Abstract
A human donor-selected immunoglobulin G for intravenous injection (IGIV) product with elevated titers against the staphylococcal fibrinogen-binding MSCRAMM proteins ClfA and SdrG (INH-A21) was tested in vitro and in vivo. INH-A21 contained a significantly increased ability to inhibit the fibrinogen-binding activity of recombinant forms of both ClfA and SdrG. Evaluation of the opsonizing potential of INH-A21 was evaluated using fluorescently labeled bacteria; this assay indicated an increase in phagocytic activity compared to normal IGIV. The prophylactic efficacy of INH-A21 against an intraperitoneal challenge of methicillin-resistant Staphylococcus epidermidis (MRSE) was evaluated in a neonatal rat model. INH-A21 was also evaluated for prophylactic and therapeutic efficacy in a rabbit model of catheter-induced aortic valve infective endocarditis caused by either MRSE or methicillin-resistant Staphylococcus aureus (MRSA). Results from the in vivo models demonstrated potent prophylactic and therapeutic efficacy against both MRSE and MRSA. These data suggest that INH-A21 may be an important tool for the prevention and treatment of staphylococcal infections, especially in high-risk populations.
Staphylococcus aureus and Staphylococcus epidermidis account for over half of all nosocomial bloodstream infections annually in the United States (30). Very-low-birth-weight (VLBW) infants are highly susceptible to such infections. Recent data indicate that VLBW infants account for approximately 1.46% of all live births in the United States, representing 58,544 infants per year (16), and in one study of infants with birth weights of 401 to 1,500 g, the rate of infection after day 3 of life was 21% (28). In fact, late-onset sepsis has become the most common cause of death among premature infants after the third day of life (27). The magnitude of the morbidity and mortality in this vulnerable patient population requires the development of new strategies to reduce this significant social and economic burden.
Passive immunization with normal human immunoglobulin G (IgG) for intravenous injection (IGIV) has been studied in numerous clinical trials to determine its effectiveness in preventing or treating nosocomial infections in premature infants. However, studies in the United States have shown only marginal benefits of treating premature infants with IGIV (14). This outcome may have resulted from inadequate levels of specific antibodies directed against prevalent neonatal pathogens (9, 10, 25). In an effort to provide an IGIV with standardized levels of protective antibodies against the most prevalent bacterial pathogens in neonatal sepsis, we have screened plasma donors for the presence of elevated levels of antibodies directed against a family of adhesin proteins that are expressed on the surface of most S. aureus and S. epidermidis clinical strains (17, 19). Some adhesins, known as MSCRAMM (microbial surface components recognizing adhesive matrix molecules), are responsible for the binding of staphylococci to human extracellular matrix proteins and also to surgical implants, such as catheters, artificial joints, and vascular grafts (11, 12, 21, 22). Microbial adherence has been recognized as the initiating event in most infectious processes. Therefore, interfering with this critical step in the pathogenesis of infection should lead to a reduction in the severity and incidence of disease.
We previously reported that a polyclonal S. aureus immunoglobulin (SA-IGIV) that contained elevated levels of antibodies to S. aureus clumping factor A (ClfA), a fibrinogen-binding MSCRAMM, has multiple in vitro and in vivo activities that suggest it may have therapeutic potential (29). Specifically, in both early and well-established methicillin-resistant S. aureus (MRSA) infective endocarditis, the addition of SA-IGIV to a standard antibiotic regimen (vancomycin) increased bacterial clearance in rabbits. We now report the in vitro and in vivo biological activities of a similar polyclonal specific immune globulin, INH-A21, that contains elevated levels of protective antibodies that recognize heterologous strains of both S. aureus and S. epidermidis.
MATERIALS AND METHODS
Bacteria.
S. epidermidis strain ATCC 35984 (RP-62A) was obtained from the American Type Culture Collection (Manassas, VA). S. epidermidis strain HB (Nilsson) was provided by Timothy Foster of Trinity College, Dublin, Ireland. F40802 is an S. epidermidis strain isolated from the blood of a neonatal intensive care unit (NICU) patient. S. aureus strain 67-0 is a methicillin-resistant wound isolate (MRSA 67-0) previously shown to be virulent in an animal model of infective endocarditis (IE) (3) that was provided by Henry Chambers, University of California San Francisco and San Francisco General Hospital.
Recombinant MSCRAMM proteins.
Clf40 is a recombinant polypeptide of the A-domain of ClfA from S. aureus strain Newman corresponding to amino acids 40 to 559, which represents the fibrinogen-binding domain of full-length ClfA (18). SdrG A-domain (SdrG-A) is a recombinant polypeptide corresponding to the fibrinogen-binding A-domain of the SdrG protein (amino acids 50 to 597) from S. epidermidis strain K28 (6). Both recombinant proteins were expressed with N-terminal hexahistidine sequences and were purified from Escherichia coli lysates by metal affinity chromatography on a chelating Sepharose Fast Flow resin (Amersham Biosciences, Piscataway, NJ) and further purified by Q Sepharose high-pressure chromatography (Amersham Biosciences, Piscataway, NJ).
IGIV preparations.
Panglobulin (ZLB Bioplasma AG, Bern, Switzerland) was purchased from the American Red Cross Blood Services. INH-A21 is an IGIV produced by screening plasma donors for antibodies to Clf40 and SdrG-A by enzyme-linked immunosorbent assay (ELISA) and selecting those donors with the highest antibody levels to produce a plasma pool. The final product contains approximately fivefold greater ClfA- and SdrG-specific antibodies than do standard commercial IGIV products as measured by ELISA. To prepare INH-A21, fresh-frozen plasma donations were thawed, filtered through a 1.2-μm serum capsule (Pall-Gelman, East Hills, NY), and collected in a sterile 1-liter bottle. The plasma was sterile filtered through a 0.2-μm culture capsule (Pall-Gelman, East Hills, NY). The IgG was purified by Protein G Fast Flow affinity chromatography (Amersham Pharmacia Biotech, Piscataway, NJ) using an AKTAexplorer equipped with XK columns. Plasma was applied to the column at a flow rate of approximately 15 ml/min, and the column was rinsed with 5 column volumes of 20 mM Na2HPO4, pH 7.0. IgG was eluted with 0.1 M glycine, pH 2.7. The eluate was neutralized by collecting it in 2 M Tris, pH 8.0. IgG fractions were combined and dialyzed three times against 4 liters of 10 mM NaH2PO4, pH 6.0. The dialyzed IgG solution was clarified by centrifugation and was concentrated to >55 mg/ml in a stirred-cell concentrator with a 30,000 MWCO membrane (Pierce, Rockford, IL). The concentrated IgG was formulated at 50 to 60 mg/ml protein in 0.15 M glycine, 0.1 M NaCl, 0.02% Tween 80, pH 6.2, and sterile filtered using a 0.2-μm filter.
ELISA-based protein inhibition assays.
Ninety-six-well microtiter plates were coated with 1 μg/ml Clf40 or SdrG-A in phosphate-buffered saline and incubated for 2 h at room temperature. Plates were washed and blocked with 1% bovine serum albumin for 1 h, washed, and incubated with antibody dilutions for 1 h at room temperature. Following incubation, 20 μg/ml human fibrinogen (Enzyme Research Lab, South Bend, IN) was added. Plates were incubated for 1 hour at 37°C and washed, and goat anti-fibrinogen horseradish peroxidase (Abcam, Cambridge, MA) conjugate was added. Plates were then washed, and 2,2′-azino-bis(3-ethylbenzothiazoline)-6 sulfonic diammonium salt (ABTS) substrate was added. Substrate was incubated at room temperature for 10 min, and the reaction was stopped by the addition of 10% sodium dodecyl sulfate. Absorbance was read at 405 nm using a SPECTRAmax M2 plate reader.
OP assay.
The opsonophagocytosis (OP) assay was performed as previously described with modifications (7). S. aureus strain 67-0 and S. epidermidis strain F40802 were labeled with the fluorescent dye 5-(and-6)-carboxyfluorescein, succinimidyl ester [5(6)-FAM, SE] and Syto 13 green fluorescent nucleic acid stain (Molecular Probes/Invitrogen, Carlsbad, CA). The bacterial cells were fixed in 2% paraformaldehyde and stored in 16% glycerol frozen stocks at −80°C. The assay was performed by preparing serial twofold dilutions of antibody in gelatin Veronal buffer (GVB) with Ca2+ and Mg2+ (Sigma-Aldrich, St. Louis, MO). Ten microliters of the IgG dilutions was added to a 96-well tissue culture plate (Corning Inc., Corning, NY). The labeled bacterial cells were thawed and diluted in GVB. Bacterial cells (2 × 105) in 20 μl were added to each well containing IgG dilutions or buffer-only controls. The plate was incubated for 30 min at 37°C with 250-rpm rotation to allow for opsonization of bacterial cells. Ten microliters of cold GVB was added to the plate followed by 105 RAW 264.7 cells (ATCC, TIB-71) in 40 μl of cold GVB. The plate was incubated for 30 min at 37°C with 250-rpm rotation to allow for phagocytosis. Eighty microliters of ice-cold 2% paraformaldehyde in GVB with EDTA was added to each well to terminate phagocytosis and allow for acquisition on a FACSCalibur flow cytometer. Opsonization was monitored as an increase in fluorescence intensity of the phagocytic cells.
Rodent model of S. epidermidis infection.
The rodent model was performed in accordance with the institutional policies of Inhibitex, Inc. Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Three- to 6-day-old newborn rats (7 to 11 g) were injected intraperitoneally (i.p.) with 1,000 mg/kg of body weight of INH-A21 (n = 11), 1,000 mg/kg of normal IGIV (Panglobulin) (n = 10), or an equal volume of buffer (n = 10). To prepare bacteria for challenge, 16 conical tubes, each containing 10 ml of tryptic soy broth, were inoculated with 100 μl of an overnight culture of S. epidermidis strain HB. The cultures were incubated at 37°C with shaking until mid-log phase. The cultures were centrifuged, and the pellets were washed once with 10 ml of sterile saline and then resuspended in 20 ml of tryptic soy broth. Twenty hours after antibody administration, the rats were challenged with an i.p. injection of 3.8 × 108 CFU, and survival was monitored for 7 days.
Rabbit model of IE.
The rabbit model was performed in accordance with the institutional policies of the Los Angeles Biomedical Research Institute at Harbor-UCLA. Female outbred New Zealand White rabbits (Irish Farms, Corona, CA), weighing 2.2 to 2.5 kg each, underwent carotid artery-to-left ventricle catheterization as previously described to induce sterile aortic valve vegetations (31). For studies of prophylaxis, animals were infused intravenously (i.v.) with 300 mg/kg of INH-A21 or 300 mg/kg of normal IGIV (Panglobulin) at 24 h postcatheterization. Twenty-four hours later, the rabbits were challenged with a 90% infective dose inoculum (as determined in pilot studies) of methicillin-resistant S. epidermidis (MRSE) ATCC 35984 (5.9 × 105 CFU) or MRSA 67-0 (5.8 × 105) by i.v. injection via the marginal ear vein. For therapeutic studies, the bacterial challenge (7.9 × 105 CFU MRSE ATCC 35984 or 7.6 × 105 CFU MRSA 67-0) was given 24 h postcatheterization. Antibody doses of 100, 200, or 300 mg/kg were administered i.v. 24 h after bacterial challenge. Previous findings with normal IGIV in pilot studies of established experimental endocarditis caused by the same MRSA 67-0 strain as used in the present study showed that normal IGIV had little impact on the disease outcomes. Thus, we utilized buffer as the control in the therapeutic experiments to simplify the animal experimentation. Rabbits were sacrificed, and the aortic valve vegetations, kidneys, and blood were harvested aseptically as the animals became moribund or at 72 h postchallenge. Only animals with correct transaortic valve catheter position and macroscopic vegetations were included in the final analyses. Samples were quantitatively cultured as previously described (29). Quantitative culture data (infection severities) were expressed as either log10 CFU/ml for blood cultures or log10 CFU/g tissue for cardiac vegetations and renal abscesses. The incidence of infection was defined as the percentage of tissue samples with culturable staphylococci. One sample of each tissue was collected per animal.
Statistics.
All statistical analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). For the rat neonate model, survival fractions were calculated using the product limit method (Kaplan-Meier), and the resulting curves were compared for significance using the Mantel-Haenszel log rank test. For the rabbit IE model, proportional data between groups (e.g., proportion of tissues rendered culture negative) were analyzed using Fisher's exact test. Continuous data (e.g., bacterial densities) were analyzed by Kruskal-Wallis analysis of variance with Dunn's post hoc correction for multiple comparisons between groups of three or more. For comparisons between two groups, a Mann-Whitney t test was performed. For all comparisons, a P value of <0.05 was considered statistically significant.
RESULTS
Inhibition of fibrinogen binding by INH-A21.
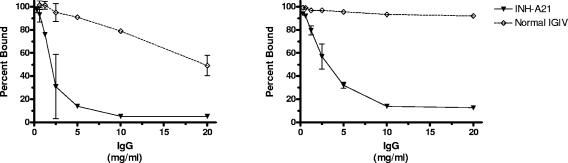
INH-A21 was produced to possess elevated titers of ClfA- and SdrG-specific antibodies compared to other normal IGIV products. An ELISA utilizing recombinant forms of ClfA and SdrG (Clf40 and SdrG-A) was used to determine if the increased titer in INH-A21 resulted in a greater capacity to inhibit the fibrinogen-binding activity of the MSCRAMM proteins. In Fig. 1, the inhibitory activity of INH-A21 was compared with a normal IGIV product (Panglobulin). At a concentration of 10 mg/ml, INH-A21 was able to inhibit Clf40 binding to human fibrinogen by 95%, whereas the same concentration of normal IGIV resulted in only 21% inhibition (Fig. 1A). Likewise, at a concentration of 10 mg/ml, INH-A21 inhibited SdrG-A binding to fibrinogen by 86% while normal IGIV only achieved 7% inhibition at that concentration (Fig. 1B). Similarly, INH-A21 was able to inhibit fibrinogen binding of both S. aureus and S. epidermidis in whole-bacteria adherence assays (data not shown).
FIG. 1.
INH-A21 IGIV inhibits fibrinogen-binding activity of Clf40 and SdrG-A. Microtiter plates were coated with Clf40 (left) or SdrG-A (right) recombinant proteins. Serial dilutions of INH-A21 IGIV (solid triangles) or normal IGIV (open diamonds) were incubated on the plates, and the capacity for Clf40 and SdrG-A to bind fibrinogen was then measured. The percentage of fibrinogen bound compared to controls lacking the antibody inhibitors is indicated. Symbols indicate the mean ± standard deviation of duplicate determinations.
Opsonic activity of INH-A21.
An OP assay was performed with fluorescently labeled S. aureus and S. epidermidis. The labeled bacteria were opsonized with increasing concentrations of INH-A21 or normal IGIV (Panglobulin) and incubated with the murine phagocytic cell line RAW 264.7. Phagocytosis of the fluorescent bacteria was monitored by flow cytometry. As shown in Fig. 2, opsonization by INH-A21 resulted in a concentration-dependent uptake of labeled bacteria by the phagocytes. The level of uptake observed with INH-A21 was consistently greater than the uptake achieved with identical concentrations of normal IGIV. Control experiments performed in the presence of the quenching agent, trypan blue, showed little reduction in overall fluorescence, indicating that the majority of bacteria associated with the RAW 264.7 cells were internalized (data not shown).
FIG. 2.
Opsonic activity of INH-A21 compared to normal IGIV. Fluorescently labeled bacteria were opsonized with increasing concentrations of either INH-A21 or normal IGIV. The opsonized bacteria were then incubated with the murine macrophage cell line RAW 264.7, and phagocytosis was monitored by flow cytometry. The percentage of fluorescent phagocytic cells observed at each antibody concentration is plotted in the graphs. Solid line and open triangles, opsonization with INH-A21; dashed line and filled circles, opsonization with normal IGIV. Symbols represent the means and standard deviations of duplicate determinations.
Protection against S. epidermidis infection in neonatal rats.
The protective efficacies of INH-A21 and normal IGIV were compared in a suckling rat model of S. epidermidis infection intended to emulate the immature immune system of a neonate. By day 3 after i.p. challenge, 100% of the animals receiving normal IGIV or buffer succumbed to the S. epidermidis infection (Fig. 3). In contrast, only 28% of the animals receiving INH-A21 had a lethal outcome by day 7. A single administration of INH-A21 provided significantly better protection in the neonatal rats than both normal IGIV (P = 0.0003) and buffer (P = 0.0002).
FIG. 3.
Protective activity of INH-A21 IGIV compared to normal IGIV in neonatal rats. Three- to 4-day-old Sprague-Dawley rats were injected i.p. with 1,000 mg/kg of INH-A21 (n = 11), 1,000 mg/kg of normal IGIV (n = 10), or an equal volume of buffer (n = 10). Animals were injected i.p. 20 h later with 3.8 × 108 S. epidermidis strain HB cells. Survival was recorded for 7 days. Survival fractions were calculated using Kaplan-Meier analysis, and the resulting curves were compared for significance using the Mantel-Haenszel log rank test of INH-A21 versus buffer (P = 0.0002) and INH-A21 versus normal IGIV (P = 0.0003).
Prophylactic efficacy against staphylococcal IE.
A rabbit model of IE was used to simulate clinical infections that include a central venous catheter, bacteremia, and hematogenous seeding to distal organs (3). INH-A21 (300 mg/kg) and normal IGIV (300 mg/kg) were each administered by a single intravenous injection to catheterized rabbits with sterile aortic valve vegetations, followed 24 h later by i.v. challenge with the heterologous staphylococcal strains MRSE ATCC 35984 or MRSA 67-0. Cardiac vegetations, kidneys, and blood were harvested 48 h after challenge. INH-A21 significantly reduced the frequency of staphylococcal infection in cardiac valve vegetations when animals were challenged with either MRSE ATCC 35984 (27% infected, P = 0.0094) (Fig. 4A) or MRSA 67-0 (7% infected, P = 0.0086) (Fig. 4B). By comparison, in groups given normal IGIV, 88% of MRSE-challenged animals (Fig. 4A) and 63% of MRSA-challenged rabbits (Fig. 4B) had infected valvular vegetations. Control animals challenged with MRSE also developed detectable bacteremia (Fig. 4A), whereas none of the animals receiving INH-A21 had detectable MRSE in their bloodstream (P = 0.0017). Regardless of the treatment group, rabbits that were infected with MRSA did not develop sustainable bacteremia (data not shown). We postulate that the infecting inoculum of MRSA used in the study (selected at the 90% infective dose level to reduce postchallenge mortality) was too low to maintain a detectable level of bacteremia. Hematogenous dissemination of staphylococci to the kidneys appeared to correlate with the incidence of bacteremia. Thus, 63% of the normal IGIV-treated rabbits infected with MRSE had detectable bacteria in their kidneys (Fig. 4A). In contrast, only 20% of the INH-A21-treated rabbits had detectable MRSE in their kidneys. Animals challenged with MRSA had low levels of hematogenous seeding, with 25% of the control group and 0% of the INH-A21-treated animals having detectable bacteria in their kidneys (Fig. 4B). To compare the number of CFU per gram recovered from tissues, samples with no detectable infection were assigned a value based on the limit of detection and the weight of the tissue sample. Using this method, the difference in bacterial density in vegetations infected with S. epidermidis was also found to be significantly different between the control and INH-A21-treated groups (P = 0.0007). To determine if the reduction in bacterial counts in the INH-A21-treated animals was simply due to a direct effect of the presence of the antibody, a control experiment was performed in which live S. aureus and S. epidermidis organisms were mixed with increasing concentrations of INH-A21 and incubated for 60 min at 37°C. A control run in the absence of INH-A21 was also prepared. The number of live bacteria remaining at the end of the incubation was then quantitated by plating. Incubation with INH-A21 had no effect on plating efficiency (data not shown).
FIG. 4.
Prophylactic efficacy of INH-A21 in a rabbit IE model. Female outbred New Zealand White rabbits were treated prophylactically with 300 mg/kg of INH-A21 (n = 15) or 300 mg/kg of normal IGIV (Panglobulin) (n = 8). Twenty-four hours later, the animals were challenged i.v. with either 5.9 ×105 CFU of MRSE 35984 (A) or 5.8 ×105 CFU of MRSA 67-0 (B). Forty-eight hours postchallenge, blood, cardiac vegetations, and kidneys were removed and cultured. Symbols indicate results from individual tissue samples. Horizontal lines indicate group medians. Detection limits in the blood samples were 1 colony/ml. Detection limits in the tissues were dependent on tissue weight and averaged 17 CFU/g for vegetations and 6 CFU/g for kidneys. Infection rates in tissues from INH-A21 treatment groups were significantly reduced in the following groups: cardiac vegetations (P = 0.0094); blood (P = 0.0017) (S. epidermidis challenge) (A); cardiac vegetations (S. aureus challenge) (P = 0.0086) (B). Bacterial densities were also significantly reduced compared to controls in cardiac vegetations (P = 0.007) of animals challenged with S. epidermidis (A). Significant P values are indicated on the graphs.
Therapeutic efficacy of INH-A21 in established IE.
The effect of treating established infections with INH-A21 was studied using the rabbit IE model. Aortic valve vegetations were induced by catheterization followed by bacterial challenge to produce IE. Twenty-four hours postchallenge, animals were treated i.v. with single INH-A21 doses of 100, 200, or 300 mg/kg. Control animals received mock injections of buffer. Treatment with 300 mg/kg INH-A21 resulted in dramatic reductions in MRSE densities in the blood (P < 0.01), kidney (P < 0.01), and cardiac valve vegetations (P < 0.001) compared to controls (Fig. 5). The incidence of MRSE bacteremia was also reduced in rabbits infused with 300 mg/kg compared to controls (P = 0.015). The two lower doses of INH-A21 produced modest reductions in the target tissues, although significant reductions were observed in the vegetations of the 200 mg/kg-treated cohort (P < 0.05).
FIG. 5.
Treatment of established S. epidermidis (MRSE) IE. New Zealand White rabbits were catheterized to induce cardiac vegetations and injected i.v. 24 h later with 7.9 ×105 CFU MRSE 35984. Twenty-four hours after challenge, the animals were treated with 100, 200, or 300 mg/kg INH-A21 or buffer control (buffer group, n = 5; all other groups, n = 6). Samples of vegetations, kidneys, and blood were harvested and cultured. Symbols indicate results from individual tissue samples. Horizontal lines indicate group medians. Bacterial counts were significantly reduced in blood and kidney samples at 300 mg/kg (P < 0.01). Bacterial density was significantly lower in cardiac vegetations at 200 mg/kg (P < 0.05) and 300 mg/kg (P < 0.001). The incidence of bacteremia was also significantly reduced in the 300 mg/kg cohort (P = 0.015). Significant P values are indicated on the graphs.
Experimental IE established with MRSA and treated with escalating concentrations of INH-A21 produced outcomes similar to the MRSE studies. As shown in Fig. 6, bacterial densities were significantly lower in the blood of animals treated with 200 and 300 mg/kg of INH-A21 (P < 0.05) than in controls. A significant reduction in the bacterial densities of cardiac vegetations was observed at 300 mg/kg INH-A21 (P < 0.05) and in kidneys at the 200 mg/kg INH-A21 dose (P < 0.05).
FIG. 6.
Treatment of established S. aureus (MRSA) IE. New Zealand White rabbits were catheterized to induce cardiac vegetations and injected i.v. 24 h later with 7.6 ×105 CFU MRSA 67-0. Twenty-four hours after challenge, the animals were treated with 100, 200, or 300 mg/kg INH-A21 or buffer control (buffer group, n = 4; all other groups, n = 6). Samples of vegetations, kidneys, and blood were harvested and cultured. Symbols indicate results from individual tissue samples. Horizontal lines indicate group medians. Bacterial counts were significantly reduced in blood samples at doses of 200 and 300 mg/kg (P < 0.05), in kidney samples of animals treated with 200 mg/kg (P < 0.05), and in cardiac vegetations of animals treated with 300 mg/kg (P < 0.05). Significant P values are indicated on the graphs.
DISCUSSION
Newborn infants, especially premature infants weighing ≤1,500 g, are highly susceptible to overwhelming, hospital-acquired bacterial and viral infections (27, 28). The risk of infection (especially endovascular) is increased by invasive procedures commonly used to treat complications of prematurity in the NICU, such as insertion of central venous catheters, peripheral catheters, mechanical ventilation, and total parenteral nutrition (27, 28). Major efforts in neonatal care in the early 1990s resulted in a decrease in morbidity and mortality; however, since 1995, no additional substantive improvements in outcomes have been made in the NICU (13). Because late-onset sepsis is a major contributor to both mortality and morbidity, a novel approach based on reducing the most prevalent causes of infection may have a profound beneficial outcome on the health of VLBW infants.
Replacement therapy with normal human IGIV has been successful in reducing the incidence and severity of acute infections in patients with inherited and acquired antibody deficiencies (25). Premature infants have low antibody levels due to insufficient transfer of maternal antibodies across the placenta before 32 weeks of gestation and because endogenous IgG synthesis does not begin until approximately 24 weeks after birth (2). Thus, prophylaxis with IGIV to raise serum IgG levels and to reduce the incidence of nosocomial infections in premature infants has been the subject of many studies. Clinical trials using normal IGIV in VLBW neonates, however, have produced mixed results. Baker and colleagues (1) conducted a randomized, double-blind, placebo-controlled clinical trial of IGIV in 588 neonates weighing from 500 to 1,750 g at birth. IGIV significantly reduced the risk of nosocomial infections by 30% compared to placebo recipients. The majority of confirmed bacterial infections were caused by coagulase-negative staphylococci and S. aureus. In contrast, Fanaroff and coworkers (8) randomized 2,416 neonates to receive a dose of 900 mg/kg IGIV for infants weighing 501 to 1,000 g at birth. In this study, prophylaxis with IGIV failed to reduce the incidence of nosocomial infections. In fact, meta-analysis of over 19 clinical studies indicated that normal IGIV provided only a marginal prophylactic benefit to the low-birth-weight neonate (14, 20).
Normal human IGIV contains a wide variety of antibodies, although since it is derived from healthy adults from the general population, the concentration of any one specific antibody is usually quite low (25). Although most clinical trials measured circulating IgG levels as surrogates for antibody activities, determinations of specific antibody activities in vivo were not generally performed. In retrospect, it is apparent that insufficient attention was given to measurements of protective antibody activities in IGIV preparations used in the clinical trials.
In an attempt to create an IGIV preparation with elevated levels of protective antibodies against both S. aureus and S. epidermidis (the most common sources of neonatal sepsis in VLBW infants), INH-A21 was prepared with plasma collected from donors with elevated antibody levels recognizing ClfA and SdrG. Fibrinogen binding mediated by ClfA and SdrG has been shown to be a key virulence factor for endovascular and biomaterial-related infections (4, 15, 23, 26). Inhibition of fibrinogen binding would likely interfere with the capacity of staphylococci to spread hematogenously and bind to sites of vascular damage. Indeed, polyclonal antibodies that recognize these proteins have previously been shown to be protective in animal models of staphylococcal infection (24, 29). In particular, an IGIV product with increased antibody titers recognizing ClfA has been shown to have therapeutic efficacy against MRSA infection in a rabbit IE model when used in combination with vancomycin (29). The data presented here demonstrate that a single IGIV preparation with increased titers against ClfA and SdrG is capable of providing both prophylactic and therapeutic protection against infection mediated not only by S. aureus but also against S. epidermidis. Moreover, the efficacy of this IGIV preparation against S. epidermidis was confirmed in two distinct models of vascular infection, neonatal rat sepsis and rabbit IE.
The biological mechanism(s) of action of INH-A21 most likely reflects both antiadhesion and opsonic properties of these antibodies. In an ELISA, INH-A21 substantially inhibited the fibrinogen-binding activity associated with recombinant forms of the ClfA and SdrG proteins. These antibodies may therefore inhibit the ability of staphylococci to colonize and replicate during the early course of infection and thereby reduce the bacterial burden and the overall severity of disease. This hypothesis is supported by data from the prophylactic and therapeutic studies of INH-A21 showing reductions in the number staphylococci recovered from the aortic vegetations as well as the kidneys. Additionally, antibodies present in INH-A21 were shown to induce opsonophagocytic uptake of both S. aureus and S. epidermidis by a murine phagocytic cell line in vitro. As a surrogate monitor of in vivo opsonic activity, the in vitro studies indicated that antibodies in INH-A21 can act as opsonins that would enable recognition and clearance of bacteria by phagocytic cells. The dramatic reduction in the quantity of MRSA and MRSE organisms in the bloodstream of the rabbits with established IE may reflect this opsonophagocytic activity of INH-A21 in vivo. However, in the present in vivo studies, it is not possible to distinguish whether the reduction in bacteria is due to prevention of seeding of the valve, postseeding clearance, more rapid intravascular clearing, and/or more rapid or extensive clearing by the reticuloendothelium system. It is possible that a composite of all these effects is involved.
The production of a specific immune globulin produced by screening donors for naturally occurring staphylococcal antibodies directed against MSCRAMM proteins may prove to be an essential intervention strategy in the prevention, and possibly the treatment, of serious staphylococcal infections. Recently, results of a multicenter phase II clinical trial for the prevention of nosocomial infections in very-low-birth-weight infants using a good manufacturing procedure-manufactured version of INH-A21 have been published (5). At a dose of 750 mg/kg, a potential to reduce sepsis caused by S. aureus, candidemia, and mortality was observed. Results from ongoing clinical trials will be required to confirm these observations and assess the potential efficacy of INH-A21 against staphylococcal diseases in humans.
Acknowledgments
We thank Teresa Coleman, Matthew Davis, Dawn Gast, Kimberly Lee, Meta-Jean Ruckstuhl, and Amy Schneider (Inhibitex, Inc.) and Yin Li Chia (Los Angeles Biomedical Research Institute) for their technical expertise and valuable input to these studies.
This work was funded entirely by Inhibitex, Inc.
REFERENCES
- 1.Baker, C., M. Melish, R. Hall, D. Casto, U. Vasan, L. Givner, and the Multicenter Group for the Study of Immune Globulin in Neonates. 1992. Intravenous Immune Globulin for the Prevention of Nosocomial Infection in Low-Birth-Weight Neonates. N. Engl. J. Med. 327:213-219. [DOI] [PubMed] [Google Scholar]
- 2.Baker, C. J., M. A. Rench, F. J. Noya, J. A. Garcia-Prats, and the Neonatal IVIG Study Group. 1990. Role of intravenous immunoglobulin in prevention of late-onset infection in low-birth-weight neonates. Rev. Infect. Dis. 12(Suppl. 4):S463-S469. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, A. S., C. Li, and M. Ing. 1998. Efficacy of trovafloxacin, a new quinolone antibiotic, in experimental staphylococcal endocarditis due to oxacillin-resistant strains. Antimicrob. Agents Chemother. 42:1837-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer, A. S., P. M. Sullam, M. Ramos, C. Li, A. L. Cheung, and M. R. Yeaman. 1995. Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect. Immun. 63:3634-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom, B., R. Schelonka, T. Kueser, W. Walker, E. Jung, D. Kaufman, K. Kesler, D. Roberson, J. Patti, and S. Hetherington. 2005. Multicenter study to assess safety and efficacy of INH-A21, a donor-selected human staphylococcal immunoglobulin, for prevention of nosocomial infections in very low birth weight infants. Pediatr. Infect. Dis. J. 24:858-866. [DOI] [PubMed] [Google Scholar]
- 6.Davis, S. L., S. Gurusiddappa, K. W. McCrea, S. Perkins, and M. Hook. 2001. SdrG, a fibrinogen-binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bbeta chain. J. Biol. Chem. 276:27799-27805. [DOI] [PubMed] [Google Scholar]
- 7.Dryla, A., S. Prustomersky, D. Gelbmann, M. Hanner, E. Bettinger, B. Kocsis, T. Kustos, T. Henics, A. Meinke, and E. Nagy. 2005. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin. Diagn. Lab. Immunol. 12:387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanaroff, A., S. Korones, L. Wright, et al. 1994. A controlled trial of intravenous immune globulin to reduce nosocomial infections in very-low-birth-weight neonates. N. Engl. J. Med. 330:1107-1113. [DOI] [PubMed] [Google Scholar]
- 9.Fischer, G. W. 1988. Therapeutic uses of intravenous gammaglobulin for pediatric infections. Pediatr. Clin. N. Am. 35:517-533. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, G. W. 1992. Uses of intravenous globulin to prevent or treat infections. Adv. Pediatr. Infect. Dis. 7:85-108. [PubMed] [Google Scholar]
- 11.Foster, T. J., and M. Höök. 1998. Surface proteins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 12.Foster, T. J., and D. McDevitt. 1994. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol. Lett. 118:199-205. [DOI] [PubMed] [Google Scholar]
- 13.Horbar, J. D., G. J. Badger, J. H. Carpenter, A. A. Fanaroff, S. Kilpatrick, M. LaCorte, R. Phibbs, and R. F. Soll. 2002. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics 110:143-151. [DOI] [PubMed] [Google Scholar]
- 14.Jenson, H., and B. Pollock. 1997. Meta-analysis of the effectiveness of intravenous immune globulin for prevention and treatment of neonatal sepsis. Pediatrics 99:1-11. [DOI] [PubMed] [Google Scholar]
- 15.Klug, D., F. Wallet, S. Kacet, and R. J. Courcol. 2003. Involvement of adherence and adhesion Staphylococcus epidermidis genes in pacemaker lead-associated infections. J. Clin. Microbiol. 41:3348-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin, J., B. Hamilton, P. Sutton, S. Ventura, F. Menacker, and M. Munson. 2003. Births: final data for 2002. Natl. Vital Stat. Rep. 52:1-113. [PubMed] [Google Scholar]
- 17.McCrea, K. W., O. Hartford, S. Davis, D. N. Eidhin, G. Lina, P. Speziale, T. J. Foster, and M. Hook. 2000. The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis. Microbiology 146:1535-1546. [DOI] [PubMed] [Google Scholar]
- 18.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1995. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol. Microbiol. 16:895-907. [DOI] [PubMed] [Google Scholar]
- 19.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 20.Ohlsson, A., and J. B. Lacy. 2004. Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants. Cochrane Database Syst. Rev. [Online.] doi: 10.1002/14651858.CD000361.pub2. [DOI] [PubMed]
- 21.Patti, J. M., B. L. Allen, M. J. Mcgavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Ann. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 22.Patti, J. M., and M. Hook. 1994. Microbial adhesins recognizing extracellular matrix macromolecules. Curr. Opin. Cell Biol. 6:752-758. [DOI] [PubMed] [Google Scholar]
- 23.Pei, L., and J. I. Flock. 2001. Lack of fbe, the gene for a fibrinogen-binding protein from Staphylococcus epidermidis, reduces its adherence to fibrinogen coated surfaces. Microb. Pathog. 31:185-193. [DOI] [PubMed] [Google Scholar]
- 24.Rennermalm, A., M. Nilsson, and J. I. Flock. 2004. The fibrinogen binding protein of Staphylococcus epidermidis is a target for opsonic antibodies. Infect. Immun. 72:3081-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiff, R. 1994. Intravenous gammaglobulin, 2: pharmacology, clinical uses and mechanisms of action. Pediatr. Allerg. Immunol. 5:127-156. [DOI] [PubMed] [Google Scholar]
- 26.Siboo, I. R., A. L. Cheung, A. S. Bayer, and P. M. Sullam. 2001. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoll, B. J., and N. Hansen. 2003. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin. Perinatol. 27:293-301. [DOI] [PubMed] [Google Scholar]
- 28.Stoll, B. J., N. Hansen, A. A. Fanaroff, L. L. Wright, W. A. Carlo, R. A. Ehrenkranz, J. A. Lemons, E. F. Donovan, A. R. Stark, J. E. Tyson, W. Oh, C. R. Bauer, S. B. Korones, S. Shankaran, A. R. Laptook, D. K. Stevenson, L. A. Papile, and W. K. Poole. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285-291. [DOI] [PubMed] [Google Scholar]
- 29.Vernachio, J., A. S. Bayer, T. Le, Y. L. Chai, B. Prater, A. Schneider, B. Ames, P. Syribeys, J. Robbins, and J. M. Patti. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 47:3400-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
- 31.Yeaman, M. R., J. Lee, and A. S. Bayer. 1999. Experimental candida endocarditis, p. 1709-1720. In O. Zak and M. A. Sande (ed.), Handbook of animal model infections. Academic Press, New York, N.Y.