Abstract
The alkyl phosphocholine drug miltefosine is structurally similar to natural substrates of the fungal virulence determinant phospholipase B1 (PLB1), which is a potential drug target. We determined the MICs of miltefosine against key fungal pathogens, correlated antifungal activity with inhibition of the PLB1 activities (PLB, lysophospholipase [LPL], and lysophospholipase-transacylase [LPTA]), and investigated its efficacy in a mouse model of disseminated cryptococcosis. Miltefosine inhibited secreted cryptococcal LPTA activity by 35% at the subhemolytic concentration of 25 μM (10.2 μg/ml) and was inactive against mammalian pancreatic phospholipase A2 (PLA2). At 250 μM, cytosolic PLB, LPL, and LPTA activities were inhibited by 25%, 51%, and 77%, respectively. The MICs at which 90% of isolates were inhibited (MIC90s) against Candida albicans, Candida glabrata, Candida krusei, Cryptococcus neoformans, Cryptococcus gattii, Aspergillus fumigatus, Fusarium solani, Scedosporium prolificans, and Scedosporium apiospermum were 2 to 4 μg/ml. The MICs of miltefosine against Candida tropicalis (n = 8) were 2 to 4 μg/ml, those against Aspergillus terreus and Candida parapsilosis were 8 μg/ml (MIC90), and those against Aspergillus flavus (n = 8) were 2 to 16 μg/ml. Miltefosine was fungicidal for C. neoformans, with rates of killing of 2 log units within 4 h at 7.0 μM (2.8 μg/ml). Miltefosine given orally to mice on days 1 to 5 after intravenous infection with C. neoformans delayed the development of illness and mortality and significantly reduced the brain cryptococcal burden. We conclude that miltefosine has broad-spectrum antifungal activity and is active in vivo in a mouse model of disseminated cryptococcosis. The relatively small inhibitory effect on PLB1 enzyme activities at concentrations exceeding the MIC by 2 to 20 times suggests that PLB1 inhibition is not the only mechanism of the antifungal effect.
Invasive fungal infections are associated with high rates of morbidity and mortality. The marketed antifungal drugs have limitations that include one or more of incomplete spectra of activity, toxicities, poor stability, lack of oral availability, and high cost. It is generally accepted that improved drugs that, ideally, act on different antifungal targets are needed. Virulence determinants common to pathogenic fungi are potential targets.
To this end we have purified and fully characterized cryptococcal phospholipase B (PLB1) (5, 7, 34), proved that it is a virulence determinant (6, 8), and correlated the inhibition of enzymatic activity with antifungal activity (12). PLB is a virulence determinant in Candida albicans and is produced by Aspergillus fumigatus (2, 19, 27), Scedosporium prolificans, Fusarium oxysporum, and a Mucorales sp. (L. C. Wright and T. C. Sorrell, unpublished data), suggesting that it is a potentially useful antifungal drug target. The structure and mechanism of action of PLB1 are not understood. However, secreted PLB1 is involved in the survival of cryptococci in macrophages (8), adhesion to pulmonary epithelium (12a), destruction of lung tissue, and production of eicosanoids, which modulate phagocytic activity (31).
The PLB1 protein is unique in that it contains three components with three separate activities: PLB, which removes both acyl chains simultaneously from phospholipids; lysophopholipase (LPL), which removes the single acyl chain from lysophospholipids; and lysophospholipase transacylase (LPTA), which adds an acyl chain to lysophospholipids to form a diacylphospholipid. Which of the secreted phospholipase activities is important in fungal virulence is not known. We have used cryptococcal PLB1 as the prototype for testing phospholipase inhibitors as potential antifungal agents (12).
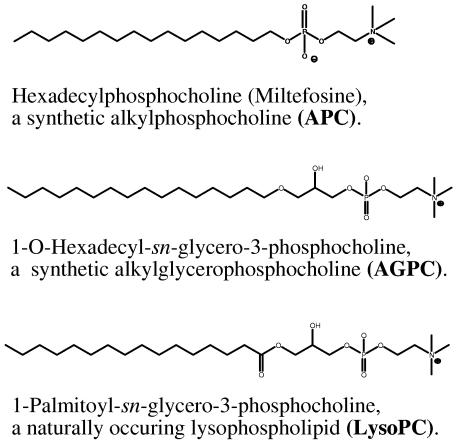
Miltefosine is an alkyl phosphocholine compound that was initially developed as an anticancer agent but that has activity against Leishmania species and Trypanosoma cruzi (1, 9, 10, 20). It has been approved in India for clinical use in leishmaniasis (37). Phosphocholines have structural similarities (Fig. 1) to the natural substrates of fungal PLB1 (e.g., phosphatidylcholine and lysophosphatidylcholine are the preferred substrates of this enzyme [7]), and alkyl-bis-phosphocholines are present in medicinal plants known for their antifungal properties (22). In mammalian tumor cells, miltefosine inhibits protein kinase C and the biosynthesis of phosphatidylcholine and sphingomyelin (14, 15, 16, 32). The mechanism(s) of its antiparasitic effect are not yet defined (9). As a group, the alkyl phosphocholines are stable molecules, unlike alkyl glycerophosphocholines and lysophospholipids (lysophosphocholine [lyso-PC]), which have previously been investigated for biomedical applications but which are chemically and metabolically labile (Fig. 1) (see reference 1 and references therein).
FIG. 1.
Structures of an alkylphosphocholine, an alkylglycerophosphocholine, and a lysophospholipid.
In this study we tested the activity of miltefosine against key fungal pathogens, including species relatively resistant to currently available drugs; correlated the antifungal activity with inhibition of PLB1 enzyme activities; and demonstrated the in vivo efficacy of miltefosine in a mouse model of cryptococcosis.
(This work was presented in part at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C., October 2004 [F. Widmer, L. Wright, D. Obando, R. Ganendren, C. Wilson, and T. Sorrell, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-2287, 2004].)
MATERIALS AND METHODS
Fungal isolates and media.
A virulent clinical isolate of Cryptococcus neoformans var. grubii (serotype A), H99, kindly supplied by Gary Cox (Duke University Medical Center, Durham, NC), was used for cell-associated phospholipase preparation and inhibition of phospholipase activities.
Preparation of cell fractions and supernatants containing secreted phospholipase activities.
Isolate H99 was grown to confluence on Sabouraud dextrose agar (SDA) in 16-cm-diameter petri dishes for 72 h at 30°C in air. Cells scraped from 10 to 20 dishes were washed sequentially with isotonic saline and imidazole buffer (10 mM imidazole, 2 mM CaCl2, 2 mM MgCl2, and 56 mM d-glucose made up in isotonic saline, pH 5.5), resuspended in a volume of this buffer that was about 10% of the cell volume, and incubated for 24 h at 37°C. The cell-free supernatant was separated by centrifugation, as described previously (6), and stored at −70°C. The residual cells were disrupted and separated by centrifugation into cytosolic and membrane fractions, as described previously (12).
Radiometric assay method for phospholipases.
Enzyme activities were measured at pH 4 as described previously (7) in a final volume of 125 μl at 37°C. For the determination of secreted PLB activity, carrier dipalmitoyl phosphatidylcholine (DPPC; final concentration, 800 μM) and 1,2-di[1-14C]palmitoyl phosphatidylcholine (20,000 dpm) were dried under nitrogen and suspended in 125 mM imidazole acetate buffer (assay buffer, pH 4.0) by sonication with a Branson 450 sonifier. The reaction time was 22 min, with 1 μg total protein used; and PLB activity was determined by the rate of decrease of the radiolabeled phosphatidylcholine (PC) substrate, with the appearance of the label in the free fatty acid. The secreted LPL and LPTA activities were measured simultaneously in a reaction mixture containing 1-[1-14C]palmitoyl lyso-PC (25,000 dpm) and carrier lyso-PC (final concentration, 200 μM) in assay buffer. The reaction time was 15 s with 1 μg of total protein, and LPL activity was measured by the rate of loss of 1-[14C]palmitoyl lyso-PC with the release of radiolabeled fatty acids. LPTA activity was estimated from the rate of formation of radiolabeled PC. The variations to these conditions for the cytosolic and membrane fraction assays described by Ganendren et al. (12) were used.
All reactions were terminated by the addition of 0.5 ml of chloroform-methanol (2:1 [vol/vol]). The reaction products were extracted by the method of Bligh and Dyer (3), separated by thin-layer chromatography, and quantified by a standard method as described previously (7). In brief, the reaction products were separated in a one-dimensional run on silica plates with chloroform-methanol-water (65:25:4 [vol/vol/vol]) as the mobile phase. Authentic, nonradioactive standards of palmitic acid, 1-palmitoyl lyso-PC, and dipalmitoyl phosphatidylcholine, were included in each run for identification of the reactants. After development, the plates were dried and stained with iodine vapor, and dark yellow spots were scraped into scintillation vials. Five milliliters of scintillation fluid was added to each vial, and the radioactivity was counted in a scintillation counter.
Protein assays.
Total protein estimations were performed by a Coomassie blue binding assay (for the supernatant containing the secreted enzymes) or the bicinchoninic acid (BCA kit; Pierce Chemical Co.) for cell-associated fractions, with bovine serum albumin (Pierce) used as a standard.
Effect of miltefosine on PLB1 enzyme activities.
Miltefosine powder (Cayman Chemical Company, Ann Arbor, MI) was prepared as a stock solution of 700 μM in assay buffer containing 5 mM EDTA. Serial 10-fold dilutions that gave concentrations of 0.07 to 70 μM were made; and 45-μl aliquots of these dilutions or the stock solution were added to make up a final volume of 125 μl containing substrate, enzyme, and buffer. The radiometric assay was carried out as described above. Inhibition was calculated from the percentage of substrate (DPPC or lyso-PC) remaining, in the case of PLB and LPL activities, or the percentage of DPPC produced, in the case of the LPTA activity. The enzyme activity in the presence of miltefosine was expressed as a percentage of that in the inhibitor-free control (normalized to 100%). All assays were done in triplicate.
Pancreatic phospholipase assay.
Porcine pancreatic phospholipase A2 was suspended in 3.2 M ammonium sulfate (2.9 mg protein/ml; Sigma, St. Louis, MO). One part of the well-mixed enzyme suspension was added to 4 parts of buffer (10 mM Tris-HCl, 10 mM CaCl2, pH 8.2 [11]). The activities and inhibition by the test compounds were then measured by the radiometric method described above, except that 25 μl of enzyme solution was used and the reaction time was 1 h. These conditions resulted in ∼60% substrate conversion in the inhibitor-free control.
Antifungal susceptibility testing.
Antifungal activity was measured by the CLSI (formerly the NCCLS) standard broth microdilution methods for yeasts (29) and filamentous fungi (30). The MIC was defined as that concentration which produced >80% inhibition of visible growth (turbidity) after 48 h of culture at 35°C for yeasts or >50% inhibition at 48 to 72 h for filamentous fungi. When >10 isolates of a fungal species were tested, the results were expressed as the minimum concentrations of test compound at which the growth of at least 50% or 90% of the isolates was inhibited (MIC50 and MIC90, respectively). All tests were performed in duplicate. The minimum fungicidal concentration (MFC) was determined by subculture of 100-μl aliquots from wells with no visible growth onto yeast extract peptone dextrose agar. The plates were incubated for 3 days at 35°C.
Kill curves.
An inoculum of 106 CFU/ml of cryptococci was prepared in RPMI broth, and 1-ml aliquots were transferred into each of four test tubes. The cells were centrifuged, the supernatants were removed, and 1 ml of one of four concentrations (700, 70, 7, and 0.7 μM) of miltefosine in RPMI medium was added to the individual tubes, respectively. Fungal cells were resuspended by vortexing. Aliquots of 100 μl were removed immediately and after 1, 3, 4, and 5 h of incubation at 35°C. The cells were pelleted by centrifugation, washed twice, plated onto SDA, and incubated for 48 h; and the number of CFU was counted.
Hemolytic activity assay.
Human blood was collected in 10-ml Vacutainer tubes containing potassium-EDTA as an anticoagulant, transferred to a 50-ml centrifuge tube, and pelleted by centrifugation at 2,000 × g for 10 min; and the cells were washed three times with 30 ml of calcium- and magnesium-free phosphate-buffered saline (PBS; GIBCO). The third supernatant was clear and colorless. The cells were stored in PBS (20 ml) at 4°C for up to 2 weeks. To test for hemolysis, 0.5 ml of the cell suspension in PBS was mixed with 0.5 ml of the test compound at concentrations of 700, 350, 175, 70, and 7 μM (final erythrocyte concentration, about 0.5 × 109 per ml). The mixtures were incubated at 37°C for 1 h with gentle shaking and centrifuged at 2,000 × g for 10 min, the supernatant was diluted 10-fold with PBS, and the optical density was measured at 540 nm. The values for 0% and 100% lysis were determined by incubating the cells with PBS or 0.1% (wt/vol) Triton X-100 in water, respectively. The assays were carried out in triplicate.
Mouse model assay.
Female BALB/c mice (specific pathogen free) were obtained from the Animal Resources Centre, Floreat Park, Western Australia, Australia, and housed in filter-top cages in a sterile hood. Forty mice were inoculated with C. neoformans H99 (50 μl sterile isotonic saline containing 106 cryptococcal cells) by injection into the tail vein on day 1 and were divided into four groups of 10 mice each. Miltefosine (in 0.5 ml water) was administered daily by gavage through a stainless steel 18-gauge feeding tube on days 1 to 5 at doses of 700 μM (group A), 350 μM (group B), and 175 μM (group C). This equates to doses of 7.2, 3.6, and 1.8 mg/kg of body weight, respectively. Group D (controls) received water by gavage. The first dose was given 60 to 90 min after inoculation of C. neoformans. The mice were monitored twice daily for signs of illness, such as anorexia, weight loss, reduced activity, ruffled fur, cranial bulging, neurological symptoms, and sluggishness. When the mice became very ill or at the conclusion of the experiment, the mice were euthanized by CO2 asphyxiation or cervical dislocation. In preliminary experiments, severe illness was inevitably followed by death within a few hours; and in line with the Western Sydney Area Health Service Animal Care and Ethics Committee requirements, the animals were euthanized. This time was recorded as the time of death from cryptococcosis for construction of the survival curves. After euthanasia, the lungs and brains of all animals were weighed and homogenized in sterile isotonic saline by hand with a sterile mortar and pestle. The volumes of the homogenates were noted. Dilutions were made in saline, plated onto SDA (100 μl per plate), and incubated at 30°C for 72 h. The results were expressed as the numbers of CFU/g of tissue.
RESULTS
Effect of miltefosine on fungal and pancreatic phospholipases.
Miltefosine inhibited the activities of all three components of secreted and cytosolic cryptococcal PLB1 at high concentrations (250 μM), with lesser but still significant inhibition of LPTA at 25 μM (Table 1). Membrane-bound LPTA was also significantly inhibited at this concentration. Porcine pancreatic phospholipase A2 (PLA2) activity (data not shown) was inhibited by 12% at 250 μM and 0% at 25 μM, indicating the there is some selectivity of the effect of miltefosine on cryptococcal LPTA activity.
TABLE 1.
Inhibition of the activities of Cryptococcal neoformans (strain H99) PLB1 by miltefosine
| Enzyme fraction | % Inhibition at the indicated concna
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| LPL
|
LPTA
|
PLB
|
|||||||
| 2.5 μM | 25 μM | 250 μM | 2.5 μM | 25 μM | 250 μM | 2.5 μM | 25 μM | 250 μM | |
| Secretory enzyme | 0 | 0 | 34.3b (2.3) | 6.8b (2.7) | 35.0b (4.0) | 80.0b (2.9) | 0 | 0 | 31.7b (3.8) |
| Cytosolic enzyme | 0 | 0 | 50.7b (1.8) | 0 | 14.3b (2.7) | 77.0b (0.6) | 0 | 0 | 25.0b (4.3) |
| Membrane-bound enzyme | 0 | 0 | 0 | 0 | 11.5b (1.5) | 31.0b (1.0) | 0 | 0 | 0 |
Data are expressed as the means (standard error of the means) of at least three assays.
Significantly different (P < 0.01) from the results for the inhibitor-free controls by the Dunnett multiple-comparison test.
In vitro antifungal activity of miltefosine against pathogenic fungi.
The MIC50, MIC90, and MFC data for miltefosine tested against different species of pathogenic yeasts and filamentous fungi are summarized in Table 2. The MFC values for miltefosine are close to the MIC90, indicating that miltefosine is fungicidal. Notably, its activity against emerging pathogenic fungi, such as Scedosporium prolificans and some strains in the class Zygomycetes, that are relatively resistant to marketed drugs is similar to that against Candida species and Cryptococcus neoformans. The MICs of fluconazole, itraconazole, voriconazole, amphotericin B, and flucytosine against randomly selected isolates were also determined (Table 2). The MICs of miltefosine were similar to or lower than those of fluconazole for the Candida and Cryptococcus species tested and were generally similar to or lower than those of the other three antifungals for most Aspergillus, Fusarium, and Scedosporium isolates tested.
TABLE 2.
Antifungal activity of miltefosine
| Fungal species | Drug | No. of tests | MIC (μg/ml)
|
MFC (μg/ml) | ||
|---|---|---|---|---|---|---|
| Rangea | 50% | 90% | ||||
| Candida albicans | Miltefosine | 14 | 1.0-2.0 | 2.0 | 2.0 | 4.0 |
| Amphotericin B | 7 | 0.06-0.125 | 0.06 | |||
| Fluconazole | 7 | 0.25-8.0 | 1.0 | |||
| Flucytosine | 7 | 0.03-0.125 | 0.06 | |||
| Itraconazole | 7 | 0.03-0.125 | 0.06 | |||
| Voriconazole | 7 | 0.008-0.5 | 0.03 | |||
| Candida parapsilosis | Miltefosine | 10 | 2.0-8.0 | 4.0 | 8.0 | 8.0 |
| Amphotericin B | 5 | 0.06-0.25 | 0.25 | |||
| Fluconazole | 5 | 0.5-4.0 | 2.0 | |||
| Flucytosine | 5 | 0.03-0.25 | 0.06 | |||
| Itraconazole | 5 | 0.06-0.125 | 0.06 | |||
| Voriconazole | 5 | 0.008-0.125 | 0.03 | |||
| Candida glabrata | Miltefosine | 10 | 2.0-4.0 | 2.0 | 4.0 | 4.0 |
| Amphotericin B | 5 | 0.125-0.25 | 0.125 | |||
| Fluconazole | 5 | 16.0-256.0 | 32.0 | |||
| Flucytosine | 5 | 0.03-0.125 | 0.06 | |||
| Itraconazole | 5 | 0.5-16.0 | 2.0 | |||
| Voriconazole | 5 | 0.25-4.0 | 0.5 | |||
| Candida krusei | Miltefosine | 10 | 2.0-4.0 | 2.0 | 2.0 | 4.0 |
| Amphotericin B | 5 | 0.25-0.5 | 0.25 | |||
| Fluconazole | 5 | 64.0 | 64.0 | |||
| Flucytosine | 5 | 8.0-16.0 | 8.0 | |||
| Itraconazole | 5 | 0.25-1.0 | 0.5 | |||
| Voriconazole | 5 | 0.25-1.0 | 0.25 | |||
| Candida tropicalis | Miltefosine | 8 | 2.0-4.0 | 4.0 | 4.0 | |
| Amphotericin B | 6 | 0.125-0.25 | 0.125 | |||
| Fluconazole | 6 | 2.0-32.0 | 2.0 | |||
| Flucytosine | 6 | 0.06->64.0 | 0.06 | |||
| Itraconazole | 6 | 0.25-0.5 | 0.25 | |||
| Voriconazole | 6 | 0.06-1.0 | 0.06 | |||
| Cryptococcus neoformans | Miltefosine | 29 | 0.25-4.0 | 0.5 | 2.0 | 8.0 |
| Amphotericin B | 15 | 0.016-0.25 | 0.125 | 0.25 | ||
| Fluconazole | 15 | 0.5-8.0 | 4.0 | 8.0 | ||
| Flucytosine | 15 | 0.5-2.0 | 1.0 | 2.0 | ||
| Itraconazole | 15 | 0.008-0.25 | 0.125 | 0.125 | ||
| Voriconazole | 15 | 0.008-0.06 | 0.03 | 0.06 | ||
| Cryptococcus gattii | Miltefosine | 38 | 0.5-2.0 | 2 | 2.0 | 4.0 |
| Amphotericin B | 23 | 0.06-0.125 | 0.125 | 0.25 | ||
| Fluconazole | 23 | 4.0-16.0 | 8.0 | 16.0 | ||
| Flucytosine | 23 | 0.25-2.0 | 1.0 | 2.0 | ||
| Itraconazole | 23 | 0.06-0.25 | 0.125 | 0.125 | ||
| Voriconazole | 21 | 0.03-0.25 | 0.06 | 0.25 | ||
| Aspergillus fumigatus | Miltefosine | 10 | 2.0 | 2.0 | 2.0 | 4.0 |
| Amphotericin B | 5 | 0.125-0.5 | 0.25 | |||
| Flucytosine | 5 | 32.0-64.0 | 64.0 | |||
| Itraconazole | 5 | 2.0-16.0 | 4.0 | |||
| Voriconazole | 5 | 0.06-0.25 | 0.25 | |||
| Aspergillus flavus | Miltefosine | 10 | 2.0-16.0 | 8.0 | 16.0 | 16.0 |
| Amphotericin B | 5 | 1.0-16.0 | 2.0 | |||
| Flucytosine | 5 | 2.0-64.0 | 64.0 | |||
| Itraconazole | 5 | 0.25-16.0 | 0.5 | |||
| Voriconazole | 5 | 0.25-1.0 | 0.5 | |||
| Aspergillus terreus | Miltefosine | 10 | 2.0-8.0 | 4.0 | 8.0 | 16.0 |
| Amphotericin B | 5 | 0.25-1.0 | 0.5 | |||
| Flucytosine | 5 | 2.0-64.0 | 32.0 | |||
| Itraconazole | 5 | 0.016-0.25 | 0.25 | |||
| Voriconazole | 5 | 0.25-0.5 | 0.5 | |||
| Fusarium solani | Miltefosine | 10 | 2.0-4.0 | 4.0 | 4.0 | 4.0 |
| Amphotericin B | 5 | 1.0-8.0 | 4.0 | |||
| Flucytosine | 5 | 64.0 | 64.0 | |||
| Itraconazole | 5 | 16.0 | 16.0 | |||
| Voriconazole | 4 | 1.0-4.0 | 2.0 | |||
| Scedosporium apiospermum | Miltefosine | 10 | 2.0-4.0 | 4.0 | 4.0 | 4.0 |
| Amphotericin B | 5 | 2.0-8.0 | 4.0 | |||
| Flucytosine | 5 | 64.0 | 64.0 | |||
| Itraconazole | 5 | 0.5-1.0 | 0.5 | |||
| Voriconazole | 5 | 0.125-0.25 | 0.125 | |||
| Scedosporium prolificans | Miltefosine | 10 | 4.0 | 4.0 | 4.0 | 4.0 |
| Amphotericin B | 4 | 4.0-8.0 | 4.0 | |||
| Flucytosine | 4 | 64.0 | 64.0 | |||
| Itraconazole | 4 | 16.0 | 16.0 | |||
| Voriconazole | 4 | 4.0 | 4.0 | |||
| Bipolaris australiensis | Miltefosine | 6 | 2.0-4.0 | 4.0 | 4.0 | 8.0 |
| Amphotericin B | 1 | 0.03 | ||||
| Flucytosine | 1 | 64.0 | ||||
| Itraconazole | 1 | 0.25 | ||||
| Voriconazole | 1 | 0.5 | ||||
| Exophiala jeanselmei | Miltefosine | 2 | 2.0-4.0 | 4.0 | ||
| Amphotericin B | 1 | 2.0 | ||||
| Flucytosine | 1 | 64.0 | ||||
| Itraconazole | 1 | 0.5 | ||||
| Voriconazole | 1 | 0.5 | ||||
| Exophiala spinifera | Miltefosine | 2 | 2.0 | 8.0 | ||
| Amphotericin B | 1 | 0.25 | ||||
| Flucytosine | 1 | 4.0 | ||||
| Itraconazole | 1 | 0.125 | ||||
| Voriconazole | 1 | 0.5 | ||||
| Paecilomyces lilacinus | Miltefosine | 8 | 2.0-4.0 | 2.0 | 4.0 | |
| Amphotericin B | 3 | 2.0-16.0 | 2.0 | |||
| Flucytosine | 3 | 64.0 | 64.0 | |||
| Itraconazole | 3 | 0.25-0.5 | 0.5 | |||
| Voriconazole | 3 | 0.06-0.125 | 0.06 | |||
| Absidia corymbifera | Miltefosine | 6 | 2.0-16.0 | 2.0 | 16.0 | |
| Amphotericin B | 3 | 0.125-16.0 | 0.25 | |||
| Flucytosine | 3 | 64.0 | 64.0 | |||
| Itraconazole | 3 | 0.5-4.0 | 0.5 | |||
| Voriconazole | 2 | 16.0 | ||||
| Rhizopus spp. | Miltefosine | 4 | 2-16 | 8.0 | 16.0 | |
| Amphotericin B | 1 | 0.06 | ||||
| Flucytosine | 1 | 64.0 | ||||
| Itraconazole | 1 | 2.0 | ||||
| Voriconazole | 1 | 4.0 | ||||
| Cunninghamella bertholletiae | Miltefosine | 4 | 2.0-4.0 | 2.0 | 4.0 | |
The range of MICs is given when <10 isolates were tested.
Killing curves for miltefosine against C. neoformans.
The rates of killing of strain H99 of C. neoformans by different concentrations of miltefosine (from 0.28 to 280 times the MIC) were determined over a 5-h period. As shown in Table 3, killing at concentrations 280 times the MIC occurred at time zero, which was actually 9 min (the limit of determination due to processing required for the assay).
TABLE 3.
In vitro rate of killing of Cryptococcus neoformans H99 by miltefosine
| Concn of miltefosine (μM) | Viable cryptococci (CFU/ml) at the following time of exposure (h)a:
|
||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 4 | 5 | |
| 700 (285 μg/ml, or 0.0285%) | 0 | 0 | 0 | 0 | 0 |
| 7.0 (2.8 μg/ml, or 0.00028%) | 1.95 × 106 | 1.23 × 106 | 1.5 × 105 | 2.7 × 104 | 2.0 × 104 |
| 0.7 (0.28 μg/ml) | 2.31 × 106 | 2.10 × 106 | 1.9 × 106 | 1.9 × 106 | 1.9 × 106 |
The number of CFU (drug-free control) at zero time was 2.5 × 106/ml.
Hemolytic activity of miltefosine.
As shown in Table 4, there was no evidence of hemolysis until concentrations of miltefosine well above the MIC of pathogenic fungi were reached, e.g., 20% hemolysis at 87.5 μM (35.5 μg/ml). This is probably an overestimate, because the erythrocyte concentration in the assays was half that in normal blood.
TABLE 4.
Hemolytic activity of miltefosine
| % Lysis at the following miltefosine concn:
| ||||
|---|---|---|---|---|
| 350 μM (142.5 μg/ml) | 175 μM (71.2 μg/ml) | 87.5 μM (35.5 μg/ml) | 35 μM (14.2 μg/ml) | 3.5 μM (1.4 μg/ml) |
| 100.0 | 92.5 | 20.5 | 0 | 0 |
Efficacy of miltefosine in a mouse model of cryptococcosis.
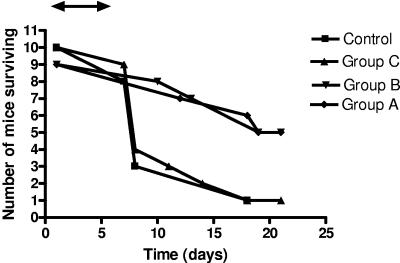
Survival curves for mice treated with three different doses of miltefosine and controls are shown in Fig. 2. The rates of survival of mice treated with 7.2 mg/kg (dose A) and 3.6 mg/kg (dose B) of miltefosine daily for 5 days after infection were significantly better than those of mice treated with 1.8 mg/kg (dose C), which died at the same rate as the controls. The fungal burdens in the brains and the lungs of the treated and the test mice are summarized in Table 5. The organ burdens at the time of death were not statistically different for any of the groups for mice that died during the experiment. The burdens were reduced in mice treated with 3.6 mg/kg or 7.2 mg/kg of miltefosine when they were culled at 21 days compared with those in the control mice and animals treated with the lowest dose of miltefosine (1.8 mg/kg/day).
FIG. 2.
Survival of mice infected with C. neoformans without treatment (controls) or treated orally by gavage for 5 days with 0.5 ml of 700 μM (A), 350 μM (B), or 175 μM (C) of miltefosine. The double-ended arrow indicates the duration of miltefosine therapy.
TABLE 5.
Lung and brain burdens of cryptococci
| Time and parameter | Controls | Group C (1.8 mg/kg miltefosine) | Group B (3.6 mg/kg miltefosine) | Group A (7.2 mg/kg miltefosine) |
|---|---|---|---|---|
| At death due to cryptococcosis | ||||
| No. of animals | 9 | 9 | 4 | 4 |
| Brain burden (CFU/g [107])a | 27.3 ± 4.7 | 20.7 ± 2.6 | 17 ± 6.1b | 14.7 ± 1.8b |
| Lung burden (CFU/g [107])a | 20.4 ± 7.3 | 23 ± 8.2 | 52.6 ± 49b | 86 ± 32b |
| At culling on day 21 | ||||
| No. of animals | 1e | 1 | 5 | 5 |
| Brain burden (CFU/g [107])a | 0.002 | 10.0 | 5.2 ± 4.8c>,d | 2.2 ± 1.9c>,d |
| Lung burden (CFU/g [107])a | Nil | 7.3 | 2.7 ± 2.3c>,d | 6.4 ± 6.4c>,d |
Data are the means ± standard errors of the means.
Not significantly different from the results for the control group or group C by nonparametric analysis of variance.
Significantly different from the results for the controls at death.
Significantly different from the results for group C at death (P < 0.05 by nonparametric analysis of variance).
Cryptococcal infection was not regarded as established in this mouse.
DISCUSSION
Enzyme inhibition and mode of action.
Subhemolytic concentrations of miltefosine showed dose-dependent inhibition of the LPTA activity of cryptococcal PLB1 and did not inhibit porcine pancreatic PLA2. This suggests that the effect was due to selective enzyme inhibition rather than a nonspecific detergent-like action. The association of inhibition of LPTA activity with antifungal activity in vitro and in vivo is interesting, since PLB activity, rather than LPL or LPTA activity, has been shown to be required for adhesion to a lung epithelial cell line (12a). It has been proposed that cell-associated LPTA is involved in membrane synthesis, remodeling, and repair (23); hence, miltefosine may exert an antifungal effect by interfering with cryptococcal cell wall or cell membrane biochemistry. Since PLB1 is essential for the adhesion of cryptococci to mammalian cells and for the hematogenous dissemination of infection (35), inhibition by miltefosine may prevent such dissemination. In addition, inhibition of both the secretory and the cell-associated LPL and LPTA activities may prevent the utilization and detoxification of the free fatty acids and lysophospholipids formed from host membranes during tissue invasion. Moreover, miltefosine is active against Leishmania donovani, other Leishmania species, and Trypanosoma cruzi in cell culture (for a review, see the work of Croft et al. [9]). There is evidence that phospholipases are active in both of these parasites and that they can be inhibited by alkylphosphonates (13, 28, 33).
Inhibition of LPTA could be important for the prevention of survival of fungi under the stressful conditions and poor nutrition encountered during invasion of the mammalian host, but it is unlikely to be the only mechanism of the antifungal effect of miltefosine, as 50% inhibition of the enzyme in vitro required concentrations greater than 25 μM, compared with an MIC and an MFC of 4 μM each. Effects on alternative biochemical pathways have been described in mammalian tumor cell lines (32). The antiproliferative activity of miltefosine was correlated with the inhibition of translocation of CTP:phosphocholine-cytidylyltransferase from an inactive cytosolic form to an active membrane-bound form, resulting in reduced synthesis of the abundant membrane phospholipid, phosphatidylcholine (14, 16). Furthermore, sphingomyelin synthesis was inhibited, resulting in increased levels of intracellular ceramide and the induction of apoptosis (39). Several effects of miltefosine and another phosphocholine, edelfosine, have been described in Leishmania, including inhibition of PC synthesis, perturbation of ether lipid metabolism, glycosylphosphatidylinositol anchor biosynthesis and signal transduction, and inhibition of choline uptake (9, 24, 40). While it is unknown whether choline uptake is critical for fungal viability, we have preliminary data that C. neoformans takes up and incorporates choline into phospholipids (L. C. Wright, unpublished data). Inhibition of phosphatidylinositol-phospholipase C by miltefosine has been reported in T. cruzi epimastigotes (24).
In vitro and in vivo activities.
The broad spectrum of activity against pathogenic fungi with fungicidal concentrations within the range of those of amphotericin B suggests that the alkylphosphocholine class of compounds can be exploited for the development of a new class of antifungal drugs. It is notable that the MICs of miltefosine were similar to those of amphotericin B against relatively resistant or highly resistant fungi, namely, some members of the class Zygomycetes, Fusarium solani, and Scedosporium prolificans. Amphotericin B is the only currently marketed drug with activity against the Zygomycetes, but the responses are suboptimal even with prolonged treatment with high doses of lipid formulations of amphotericin B in combination with extensive surgical debridement. There is evidence that zygomycoses have become more common in immunosuppressed patients with acute leukemia and recipients of hematopoietic stem cell transplants (HSCT), possibly related to the increasing use of voriconazole, which has no activity against this group of fungi (21). Scedosporium spp. and Fusarium spp. are emerging as pathogens, especially in heavily immunosuppressed hosts. Scedosporium prolificans infections have been reported most commonly from Australia and Spain and result in a high rate of mortality among patients with acute leukemia and recipients of HSCT. Current antifungals are ineffective. Azole drugs plus terbinafine exhibit synergistic activity in vitro (26) and have successfully been used in clinical practice (17), but the mortality rate in recipients of HSCT remains high (18) and additional agents are needed.
The absorption, distribution, and metabolism of miltefosine have been studied in rats and mice (4, 25, 38). Serum concentrations of 110 μM were achieved in rats after 2 weeks of daily dosing with 10 mg/kg. This is 5 to 20 times the MIC90 for miltefosine against fungi causing invasive mycoses. We showed in a mouse model of cryptococcosis that the oral administration of miltefosine for 5 days following infection increased survival and reduced the brain and lung cryptococcal burdens. This was achieved with relatively low doses of 7.2 and 3.6 mg/kg/day of miltefosine and confirmed the potential of this drug for the treatment of invasive mycoses, including intracerebral infections.
Although miltefosine shows promise as an antifungal drug and is approved for use in humans with leishmaniasis, it has disadvantages. The parent compound has a high incidence of gastrointestinal side effects (30% incidence of usually mild nausea and vomiting in a study of treatment of visceral leishmaniasis); a lesser incidence of hepatotoxicity, with typically transient increases in liver enzyme levels; and occasional rashes, including rare instances of Stevens-Johnson syndrome (36, 37). Nausea and vomiting precluded its long-term use in patients with cancer (36). High doses are teratogenic in rats; and although congenital abnormalities have not been reported in humans when the male partner was taking miltefosine (37), the drug is contraindicated in pregnancy. Although the MICs of miltefosine are similar to those of amphotericin B, they are relatively high. The primary molecular target(s) of the drug and the mechanism(s) of its biological effect(s) on fungi remain undetermined.
We conclude that miltefosine is a fungicidal, orally active compound which is effective in vitro against common as well as resistant and emerging pathogens. Although it has significant side effects, it provides an alternative to current agents for the treatment of drug-resistant species such as Scedosporium prolificans. Overall, miltefosine is less toxic than amphotericin B. Miltefosine is therefore a suitable lead compound for the synthesis of more effective and less toxic antifungal derivatives.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (grant 211040) and an infrastructure grant to the Centre for Infectious Diseases and Microbiology and Westmead Millennium Institute from the New South Wales Department of Health. T. C. Sorrell's work is also supported by a Centre of Clinical Research Excellence program grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Agresta, M., P. D'Arrigo, E. Fasoli, D. Losi, G. Pedrocchi-Fantoni, S. Riva, S. Servi, and D. Tessaro. 2003. Synthesis and antiproliferative activity of alkylphosphocholines. Chem. Phys. Lipids 126:201-210. [DOI] [PubMed] [Google Scholar]
- 2.Birch, M., G. Robson, D. Law, and D. W. Denning. 1996. Evidence of multiple extracellular phospholipase activities of Aspergillus fumigatus. Infect. Immun. 64:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bligh, E. C., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 4.Breiser, A., D.-J. Kim, E. Fleer, D. Damenz, A. Drube, M. Berger, G. A. Nagel, H. Eibl, and C. Unger. 1987. Distribution and metabolism of hexadecylphosphocholine in mice. Lipids 22:925-926. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S. C., L. C. Wright, R. T. Santangelo, M. Muller, V. R. Moran, P. W. Kuchel, and T. C. Sorrell. 1997. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect. Immun. 65:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, S. C., M. Muller, J. Z. Zhou, L. C. Wright, and T. C. Sorrell. 1997. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J. Infect. Dis. 175:414-420. [DOI] [PubMed] [Google Scholar]
- 7.Chen, S. C., L. C. Wright, J. C. Golding, and T. C. Sorrell. 2000. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 347:431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, G. M., H. C. McDade, S. C. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Cassadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166-175. [DOI] [PubMed] [Google Scholar]
- 9.Croft, S. L., K. Seifert, and M. Duchene. 2003. Antiprotozoal activities of phospholipid analogues. Mol. Biochem. Parasitol. 126:165-172. [DOI] [PubMed] [Google Scholar]
- 10.De Castro, S. L., R. M. Santa-Rita, J. A. Urbina, and S. L. Croft. 2004. Antiprotozoal lysophospholipid analogues: a comparison of their activity against trypanosomatid parasites and tumor cells. Mini-Rev. Med. Chem. 4:141-151. [DOI] [PubMed] [Google Scholar]
- 11.De Haas, G. H., M. G. Van Oort, R. Dijkman, and R. Verger. 1989. Phospholipase A2 inhibitors: monoacyl, monoacylamino-glycero-phosphocholines. Biochem. Soc. Trans. 17:274-276. [DOI] [PubMed] [Google Scholar]
- 12.Ganendren, R., F. Widmer, V. Singhal, C. Wilson, T. Sorrell, and L. Wright. 2004. In vitro antifungal activity of inhibitors of phospholipases from the fungal pathogen Cryptococcus neoformans. Antimicrob. Agents Chemother. 48:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Ganendren, R., L. C. Wright., et al. Microbes Infect., in press.
- 13.Gautam, C. 1986. Lipase and phospholipases of Leishmania donovani promastigotes. IRCS J. Med. Sci. 14:1091-1092. [Google Scholar]
- 14.Geilen, C. C., A. Haase, T. Wieder, D Arndt, R. Zeisig, and W. Reutter. 1994. Phospholipid analogues: side chain- and polar head group-dependent effects on phosphatidylcholine biosynthesis. J. Lipid Res. 35:625-632. [PubMed] [Google Scholar]
- 15.Geilen, C. C., R. Haase, K. Buchner, T. Wieder, F. Hucho, and W. Reutter. 1991. The phospholipid analogue, hexadecylphosphocholine, inhibits protein kinase C in vitro and antagonises phorbol ester-stimulated cell proliferation. Eur. J. Cancer 27:1650-1653. [DOI] [PubMed] [Google Scholar]
- 16.Geilen, C. C., T. Wieder, and W. Reutter. 1991. Hexadecylphosphocholine inhibits translocation of CTP:choline-phosphate cytidylyltransferase in Madin-Darby canine kidney cells. J. Biol. Chem. 267:6719-6724. [PubMed] [Google Scholar]
- 17.Howden, B. P., M. A. Slavin, A. P. Schwarer, and A. M. Mijch. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 22:111-113. [DOI] [PubMed] [Google Scholar]
- 18.Husain, S., P. Munoz, G. Forrest, B. D. Alexander, J. Somani, K. Brenan, M. M. Wagener, and N. Singh. 2005. Infections due to Scedosporium apiospermum and Scedosporiumprolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 40:89-99. [DOI] [PubMed] [Google Scholar]
- 19.Ivanovska, N. 2003. Phospholipases as a factor of pathogenicity in microorganisms. J. Mol. Catalysis B: Enzymatic 22:357-361. [Google Scholar]
- 20.Kaminsky, R. 2002. Miltefosine zentaris. Curr. Opin. Investig. Drugs 3:550-554. [PubMed] [Google Scholar]
- 21.Kontoyiannis, D. P., M. S. Lionakis, R. E. Lewis, G. Chamilos, M. Healy, C. Perego, A. Safdar, H. Kantarjian, R. Champlin, T. J. Walsh, and I. I. Raad. 2005. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J. Infect. Dis. 191:1350-1360. [DOI] [PubMed] [Google Scholar]
- 22.Lu, Q., R. B. Ubillas, L. G. Dubenko, J. M. Dener, J. Litvak, P.-W. Phuan, M. Flores, Z.-J. Ye, E. Gerber, T. Truong, and D. E. Bierer. 1999. Synthetic analogues of irlbacholine: a novel antifungal plant metabolite isolated from Irlbachia alata. J. Nat. Prod. 62:824-828. [DOI] [PubMed] [Google Scholar]
- 23.Lux, H., H. Norton, T. Klenner, D. Hart, and F. R. Opperdos. 2000. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in Leishmania. Mol. Biochem. Parasitol. 111:1-14. [DOI] [PubMed] [Google Scholar]
- 24.Lux, H., D. T. Hart, P. J. Parker, and T. Klenner. 1996. Ether lipid metabolism, GPI anchor biosynthesis, and signal transduction are putative targets for anti-leishmanial alkyl phospholipid analogues. Adv. Exp. Med. Biol. 416:201-211. [DOI] [PubMed] [Google Scholar]
- 25.Marschner, N., J. Kotting, H. Eibl, and C. Unger. 1992. Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother. Pharmacol. 31:18-22. [DOI] [PubMed] [Google Scholar]
- 26.Meletiadis, J., J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirbod, F., Y. Banno, M. A. Ghannoum, A. S. Ibrahim, S. Nagashima, Y. Kitajhima, G. T. Gole, and Y. Nozava. 1995. Purification and characterization of lysophospholipase- transacylase (h-LPTA) from a highly virulent strain of Candida albicans. Biochim. Biophys. Acta 1257:181-188. [DOI] [PubMed] [Google Scholar]
- 28.Morris, J. C., L. Ping-Shen, H.-X. Zhai, T.-Y. Shen, and K. Mensa-Wilmot. 1998. Inhibition of GPI phospholipase C from Trypanosoma brucei by fluoro-inositol dodecylphosphonates. Biochem. Biophys. Res. Commun. 244:873-876. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution susceptibility testing of yeasts: approved standard. NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution susceptibility testing of filamentous fungi: approved standard. NCCLS document M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 31.Noverr, M. C., G. M. Cox, J. R. Perfect, and G. B. Huffnagle. 2003. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 71:1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rybczynska, M., M. Spitaler, N. G. Knebel, G. Boeck, H. Grunicke, and J. Hofmann. 2001. Effects of miltefosine on various biochemical parameters in a panel of tumor cell lines with different sensitivities. Biochem. Pharmacol. 62:765-772. [DOI] [PubMed] [Google Scholar]
- 33.Sage, L., P. N. Hambrey, G. M. Werchola, A. Mellors, and I. R. Tizard. 1981. Lysophospholipase 1 in Trypanosoma brucei. Tropenmed. Parasitol. 32:215-220. [PubMed] [Google Scholar]
- 34.Santangelo, R. T., M. H. Nouri-Sorkhabi, T C. Sorrell, M. Cagney, S. C. Chen, P. W. Kuchel, and L. C. Wright. 1999. Biochemical and functional characterisation of secreted phospholipase activities from Cryptococcus neoformans in their naturally occurring state. J. Med. Microbiol. 48:731-740. [DOI] [PubMed] [Google Scholar]
- 35.Santangelo, S., H. Zoellner, T. C. Sorrell, C. Wilson, C. Donald, J. Djordjevic, Y. Shounan, and L. Wright. 2004. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 72:2229-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sindermann, H., S. L. Croft, K. R. Engel, W. Bommer, H. J. Eibl, C. Unger, and J. Engel. 2004. Miltefosine (Impavido): the first oral treatment against leishmaniasis. Med. Microbiol. Immunol. 193:173-180. [DOI] [PubMed] [Google Scholar]
- 37.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fischer, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 38.Unger, C., E. Fleer, W. Damenz, P. Hilgard, G. Nagel, and H. Eibl. 1991. Hexadecylphosphocholine: determination of serum concentrations in rats. J. Lipid Mediators 3:71-78. [PubMed] [Google Scholar]
- 39.Wieder, T., C. E. Orfanos, and C. C. Geilen. 1998. Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine. J. Biol. Chem. 273:11025-11031. [DOI] [PubMed] [Google Scholar]
- 40.Zuffrey, R., and C. B. Mamoun. 2002. Choline transport in Leishmania major promastigotes and its inhibition by choline and phosphocholine analogues Mol. Biochem. Parasitol. 125:127-134. [DOI] [PubMed] [Google Scholar]