Abstract
The administration of doxycycline prior to bleomycin in mice attenuated pulmonary fibrosis. Bronchoalveolar neutrophil influx and gelatinase activity, but not caseinolytic activity, were attenuated by doxycycline. Established fibrosis was not affected by doxycycline. Thus, doxycycline might be useful for slowing down pulmonary fibrosis by biological activity other than antibacterial activity.
Several antibiotics are known to exert biological effects other than by their own antibacterial activities. For instance, macrolides are known to have an inhibitory effect on inflammation by blocking interleukin-8 production in bronchial epithelial cells or activated neutrophils (1, 19). Tetracycline also has an anti-inflammatory effect via a decreased release of neutrophil chemotactic factors or reactive oxygen species (5, 14).
Several lines of evidence indicate that matrix metalloproteinases (MMPs) play a critical role in pulmonary fibrosis. MMP-9 activation was observed in the alveolar macrophages from pulmonary fibrosis patients (16) and in the bronchoalveolar lavage (BAL) fluids from bleomycin-induced pulmonary fibrosis (2, 4, 7, 12).
Doxycycline (DOXY) is an antibiotic widely used for attenuating MMP activity (8). However, it remains unknown as to whether DOXY influences pulmonary fibrosis, in spite of the wide usage of DOXY for the inducible overexpression of transgenes, such as the urokinase gene, in a pulmonary fibrosis study (23). We hypothesized that DOXY affects the development of pulmonary fibrosis through biological effects, such as gelatinase reduction. To confirm this hypothesis, the influence of DOXY was tested using a mouse model of pulmonary fibrosis induced by bleomycin.
Female C57BL/6 mice were intratracheally administered a single bleomycin dose of 1.0 U/ml (Pharmacia, Kalamazoo, MI) in 50 μl of sterile saline. DOXY was a kind gift from Pfizer Pharmaceutical Ltd. (Groton, CT). A 0.01-mg/ml dose of DOXY in drinking water, approximately 2 mg/kg, was given every day from 1 day before bleomycin administration to 28 days after. In another study, the start of DOXY administration was from 10 days after bleomycin. That study was approved by the Institutional Animal Care and Use Committee.
BAL was performed on day 7 after bleomycin instillation as previously described (9, 10). The lung sections on day 28 were stained with hematoxylin and eosin or Sirius red. Morphological evaluation of the lung sections was done by grade scoring on a scale of 0 (normal lung) to 8 (total fibrous obliteration of the field) as described elsewhere (24). A hydroxyproline assay or detection of the gelatinolytic or caseinolytic activity was performed as previously described (9, 10). Immunoblotting was done with gelatinase B (MMP-9) antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Reverse transcription-PCR was done using the primers of gelatinase B as follows: sense, 5′-GAGCACGGCAACGGAGAAGG-3′; antisense, 5′-CGAAGGGGAAGACGCACAGC-3′.
The data are expressed as the means ± standard errors. Analysis of variance was performed, followed by a post hoc analysis (Fisher PLSD test) to adjust for multiple comparisons. A P value of <0.05 was considered to indicate a significant difference.
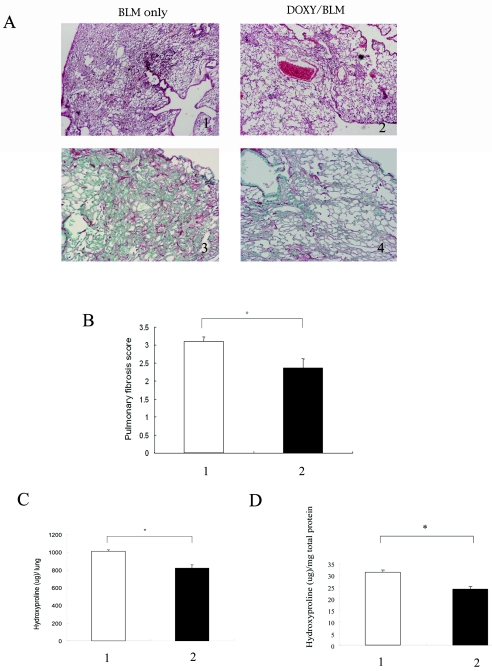
Bleomycin administration resulted in the development of extensive fibrosis areas in the lung on day 28 after bleomycin treatment. Collagen fiber accumulation was also confirmed by Sirius red staining (Fig. 1A). DOXY administration attenuated pulmonary fibrosis, which was confirmed by lung histology as well as by morphometry (Fig. 1A and B). DOXY administration caused a decrease both in the hydroxyproline content per total lung and in the hydroxyproline content per mg total protein (Fig. 1C).
FIG.1.
Effect of doxycycline on bleomycin-induced pulmonary fibrosis. At 28 days after bleomycin administration, the lungs were obtained from either the DOXY-treated mice or water-treated mice. (A) Lung histology. BLM only, lungs from the mice at day 28 after bleomycin administration with water; DOXY/BLM, lungs from the mice at day 28 after bleomycin with DOXY. 1 and 3, staining with hematoxylin and eosin (magnification, ×40); 2 and 4, Sirius red staining (magnification, ×100). (B) The pulmonary fibrosis score after DOXY administration. The pulmonary fibrosis score was measured by the method described in the text. Lane 1 (white bar), bleomycin with water; lane 2 (black bar), bleomycin with DOXY. The asterisk indicates a significant difference. (C and D) The hydroxyproline content after DOXY administration. The hydroxyproline content is expressed per lung (C) and per mg total protein (D). Lane 1, bleomycin with water; lane 2, bleomycin with DOXY. Each group consisted of five mice. The asterisk indicates a significant difference.
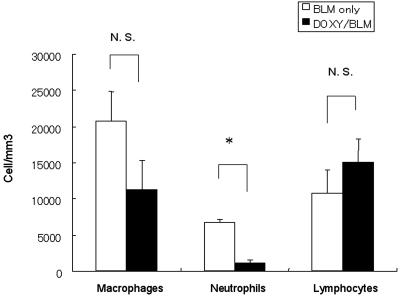
The BAL fluids on day 7 showed the number of neutrophils to have significantly decreased, although the total number of cells in the BAL fluids remained unchanged after DOXY administration (Fig. 2). Gelatinolytic and caseinolytic activities were evaluated using BAL fluids on day 7. DOXY administration reduced gelatinolytic activity (bleomycin only, 26.10 ± 2.79 arbitrary units; bleomycin with DOXY, 6.64 ± 0.42 arbitrary units; P < 0.01) but not the caseinolytic activity (bleomycin only, 7.68 ± 1.41 arbitrary units; bleomycin with DOXY, 9.01 ± 0.09 arbitrary units; P = 0.22) associated with the attenuation of bleomycin-induced pulmonary fibrosis (Fig. 3A and D).
FIG. 2.
Effect of doxycycline on neutrophil recruitment into the alveoli. The cell counts were determined in the BAL fluids from saline- or bleomycin-treated mice. A substantial neutrophil accumulation was observed in the untreated bleomycin instillation mice (white bar). This accumulation was significantly smaller in the DOXY-treated mice (black bar) than in the control mice. Each group consisted of four mice. The asterisk indicates a significant difference. N. S. indicates no significant difference.
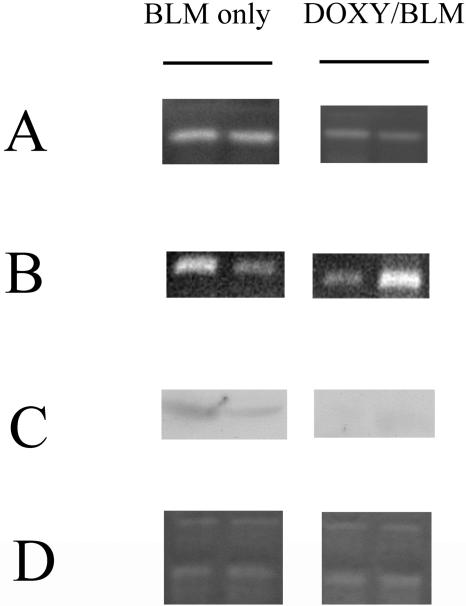
FIG. 3.
Effect of doxycycline on gelatinolytic activity and gelatinase B mRNA expression. (A) BAL fluids (40 μl) were analyzed by gelatin zymography. Left lane, the BAL fluid from bleomycin-treated mice on day 7; right lane, the BAL fluid from bleomycin-treated and DOXY-coadministered mice on day 7. Two samples from individual mice were shown in each lane. DOXY inhibited gelatinase. (B) Gelatinase B mRNA expression in the inflammatory cells from BAL fluids. Total RNA was extracted from the inflammatory cells of BAL fluids. The lanes were identical to the lanes in panel A. DOXY did not reduce the mRNA expression of gelatinase B. (C) Western blotting with anti-gelatinase B antibody. Proteins were extracted from inflammatory cells of BAL fluids. The lanes were identical to the lanes in panel A. DOXY reduced the production of gelatinase B. (D) The BAL fluid (40 μl) was analyzed by casein zymography. The lanes were identical to the lanes in panel A. DOXY did not inhibit the caseinolytic activity. Inhibition of gelatinase, mRNA expression, proteins of gelatinase B, and caseinolytic activity by DOXY were assessed by gel scanning densitometry as described in the text. Each group consisted of four mice.
RNA and protein were extracted from inflammatory cells, mainly macrophages, of BAL fluids after bleomycin administration with or without DOXY. Western blotting revealed that DOXY administration reduced the production of gelatinase B (bleomycin only, 19.17 ± 4.66 arbitrary units; bleomycin with DOXY, 5.32 ± 0.84 arbitrary units; P < 0.05) as shown in Fig. 3C. In contrast, reverse transcription-PCR analysis did not demonstrate any difference in gelatinase B mRNA expression between DOXY and the control group (bleomycin only, 44.7 ± 21.3 arbitrary units; bleomycin with DOXY, 77.6 ± 26.0 arbitrary units; P = 0.19) as shown in Fig. 3B. These results indicated that DOXY reduced protein synthesis after transcription or posttranslational modification. By this process, DOXY was able to decrease gelatinase levels in BAL fluids. Gelatinase could help induce pulmonary fibrosis by facilitating fibroblast migration into the interstitium of the lung (7). Therefore, reducing the amount of gelatinase might thus play a role in attenuating pulmonary fibrosis.
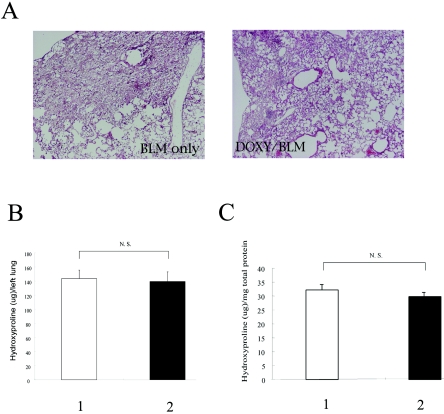
Another concern was raised regarding whether the later administration of DOXY could cause the degree of established fibrosis to decrease. DOXY was started from day 10 after bleomycin administration, when the inflammation induced by bleomycin began to subside (11). In this case, the right lungs were histologically examined, and the left lungs were evaluated for the presence of hydroxyproline. Lung histology was not affected by the late administration of DOXY as shown in Fig. 4. In addition, neither the hydroxyproline content in the left lungs nor the hydroxyproline per mg total protein changed after DOXY administration. Similar findings were observed in a macrolide study. In 14-membered ring macrolides, pretreatment was reported to attenuate pulmonary fibrosis more efficiently than posttreatment (17). Taken together, the control of inflammation induced by bleomycin could be one of the relevant factors for the attenuation of pulmonary fibrosis.
FIG. 4.
Effect of late administration of doxycycline on pulmonary fibrosis. (A) Lung histology. BLM only, lungs from the mice on day 28 after bleomycin administration with water; DOXY/BLM, lungs from the mice on day 28 after bleomycin with DOXY. (B and C) The hydroxyproline content after DOXY administration. Hydroxyproline content is expressed per left lung (B) and per mg total protein (C). Lane 1, bleomycin with water; lane 2, bleomycin with DOXY. Each group consisted of five mice. N. S. indicates no significant difference.
DOXY can also suppress inflammation, and alveolar inflammation is thought to underlie the development of pulmonary fibrosis in several forms of diffuse lung diseases (20). For instance, a decrease of tumor necrosis factor alpha production after lipopolysaccharide injection has been reported (22). Moreover, DOXY inhibits both the release of reactive oxygen species and apoptosis (12). In addition, DOXY decreases neutrophil chemotaxis (13). Reactive oxygen species, apoptosis, and neutrophil chemotaxis are known to contribute to the development of pulmonary fibrosis (3, 15, 21). Although some reports have documented that neutrophil depletion resulted in the progression of pulmonary fibrosis (6, 18), an inhibition of neutrophil recruitment has been reported to attenuate pulmonary fibrosis (3), similar to the findings observed in this study. We therefore consider DOXY to be a potentially useful therapeutic modality for pulmonary fibrosis in a clinical setting in spite of the limitations of such treatment against fibrogenesis.
Acknowledgments
We thank Pfizer Inc. for providing the kind gift of doxycycline. We also appreciate the assistance of Brian Quinn for editing the English usage.
REFERENCES
- 1.Abe, S., H. Nakamura, S. Inoue, H. Takeda, H. Saito, S. Kato, N. Mukaida, K. Matsushima, and H. Tomoike. 2001. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding sites in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 22:51-60. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, J. J., and R. M. Senior. 2003. Matrix metalloproteinase-9 in lung remodeling. Am. J. Respir. Cell Mol. Biol. 28:12-24. [DOI] [PubMed] [Google Scholar]
- 3.Azoulay, E., S. Herigault, M. Levame, L. Brochard, B. Schlemmer, A. Harf, and C. Delclaux. 2003. Effect of granulocyte colony-stimulating factor on bleomycin-induced acute lung injury and pulmonary fibrosis. Crit. Care Med. 31:1442-1448. [DOI] [PubMed] [Google Scholar]
- 4.Bakowska, J., and I. Y. Adamson. 1998. Collagenase and gelatinase activities in bronchoalveolar lavage fluids during bleomycin-induced lung injury. J. Pathol. 185:319-323. [DOI] [PubMed] [Google Scholar]
- 5.Carney, D., C. Lutz, A. Picone, L. Gatto, N. Ramamurthy, L. Golub, S. Simon, B. Searles, A. Paskanik, K. Snyder, C. Finck, H. Schiller, and G. Nieman. 1999. Matrix metalloproteinase inhibitor prevents acute lung injury after cardiopulmonary bypass. Circulation 100:400-406. [DOI] [PubMed] [Google Scholar]
- 6.Clark, J. G., and C. Kuhn III. 1982. Bleomycin-induced pulmonary fibrosis in hamsters: effect of neutrophil depletion on lung collagen synthesis. Am. Rev. Respir. Dis. 126:737-739. [DOI] [PubMed] [Google Scholar]
- 7.Corbel, M., S. Caulet-Maugendre, N. Germain, S. Molet, V. Lagente, and E. Boichot. 2001. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J. Pathol. 193:538-545. [DOI] [PubMed] [Google Scholar]
- 8.Curci, J., D. Mao, D. Bohner, B. Allen, B. Rubin, J. Reilly, G. Sicard, and R. Thompson. 2000. Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J. Vasc. Surg. 31:325-342. [DOI] [PubMed] [Google Scholar]
- 9.Fujita, M., J. M. Shannon, C. G. Irvin, K. A. Fagan, C. Cool, A. Augustin, and R. J. Mason. 2001. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L39-L49. [DOI] [PubMed] [Google Scholar]
- 10.Fujita, M., J. M. Shannon, O. Morikawa, J. Gauldie, N. Hara, and R. J. Mason. 2003. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am. J. Respir. Cell Mol. Biol. 29:669-676. [DOI] [PubMed] [Google Scholar]
- 11.Fujita, M., J. M. Shannon, H. Ouchi, D. R. Voelker, Y. Nakanishi, and R. J. Mason. 2005. Serum surfactant protein D is increased in acute and chronic inflammation in mice. Cytokine 31:25-33. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda, Y., M. Ishizaki, S. Kudoh, M. Kitaichi, and N. Yamanaka. 1998. Localization of matrix metalloproteinases-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab. Investig. 78:687-698. [PubMed] [Google Scholar]
- 13.Iwasaki, H., H. Inoue, Y. Mituke, A. Badran, S. Ikegaya, and T. Ueda. 2002. Doxycycline induces apoptosis by way of caspase-3 activation with inhibition of matrix metalloproteinase in human T-lymphoblastic leukemia CCRF-CEM cells. J. Lab. Clin. Med. 140:382-386. [DOI] [PubMed] [Google Scholar]
- 14.Jain, A., L. Sangal, E. Basal, G. P. Kaushal, and S. K. Agarwal. 2002. Anti-inflammatory effects of erythromycin and tetracycline on Propionibacterium acnes induced production of chemotactic factors and reactive oxygen species by human neutrophils. Dermatol. Online J. 8:2. [Online.] http: //dermatology.cdlib.org/DOJvol8num2/original/antibiotics2/jain.html. [PubMed] [Google Scholar]
- 15.Kuwano, K., N. Hagimoto, T. Maeyama, M. Fujita, M. Yoshimi, I. Inoshima, N. Nakashima, N. Hamada, K. Watanabe, and N. Hara. 2002. Mitochondria-mediated apoptosis of lung epithelial cells in idiopathic interstitial pneumonias. Lab. Investig. 82:1695-1706. [DOI] [PubMed] [Google Scholar]
- 16.Lemjabbar, H., P. Gosset, E. Lechapt-Zalcman, M. L. Franco-Montoya, B. Wallaert, A. Harf, and C. Lafuma. 1999. Overexpression of alveolar macrophage gelatinase B (MMP-9) in patients with idiopathic pulmonary fibrosis. Effect of steroid and immunosuppressive treatment. Am. J. Respir. Cell Mol. Biol. 20:903-913. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y., A. Azuma, S. Takahashi, J. Usuki, K. Matsuda, A. Aoyama, and S. Kudoh. 2002. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule 1 messenger RNA induction and leukocyte migration: role in preventing lung injury and fibrosis in bleomycin-challenged mice. Chest 122:2137-2145. [DOI] [PubMed] [Google Scholar]
- 18.Nettelbladt, O., K. Lundberg, A. Tengblad, and R. Hallgren. 1990. Accumulation of hyaluronan in bronchoalveolar lavage fluid is independent of iron-, complement- and granulocyte-depletion in bleomycin-induced alveolitis in the rat. Eur. Respir. J. 3:765-771. [PubMed] [Google Scholar]
- 19.Oishi, K., F. Sonoda, S. Kobayashi, A. Iwagaki, T. Nagatake, and K. Matsushima. 1994. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect. Immun. 62:4145-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens, G. R., I. L. Paradis, S. Gryzan, T. A. Medsger, Jr., W. P. Follansbee, H. A. Klein, and J. H. Dauber. 1986. Role of inflammation in the lung disease of systemic sclerosis: comparison with idiopathic pulmonary fibrosis. J. Lab. Clin. Med. 107:253-260. [PubMed] [Google Scholar]
- 21.Piguet, P. F., M. A. Collart, G. E. Grau, Y. Kapanci, and P. Vassalli. 1989. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J. Exp. Med. 170:655-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapira, L., V. Barak, W. A. Soskolne, A. Halabi, and A. Stabholz. 1998. Effects of tetracyclines on the pathologic activity of endotoxin: in vitro and in vivo studies. Adv. Dent. Res. 12:119-122. [DOI] [PubMed] [Google Scholar]
- 23.Sisson, T. H., K. E. Hanson, N. Subbotina, A. Patwardhan, N. Hattori, and R. H. Simon. 2002. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L1023-L1032. [DOI] [PubMed] [Google Scholar]
- 24.Tanino, Y., H. Makita, K. Miyamoto, T. Betsuyaku, Y. Ohtsuka, J. Nishihira, and M. Nishimura. 2002. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L156-L162. [DOI] [PubMed] [Google Scholar]