Abstract
Recent pharmacokinetic studies that included children found that serum drug levels were low compared to those of adults for whom the same dosages were used. This study aimed to characterize the pharmacokinetics of pyrazinamide and ethambutol in Malawian children and to examine the impact of age, nutritional status, and human immunodeficiency virus (HIV) infection. We conducted a pharmacokinetic study of children treated for tuberculosis with thrice-weekly pyrazinamide (n = 27; mean age, 5.7 years) and of a separate group of children treated with thrice-weekly ethambutol (n = 18; mean age, 5.5 years) as portions of tablets according to national guidelines. Malnutrition and HIV infection were common in both groups. Blood samples were taken just prior to oral administration of the first dose, and subsequent samples were taken at intervals of 2, 3, 4, 7, 24, and 48 h after drug administration. Serum drug levels were low in all children for both drugs; in almost all cases, the maximum concentration of the drug in serum (Cmax) failed to reach the MIC for Mycobacterium tuberculosis. The Cmax of pyrazinamide was significantly lower in younger children (<5 years) than in older children. The Cmax of pyrazinamide was also lower for HIV-infected children and children with severe malnutrition, but these differences did not reach statistical significance. No differences were found for ethambutol in relation to age, HIV infection, or malnutrition, but the Cmax was <2 mg/liter in all cases. Studies of pharmacokinetic parameters and clinical outcomes obtained by using higher dosages of drugs for treatment of childhood tuberculosis are needed, and recommended dosages may need to be increased.
There are very few pharmacokinetic (PK) studies of antituberculosis (anti-TB) drugs in children (8). Dosages for children are based on weight and extrapolated from data from studies with adults, yet pharmacokinetics for children, especially young children, is likely to be different than for adults. Studies of ethambutol and pyrazinamide have found lower plasma drug levels and shorter half-lives in children than in adults using the same dosages, and the authors have suggested that dosages per kilogram of body weight need to be higher for children than for adults (36, 37). Similar conclusions were drawn from a recent study of isoniazid pharmacokinetics in South African children (29).
Until recently, the use of the same dosage recommendation as for adults may not have been an important issue, since studies that followed these schedules for children found that outcomes were very good and serious adverse events were rare (1, 2, 6, 33, 34). These data suggest that adequate levels of drug were being achieved within a range that was safe. Recent reports of outcomes for child TB, however, have found much poorer treatment response than earlier studies (5, 15, 20, 23). In these studies, human immunodeficiency virus (HIV) is the most important risk factor for poor treatment response. One reason for this may be malabsorption of oral anti-TB drugs by the HIV-infected individual, especially with advanced disease (10, 11, 26). There are no published reports on the effect of HIV on the pharmacokinetics of anti-TB drugs in children.
We aimed to characterize the pharmacokinetics of pyrazinamide and ethambutol in Malawian children treated with the standard recommended regimen for TB and to examine the impact of age, nutritional status, and HIV infection.
MATERIALS AND METHODS
Patient population.
Children admitted to Queen Elizabeth Central Hospital, Blantyre, Malawi, between August 2000 and February 2001 with a diagnosis of TB and scheduled to be started on anti-TB treatment were considered eligible and were included in the study following informed consent by their guardians. Baseline clinical data included demographic data, findings on history and examination, weight, and hematocrit. Investigations included the Mantoux test, radiography of the chest or spine when appropriate, sputum when available for smear and culture, and HIV status. Mantoux testing was performed using 0.1 ml (10 IU) of tuberculin purified protein derivative RT23 (1:1,000). Results were read between 48 and 72 h and recorded as the transverse diameter of palpable induration. An induration of 10 mm or more was regarded as positive irrespective of Mycobacterium bovis BCG or HIV status. An induration greater than 5 mm was also regarded as positive if the child was HIV infected. HIV testing using previously described methods (19) was undertaken on all children whose parents or guardians gave consent. Nutritional status was graded according to the Wellcome Classification on the basis of percentage of expected weight for age (WFA): >80% WFA was graded as normal, 60 to 80% WFA as undernutrition, and <60% WFA as marasmus. No children were receiving antiretroviral therapy at the time of enrollment, and no child had clinical evidence or a history of renal or liver disease.
Drug dosage and dosing regimen for pyrazinamide.
Pyrazinamide, in combination with isoniazid and rifampin, is recommended for the intensive phase of TB therapy in Malawi for all forms of TB (21, 35). The recommended dose for thrice-weekly therapy is 35 mg/kg of body weight, with a range of 30 to 40 mg/kg/dose. Liquid preparations are not available, and pyrizinamide (Pharmamed, Amsterdam, The Netherlands) was administered orally as 400-mg tablets or portions of 400-mg tablets three times per week, on Monday, Wednesday, and Friday, according to the recommended dosages for the children's weight ranges: 5 to 8.9 kg, 1/2 tablet; 9 to 14.9 kg, 1 tablet; 15 to 19.9 kg, 1 1/2 tablets; 20 to 24.9 kg, 2 tablets; and 25 to 39.9 kg, 3 tablets (21). Pyrazinamide was administered orally as the first dose of the initial phase of therapy at the same time each day, around 6 am, prior to breakfast. All patients received isoniazid and rifampin at the same time as pyrazinamide, but none received ethambutol.
Drug dosage and dosing regimen for ethambutol.
Ethambutol is recommended for the intensive phase of TB therapy in Malawi as one of a four-drug combination (R3H3Z3E3) for new cases of smear-positive pulmonary TB (PTB), cases of smear-negative PTB with extensive parenchymal involvement, and severe cases of extrapulmonary TB except for TB meningitis (21). The recommended dose for thrice-weekly therapy is 30 mg/kg, with a range of 25 to 35 mg/kg/dose (35). Ethambutol (Pharmamed, Amsterdam, The Netherlands) was administered orally as 400-mg tablets or portions of 400 mg tablets three times per week, on Monday, Wednesday, and Friday, according to the recommended dosages for the children's weight ranges: 5 to 8.9 kg, 1/2 tablet; 9 to 14.9 kg, 1 tablet; 15 to 24.9 kg, 1 1/2 tablets; and 25 to 34.9 kg, 2 tablets (21). Ethambutol was administered orally as the first dose of the initial phase of therapy at the same time as other prescribed anti-TB drugs, around 6 am, prior to breakfast. All patients receiving ethambutol also received isoniazid, pyrazinamide, and rifampin at the same time.
Sampling schedule.
Following informed consent, the study patient was admitted to the research ward, and an intravenous cannula was inserted for regular blood sampling. The first sample was taken just prior to oral administration (0 h) of the first dose of anti-TB therapy, and subsequent samples were taken at intervals of 2, 3, 4, 7, 24, and 48 h after drug administration. Breakfast of maize porridge and tea was usually consumed within 30 min of taking anti-TB medication, but the patient remained in bed. The 48-hour sample was taken before the administration of the next prescribed dose. Blood samples were allowed to clot, then centrifuged for 10 min, and serum was stored at −70°C. Specimens were transported at the completion of the study from Malawi to the University of Liverpool for assay. On completion of the 48-h sampling procedure, the study patient was transferred to the pediatric TB ward for ongoing management and education to encourage adherence.
Sample analysis for pyrazinamide.
Plasma pyrazinamide concentrations were determined by a fully validated high-performance liquid chromatography (HPLC) method with UV detection. Plasma samples (100 μl) were transferred to clean 1.5-ml microcentrifuge tubes followed by the addition of 200 μl of an internal standard (acetazolamide at 10 μg/ml in acetonitrile). After vortex mixing for 30 s, proteins were precipitated by centrifugation (10 min, 12,000 × g). The clear supernatant was transferred to a clean LSL tube and evaporated to dryness under a stream of nitrogen in a water bath at 37°C. Samples were reconstituted in mobile phase (300 μl) and were mixed by vortexing, and the contents were transferred to an autosampler vial. A 60-μl volume of sample was injected into the HPLC system. Chromatographic separation was achieved on a HyPurity C18 column (5 μm particle size; 150 by 4.6 mm diameter) (Thermo Electron Corporation, Runcorn, Cheshire, United Kingdom), protected by a LiChroCart precolumn guard using an isocratic mobile phase of water containing 0.06% trifluoroacetic acid and acetonitrile (95/9, vol/vol), at a flow rate of 1.2 ml/min. Analyte detection was performed on a Spectra 100 variable UV detector operating at 268 nM (Thermo Electron). The assay was linear in the range of 0 to 80 μg/ml, with a lower limit of detection of 100 ng/ml. Inter- and intraassay variabilities were less than 15%.
Sample analysis for ethambutol.
Plasma ethambutol concentrations were determined by a fully validated liquid chromatography-tandem mass spectrometry (LC-MS-MS) method. Plasma samples (200 μl) were transferred to clean 1.5-ml microcentrifuge tubes, followed by the addition of 200 μl of an internal standard (propanolol at 1 μg/ml in acetonitrile). Proteins were precipitated by the addition of 400 μl of acetonitrile followed by centrifugation (10 min, 12,000 × g). A 200-μl volume of supernatant was transferred to an autosample vial. A 2-μl volume of sample was injected into the HPLC-MS-MS system. Chromatographic separation was achieved on a HYPERSIL silica column (5 μm; 50 × 4.6 mm) (Thermo Electron Corporation, Runcorn, Cheshire, United Kingdom), protected by a precolumn guard (Si 60; 5 μm; Merck, Germany) using an isocratic mobile phase of 4 mM ammonium acetate and acetonitrile (20:80, vol/vol), at a flow rate of 0.4 ml/min. Analyte detection was performed on a TSQ7000 triple quad mass spectrometer operating in the MS-MS mode (Thermo Electron). For ethambutol, the daughter ion at 115.6 m/z produced from the parent ion at 205 m/z was used for quantitation. For the internal standard, the daughter ion at 116 m/z from the parent ion at 260 m/z was used for quantitation. The assay was linear in the range of 0 to 12.8 μg/ml, with a lower limit of detection of 100 ng/ml. Inter- and intraassay variabilities were less than 15%.
PK analysis.
The maximum concentration of the drug in serum (Cmax), the time to reach Cmax (Tmax), and the area under the concentration-time curve (AUC) were determined from the concentration-time profile of each patient by noncompartmental methods using the PK software package KINETICA (version 4.1.1; InnaPhase Corporation). AUC was estimated using the trapezoidal rule.
For pyrazinamide, concentration-time data were available for most of the children only up to 24 h and not beyond. Consequently, it is impossible to confidently estimate the terminal elimination phase, and estimations of the AUC to infinity produced an extrapolated AUC that was unacceptably large (more than 20% of the total AUC from 0 h to infinity). For this reason, the AUC to 24 h (AUC24) was determined, and we have not attempted to calculate the apparent clearance or elimination half-life (t1/2) for pyrazinamide. For 13 of the 18 children with ethambutol concentration-time profiles, we were able to define the terminal elimination phase, and detailed PK analysis has been restricted to this group.
Published MICs of pyrazinamide for drug-susceptible strains of Mycobacterium tuberculosis are 6 to 50 mg/liter (28), and those of ethambutol are 1.0 to 2.5 mg/liter (36). For intermittent dosing, reference cutoff points for Cmax are defined as low at 25 mg/liter and very low at 20 mg/liter for pyrazinamide and as low at 4 mg/liter for ethambutol (27, 36, 37).
Statistical analysis.
Data were analyzed using SPSS (version 11.0.0; SPSS Inc.). Comparisons of PK data were made using Mann-Whitney tests for HIV status, nutrition status, age, and reactivity to a tuberculin skin test (TST). Differences between groups were considered statistically significant at a Pvalue of <0.05.
Ethical approval.
The study was approved by the Research Ethics Committee, College of Medicine, University of Malawi.
RESULTS
Pyrazinamide results.
Twenty-seven children received thrice-weekly pyrazinamide as the first dose of the initial phase for TB treatment. Table 1 shows the clinical characteristics of these children. Of those with a TST result available, 4 of 18 HIV-infected children had a reactive TST compared to 2 of 7 who were not HIV infected (22% versus 29%). The mean WFA was 64% for HIV-infected children compared to 74% for non-HIV-infected children, and 63% for those with a nonreactive TST compared to 66% for those with a reactive TST.
TABLE 1.
Characteristics of study patients
| Clinical characteristic | Pyrazinamide (n = 27) | Ethambutol (n = 18) |
|---|---|---|
| Diagnosis (n) | ||
| Smear-negative PTB | 23 | 7 |
| TB adenitis | 4 | |
| Spinal TB | 5 | |
| Miliary TB | 4 | |
| Pericardial TB | 2 | |
| Mean age (range) (yr) | 5.7 (0.9-14) | 5.5 (1-12) |
| Mean wt (range) (kg) | 14.3 (6-30) | 14.8 (7-33) |
| Mean % WFA (range) | 67 (43-104) | 72 (56-102) |
| Mean dosage (range) (mg/kg) | 33 (25-48) | 33 (24-44) |
| Other TB drugs | Rifampin, isoniazid | Rifampin, isoniazid, pyrazinamide |
| TST result (n) | ||
| Reactive | 6 | 9 |
| Nonreactive | 19 | 6 |
| Not known | 2 | 3 |
| HIV status (n) | ||
| Infected | 18 | 6 |
| Noninfected | 9 | 12 |
Table 2 shows the results of analysis of the pyrazinamide pharmacokinetic profiles for the 27 study patients and the impact on pyrazinamide levels of HIV status, age, and nutritional status. The table also shows the Cmax and AUC24 normalized for the dose taken. The overall range for Cmax was wide (5.78 to 84.1 mg/liter), with 10 patients (37%) recording Cmax values below the low reference cutoff point of 25 mg/liter and 9 (33%) below the very low point of 20 mg/liter (27).
TABLE 2.
Results of pharmacokinetic analysis for pyrazinamidea
| Parameter | No. of patients | Cmax (mg/liter) | Tmax (h) | AUC24 (mg/liter/h) | Cmax/dose | AUC24/dose |
|---|---|---|---|---|---|---|
| All patients | 27 | 36.6 (19.7) | 3.4 (1.5) | 376 (328) | 1.1 (0.6) | 11.3 (10.0) |
| HIV status | ||||||
| Uninfected | 9 | 41.9 (22.9) | 2.9 (0.8) | 322 (240) | 1.2 (0.7) | 8.8 (5.6) |
| Infected | 18 | 34.0 (18.1) | 3.7 (1.7) | 411 (382) | 1.0 (0.5) | 13.1 (12.0) |
| Age | ||||||
| 0-4 yr | 15 | 27.5 (16.6)b | 3.5 (1.6) | 327 (335) | 0.9 (0.6)c | 11.2 (11.9) |
| ≥5 yr | 12 | 47.9 (17.7) | 3.3 (1.4) | 416 (333) | 1.3 (0.6) | 11.4 (8.8) |
| WFA | ||||||
| >80% | 6 | 44.3 (17.1) | 3.7 (1.8) | 496 (407) | 1.2 (0.5) | 13.4 (11.1) |
| 60-80% | 12 | 33.7 (23.2) | 3.4 (1.4) | 312 (261) | 1.1 (0.7) | 9.8 (7.8) |
| <60% | 9 | 35.4 (16.8) | 3.2 (1.6) | 337 (337) | 1.1 (0.5) | 11.2 (12.1) |
| TST result | ||||||
| Reactive | 5 | 48.6 (4.4)b | 2.8 (0.8) | 314 (214) | 1.3 (0.3) | 9.6 (8.4) |
| Nonreactive | 20 | 30.8 (18.2) | 3.6 (1.6) | 390 (378) | 1.0 (0.5) | 11.9 (11.3) |
All results are presented as means (standard deviations).
Statistically significant difference (P < 0.05).
P = 0.09.
Young age was associated with a significantly lower Cmax (Table 2), although when normalized for the dose given, the difference did not reach statistical significance (P = 0.06). Cmax values were also lower in HIV-infected children, but this difference was not significant. HIV prevalence was higher for children under the age of 5 years in this study than for older children: 13 of 15 (87%) compared to 5 of 12 (42%), respectively (P < 0.05). The mean (± standard deviation) dose received by the younger children was also lower (30.5 ± 5.4 mg/kg) than that for the older age group (35.0 ± 6.4 mg/kg), but the difference was not significant. The mean (± standard deviation) dose received by HIV-infected children was similar to that received by non-HIV-infected children (32 ± 6.3 mg/kg versus 34 ± 3.2 mg/kg, respectively). The Cmax was significantly higher in children with a reactive TST. The sample size was not large enough to examine the importance of HIV status as a confounder for young age or a nonreactive TST. Malnutrition was as common in older children as in younger children and was associated with lower values, but these were not significant.
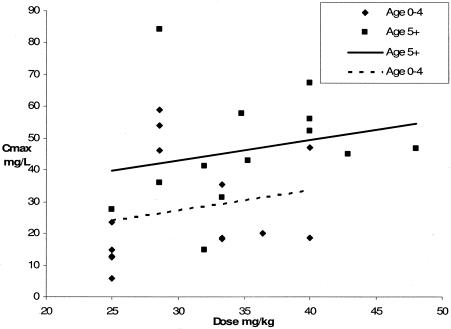
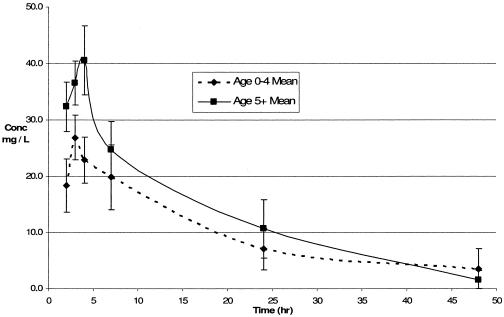
Figure 1 demonstrates the relationship between dosage in mg/kg of body weight and Cmax for both age groups. All five patients who received a dose of 25 mg/kg had admission weights of between 7.9 and 8.2 kg, for which the recommended dose is 1/2 tablet, or 200 mg. One child received a dose of 48 mg/kg, which is beyond the recommended range but in line with recommended dosing by tablets for weight groups: the child's weight was 25 kg, for which the recommended dose in Malawi is 3 tablets, or 1,200 mg. Both children who recorded Cmax values of more than 60 mg/liter were above the age of 5 years, not HIV infected, and not severely malnourished. Figure 2 shows comparisons of mean concentrations (± standard errors) with time in relation to age.
FIG. 1.
Cmax of pyrazinamide in relation to dose received. Data for children below the age of 5 years (n = 15) and for children 5 years old and older (n = 12) are compared.
FIG. 2.
Comparison of PK profiles for pyrazinamide in relation to age.
Ethambutol results.
Eighteen children received thrice-weekly ethambutol as part of the initial phase of treatment. Table 1 shows the clinical characteristics of these children. No child had a diagnosis of gastrointestinal or abdominal TB. All children received the recommended regimen for cases of severe PTB or extrapulmonary TB, R3H3Z3E3 (21). Of those who had a TST result available, 1 of 5 HIV-infected children had a reactive TST compared to 8 of 10 non-HIV-infected children. The mean WFA for HIV-infected children was 68%, compared to 75% for non-HIV-infected children. The mean WFA was the same (72%) for those with reactive or nonreactive TST.
Table 3 shows results of analysis of ethambutol pharmacokinetic profiles for 18 study patients and the impact of HIV, age, and nutritional status. The overall range for Cmax was wide (0.32 to 3.68 mg/liter), with all patients recording a Cmax below the low reference cutoff point for intermittent dosing of 4 mg/liter (36) and 11 (61%) recording a Cmax of <2 mg/liter. The range for Tmax was 2 to 7 h, and Tmax was 4 or 7 h for seven (39%) patients. No significant differences were recorded in Cmax and Tmax in relation to age, nutritional status, or TST result. Tmax was significantly later for HIV-negative children, though Cmax was the same. Three (43%) of the 7 children below the age of 5 years were HIV infected compared to 3 (27%) of the 11 older children (P = 0.6 by Fisher's exact test). The mean dose received by the younger children was the same (33 mg/kg) as that received by older children, and the mean dose received by HIV-infected children was similar to that received by non-HIV-infected children (32 mg/kg versus 34 mg/kg, respectively). Table 4 shows the volume of distribution and t1/2 data for 13 children. There were no significant differences recorded in these data in relation to age, nutritional, HIV or TST status.
TABLE 3.
Results of pharmacokinetic analysis for ethambutola
| Parameter | No. of patients | Cmax (mg/liter) | Tmax (h) | Cmax/dose |
|---|---|---|---|---|
| All patients | 18 | 1.8 (1.2) | 3.5 (1.8) | 0.04 (0.03) |
| HIV status | ||||
| Uninfected | 12 | 1.8 (1.1) | 4.2 (1.9)b | 0.04 (0.03) |
| Infected | 6 | 1.8 (1.3) | 2.2 (0.4) | 0.04 (0.03) |
| Age | ||||
| 0-4 yr | 7 | 1.8 (1.2) | 4.0 (2.2) | 0.04 (0.03) |
| ≥5 yr | 11 | 1.8 (1.2) | 3.2 (1.5) | 0.04 (0.03) |
| WFA | ||||
| >80% | 3 | 1.0 (0.9) | 4.3 (2.5) | 0.03 (0.03) |
| 60-80% | 14 | 1.9 (1.2) | 3.4 (1.7) | 0.05 (0.03) |
| <60% | 1 | 2.8 | 2.0 | 0.07 |
| TST result | ||||
| Reactive | 9 | 1.6 (1.2) | 4.3 (2.2) | 0.03 (0.03) |
| Nonreactive | 6 | 1.9 (1.2) | 2.7 (0.8) | 0.05 (0.03) |
All results are presented as means (standard deviations).
Statistically significant difference.
TABLE 4.
Volume of distribution and elimination half-life data for ethambutola
| Parameter | No. of patients | AUC∞ (mg/liter/h) | t1/2 (h) | Clearance (liters/h) | V (liters)b | AUC∞/dose |
|---|---|---|---|---|---|---|
| All patients | 13 | 22.2 (12.9) | 8.6 (4.7) | 49.0 (62.2) | 413.3 (304.3) | 0.53 (0.33) |
| HIV status | ||||||
| Uninfected | 9 | 23.8 (13.8) | 9.8 (4.8) | 34.0 (19.0) | 469.3 (351.8) | 0.57 (0.36) |
| Infected | 4 | 18.7 (11.5) | 6.0 (3.9) | 83.0 (110.8) | 287.2 (99.1) | 0.45 (0.29) |
| Age | ||||||
| 0-4 yr | 5 | 20.2 (18.9) | 6.3 (3.7) | 68.3 (101.6) | 234.6 (104.3) | 0.47 (0.45) |
| ≥5 yr | 8 | 23.5 (8.8) | 10.1 (4.9) | 37.0 (17.3) | 525.0 (339.8) | 0.58 (0.26) |
| WFA | ||||||
| ≥70% | 7 | 23.1 (9.8) | 9.7 (4.0) | 33.6 (14.2) | 510.6 (375.5) | 0.54 (0.27) |
| <70% | 6 | 21.3 (16.8) | 7.4 (5.6) | 67.0 (91.2) | 300.0 (156.1) | 0.53 (0.42) |
| TST | ||||||
| Reactive | 8 | 19.6 (16.1) | 7.4 (4.8) | 64.3 (76.7) | 457.2 (382.0) | 0.43 (0.37) |
| Nonreactive | 4 | 26.6 (3.82) | 11.1 (4.9) | 26.9 (9.5) | 382.0 (81.2) | 0.71 (0.20) |
All results are presented as means (standard deviations). No comparisons show significant differences.
V, volume of distribution.
DISCUSSION
This study provides original pharmacokinetic data for pyrazinamide and ethambutol in children. Recommended dosages for TB in children are from pharmacokinetic studies of adults, yet there are likely to be important age-related differences in drug absorption, metabolism, and clearance. We have found that serum drug levels achieved using intermittent pyrazinamide or ethambutol therapy at recommended doses are very low in Malawian children. Only 2 of 27 children in our study recorded a maximum serum pyrazinamide concentration above the median of 66 mg/liter recorded using intermittent dosing for North American adults and children (37). Both these children were 5 years old or older, not HIV infected, and not severely malnourished. For ethambutol, none of the children in our study recorded a maximum concentration in serum above what is considered the low cutoff point of 4 mg/liter for intermittent dosing from earlier studies of adults (36).
Studies have found lower concentrations and delayed absorption of anti-TB drugs in children compared to adults receiving the same dose (29, 36, 37). An important recent study of 64 South African children under the age of 13 years (median age, 3.8 years) found that younger children eliminate isoniazid faster than older children and that children require a higher mg/kg dose to achieve concentrations comparable to those for adults (29). An earlier study compared mean Cmax values among 28 European children of different age ranges who received 35 mg/kg ethambutol. That study found lower levels for the younger children—1.5 mg/liter for 2- to 5-year-olds compared to 2.3 mg/liter for 6- to 9-year-olds and 3.0 mg/liter for 10- to 14-year-olds (14). We did not find a similar trend with ethambutol.
We did find that younger children reached significantly lower serum pyrazinamide concentrations than older children. There may be confounders. Younger children in the pyrazinamide arm of the study had a significantly higher HIV prevalence and received lower mean drug dosages than older children. The sample size was not large enough to allow multivariate analysis. There is some evidence that adults with HIV/AIDS do not absorb some anti-TB drugs, especially rifampin, as well as non-HIV-infected patients (10, 11, 26). Absorption might be especially reduced in HIV-infected individuals with severe immunosuppression and HIV-related enteropathy, but we did not perform CD4 cell counts. Pyrazinamide and ethambutol levels were not significantly lower in HIV-infected or severely malnourished children in our study. A recent study of 48 HIV-infected adults with TB in the United States, 75% with a CD4 cell count of <200/mm3, found that adequate concentrations were achieved with intermittent dosing of pyrazinamide (27).
We compared pharmacokinetic parameters in relation to malnutrition. Levels of pyrazinamide but not ethambutol were lower for more malnourished children, but these differences did not reach the level of significance. Severe malnutrition was as common in older children as in younger children, and HIV prevalence was not significantly higher in children with marasmus. Previous studies of isoniazid and rifampin in children have been carried out to examine the impact of malnutrition and did not find a major effect (30-32).
It is known that reduced absorption occurs if the drugs are taken with a meal, especially a high-fat meal (24, 25). We are not sure what impact the taking of a low-fat meal around 30 min after the drugs had on absorption in our study group. The practice in this study was consistent with the usual practice in Malawian hospitals when anti-TB treatment is administered.
Important reasons for undertaking this study were the worsening outcomes for child TB in Malawi and elsewhere in the region and the relatively recent recommendation for ethambutol usage for all childhood age groups. The death rate for 2,739 Malawians treated for TB in 1998 was 17%, and the outcome was unknown for an additional 21% (13). Evidence from the region including Malawi suggests that coinfection with HIV is a major reason for the high death rate (15, 17, 18, 23). Malabsorption of anti-TB drugs may be one reason for poor outcomes for HIV-infected children, but there are likely to be others, such as wrong diagnosis (17), coinfection with other pathogens (4, 15), inadequate dosages received (12), and poorer compliance (9). Another factor contributing to poor outcome may be that ethambutol is not as effective as rifampin in the continuation phase of therapy (16). Ethambutol replaced thiacetazone in regions of HIV endemicity because thiacetazone caused severe and often fatal adverse reactions in HIV-infected adults and children (3, 22). There were concerns about the use of ethambutol for young children because of their inability to report the early symptoms of optic neuritis, the most important side effect, that can lead to blindness. This is a dose-related side effect, so this risk is considered negligible if recommended doses are used (7). Ethambutol is now recommended and commonly used for children of all ages in standard regimens (21, 35). This study suggests an important potential problem with ethambutol in that currently recommended doses result in inadequate therapeutic drug levels rather than any risk of toxicity.
In the majority of developing countries, where most childhood TB occurs, anti-TB therapy is available only in tablet form. This means that the same portions of tablets are given to all children within a particular age range (21). This is a potential problem, especially for children with low weights. For example, children weighing 5.0 kg and 8.9 kg receive the same dose. Figure 1 shows that all five patients between 7.9 and 8.2 kg (who received a recommended dose of 200 mg, or 25 mg/kg [21]) recorded maximum serum drug concentrations of <30 mg/liter. These recommendations may need to be revised.
We examined the relationship between a reactive TST and drug levels. This is because a reactive TST is likely to be a readily available surrogate marker for immunocompetence in regions where CD4 counts are not available. Although numbers were small, the maximum pyrazinamide concentration was significantly higher in children with reactive TSTs, but there was no difference for ethambutol. A nonreactive TST can be due to immunosuppression due to advanced HIV disease or severe malnutrition, but it may also indicate a wrong diagnosis. Therefore, the potential use of the TST result in determining drug dosage would be limited to those with a positive result.
In conclusion, this pharmacokinetic study has found poor absorption of pyrazinamide and ethambutol in Malawian children. It has also found that low serum drug levels are common using intermittent therapy at recommended doses and that young age is an important risk factor for low levels. Studies areneeded that compare pharmacokinetic parameters using higher doses and that measure the impact of higher doses on outcome, as well as the incidence of adverse reactions.
Acknowledgments
S.M.G. and D.J.B. are supported by the Wellcome Trust, United Kingdom, and drug analysis was performed under the Wellcome Trust-funded LOTLink award. We acknowledge the Research Development Fund of the University of Liverpool and S. B. Squire of the TB Knowledge Programme, Liverpool School of Tropical Medicine, for providing support for S.N. to receive training in drug analysis at the University of Liverpool.
We thank I. G. Edwards for assistance with proposal development.
There were no conflicts of interest.
REFERENCES
- 1.Al Dossary, F. S., L. T. Ong, A. G. Correa, and J. R. Starke. 2002. Treatment of childhood tuberculosis with a six month directly observed regimen of only two weeks of daily therapy. Pediatr. Infect. Dis. J. 21:91-97. [DOI] [PubMed] [Google Scholar]
- 2.Biddulph, J. 1990. Short course chemotherapy for childhood tuberculosis. Pediatr. Infect. Dis. J. 9:794-801. [DOI] [PubMed] [Google Scholar]
- 3.Chintu, C., C. Luo, G. Bhat, M. Raviglione, H. DuPont, and A. Zumla. 1993. Cutaneous hypersensitivity reactions due to thiacetazone in the treatment of tuberculosis in Zambian children infected with HIV-I. Arch. Dis. Child. 68:665-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chintu, C., V. Mudenda, S. Lucas, A. Nunn, K. Lishimpi, D. Maswahu, F. Kasolo, P. Mwaba, G. Bhat, H. Terunuma, and A. Zumla. 2002. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 360:985. [DOI] [PubMed] [Google Scholar]
- 5.Espinal, M. A., A. L. Reingold, G. Perez, E. Camilo, S. Soto, E. Cruz, N. Matos, and G. Gonzalez. 1996. Human immunodeficiency virus infection in children with tuberculosis in Santo Domingo, Dominican Republic: prevalence, clinical findings, and response to antituberculosis treatment. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:155-159. [DOI] [PubMed] [Google Scholar]
- 6.Gocmen, A., U. Ozcelic, N. Kiper, M. Toppare, S. Kaya, R. Cengizlier, and F. Cetinkaya. 1993. Short course intermittent chemotherapy in childhood tuberculosis. Infection 21:324-327. [DOI] [PubMed] [Google Scholar]
- 7.Graham, S. M., H. M. Daley, A. Banerjee, F. M. Salaniponi, and A. D. Harries. 1998. Ethambutol in tuberculosis: time to reconsider? Arch. Dis. Child. 79:274-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham, S. M., R. P. Gie, H. S. Schaaf, J. B. Coulter, M. A. Espinal, and N. Beyers. 2004. Childhood tuberculosis: clinical research needs. Int. J. Tuberc. Lung Dis. 8:648-657. [PubMed] [Google Scholar]
- 9.Graham, S. M., and A. D. Harries. 1999. Childhood TB/HIV co-infection: correction, confusion and compliance. Int. J. Tuberc. Lung Dis. 3:1144. [PubMed] [Google Scholar]
- 10.Gurumurthy, P., G. Ramachandran, A. K. Hemanth Kumar, S. Rajasekaran, C. Padmapriyadarsini, S. Swaminathan, S. Bhagavathy, P. Venkatesan, L. Sekar, A. Mahilmaran, N. Ravichandran, and P. Paramesh. 2004. Decreased bioavailability of rifampin and other antituberculosis drugs in patients with advanced human immunodeficiency virus disease. Antimicrob. Agents Chemother. 48:4473-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurumurthy, P., G. Ramachandran, A. K. Hemanth Kumar, S. Rajasekaran, C. Padmapriyadarsini, S. Swaminathan, P. Venkatesan, L. Sekar, S. Kumar, O. R. Krishnarajasekhar, and P. Paramesh. 2004. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin. Infect. Dis. 38:280-283. [DOI] [PubMed] [Google Scholar]
- 12.Harries, A. D., F. Gausi, and F. M. Salaniponi. 2004. Prescriptions and dosages of anti-tuberculosis drugs in the National Tuberculosis Control Programme of Malawi. Int. J. Tuberc. Lung Dis. 8:724-729. [PubMed] [Google Scholar]
- 13.Harries, A. D., N. J. Hargreaves, S. M. Graham, C. Mwansambo, P. Kazembe, R. L. Broadhead, D. Maher, and F. M. Salaniponi. 2002. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. Int. J. Tuberc. Lung Dis. 6:424-431. [PubMed] [Google Scholar]
- 14.Hussels, H., U. Kroening, and K. Magdorf. 1973. Ethambutol and rifampicin serum levels in children: second report on the combined administration of ethambutol and rifampicin. Pneumonologie 149:31-38. [DOI] [PubMed] [Google Scholar]
- 15.Jeena, P. M., P. Pillay, T. Pillay, and H. M. Coovadia. 2002. Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa. Int. J. Tuberc. Lung Dis. 6:672-678. [PubMed] [Google Scholar]
- 16.Jindani, A., A. J. Nunn, and D. A. Enarson. 2004. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet 364:1244-1251. [DOI] [PubMed] [Google Scholar]
- 17.Kiwanuka, J., S. M. Graham, J. B. Coulter, J. S. Gondwe, N. Chilewani, H. Carty, and C. A. Hart. 2001. Diagnosis of pulmonary tuberculosis in children in an HIV-endemic area, Malawi. Ann. Trop. Paediatr. 21:5-14. [PubMed] [Google Scholar]
- 18.Madhi, S. A., R. E. Huebner, L. Doedens, T. Aduc, D. Wesley, and P. A. Cooper. 2000. HIV-1 co-infection in children hospitalised with tuberculosis in South Africa. Int. J. Tuberc. Lung Dis. 4:448-454. [PubMed] [Google Scholar]
- 19.Molyneux, E. M., A. L. Walsh, H. Forsyth, M. Tembo, J. Mwenechanya, K. Kayira, L. Bwanaisa, A. Njobvu, S. Rogerson, and G. Malenga. 2002. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomised controlled trial. Lancet 360:211-218. [DOI] [PubMed] [Google Scholar]
- 20.Mukadi, Y. D., S. Z. Wiktor, I. M. Coulibaly, D. Coulibaly, A. Mbengue, A. M. Folquet, A. Ackah, M. Sassan-Morokro, D. Bonnard, C. Maurice, C. Nolan, J. K. Kreiss, and A. E. Greenberg. 1997. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Cote d'Ivoire. AIDS 11:1151-1158. [DOI] [PubMed] [Google Scholar]
- 21.National Tuberculosis Control Programme, Malawi. 2002. Manual of the National Tuberculosis Control Programme of Malawi. Ministry of Health and Population, Lilongwe, Malawi.
- 22.Nunn, P., D. Kibuga, S. Gathua, R. Brindle, A. Imalingat, K. Wasunna, S. Lucas, C. Gilks, M. Omwega, J. Were, et al. 1991. Cutaneous hypersensitivity reactions due to thiacetazone in HIV-1 seropositive patients treated for tuberculosis. Lancet 337:627-630. [DOI] [PubMed] [Google Scholar]
- 23.Palme, I. B., B. Gudetta, J. Bruchfeld, L. Muhe, and J. Giesecke. 2002. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr. Infect. Dis. J. 21:1053-1061. [DOI] [PubMed] [Google Scholar]
- 24.Peloquin, C. A., A. E. Bulpitt, G. S. Jaresko, R. W. Jelliffe, J. M. Childs, and D. E. Nix. 1999. Pharmacokinetics of ethambutol under fasting conditions, with food, and with antacids. Antimicrob. Agents Chemother. 43:568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peloquin, C. A., R. Namdar, M. D. Singleton, and D. E. Nix. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12-18. [DOI] [PubMed] [Google Scholar]
- 26.Peloquin, C. A., A. T. Nitta, W. J. Burman, K. F. Brudney, J. R. Miranda-Massari, M. E. McGuinness, S. E. Berning, and G. T. Gerena. 1996. Low antituberculosis drug concentrations in patients with AIDS. Ann. Pharmacother. 30:919-925. [DOI] [PubMed] [Google Scholar]
- 27.Perlman, D. C., Y. Segal, S. Rosenkranz, P. M. Rainey, C. A. Peloquin, R. P. Remmel, K. Chirgwin, N. Salomon, and R. Hafner. 2004. The clinical pharmacokinetics of pyrazinamide in HIV-infected persons with tuberculosis. Clin. Infect. Dis. 38:556-564. [DOI] [PubMed] [Google Scholar]
- 28.Salfinger, M., and L. B. Heifets. 1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob. Agents Chemother. 32:1002-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaaf, H. S., D. P. Parkin, H. I. Seifart, C. J. Werely, P. B. Hesseling, P. D. van Helden, J. S. Maritz, and P. R. Donald. 2005. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch. Dis. Child. 90:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifart, H. I., P. R. Donald, J. N. De Villiers, D. P. Parkin, and P. P. Jaarsveld. 1995. Isoniazid elimination kinetics in children with protein-energy malnutrition treated for tuberculous meningitis with a four-component antimicrobial regimen. Ann. Trop. Paediatr. 15:249-254. [DOI] [PubMed] [Google Scholar]
- 31.Seth, V., A. Beotra, A. Bagga, and S. Seth. 1992. Drug therapy in malnutrition. Indian Pediatr. 29:1341-1346. [PubMed] [Google Scholar]
- 32.Seth, V., A. Beotra, S. D. Seth, O. P. Semwal, S. Kabra, Y. Jain, and S. Mukhopadhya. 1993. Serum concentrations of rifampicin and isoniazid in tuberculosis. Indian Pediatr. 30:1091-1098. [PubMed] [Google Scholar]
- 33.Te Water Naude, J. M., P. R. Donald, G. D. Hussey, M. A. Kibel, A. Louw, D. R. Perkins, and H. S. Schaaf. 2000. Twice weekly vs. daily chemotherapy for childhood tuberculosis. Pediatr. Infect. Dis. J. 19:405-410. [DOI] [PubMed] [Google Scholar]
- 34.Tsakalidis, D., P. Pratsidou, A. Hitoglou-Makedou, G. Tzouvelekis, and I. Sofroniadis. 1992. Intensive short course chemotherapy for treatment of Greek children with tuberculosis. Pediatr. Infect. Dis. J. 11:1036-1042. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. 2003. Treatment of tuberculosis: guidelines for national programmes. World Health Organization, Geneva, Switzerland.
- 36.Zhu, M., W. J. Burman, J. R. Starke, J. J. Stambaugh, P. Steiner, A. E. Bulpitt, D. Ashkin, B. Auclair, S. E. Berning, R. W. Jelliffe, G. S. Jaresko, and C. A. Peloquin. 2004. Pharmacokinetics of ethambutol in children and adults with tuberculosis. Int. J. Tuberc. Lung Dis. 8:1360-1367. [PubMed] [Google Scholar]
- 37.Zhu, M., J. R. Starke, W. J. Burman, P. Steiner, J. J. Stambaugh, D. Ashkin, A. E. Bulpitt, S. E. Berning, and C. A. Peloquin. 2002. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy 22:686-695. [DOI] [PubMed] [Google Scholar]