. A. Kartsonis, A. Ngai, A. Taylor, T. F. Patterson, D. W. Denning, and T. J. Walsh. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563-1571. [DOI] [PubMed] [Google Scholar]
- 19.Marco, F., M. A. Pfaller, S. A. Messer, and R. N. Jones. 1998. In vitro activity of a new triazole antifungal agent, Sch 56592, against clinical isolates of filamentous fungi. Mycopathologia 141:73-77. [DOI] [PubMed] [Google Scholar]
- 20.Musa, M. O., A. Al Eisa, M. Halim, E. Sahovic, M. Gyger, N. Chaudhri, F. Al Mohareb, P. Seth, M. Aslam, and M. Aljurf. 2000. The spectrum of Fusarium infection in immunocompromised patients with haematological malignancies and in non-immunocompromised patients: a single institution experience over 10 years. Br. J. Haematol. 108:544-548. [DOI] [PubMed] [Google Scholar]
- 21.Ng, T. T., and D. W. Denning. 1995. Liposomal amphotericin B (AmBisome) therapy in invasive fungal infections. Evaluation of United Kingdom compassionate use data. Arch. Intern. Med. 155:1093-1098. [PubMed] [Google Scholar]
- 22.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2002. In vitro activities of investigational triazoles against Fusarium species: effects of inoculum size and incubation time on broth microdilution susceptibility test results. Antimicrob. Agents Chemother. 46:3298-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2001. In vitro activities of posaconazole (Sch 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:2862-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ullmann, A. J., C. P. Heussel, and O. A. Cornely. 2002. Voriconazole versus liposomal amphotericin B for empirical antifungal therapy. N. Engl. J. Med. 346:1745-1747. [PubMed] [Google Scholar]
- 29.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. de Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 30.Walsh, T. J., R. W. Finberg, C. Arndt, J. Hiemenz, C. Schwartz, D. Bodensteiner, P. Pappas, N. Seibel, R. N. Greenberg, S. Dummer, M. Schuster, J. S. Holcenberg, et al. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 340:764-771. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 32.Walsh, T. J., P. Pappas, D. J. Winston, H. M. Lazarus, F. Petersen, J. Raffalli, S. Yanovich, P. Stiff, R. Greenberg, G. Donowitz, M. Schuster, A. Reboli, J. Wingard, C. Arndt, J. Reinhardt, S. Hadley, R. Finberg, M. Laverdiere, J. Perfect, G. Garber, G. Fioritoni, E. Anaissie, and J. Lee. 2002. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 346:225-234. [DOI] [PubMed] [Google Scholar]
- 33.Walsh, T. J., H. Teppler, G. R. Donowitz, J. A. Maertens, L. R. Baden, A. Dmoszynska, O. A. Cornely, M. R. Bourque, R. J. Lupinacci, C. A. Sable, and B. E. dePauw. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 351:1391-1402. [DOI] [PubMed] [Google Scholar]