Abstract
Theiler's murine encephalomyelitis virus (TMEV) belongs to the family Picornaviridae and causes demyelinating disease in the spinal cords of infected mice. Although immune responses have been shown to play an important role in demyelination, the precise effector mechanism(s) is unknown. Potentially autoreactive cytotoxic cells could contribute to the destruction. We tested whether an autoreactive cell induced by TMEV infection mediated cytotoxicity by using a 5-h 51Cr release assay in SJL/J mice. Spleen cells from TMEV-infected mice were stimulated with irradiated TMEV antigen-presenting cells and used as effector cells. The effector cells differed from conventional cytotoxic T cells since these cells could kill both TMEV-infected and uninfected syngeneic or semisyngenic cell lines (PSJLSV and BxSF11gSV) but could not kill an allogeneic cell line (C57SV). The TMEV-induced autoreactive cells were also different from conventional natural killer (NK) cells or lymphokine-activated killer (LAK) cells, because they could kill neither NK cell-sensitive YAC-1 nor NK cell-resistant P815 and EL4 cells. Induction of autoreactive cells was not detected in vaccinia virus infection. The autoreactive killing required direct cell-to-cell contact and was mediated by a Fas-FasL pathway but not by a perforin pathway. The phenotype of the killer cells was CD3+ CD4− CD8+. Intracerebral inoculation of the effector cells into naive mice caused meningitis and perivascular cuffing not only in the brain parenchyma but also in the spinal cord, with no evidence of viral antigen-positive cells. This is the first report demonstrating that TMEV can induce autoreactive cytotoxic cells that induce central nervous system pathology.
Multiple sclerosis (MS) is the major cause of demyelinating disease of the central nervous system (CNS) in humans. Although the cause of MS is unknown, epidemiologic evidence points to an infectious etiology. Experimental viral animal models also support a viral hypothesis for MS, where inflammatory demyelination is a prominent feature of persistent viral infection (18). These experimental models include Theiler's murine encephalomyelitis virus (TMEV) infection of mice, its natural host (9, 51, 52).
TMEV belongs to the family Picornaviridae and causes extensive demyelinating disease in the spinal cords of the infected mice (51, 52). Although the precise mechanism(s) of demyelination in TMEV infection is not clear, demyelination can result either from direct viral infection of oligodendrocytes or myelin-forming cells or from immune mediated mechanisms. For a hypothesis of immune mediation, several effector mechanisms have been proposed, including delayed-type hypersensitivity responses initiated by TMEV-specific CD4+ Th1 cells and anti-TMEV antibody responses cross-reactive with the myelin component galactocerebroside (reviewed in references 51 and 52). While CD8+ T cells have been demonstrated to be important in viral clearance (29, 30), CD8+ T cells may also be critical effector cells during the chronic TMEV-induced demyelinating phase of infection. Several reports have provided evidence showing parenchymal infiltration of CD8+ cells in demyelinating lesions, diminution of demyelination in CD8-depleted mice infected with TMEV, and an upregulation of major histocompatibility complex (MHC) class I molecules in the CNS in TMEV-infected mice (reviewed in reference 9).
Organ-specific autoimmune diseases have been associated with MHC class II-restricted Th1 type responses. A prototype model for CNS organ-specific autoimmune disease is experimental allergic (autoimmune) encephalomyelitis (EAE), another animal model for MS. To date only MHC class II-restricted CD4+ T-cell responses have been extensively investigated both in MS and its animal models, although clinical and experimental evidence indicates that MHC class I-restricted CD8+ T-cell responses could also be involved in the pathogenesis of demyelinating disease (reviewed in reference 56). CD8+ cytotoxic T lymphocytes (CTLs) have been associated with only a few autoimmune diseases, such as polymyositis, inclusion body myositis (33), and experimental myocarditis induced by coxsackievirus B3, which is also a member of the family Picornaviridae (12, 16).
T-cell cytotoxicity is one mechanism that could result in primary oligodendrocyte destruction during demyelinating disease. In both MS and TMEV infection, apoptosis of oligodendrocytes has been demonstrated (7, 51, 52). However, reports of CTLs with autoreactivity have been rare. Encephalitogenic myelin basic protein-specific CD4+ T cells having in vitro cytotoxic activity against oligodendrocytes, myelin basic protein-pulsed astrocytes, macrophages and cerebral vascular endothelial cells have been observed (22; reviewed in reference 40). Since these CD4+ T cells are restricted by MHC class II antigens, with the exception of bystander killing, MHC class II expression by oligodendrocytes would be a prerequisite for oligodendrocyte-directed cytotoxicity. However, under in vitro conditions, oligodendrocytes can be induced by gamma interferon (IFN-γ) to express MHC class I antigen but are refractory to class II induction (13). Therefore, since conventional CTL responses are mediated by MHC class I-restricted CD8+ T cells, it would not be surprising if CD8+ autoreactive T cells cytotoxic for oligodendrocytes could contribute to immune-mediated demyelination. To date, Biddison and colleagues have suggested that this could occur in MS (15, 50).
We undertook to study cell-mediated cytotoxic responses in SJL/J mice, which are genetically susceptible to TMEV-induced demyelination. Unexpectedly, we found that spleen cells from TMEV-infected mice exhibited a high level of cytotoxicity against uninfected syngeneic target cells, but not against allogeneic cells, after in vitro stimulation with TMEV-infected antigen-presenting cells (TMEV-APCs). The autoreactive cytotoxic cells were CD3+ CD8+ and were different from conventional natural killer (NK) cells or lymphokine-activated killer (LAK) cells. Cell lysis required direct cell-to-cell contact and was mediated by the Fas-FasL pathway. Adoptive transfer of TMEV-induced autoreactive cells into the brains of naive mice resulted in lesions in the spinal cord. Therefore, these TMEV-induced autoreactive cells could play an effector role in CNS pathology in vivo.
MATERIALS AND METHODS
Generation of effector cells.
Female SJL/J mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Mice were infected intracerebrally (i.c.) (2 × 105 PFU) or intravenously (i.v.) (1 × 106 PFU) with the DA strain of TMEV. In most experiments autoreactive cells were induced by the i.v. route of infection. Three weeks later, spleen cells were isolated from the mice and treated with an NH4Cl red blood cell-lysing solution. The spleen cells from infected or control mice were stimulated in vitro with TMEV-APCs for 6 days. TMEV-APCs were made from whole spleen cells infected with DA virus at a multiplicity of infection (MOI) of 1 and irradiated with 2,000 rads by using a 137Cs irradiator. After in vitro stimulation, the cells were washed three times and used as effector cells in 5-h short-term 51Cr release assays. For induction of vaccinia virus-specific CTLs, mice were inoculated i.v. with 5 × 106 PFU of VVsc11 virus, a recombinant vaccinia virus encoding β-galactosidase (3).
Target cells.
Three simian virus 40-transformed murine fibroblast cell lines were used as target cells in the CTL assays. C57SV (H2b), PSJLSV (PSJL) (H2s), and BxSF11gSV (BxSF1) (H2b/s) were kindly provided by Barbara Knowles (Jackson Laboratory) (23, 28, 29). TMEV-infected and uninfected cell lines used in these experiments express MHC class I antigens but do not express detectable levels of MHC class II antigens by immunoperoxidase staining for class II (30). MHC class I expression on the cell lines was verified by flow cytometry with a monoclonal antibody that recognizes MHC class I molecules, M1/42 (44, 48). The M1/42 cell line was obtained from the American Type Culture Collection (Manassas, Va.). In some experiments, cells were infected with DA virus at an MOI of 5 for 18 to 24 h (DAL-PSJL) or were pulsed at an MOI of 10 either with DA virus (DAs-PSJL) or with VVsc11 virus (VV-PSJL) during the 51Cr loading of the target cells. We also transfected the PSJL cell line with cDNAs encoding the DA virus capsid proteins, VP1, VP2, VP3, and VP4, and designated them PSJL-VP1, PSJL-VP2, PSJL-VP3, and PSJL-VP4, respectively.
51Cr release assay.
Target cell lysis was assessed by a 51Cr release assay as described previously (54). Target cells were labeled with Na251CrO4 (New Life Science Products, Inc., Boston, Mass.) for 1 h. The target cells (104) were placed in wells of 96-well round-bottomed microtiter plates. Effector cells were used at a concentration of 106/100 μl, and threefold serial dilutions were made to provide effector-to-target cell (E/T) ratios of 100:1, 33:1, 11:1, and 3:1. After a 5-h incubation, the radioactivity released from the cells was measured in a model 20/20 gamma counter (Iso-Data, Inc., Palatine, Ill.). The percentage of target cell lysis was calculated by using the following equation: % lysis = [(experimental release cpm − spontaneous release cpm)/(maximal release cpm − spontaneous release cpm)] × 100.
In cold target inhibition experiments, 106 effector cells were incubated for 5 h with 104 51Cr-labeled PSJL cells in the presence of various numbers of nonlabeled cold inhibitor PSJL, BxSF1, C57SV, or EL4 cells (37).
To analyze a pathway for cell lysis, effector cells were preincubated with either brefeldin A (PharMingen, San Diego, Calif.) or concanamycin A (Sigma, St. Louis, Mo.) as an inhibitor for a Fas-FasL or a perforin pathway, respectively (11, 21, 26). A negative control group was incubated with dimethyl sulfoxide, since both brefeldin A and concanamycin A were dissolved in dimethyl sulfoxide as stock solutions. After a 2-h incubation, effector cells were added to the 51Cr-labeled PSJL target cells. Cells were treated with 0.016 to 10 μg of brefeldin A per ml, and MHC class I expression was measured by flow cytometry with the anti-MHC class I antibody M1/42. No decrease in MHC class I expression was observed over this concentration range of brefeldin A.
Assay for NK cell activity.
Since SJL/J mice are known to have a “low” NK phenotype (20), we induced NK cells in BALB/c mice, either in vitro or in vivo, using poly(I:C) (Sigma). Poly(I:C) was dissolved in Hanks' balanced salt solution at a concentration of 1 mg/ml and stored at −20°C. Mice were injected intraperitoneally with 100 μl of poly(I:C) (1 mg/ml) 1 day prior to spleen cell harvests (20). Spleen cells were isolated from poly(I:C)-injected mice, washed, and used as effector NK cells. Alternatively, spleen cells were isolated from naive mice, and 108 spleen cells were stimulated overnight with 100 μg of poly(I:C) per ml in 1 ml of RPMI 1640 medium supplemented with antibiotics, 1% fetal bovine serum, and 20 mM HEPES (6, 58). LAK cells were induced from the spleens of SJL/J mice as described previously (24). The spleen cells were isolated and cultured at 2 × 106 cells/ml with 1,000 U of interleukin-2 (IL-2) per ml for 6 days. Bacterial DNA containing CpG motifs was also used for induction of NK cell activity in SJL/J mice as described previously (54). We incubated spleen cells at a concentration of 2 × 106 cells/ml with 5 μg of plasmid pCMV DNA (Clontech, Palo Alto, Calif.) per ml for 6 days.
The NK cell-sensitive YAC-1 and NK cell-resistant P815 cell lines were kindly provided by Raymond M. Welsh (Department of Pathology, University of Massachusetts Medical Center, Worcester). NK cell-resistant EL4 cells were purchased from the American Type Culture Collection. At the indicated E/T ratios, NK cells with YAC-1, P815, or EL4 target cells were incubated in triplicate for 5 h.
Double-chamber experiments.
Double-chamber experiments were carried out using Transwell-Clear tissue culture-treated polyester membrane chambers (pore size, 0.4 μm; Costar, Cambridge, Mass.) (55). Effector cells (4 × 106) were placed in the lower compartment of the Transwell chamber (i.e., in the main well) in a total volume of 0.6 ml. 51Cr-labeled PSJL cells (4 × 104) in 0.1 ml were placed in the upper compartment of the Transwell chamber. Five hours after culture, the total culture mixture of 0.7 ml was collected and centrifuged; the radioactivity in 0.3 ml of supernatant was counted, and the percent specific lysis was calculated.
Phenotypic characterization of the autoreactive cell.
The blocking analyses were carried out at an E/T ratio of 100 in the presence of anti-CD3, anti-CD4, or anti-CD8 antibody. Both anti-CD4 antibody (GK1.5) and anti-CD8 antibody (2.43) are blocking antibodies (5, 30, 43), while the anti-CD3 antibody, (145-2C11) has been reported to either block or enhance cytotoxicity when used in cytotoxicity assays (17, 25). TMEV-induced autoreactive cells were preincubated with monoclonal antibody or medium alone for 30 min, after which 51Cr-labeled PSJL target cells were added. We incubated the cultures for 5 h and determined the amount of 51Cr released into the supernatant.
In additional experiments, anti-CD4 or -CD8 antibody and LOW-TOX-M rabbit complement (Cedarlane, Hornby, Ontario, Canada) were added to effector cells to deplete T-cell subsets from the effector cell population. CD3+ T cells were enriched or purged with either a murine CD3+-T-cell enrichment cocktail or a murine CD3+-T-cell purging cocktail, respectively, according to the protocols of the manufacturer (StemCell Technologies, Vancouver, British Columbia, Canada).
Adoptive transfer of autoreactive cells.
We induced autoreactive cells from spleens of TMEV-infected mice, which was followed by in vitro stimulation with TMEV-APCs. Cytotoxic activity of the transferred cells for syngeneic cells was confirmed by using a 51Cr assay, and no live virus was detected in the inocula by viral plaque assay (the lower limit of the viral plaque assay is 5 PFU/ml). As a negative control, we used either normal spleen cells or in vitro-TMEV-APC-stimulated spleen cells from mock-infected mice. To five naive mice in each group, 2 × 107 cells i.v. or 2 × 106 cells i.c. at the right cerebral hemisphere were adoptively transferred. Mice were weighed daily, killed 6 or 10 days after cell transfer, and perfused with 4% paraformaldehyde. Brains and spinal cords were embedded in paraffin, and CNS sections were stained with Luxol fast blue. TMEV antigen-positive cells were detected by the avidin-biotin peroxidase complex technique with hyperimmune rabbit serum to DA virus (53, 54).
RESULTS
Autoreactive cytotoxicity correlates with H2 haplotype.
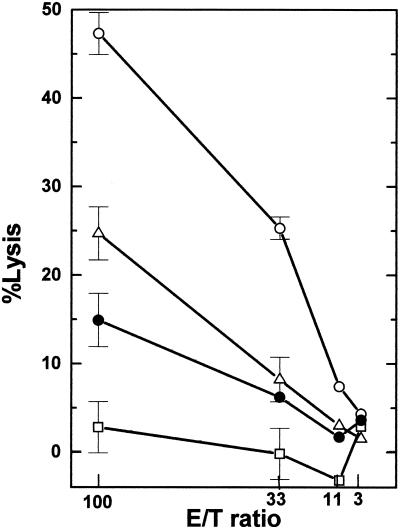
Autoreactive cytotoxic cells were induced in TMEV-infected SJL/J mice, which are susceptible to TMEV-induced demyelinating disease. Spleen cells isolated from TMEV-infected mice were stimulated in vitro with TMEV-APCs for 6 days and used as effector cells in a 5-h short-term 51Cr release assay. Using syngeneic and allogeneic target cells, we first tested whether the TMEV-induced autoreactive cells were different from classical CTLs. Three murine fibroblast cell lines were used: a syngeneic cell line, PSJL (H2s), derived from SJL/J mice; an allogeneic cell line, C57SV (H2b), derived from C57BL/6/J mice; and BxSF1 (H2b/s), derived from an F1 mouse resulting from a cross between C57BL/6 and SJL/J mice. All target cells were uninfected. The largest amount of killing was observed with syngeneic cells, and the degree of the killing correlated with the H2 haplotype of the target (Fig. 1). If the killer cells were classical virus-specific CTLs, they would not be expected to kill naive uninfected syngeneic cells. A primary SJL/J astrocyte cell line that was also used as a target cell was killed the least. However, if the astrocytes were treated for 48 h with IFN-γ, killing was enhanced by greater than 80% (data not shown). Incubation of target cells with IFN-γ also significantly increased the killing of BxSF1 cells (108%) but had only a modest effect on the killing of C57SV cells (27%) (data not shown).
FIG. 1.
TMEV-induced cytotoxic responses against various cell lines. Three weeks after TMEV infection, spleen cells were isolated from SJL/J mice, incubated with TMEV-APCs for 6 days, and used as effector cells in a 51Cr release assay. As target cells, three mouse fibroblast cell lines were used: syngeneic PSJL (H2s) (○), BxSF1 (H2b/s) (▵), and allogeneic target C57SV (H2b) (•). PSJL and C57SV cells were derived from SJL/J mice and C57BL mice, respectively. BxSF1 cells were derived from F1 mice resulting from a cross between SJL/J and C57BL mice. We detected high and moderate autoreactive cytotoxic responses against PSJL and BxSF1 cells but much lower cytotoxicity against C57SV cells. An astrocyte cell line (□) was also used as a target and was killed the least. Results are representative of those from 10 independent experiments. Error bars indicate standard errors.
Killing does not require recognition of TMEV antigen.
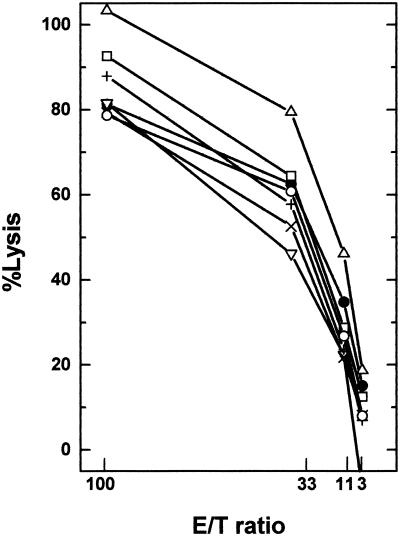
CD4+ and CD8+ T-cell and B-cell epitopes are encoded within the capsid proteins of TMEV and play important roles in the pathogenesis of TMEV-induced demyelinating disease (49, 52). Therefore, we hypothesized that the putative epitope on uninfected PSJL cells that is recognized by TMEV-induced autoreactive cells could be identical to or have molecular mimicry with the TMEV-encoded proteins, particularly the capsid proteins. If this were the case, TMEV-infected or TMEV capsid-expressing cells may be more efficiently killed than uninfected cells. As targets, we used uninfected PSJL cells, PSJL cells pulsed with DA virus at an MOI of 10 (DAs-PSJL), PSJL cells infected with DA virus at an MOI of 5 for 18 to 24 h (DAL-PSJL), and PSJL cells expressing the VP1, VP2, VP3, and VP4 capsid proteins of TMEV (PSJL-VP1, PSJL-VP2, PSJL-VP3, and PSJL-VP4, respectively). TMEV-induced autoreactive cytotoxic cells were used as effector cells in a 51Cr release assay. Although TMEV-infected target cells showed slightly higher killing, uninfected PSJL cells showed a similarly high level of lysis as the other cell lines (Fig. 2). These data suggest that the TMEV-induced autoreactive cells do not recognize TMEV antigen for killing and therefore differ from conventional virus-specific CTLs.
FIG. 2.
Cytotoxicity against target cells that express TMEV antigens. In a 51Cr release assay, we used TMEV-induced autoreactive cells as effector cells. As target cells, we used uninfected PSJL cells (•), PSJL cells pulsed with TMEV at an MOI of 10 (DAs-PSJL) (▵), PSJL cells infected with DA virus at an MOI of 5 for 18 to 24 h (DAL-PSJL) (□), and PSJL cell lines transfected with TMEV capsid proteins VP1, VP2, VP3, and VP4 (PSJL-VP1 [▿], PSJL-VP2 [×], PSJL-VP3 [+], and PSJL-VP4 [○], respectively). Although TMEV-infected targets showed slightly higher killing, uninfected PSJL cells showed killing similar to that of other cell lines. Results are representative of those from four independent experiments.
Induction of autoreactive cells is specific for TMEV infection.
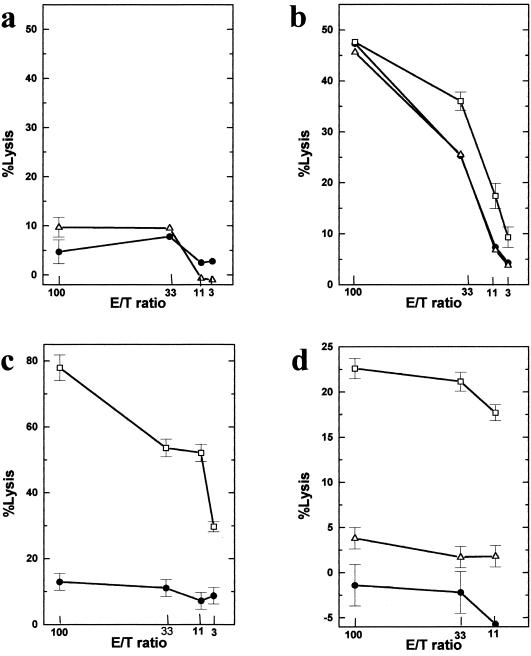
To confirm that the cytotoxicity of the uninfected syngeneic PSJL cells was not a property of CTLs in general, we tested whether uninfected PSJL cells could be killed by vaccinia virus-specific CTLs. Vaccinia virus-specific CTLs were induced in mice, and the spleen cells were isolated and used in a primary CTL assay ex vivo. With no prior in vitro stimulation, the cells could kill vaccinia virus-infected PSJL (VV-PSJL) cells but not uninfected PSJL cells (Fig. 3c). Therefore, lysis of uninfected PSJL cells by conventional CTLs did not occur. In additional experiments, vaccinia virus-specific CTLs were stimulated with TMEV-APCs in vitro to see whether stimulation with TMEV-APCs was sufficient to generate autoreactive cells. However, no cytotoxicity against uninfected PSJL or TMEV-infected PSJL (TMEV-PSJL) cells by vaccinia virus-specific CTLs stimulated with TMEV-APCs was observed (Fig. 3d). On the other hand, in a primary CTL assay, spleen cells from TMEV-infected mice killed neither uninfected PSJL nor TMEV-PSJL cells (Fig. 3a). Similarly, when CNS mononuclear cells were isolated from TMEV-infected mice and used in a primary CTL assay with uninfected PSJL cells as targets, no killing was observed (4% killing at an E/T ratio of 100:1). However, after a 6-day in vitro secondary stimulation with TMEV-APCs, the cells from TMEV-infected mice killed not only TMEV-PSJL cells but also uninfected PSJL cells and VV-PSJL cells (Fig. 3b). In contrast, when spleen cells from TMEV-infected mice were incubated with uninfected APCs for 6 days, the cells showed only minimal cytotoxicity against uninfected PSJL cells (8 and 1% lysis at E/T ratios of 100 and 33, respectively [average from two experiments]).
FIG. 3.
Generation of autoreactive cells. (a) Three weeks after i.v. infection with TMEV, spleen cells were isolated and used as effector cells in a primary CTL assay. The spleen cells did not show significant cytotoxicity towards either 51Cr-labeled uninfected PSJL cells (•) or TMEV-infected PSJL cells (TMEV-PSJL cells [▵]). (b) The spleen cells were further incubated with TMEV-APCs for 6 days and were used as effector cells in a secondary CTL assay. The effector cells showed significant cytotoxicity not only to TMEV-PSJL cells but also to uninfected PSJL or vaccinia virus-infected PSJL (VV-PSJL [□]) cells. (c) Three weeks after infection with vaccinia virus, spleen cells were isolated and used as effector cells in a primary CTL assay. The effector cells killed VV-PSJL cells but could not kill uninfected PSJL cells. (d) The spleen cells in panel c were incubated with TMEV-APCs for 6 days in vitro and then used as effector cells in a secondary CTL assay. The effector cells killed only VV-PSJL cells. Results are representative of those from three independent experiments. Error bars indicate standard errors.
Comparison of poly(I:C)-induced NK cells, LAK cells, and DNA-induced NK cells.
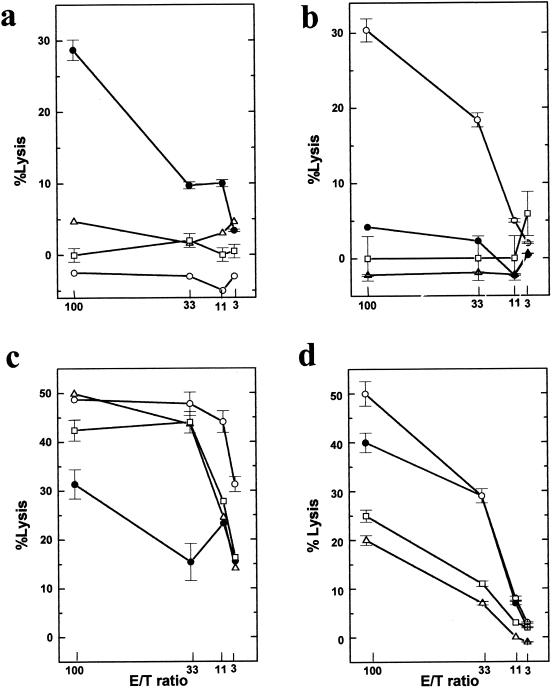
The data thus far suggested that this killer cell is not a conventional CTL. Another possibility is that the killer cell is an NK cell. To determine whether these cells were functionally similar to NK cells, we tested for NK cell cytotoxic activity. TMEV-induced autoreactive cells (Fig. 4a) were compared with various lymphocytes with NK cell activity. First, NK cells were induced with poly(I:C) either in vitro or in vivo (Fig. 4b). Second, in vitro LAK cells were generated by culturing spleen cells with IL-2 (Fig. 4c). Third, spleen cells were stimulated with bacterial DNA encoding CpG motifs to stimulate NK cell activity (Fig. 4d) (54, 58). As targets, we used the NK-sensitive cell line YAC-1, the NK-resistant cell lines P815 and EL4, and the syngeneic cell line PSJL.
FIG. 4.
Autoreactive cells are not NK cells. (a) TMEV-induced autoreactive cells were compared with various cells with NK activity. (b) NK cells were induced by intraperitoneal injection of poly(I:C). (c) LAK cells were induced by culture with IL-2 in vitro. (d) Spleen cells were incubated with bacterial DNA to induce NK cell activity. As target cells, we used the NK-sensitive cell line YAC-1 (○), the NK-resistant cell lines P815 (▵) and EL4 (□), and the syngeneic cell line PSJL (•). Poly(I:C)-induced NK cells, LAK cells and DNA-induced NK cells killed the NK-sensitive cell line, YAC-1, whereas TMEV-induced autoreactive cells did not kill YAC-1. While poly(I:C)-induced NK cells did not kill PSJL cells, LAK cells and DNA-induced NK cells killed not only PSJL cells but also the NK-resistant cell lines, P815 and EL4. Results are representative of those from four independent experiments. Error bars indicate standard errors.
As expected, the NK-sensitive cell line, YAC-1, was efficiently killed by all effector cells with NK activity, i.e., poly(I:C)-induced NK, LAK, and DNA-induced NK cells. However, TMEV-induced autoreactive cells did not kill YAC-1 cells. The NK-resistant cell lines, P815 and EL4, were killed neither by TMEV-induced autoreactive cells nor by poly(I:C)-induced NK cells, but they were killed by LAK cells and DNA-induced NK cells. While LAK cells and DNA-induced NK cells killed PSJL cells, poly(I:C)-induced NK cells did not kill PSJL cells. We found no difference in killing between poly(I:C)-induced NK cells induced in vitro and poly(I:C)-induced NK cells induced in vivo (data not shown). These results indicate that the autoreactive cell is not an NK cell.
Cold target inhibition suggests haplotype specificity and a requirement for cell-to-cell contact.
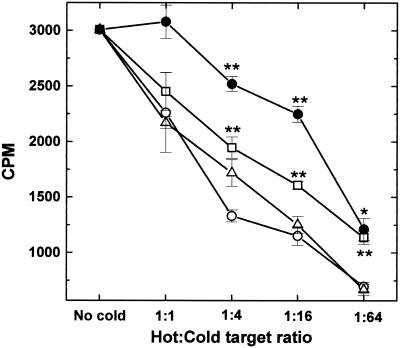
The haplotype specificity of the autoreactive cells was also supported by cold target inhibition studies. Cytolysis of 51Cr-labeled PSJL cells (H2s) by autoreactive cells was more efficiently inhibited by the addition of either cold PSJL or cold BxSF1 cells (H2b/s) than by the addition of cold allogeneic C57SV cells (H2b) (Fig. 5). Cold EL4 (H2b) cells inhibited the least. However, at a hot/cold target cell ratio of 1:64, even EL4 cells inhibited killing. A similar dose-dependent inhibition of CTL killing by specific as well as nonspecific cold target cells, particularly where killing was dependent on bystander mechanisms, has been reported by Ozaki et al. (37). This inhibition could be explained in two ways. First, the cold target inhibition could be due to interference with effector-to-target contact or proximity. Thus, a large number of the low-affinity nonspecific cold target cells could compete with the specific target cells. Second, the cold target cells could absorb or could result in the consumption of soluble cytotoxic factors.
FIG. 5.
Cold target inhibition experiments. During a 51Cr release assay, TMEV-induced autoreactive cells and 51Cr-labeled PSJL cells (hot target) were mixed at an E/T ratio of 100 with various numbers of nonlabeled cells (cold target). Cold PSJL (○) and BxSF1 (H2b/s) (▵) cells inhibited autoreactive killing more effectively than H2b cell lines C57SV (□) and EL4 (•). ∗, P < 0.05; ∗∗, P < 0.01 (compared with cold PSJL cells, as determined by analysis of variance). Results are representative of those from two independent experiments. Error bars indicate standard errors.
Cytolysis requires cell-to-cell contact and is not mediated by a soluble factor.
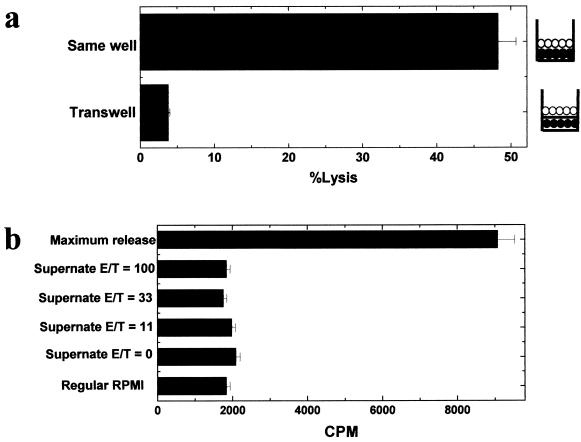
Killing or lysis of cells by immune effector cells can be caused by the interaction of membrane-bound effector molecules or by the release of soluble factors. The production of death-inducing factors by effector cells could be upregulated after binding of the target cell or be preformed, such as perforin granules. To test the need for cell-to-cell contact, experiments using Transwell chambers were conducted. Transwell chambers are wells separated by a membrane (0.4-μm pore size), where soluble factors can flow between the chambers but cells cannot. In a 51Cr release assay, we used TMEV-induced autoreactive cells and syngeneic PSJL cells as effector and target cells, respectively. Effector cells were placed in the lower chamber, and 51Cr-labeled target cells were placed either together in the same chamber (well) as the effector cells or separated in the upper compartment (Fig. 6a). When effector and target cells were placed in a single well together, a high level of killing was observed. However, when a membrane separated cells, no lysis of the labeled target cells occurred. In our experiment, the effector cell population was mixed with TMEV-APCs. Therefore, it is conceivable that the APCs could stimulate the effector cells, leading to the production of soluble or membrane-bound death-inducing molecules without the binding to target cells as was reported in bystander killing studies by Wang et al. (55). However, the Transwell experiments exclude the possibility that soluble cytotoxic factors produced in a bystander fashion are responsible for the killing we observe.
FIG. 6.
Cell-to-cell contact is required for killing. In a 51Cr release assay, we used TMEV-induced autoreactive cells as effector cells and the syngeneic PSJL cell line as target cells at an E/T ratio of 100. (a) Double-chamber experiments. Effector cells (•) were placed in the lower compartment of the Transwell chamber, and 51Cr-labeled target cells (○) were placed either in the same compartment as the effector cells or in the upper compartment of the Transwell chamber. Target cell lysis did not occur when effector and target cells were separated. (b) After a 5-h incubation of TMEV-induced autoreactive cells with syngeneic PSJL cells at E/T ratios of 100, 33, and 11 or with no effector cells, supernatants were harvested from the cultures. 51Cr-labeled PSJL cells were incubated with the supernatants, regular RPMI 1640 medium, or Triton X-100 (maximum release), for 5 h. No cytotoxic activity was seen in the supernatants. Results are representative of those from two independent experiments. Error bars indicate standard errors.
The Transwell experiments still left open the possibility that a cytotoxic soluble factor could be released from the effector cells after binding to the target cells, resulting in the lysis of the target cell. Therefore, supernatant fluids from the cultures were harvested after a 5-h incubation of TMEV-induced autoreactive cells with syngeneic PSJL cells at E/T ratios of 100, 33, and 11 or with no effector cells. 51Cr-labeled PSJL cells were then incubated with culture supernatants, RPMI 1640 medium (negative control), or a Triton X-100 solution (maximum release). No cytotoxic activity could be attributed to or was found in the supernatant fluids (Fig. 6b). These data confirm our experiments showing that cytolysis requires cell-to-cell contact and is not mediated by a soluble factor.
Cytotoxicity is mediated through a Fas-FasL pathway but not by a perforin pathway.
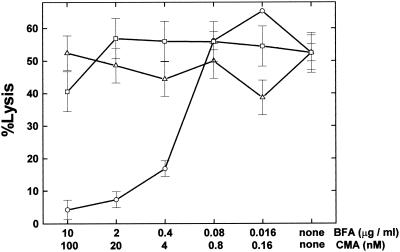
Kagi et al. (19) and Lowin et al. (31) demonstrated that perforin- and Fas-based mechanisms account for all T-cell mediated cytotoxicity in short-term CTL assays. In our experiments, lysis of target cells required only a short-term incubation with effector cells, i.e., less than 5 h. Therefore, a Fas-FasL- or perforin-dependent pathway most likely mediates the killing. Kataoka et al. (21) clearly demonstrated that brefeldin A and concanamycin A are selective inhibitors that block Fas-based cytotoxicity and perforin-based killing, respectively. Since then, brefeldin A and concanamycin A have been used to clarify the contributions of the two distinct cytolytic pathways (11, 26). To help distinguish between the two pathways, we tested inhibitors for each. TMEV-induced autoreactive cells were preincubated with brefeldin A, concanamycin A, or vehicle alone. We incubated effector cells with 51Cr-labeled PSJL target cells for 5 h. Only brefeldin A showed a dose-dependent inhibition of the killing (Fig. 7). The killing was also inhibited by incubation with anti-Fas antibody (data not shown). This suggests that the killing is mediated by a Fas-FasL pathway but not by the perforin pathway.
FIG. 7.
The Fas-mediated pathway is favored. Two hours prior to the 51Cr release assay, TMEV-induced autoreactive cells were incubated with brefeldin A (BFA) (○), concanamycin A (CMA) (▵), or vehicle (□). Brefeldin A and concanamycin A are known to inhibit Fas- and perforin-mediated cytotoxicities, respectively. Effector cells together with 51Cr-labeled PSJL target cells were cultured for 5 h. Only brefeldin A showed dose-dependent inhibition of autoreactive killing. Results are representative of those from two independent experiments. Error bars indicate standard errors.
Killing is mediated by CD3+ CD8+ T cells.
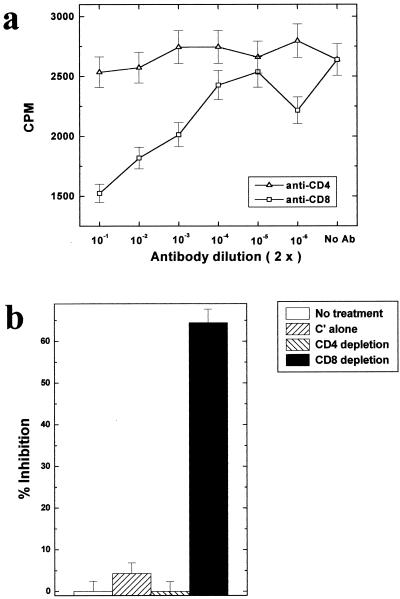
We then investigated the cell surface phenotype of the cells mediating the killing. TMEV-induced autoreactive cells were incubated with 51Cr-labeled PSJL at an E/T ratio of 100 in the presence of anti-CD3, anti-CD4, anti-CD8, or no antibody. Figure 8a shows that the killing was significantly inhibited by addition of anti-CD8 antibody but not by addition of anti-CD4 antibody. In contrast, anti-CD3 antibody enhanced the killing (data not shown). This antibody has been previously reported to either block or enhance cytotoxicity when added to killing assays (17, 25). We further confirmed the phenotype of effector cells by depletion of T-cell subsets from effector cell population, using anti-CD4 or anti-CD8 antibody and complement. Removal of CD8+ T cells resulted in a marked decrease in killing, while removal of CD4+ T cells showed no decrease (Fig. 8b). In addition, using StemSep cocktails, we were able to either enrich for or purge CD3+ T cells from the effector cell population. CD3 enrichment enhanced the killing, whereas CD3 depletion suppressed the killing of PSJL target cells (data not shown). Thus, the autoreactive cytotoxic cells appear to be CD3+ CD4− CD8+.
FIG. 8.
Anti-CD8 inhibits killing. (a) TMEV-induced effector cells were incubated with PSJL target cells at an E/T ratio of 100 in the presence of anti-CD4 (▵), anti-CD8 (□), or no antibody (No Ab). Both anti-CD4 and anti-CD8 antibodies are blocking antibodies (5, 30) when added to CTL assays. Although anti-CD4 antibody showed no effect, anti-CD8 antibody significantly inhibited the killing (the spontaneous release of PSJL target cells without effector cells was 1,137 cpm). (b) CD4+ or CD8+ T cells were depleted from the effector cell population by using anti-CD4 or anti-CD8 antibody with complement. In the control group, effector cells received no treatment or treatment with complement alone (C′ alone). Results are representative of those from two independent experiments. Error bars indicate standard errors.
Adoptive transfer of autoreactive cells induces CNS inflammation.
Finally, we investigated whether TMEV-induced autoreactive cells could participate in vivo in CNS inflammation. We generated autoreactive cells in vitro and adoptively transferred the cells into naive mice by either the i.v. or i.c. route. As a negative control, we used normal spleen cells or spleen cells from mice mock infected with phosphate-buffered saline instead of DA virus. Mice were killed either on day 6 or 10, and CNS tissue was examined. Although no mice showed obvious clinical signs, the group that received autoreactive cells i.c. showed less weight gain than the other groups (mean weight gains and standard errors on day 6 [in grams per mouse] were as follows: control cell i.v., 1.04 ± 0.22; control cell i.c., 0.5 ± 0.19; autoreactive cell i.v., 0.95 ± 0.24; autoreactive cell i.c., −0.01 ± 0.13 [P < 0.05 by analysis of variance]).
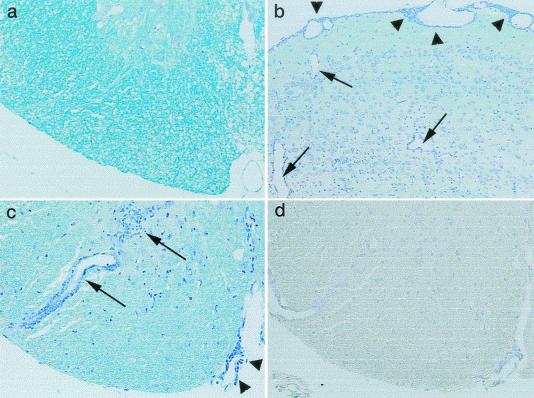
Histologically, i.v. transfer of normal or mock-infected cells into 7 control mice or of TMEV-induced autoreactive cells into 10 mice did not induce CNS changes. In eight mice that received control spleen cells i.c., a mild meningitis was detected only at the injection site. In contrast, in 8 of 10 mice receiving TMEV-induced autoreactive cells i.c., perivascular cuffing and meningitis were found distant from the injection site, including bilateral lesions of the cerebral cortex, corpus callosum, hippocampus, thalamus, pontine tegmentum, and gray and white matter of the spinal cord (Fig. 9b and c). Using immunohistochemistry, no viral antigen-positive cells were found in the lesions or in other parts of the CNS (Fig. 9d). This suggests that the autoreactive cells themselves can induce brain pathology in vivo.
FIG. 9.
Neuropathology caused by i.c. adoptive transfer of TMEV-induced autoreactive cells. Naive mice received control cells (a) or autoreactive cells induced in TMEV-infected mice (b, c, and d). Mice were killed 10 days after cell transfer. No lesions were found in the spinal cords of mice receiving control cells (a). In contrast, adoptive transfer of autoreactive cells caused meningitis (arrowheads) and parenchymal inflammation (arrows) not only in the brain (b) (the cerebral cortex) but also in the spinal cord away from the injection site (c). Viral antigen-positive cells were not detected in consecutive sections of the CNS (d). (a to c) Luxol fast blue stain; (d) immunohistochemistry for TMEV antigens. Magnifications, ×140 (a, c, and d) and ×70 (b).
DISCUSSION
The phenomenon of TMEV-induced autoreactive cytotoxicity described here is unlike any previously reported phenomena of virus-associated cytotoxicity. Yang et al. (59, 60) demonstrated that polyclonal stimulation of CD8+ CTLs occurred during infection with a variety of viruses, i.e., lymphocytic choriomeningitis, murine cytomegalovirus, Pichinde virus, and vaccinia virus. The CTL responses were allospecific but not autoreactive or xenoreactive. Holmes et al. (14) and Welsh et al. (57) demonstrated that spleen cells from uninfected mice selectively lysed BALB/c fibroblasts infected with mouse hepatitis virus, a murine coronavirus. These virus killer (VK) cells differed from conventional NK cells and T cells. Since VK cells were positive for B-cell markers, immunoglobulin G (IgG), IgM, IgD, and B220 (CD45R), VK cells appeared to be cytotoxic B cells. VK cells were not cytotoxic for uninfected cells, and SJL/J mice lacked VK cell activity. Therefore, TMEV-induced autoreactive cytotoxic cell activity differs from either the CTL activity seen in lymphocytic choriomeningitis infection or the VK cell activity seen in mouse hepatitis virus infection.
The SJL/J mouse is a genetically low-NK cell strain, and cytotoxic activity cannot be augmented by using conventional NK inducers (20). In this report, however, we demonstrated that both bacterial DNA and IL-2 were able to induce NK cells or LAK cells, which killed the NK-sensitive target YAC-1. Induction of unique cytotoxicity in SJL/J mice has also been reported by two groups, i.e., reticulum cell sarcoma-induced NK cells (27) and oligodendrocyte-specific autoreactive T-cell clone, C2 (17). Reticulum cell sarcoma-induced NK cells were CD8− and could kill YAC-1 cells. The C2 cell was CD8+ and could kill not only syngeneic murine oligodendrocytes but also rat oligodendrocytes. This killing was blocked by anti-myelin oligodendrocyte glycoprotein antibody but not by anti-class I (H-2K) or anti-class II antibody. Our TMEV-induced autoreactive cells did not show cytotoxicity against YAC-1 or xenogeneic cells (data not shown), and thus they are different from other cytotoxic cells reported to be induced in SJL/J mice.
Another candidate cell population for the TMEV-induced autoreactive cells is the NKT cell. NKT cells have been demonstrated to be autoreactive and exhibit potent lytic activity (2). Originally, SJL/J mice were reported to be deficient in NKT cells (61). However, Murakami and Paul (34) reported that there is an age-dependent appearance of NKT1.1+ T cells in livers of SJL/J mice. Conversely, Beutner et al. (4) found NKT-like cells that were IL-2Rβ+ but NK1.1− in the livers of SJL/J mice. Although the cytotoxic activity of these NKT cells is not known, the low numbers of these cells present in the spleens of SJL/J mice may explain why we detect cytotoxic activity only after an in vitro secondary stimulation.
In TMEV infection, both CD4+ (38) and CD8+ (9, 29) T cells have been demonstrated to kill TMEV-infected or TMEV antigen-pulsed targets but not uninfected syngeneic targets. Lin et al. (28, 29) demonstrated nonspecific cytotoxicity associated with CNS-infiltrating cells from either resistant or susceptible mouse strains infected with TMEV. The killer cells could lyse an anti-CD3 antibody-secreting hybridoma cell line and the xenogeneic cell line Jurkat. Here, CD8+ T cells and a perforin pathway mainly mediated the cytotoxicity. The killer cell could not lyse syngeneic target cells. Thus, although this cell appears to have an non-antigen-specific killing phenotype, its characteristics are different from those of the autoreactive cells described here.
Thus far, we do not know what is being recognized on the target cells by the TMEV-induced autoreactive cells. One possibility is that various self-peptides presented by MHC molecules are recognized. Enhancement of killing of BxSF1 cells and astrocytes after IFN-γ treatment supports this hypothesis, since IFN-γ is known to upregulate MHC molecules on various cells. Using flow cytometry, we could not detect the presence of MHC class II molecules on PSJL cells with anti-class II antibody prior to or after IFN-γ treatment (data not shown). Therefore, MHC class II-presented peptides are unlikely target epitopes. In contrast, an anti-class I antibody, M1/42, showed that PSJL cells had high levels of class I on their surface without stimulation. IFN-γ treatment significantly increased the amount of class I on both BxSF1 cells and the primary astrocytes, correlating with their ability to be killed by the TMEV-induced autoreactive cells (data not shown). On the other hand, we found that syngeneic concanavalin A-stimulated lymphoblasts were resistant to killing by TMEV-induced autoreactive cells (0 and 3% lysis in two experiments [data not shown]). This implies that not all syngeneic cells, including those that express MHC class I molecules, are capable of being killed by the autoreactive cells.
We showed that TMEV-induced autoreactive cytotoxicity is most likely mediated by a Fas-FasL pathway but not by a perforin pathway. In TMEV infection, perforin-deficient mice have been reported to develop exacerbation and/or prolongation of acute polioencephalomyelitis followed by demyelinating disease in spite of the C57BL/6 genetic background, which is normally resistant to demyelinating disease (35, 39). Therefore, although the perforin-mediated pathway seems to play an important role in virus clearance and gray matter inflammation during the acute stage of TMEV infection, it is not required for induction of demyelinating disease. On the other hand, TMEV induces chronic brain pathology instead of demyelination in the spinal cord white matter in lpr (Fas mutation) and gld (FasL mutation) mice having a resistant B6 genetic background (35).
TMEV-induced autoreactive cytotoxic cells could play a role in vivo as either effector or regulatory cells. Potentially, the CD8+ autoreactive cells could induce cell death of Fas+ oligodendrocytes directly if oligodendrocytes express class I molecules on the surface or indirectly without expression of class I molecules in a bystander manner. This could lead to demyelination during TMEV infection. Fas-dependent bystander lysis has been reported to be caused not only by CD4+ T cells (47, 55) but also by CD8+ T cells (1). Although this hypothesis needs to be substantiated, supportive data suggest that this could occur: (i) in TMEV infection, we demonstrated apoptosis of oligodendrocytes, which are myelin-forming cells (41, 51, 53); (ii) class I molecules are known to be inducible on oligodendrocytes (13); (iii) FasL+ infiltrates and Fas+ oligodendrocytes are detected in MS (8, 10, 62; reviewed in reference 42); and (iv) oligodendrocytes are susceptible to Fas-mediated killing in vitro (10).
Within this context, a possible scenario for demyelination by TMEV-induced autoreactive cells is that first APCs present TMEV capsid proteins to CD3+ CD8+ T cells. These activated cells now express FasL on the surface and recognize Fas+ cells together with self-antigen, possibly through molecular mimicry between TMEV capsid proteins and host proteins. If oligodendrocytes express self-antigen with Fas on its surface, this would lead to oligodendrocyte apoptosis and demyelination. Alternatively, if some other host cell presents self-antigens near Fas+ oligodendrocytes, this will also lead to oligodendrocyte death in a bystander manner.
Alternatively, CD8+ cytotoxic autoreactive T cells could play a suppressive role. Pathogenesis of autoimmune disease in some animal models involves defects in immunoregulation, where immune responses to self are normally downregulated. Fas-defective lpr mice and FasL-defective gld mice are known to develop a systemic lupus erythematosus-like autoimmune disease with accumulation of lymphocytes in the periphery (32). MS has also been associated with loss of negative regulation, including suppressor cells and regulatory cells (reviewed in references 40 and 56). In some EAE models, CD8+ T suppressor cells have been reported, although their actual role and mechanism of action are controversial (reviewed in references 40 and 51). Sun et al. (45) demonstrated that CD8+ regulatory (suppressor) T cells could kill the encephalitogenic T-cell line (S1) in vitro and suppress EAE in vivo. The S1-specific regulatory cells seem to be FasL+ and lytic for a FasL-sensitive mouse lymphoma cell line, A20, although soluble Fas was able to only partially inhibit cytolysis of S1 cells (46). In TMEV infection, Nicholson et al. (36) suggested that the resistance of BALB/c mice to demyelinating disease might be due to regulatory CD8+ T cells rather than TMEV-specific cytotoxic CD8+ T cells, while the mechanism of regulation was not defined.
The autoreactive cells in TMEV infection might act as regulatory cells that suppress effector cells in TMEV-induced demyelination. Although the precise mechanism(s) of demyelination is not clear, possible effector cells include TMEV-specific CD4+ and CD8+ T cells, myelin-specific Th1 cells generated by determinant spreading, TMEV-specific B cells that produce myelinotoxic antibody, and macrophages (reviewed in references 9, 51, and 52). In this study, we detected autoreactive cells only after in vitro stimulation and not in a primary CTL assay. Therefore, a failure to produce regulatory CTLs in vivo in susceptible SJL/J mice could be a key factor for survival of effector cells that might be otherwise killed in resistant mice. We are currently investigating whether we can detect TMEV-induced autoreactive cells in a primary CTL assay in TMEV-resistant mice.
Potentially, our inability to detect killing by the autoreactive cells in primary CTL assays could be due to insufficient numbers of cells. Moreover, cytotoxicity in freshly isolated killer cells has been reported to require different conditions. Arase et al. (2) showed that detection of cytotoxicity of freshly isolated NKT cells required a longer incubation period for the CTL assay than was necessary with NKT cells that were stimulated in vitro.
To further characterize the TMEV-induced autoreactive cells, we are in the process of cloning these cells. Thus far, in order to obtain these cells we had to select either positively or negatively for CD8+ T cells and then clone these cells by limiting dilution. Several clones and T-cell hybridomas which can kill both TMEV-infected and syngeneic cells have been generated. Investigation to compare the phenotypes of these cell lines with those in the bulk cultures described above is under way. These cell lines should provide us with important tools to further investigate the nature of the autoreactivity generated by TMEV infection.
Acknowledgments
We thank Michael Wang, Jane E. Libbey, Caroline I. B. Kurtz, and Neal D. Tolley for many helpful discussions and Timothy S. Alexander, Jana Blackett, Thomas S. Cannon, Kornelia Edes, and Amy Perou for their technical assistance. We also thank Raymond M. Welsh and Barbara R. Knowles for their generous gifts of cell lines. We are grateful to Kathleen Borick for preparation of the manuscript.
This work was supported by NIH grant NS34497.
REFERENCES
- 1.Ando, K., K. Hiroishi, T. Kaneko, T. Moriyama, Y. Muto, N. Kayagaki, H. Yagita, K. Okumura, and M. Imawari. 1997. Perforin, Fas/Fas ligand, and TNF-α pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J. Immunol. 158:5283-5291. [PubMed] [Google Scholar]
- 2.Arase, H., N. Arase, Y. Kobayashi, Y. Nishimura, S. Yonehara, and K. Onoe. 1994. Cytotoxicity of fresh NK1.1+ T cell receptor α/β+ thymocytes against a CD4 +8+ thymocyte population associated with intact Fas antigen expression on the target. J. Exp. Med. 180:423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, L. A., J. L. Whitton, Y. Wada, and R. S. Fujinami. 1993. Enhancement of autoimmune disease using recombinant vaccinia virus encoding myelin proteolipid protein. J. Neuroimmunol. 44:15-25. [DOI] [PubMed] [Google Scholar]
- 4.Beutner, U., P. Launois, T. Ohteki, J. A. Louis, and H. R. MacDonald. 1997. Natural killer-like T cells develop in SJL mice despite genetically distinct defects in NK1.1 expression and in inducible interleukin-4 production. Eur. J. Immunol. 27:928-934. [DOI] [PubMed] [Google Scholar]
- 5.Dialynas, D. P., Z. S. Quan, K. A. Wall, A. Pierres, J. Quintáns, M. R. Loken, M. Pierres, and F. W. Fitch. 1983. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J. Immunol. 131:2445-2451. [PubMed] [Google Scholar]
- 6.Djeu, J. Y., J. A. Heinbaugh, H. T. Holden, and R. B. Herberman. 1979. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J. Immunol. 122:175-181. [PubMed] [Google Scholar]
- 7.Dowling, P., W. Husar, J. Menonna, H. Donnenfeld, S. Cook, and M. Sidhu. 1997. Cell death and birth in multiple sclerosis brain. J. Neurol. Sci. 149:1-11. [DOI] [PubMed] [Google Scholar]
- 8.Dowling, P., G. Shang, S. Raval, J. Menonna, S. Cook, and W. Husar. 1996. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J. Exp. Med. 184:1513-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drescher, K. M., L. R. Pease, and M. Rodriguez. 1997. Antiviral immune responses modulate the nature of central nervous system (CNS) disease in a murine model of multiple sclerosis. Immunol. Rev. 159:177-193. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza, S. D., B. Bonetti, V. Balasingam, N. R. Cashman, P. A. Barker, A. B. Troutt, C. S. Raine, and J. P. Antel. 1996. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J. Exp. Med. 184:2361-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnon, S. J., F. A. Ennis, and A. L. Rothman. 1999. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4+ cytotoxic T-lymphocyte clones. J. Virol. 73:3623-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebhard, J. R., C. M. Perry, S. Harkins, T. Lane, I. Mena, V. C. Asensio, I. L. Campbell, and J. L. Whitton. 1998. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am. J. Pathol. 153:417-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenier, Y., T. C. Ruijs, Y. Robitaille, A. Olivier, and J. P. Antel. 1989. Immunohistochemical studies of adult human glial cells. J. Neuroimmunol. 21:103-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes, K. V., R. M. Welsh, and M. V. Haspel. 1986. Natural cytotoxicity against mouse hepatitis virus-infected target cells. I. Correlation of cytotoxicity with virus binding to leukocytes. J. Immunol. 136:1446-1453. [PubMed] [Google Scholar]
- 15.Honma, K., K. C. Parker, K. G. Becker, H. F. McFarland, J. E. Coligan, and W. E. Biddison. 1997. Identification of an epitope derived from human proteolipid protein that can induce autoreactive CD8+ cytotoxic T lymphocytes restricted by HLA-A3: evidence for cross-reactivity with an environmental microorganism. J. Neuroimmunol. 73:7-14. [DOI] [PubMed] [Google Scholar]
- 16.Huber, S. A., N. Heintz, and R. Tracy. 1988. Coxsackievirus B-3-induced myocarditis. Virus and actinomycin D treatment of myocytes induces novel antigens recognized by cytolytic T lymphocytes. J. Immunol. 141:3214-3219. [PubMed] [Google Scholar]
- 17.Jewtoukoff, V., R. Lebar, and M.-A. Bach. 1989. Oligodendrocyte-specific autoreactive T cells using an α/β T-cell receptor kill their target without self restriction. Proc. Natl. Acad. Sci. USA 86:2824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, R. T. 1998. Viral infections of the nervous system, p. 181-210. Lippincott-Raven, Philadelphia, Pa.
- 19.Kägi, D., F. Vignaux, B. Ledermann, K. Bürki, V. Depraetere, S. Nagata, H. Hengartner, and P. Golstein. 1994. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265:528-530. [DOI] [PubMed] [Google Scholar]
- 20.Kaminsky, S. G., I. Nakamura, and G. Cudkowicz. 1983. Selective defect of natural killer and killer cell activity against lymphomas in SJL mice: low responsiveness to interferon inducers. J. Immunol. 130:1980-1984. [PubMed] [Google Scholar]
- 21.Kataoka, T., N. Shinohara, H. Takayama, K. Takaku, S. Kondo, S. Yonehara, and K. Nagai. 1996. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156:3678-3686. [PubMed] [Google Scholar]
- 22.Kawai, K., and B. Zweiman. 1988. Cytotoxic effect of myelin basic protein-reactive T cells on cultured oligodendrocytes. J. Neuroimmunol. 19:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowles, B. B., M. Koncar, K. Pfizenmaier, D. Solter, D. P. Aden, and G. Trinchieri. 1979. Genetic control of the cytotoxic T cell response to SV40 tumor-associated specific antigen. J. Immunol. 122:1798-1806. [PubMed] [Google Scholar]
- 24.Lee, R. K., J. Spielman, D. Y. Zhao, K. J. Olsen, and E. R. Podack. 1996. Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J. Immunol. 157:1919-1925. [PubMed] [Google Scholar]
- 25.Leo, O., M. Foo, D. H. Sachs, L. E. Samelson, and J. A. Bluestone. 1987. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc. Natl. Acad. Sci. USA 84:1374-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, J.-H., D. Rosen, D. Ronen, C. K. Behrens, P. H. Krammer, W. R. Clark, and G. Berke. 1998. The regulation of CD95 ligand expression and function in CTL. J. Immunol. 161:3943-3949. [PubMed] [Google Scholar]
- 27.Lin, T.-Z., and N. M. Ponzio. 1991. Syngeneic B lymphoma cells provide a unique stimulus to natural killer (NK) cells in genetically low-NK SJL/J mice. J. Leukoc. Biol. 49:48-57. [DOI] [PubMed] [Google Scholar]
- 28.Lin, X., L. R. Pease, P. D. Murray, and M. Rodriguez. 1998. Theiler's virus infection of genetically susceptible mice induces central nervous system-infiltrating CTLs with no apparent viral or major myelin antigenic specificity. J. Immunol. 160:5661-5668. [PubMed] [Google Scholar]
- 29.Lin, X., L. R. Pease, and M. Rodriguez. 1997. Differential generation of class I H-2D- versus H-2K-restricted cytotoxicity against a demyelinating virus following central nervous system infection. Eur. J. Immunol. 27:963-970. [DOI] [PubMed] [Google Scholar]
- 30.Lindsley, M. D., R. Thiemann, and M. Rodriguez. 1991. Cytotoxic T cells isolated from the central nervous systems of mice infected with Theiler's virus. J. Virol. 65:6612-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowin, B., M. Hahne, C. Mattmann, and J. Tschopp. 1994. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 370:650-652. [DOI] [PubMed] [Google Scholar]
- 32.Lynch, D. H., F. Ramsdell, and M. R. Alderson. 1995. Fas and FasL in the homeostatic regulation of immune responses. Immunol. Today 16:569-574. [DOI] [PubMed] [Google Scholar]
- 33.Mantegazza, R., P. Bernasconi, P. Confalonieri, and F. Cornelio. 1997. Inflammatory myopathies and systemic disorders: a review of immunopathogenetic mechanisms and clinical features. J. Neurol. 244:277-287. [DOI] [PubMed] [Google Scholar]
- 34.Murakami, M., and W. E. Paul. 1998. Age-dependent appearance of NK.1.1+ T cells in the livers of β2-microglobulin knockout and SJL mice. J. Immunol. 160:2649-2654. [PubMed] [Google Scholar]
- 35.Murray, P. D., D. B. McGavern, X. Lin, M. K. Njenga, J. Leibowitz, L. R. Pease, and M. Rodriguez. 1998. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J. Neurosci. 18:7306-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson, S. M., L. M. Haynes, C. L. Vanderlugt, S. D. Miller, and R. W. Melvold. 1999. The role of protective CD8+ T cells in resistance of BALB/c mice to Theiler's murine encephalomyelitis virus-induced demyelinating disease: regulatory vs. lytic. J. Neuroimmunol. 98:136-146. [DOI] [PubMed] [Google Scholar]
- 37.Ozaki, S., J. York-Jolley, H. Kawamura, and J. A. Berzofsky. 1987. Cloned protein antigen-specific, Ia-restricted T cells with both helper and cytolytic activities: mechanisms of activation and killing. Cell Immunol. 105:301-316. [DOI] [PubMed] [Google Scholar]
- 38.Palma, J. P., R. L. Yauch, S. Lang, and B. S. Kim. 1999. Potential role of CD4+ T cell-mediated apoptosis of activated astrocytes in Theiler's virus-induced demyelination. J. Immunol. 162:6543-6551. [PubMed] [Google Scholar]
- 39.Pena Rossi, C., A. McAllister, M. Tanguy, D. Kägi, and M. Brahic. 1998. Theiler's virus infection of perforin-deficient mice. J. Virol. 72:4515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pender, M. P. 1995. Experimental autoimmune encephalomyelitis, p. 26-88. In M. P. Pender and P. A. McCombe (ed.), Autoimmune neurological disease. Cambridge University Press, Cambridge, United Kingdom.
- 41.Rose, J. W., K. E. Hill, Y. Wada, C. I. B. Kurtz, I. Tsunoda, R. S. Fujinami, and A. H. Cross. 1998. Nitric oxide synthase inhibitor, aminoguanidine, reduces inflammation and demyelination produced by Theiler's virus infection. J. Neuroimmunol. 81:82-89. [DOI] [PubMed] [Google Scholar]
- 42.Sabelko-Downes, K. A., J. H. Russell, and A. H. Cross. 1999. Role of Fas-FasL interactions in the pathogenesis and regulation of autoimmune demyelinating disease. J. Neuroimmunol. 100:42-52. [DOI] [PubMed] [Google Scholar]
- 43.Sarmiento, M., A. L. Glasebrook, and F. W. Fitch. 1980. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J. Immunol. 125:2665-2672. [PubMed] [Google Scholar]
- 44.Stallcup, K. C., T. A. Springer, and M. F. Mescher. 1981. Characterization of an anti-H-2 monoclonal antibody and its use in large-scale antigen purification. J. Immunol. 127:923-930. [PubMed] [Google Scholar]
- 45.Sun, D., Y. Qin, J. Chluba, J. T. Epplen, and H. Wekerle. 1988. Suppression of experimentally induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature 332:843-845. [DOI] [PubMed] [Google Scholar]
- 46.Sun, D., J. N. Whitaker, and D. B. Wilson. 1999. Regulatory T cells in experimental allergic encephalomyelitis. II. T cells functionally antagonistic to encephalitogenic MBP-specific T cells show persistent expression of FasL. J. Neurosci. Res. 58:357-366. [PubMed] [Google Scholar]
- 47.Thilenius, A. R., K. A. Sabelko-Downes, and J. H. Russell. 1999. The role of the antigen-presenting cell in Fas-mediated direct and bystander killing: potential in vivo function of Fas in experimental allergic encephalomyelitis. J. Immunol. 162:643-650. [PubMed] [Google Scholar]
- 48.Thor, G., H. Sepulveda, S. Chada, and R. W. Dutton. 1993. Monoclonal antibody that distinguishes between a phosphorylated, β2-microglobulin-associated, and a free, nonphosphorylated, chain of MHC class I. J. Immunol. 151:211-224. [PubMed] [Google Scholar]
- 49.Tolley, N. D., I. Tsunoda, and R. S. Fujinami. 1999. DNA vaccination against Theiler's murine encephalomyelitis virus leads to alterations in demyelinating disease. J. Virol. 73:993-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsuchida, T., K. C. Parker, R. V. Turner, H. F. McFarland, J. E. Coligan, and W. E. Biddison. 1994. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc. Natl. Acad. Sci. USA 91:10859-10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsunoda, I., and R. S. Fujinami. 1996. Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus. J. Neuropathol. Exp. Neurol. 55:673-686. [DOI] [PubMed] [Google Scholar]
- 52.Tsunoda, I., and R. S. Fujinami. 1999. Theiler's murine encephalomyelitis virus, p. 517-536. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 53.Tsunoda, I., C. I. B. Kurtz, and R. S. Fujinami. 1997. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology 228:388-393. [DOI] [PubMed] [Google Scholar]
- 54.Tsunoda, I., N. D. Tolley, D. J. Theil, J. L. Whitton, H. Kobayashi, and R. S. Fujinami. 1999. Exacerbation of viral and autoimmune animal models for multiple sclerosis by bacterial DNA. Brain Pathol. 9:481-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, R., A. M. Rogers, T. L. Ratliff, and J. H. Russell. 1996. CD95-dependent bystander lysis caused by CD4+ T helper 1 effectors. J. Immunol. 157:2961-2968. [PubMed] [Google Scholar]
- 56.Wekerle, H., and H. Lassmann. 1998. McAlpine's multiple sclerosis, p. 379-407. Churchill Livingstone, London, United Kingdom.
- 57.Welsh, R. M., M. V. Haspel, D. C. Parker, and K. V. Holmes. 1986. Natural cytotoxicity against mouse hepatitis virus-infected cells. II. A cytotoxic effector cell with a B lymphocyte phenotype. J. Immunol. 136:1454-1460. [PubMed] [Google Scholar]
- 58.Yamamoto, S., E. Kuramoto, S. Shimada, and T. Tokunaga. 1988. In vitro augmentation of natural killer cell activity and production of interferon-α/β and -γ with deoxyribonucleic acid fraction from Mycobacterium bovis BCG. Jpn. J. Cancer Res. 79:866-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, H., and R. M. Welsh. 1986. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J. Immunol. 136:1186-1193. [PubMed] [Google Scholar]
- 60.Yang, H., P. L. Dundon, S. R. Nahill, and R. M. Welsh. 1989. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J. Immunol. 142:1710-1718. [PubMed] [Google Scholar]
- 61.Yoshimoto, T., A. Bendelac, J. Hu-Li, and W. E. Paul. 1995. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc. Natl. Acad. Sci. USA 92:11931-11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zipp, F., P. H. Krammer, and M. Weller. 1999. Immune (dys)regulation in multiple sclerosis: role of the CD95-CD95 ligand system. Immunol. Today 20:550-554. [DOI] [PubMed] [Google Scholar]