Abstract
In human immunodeficiency virus type 1 (HIV-1) latently infected cells, NF-κB plays a major role in the transcriptional induction of HIV-1 replication. Hence, downregulation of NF-κB activation has long been sought for effective anti-HIV therapy. Tumor necrosis factor alpha (TNF-α) stimulates IκB kinase (IKK) complex, a critical regulator in the NF-κB signaling pathway. A novel IKK inhibitor, ACHP {2-amino-6-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-4-piperidin-4-yl-nicotinonitrile}, was developed and evaluated as a potent and specific inhibitor for IKK-α and IKK-β. In this study, we examined the ability of this compound to inhibit HIV-1 replication in OM10.1 cells latently infected with HIV. When these cells were pretreated with ACHP, TNF-α-induced HIV-1 replication was dramatically inhibited, as measured by the HIV p24 antigen levels in the culture supernatants. Its 50% effective concentration was approximately 0.56 μM, whereas its 50% cytotoxic concentration was about 15 μM. Western blot analysis revealed inhibition of IκBα phosphorylation, IκBα degradation, p65 nuclear translocation, and p65 phosphorylation. ACHP was also found to suppress HIV-1 long terminal repeat (LTR)-driven gene expression through the inhibition of NF-κB activation. Furthermore, ACHP inhibited TNF-α-induced NF-κB (p65) recruitment to the HIV-1 LTR, as assessed by chromatin immunoprecipitation assay. These findings suggest that ACHP acts as a potent suppressor of TNF-α-induced HIV replication in latently infected cells and that this inhibition is mediated through suppression of IKK activity.
Although the recent progress in combination therapy against viral reverse transcriptase and protease has achieved considerable reduction of the viral load in human immunodeficiency virus type 1 (HIV-1)-infected individuals and significant improvement in survival, chemotherapy could not be terminated unless chronically infected cell populations, such as resting memory T cells and monocytes/macrophages, could be eradicated (15, 51, 53). Thus, it is crucial to inhibit HIV-1 replication in the latently infected cells. Molecular analyses of HIV-1 replication have revealed a concerted complexity that regulates the viral life cycle (52). Among the various steps of the viral life cycle, the step of transcription from HIV-1 provirus is conceived to be crucial for viral replication, since amplification of the viral genetic information is attainable only through transcription. It is through this step that HIV acquires genetic variation, thus enabling the emergence of HIV quasispecies containing clones resistant to host immune responses and anti-HIV drugs. In addition to the virus-encoded transcriptional transactivator Tat, several cellular factors are known to regulate HIV-1 transcription (29, 52). Among these host factors, nuclear factor κB (NF-κB) is known to play a major role in regulated HIV-1 gene expression (44, 48, 52).
NF-κB is an inducible cellular transcription factor that regulates a wide variety of cellular and viral gene expression, including that of HIV (6, 7, 22, 44, 48, 50, 65). Recently, two major signaling pathways leading to receptor-mediated NF-κB activation have been classified: the canonical and noncanonical (alternative) pathways. In the canonical pathway, a diverse range of stimuli, such as tumor necrosis factor alpha (TNF-α), viral and bacterial pathogens, and stress-inducing agents (24), stimulate the signal transduction pathways that lead to the activation of NF-κB. In cells, NF-κB, a hetero- or homodimer consisting of the Rel family proteins p65 (RelA), RelB, c-Rel, p50/p105, and p52/p100, resides in the cytoplasm and is complexed with an inhibitory molecule, IκB (6). Stimulation activates the IκB kinase (IKK) complex composed of two catalytic subunits, IKK-α and IKK-β, and a regulatory subunit, IKK-γ (22). IKK rapidly phosphorylates IκBα on its two NH2-terminal serine residues (Ser32 and Ser36) (21, 41, 71). Phosphorylation targets IκBα for its ubiquitination and degradation by the β-transducin repeat-containing protein ubiquitin ligase and 26S proteasome, respectively, thus allowing free NF-κB to translocate to the nucleus to activate gene expression (22). In this event, IKK-β and IKK-γ mainly regulate IκB degradation, while IKK-α is dispensable (22), although its nuclear function remains essential for the transcriptional activity of NF-κB (1, 70). The noncanonical pathway, however, is strictly dependent on the NF-κB-inducing kinase (NIK)-mediated activation of IKK-α, which phosphorylates p100, causing its inducible processing into p52 (60, 68). This IKK-β/IKK-γ-independent pathway is induced in response to stimuli, such as lymphotoxin B (18), B-cell-activating factor (16), and CD40 ligand (17). Moreover, recent reports by us and others have shown that IKK-α also phosphorylates p65 at Ser536, which is pivotal for the transcriptional competence of NF-κB when it is bound to the promoter sequence of target genes in the nucleus (27, 28, 56).
The role of NF-κB in activating HIV transcription has been extensively studied. HIV-1 replication is positively regulated by several cytokines, or T-cell activators, most of which act either completely or partially via NF-κB (4, 8, 38). NF-κB has been shown to regulate viral transcription via the two NF-κB sites located in the HIV-1 long terminal repeat (LTR) enhancer region (44) and is further enhanced through synergism with Sp1 (34, 49). In HIV-1 latently infected cells, activation of NF-κB could trigger the transcription of viral genes, including the transactivator Tat, resulting in an explosive increase in HIV replication (29, 47, 52). Treatment with compounds that block NF-κB activation inhibits HIV-1 gene expression and viral replication (3, 20, 33, 45, 59, 63, 64). Hence, downregulation of NF-κB activity by suppressing NF-κB or the signaling proteins involved in the NF-κB activation pathway, such as the IKKs (23, 30), is considered a feasible target for future anti-HIV therapy.
To control HIV-1 expression from latently infected cells, we examined the effect of a novel IKK inhibitor, 2-amino-6-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-4-piperidin-4-yl- nicotinonitrile (ACHP). ACHP was found on a massive screening to have specific inhibitory action on IKK-β and IKK-α (42, 43). The 50% inhibitory concentrations for IKK-β and IKK-α are 8.5 and 250 nmol/liter, respectively, measured by in vitro kinase assays, and those for other kinases, such as IKK-γ, Syk, and mitogen-activated protein kinase kinase kinase 4, were greater than 20 μmol/liter (42). ACHP also showed good aqueous solubility and cell permeability, thus demonstrating high bioavailability in mice and rats (43).
In this study, we demonstrate the inhibitory action of ACHP on IκBα phosphorylation and its degradation, as well as the nuclear translocation and phosphorylation of p65, resulting in the reduction of HIV production in HIV-1 latently infected cells. Furthermore, NF-κB (p65) binding to the HIV-1 LTR was also abolished by this compound. From these findings, this compound and its derivatives appear to be feasible candidates for novel anti-HIV therapy.
MATERIALS AND METHODS
Reagents.
An IKK inhibitor, ACHP, was a kind gift from T. Murata of Bayer Yakuhin Inc. (Kyoto, Japan). Human recombinant TNF-α was purchased from Roche and used at 1 ng/ml for NF-κB stimulation. Antibodies for IκBα, p65 (RelA), and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), while antibodies for phospho-IκBα (Ser32) and phospho-p65 (Ser536) were purchased from Cell Signaling Technology (Beverly, MA). The pooled sera from HIV-1-infected individuals were kindly given by T. Hamano (National Institute of Infectious Diseases, Tokyo, Japan). Horseradish peroxidase-conjugated secondary antibodies were obtained from Amersham Biosciences (Little Chalfont, United Kingdom) (rabbit and mouse) and from DAKO (DAKO A/S, Denmark) (goat).
Cell lines.
OM10.1 cells (13), a human macrophage/monocytic cell line latently infected with HIV-1, and MOLT4/IIIB cells (3), a T-cell line chronically infected with HIV-1 (IIIB strain), were used in the antiviral assays. The OM10.1 cells and MOLT4/IIIB cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin, and streptomycin at 37°C in a 5% CO2 incubator. To maintain the latency in OM10.1 cells, 20 μM of AZT was added to culture media and was removed prior to experiments (3, 58, 59, 64).
Antiviral assay.
The antiviral activity of ACHP was evaluated based on the extent of inhibition of p24 antigen production in OM10.1 cells as previously described (59, 64). Cells (2 × 105/ml) were incubated with or without ACHP for 1 h and then stimulated with TNF-α (1 ng/ml) for 24 h at 37°C. A time course experiment (until 72 h) was also conducted. On the other hand, MOLT4/IIIB cells (2 × 105/ml) were cultured in the absence or presence of ACHP with or without TNF-α stimulation. The culture supernatants were then collected and assayed for viral p24 antigen. Experiments were carried out in triplicate and repeated at least twice. The cytotoxicity of the test compound was also determined by the WST-1 method (Roche) (58).
Quantitation of HIV-1 replication.
Viral p24 antigen levels in the cell supernatants of OM10.1 and MOLT4/IIIB cells were determined using the commercial Retrotek HIV-1 p24 antigen enzyme-linked immunosorbent assay (ELISA) kit (Cellular Products, Buffalo, NY) according to the manufacturer's instructions. Assays were performed in triplicate and repeated at least twice.
Transient luciferase assay.
293 cells (1 × 105/well) were transfected with reporter plasmids using FUGENE 6 transfection reagent (Roche Applied Science) according to the manufacturer's instructions. For each transfection, 0.3 μg of reporter plasmid, CD12wt-Luc containing wild-type HIV-1 LTR (47) or CD12mut-Luc containing mutated NF-κB binding sites (59), and 0.1 μg of the internal control plasmid, phRL-TK, expressing Renilla luciferase, were used (57). Twenty-four hours after transfection, the cells were treated with ACHP for 30 min and stimulated with TNF-α (5 ng/ml) for 4 h. The transfected cells were then harvested, and the extracts were subjected to luciferase assay using the Luciferase Assay System (Promega). The luciferase activity was normalized with Renilla luciferase activity as an internal control to assess the transfection efficiency. The data are presented as the increase in luciferase activities (means ± standard deviations) relative to the control from triplicate transfections.
Preparation of whole-cell and nuclear extracts.
OM10.1 cells (1 × 106/ml) were treated with or without ACHP for 1 h and stimulated with or without TNF-α (1 ng/ml) at various times. The cells were then washed with cold phosphate-buffered saline and resuspended in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1% Triton X-100, and protease inhibitors [Roche Diagnostics GmbH, Mannheim, Germany]), incubated on ice for 10 min, and centrifuged at 15,000 rpm for 15 min. The supernatant was then collected (whole-cell extract) and stored at −80°C until it was used. In order to prepare the nuclear extract, sedimented cells were resuspended in cytoplasmic lysis buffer (Chemicon International, Tenecula, CA) and incubated for 15 min on ice. The cells were vortexed and then centrifuged at 15,000 rpm for 10 min, and the supernatant was removed. The cell pellets were washed twice with cytoplasmic buffer to remove any trace of proteins from the cytoplasmic extracts, resuspended in 20 μl of nuclear lysis buffer (Chemicon International, Tenecula, CA), and incubated on ice for 15 min. The cell suspensions were then sonicated for 10 s and centrifuged at 15,000 rpm for 10 min, and the supernatant fractions were stored at −80°C until they were used. The protein content was measured by a detergent-compatible protein assay kit (Bio-Rad, Hercules, CA).
Western blotting.
Equal amounts of the proteins (14 μg) were resolved on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel, transferred onto a polyvinylidene fluoride membrane (Millipore Corporation, MA), and reacted with specific antibodies for various proteins. Expression of HIV-1 viral proteins was examined by reaction with pooled sera from HIV-1-infected individuals. Detection of immunoreactive bands was visualized by chemiluminescence using SuperSignal (Pierce, Rockford, IL).
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed with a previously described protocol with some modifications (25). In brief, cells either with or without ACHP treatment and with or without TNF-α stimulation were cross-linked by adding formaldehyde to the medium (1% final concentration) and incubated at 37°C for 10 min. The cells were then washed with cold phosphate-buffered saline containing protease inhibitors and PMSF and lysed in SDS-lysis buffer (1% SDS, 50 mM Tris-HCl [pH 8.0], 16.7 mM NaCl, PMSF, and protease inhibitors), and the chromatin was sheared by sonication 20 times for 30 s each time at one-third of the maximum power with 1 min of cooling on ice between each pair of pulses (Bioruptor; COSMO Bio, Tokyo, Japan). The lysates were then collected after centrifugation at 15,000 rpm for 15 min, followed by the addition of specific antibodies, and the mixture was rotated at room temperature for 2 h and further incubated for another hour at 4°C. DNA samples were then precipitated with salmon sperm DNA and protein G-agarose beads (Upstate Biotechnology, Lake Placid, NY), and cross-linking of the immunoprecipitates and input DNAs was reversed by incubation at 65°C for 6 h. The DNAs were then purified using Qiaquick spin columns (QIAGEN), and PCR was performed with a HotStarTaq Master Mix kit (QIAGEN). The PCR primers used for amplifying promoters containing the NF-κB binding sites included HIV-1 LTR promoter (−176 to +61), 5′-CGA GAG CTG CAT CCG GAG TA-3′ and 5′-AGC TTT ATT GAG GCT TAA GC-3′ (37); human IκBα promoter (−316 to −15), 5′-GAC GAC CCC AAT TCA AAT CG-3′ and 5′-TCA GGC TCG GGG AAT TTC C-3′ (70); and human β-actin promoter (−980 to −915), 5′-TGC ACT GTG CGG CGA AGC-3′ and 5′-TCG AGC CAT AAA AGG CAA-3′ (70).
RESULTS
ACHP inhibited TNF-α-induced IκBα phosphorylation and degradation and p65 phosphorylation.
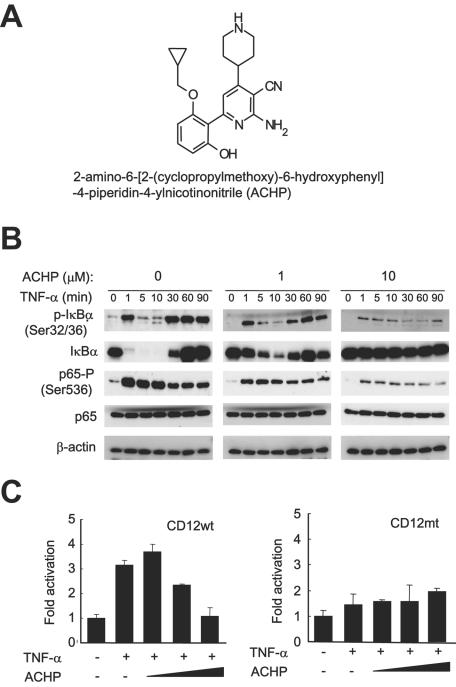
Activated IKKs target the IκBα inhibitor of NF-κB for phosphorylation on Ser32 and Ser36, leading to the rapid ubiquitylation and degradation of the inhibitor by the 26S proteasome (44). To assess whether ACHP (Fig. 1A) modulates this cascade, OM10.1 cells, a macrophage/monocyte cell model widely used in studying therapeutic interventions (3, 13, 58, 64), were stimulated with TNF-α for the indicated times in the absence or presence of ACHP and analyzed for IκBα and NF-κB (p65) expression and for their phosphorylation status by Western blot analysis.
FIG. 1.
Effect of ACHP on IκBα phosphorylation and degradation and p65 phosphorylation induced by TNF-α. (A) Chemical structure of ACHP. (B) Inhibition of phosphorylation of IκBα and p65 by ACHP. OM10.1 cells (1 × 106/ml) were pretreated with or without ACHP for an hour and then stimulated with TNF-α (1 ng/ml) for the indicated time periods. Whole-cell extracts were fractionated on 10% SDS-polyacrylamide gel electrophoresis and subjected to immunoblot analysis with the specified antibodies. Immunoblotting of β-actin indicated that equal amounts of protein were applied in each lane. (C) Inhibitory effect of ACHP on HIV-1 LTR-driven gene expression in 293 cells. A luciferase reporter plasmid containing wild-type NF-κB binding sequence (CD12wt, left) or its mutant (CD12mt, right) was transfected into 293 cells under the control of the HIV-1 LTR. After transfection, the cells were incubated in the absence or presence of ACHP for 30 min, stimulated with TNF-α (5 ng/ml) for 4 h, and harvested for luciferase assay, as described in Materials and Methods. The luciferase activity is indicated as increase relative to the untreated control (lane 1). The data are mean values plus standard deviations of triplicate experiments.
As shown in Fig. 1B, immunoblotting analysis showed that IκBα was readily phosphorylated at Ser32 and Ser36 as early as 1 min after TNF-α stimulation. Following its degradation, a massive increase was observed, indicating the activation of the IκBα gene by NF-κB (Fig. 1B, first gel, first row) (12, 14, 37, 61). However, treatment with ACHP reduced IκBα phosphorylation in a dose-dependent manner (second and third gels, first row). ACHP (10 μM) maintained the phosphorylation approximately at basal levels throughout the time course experiment (third gel, first row). Because IκBα normally undergoes degradation following phosphorylation, we proceeded to ascertain whether ACHP blocked its degradation. Remarkably, IκBα expression levels were apparently sustained at basal levels, and no degradation was observed when cells were treated with 10 μM ACHP, confirming that the preceding step, IκBα phosphorylation, was inhibited (third gel, second row).
To further explore the status of NF-κB activation in these cells, we also investigated the phosphorylation levels of the p65 subunit of NF-κB. Figure 1B (third row of each gel) shows constitutive phosphorylation of p65 at Ser536, which was markedly induced a minute after TNF-α treatment and gradually decreased in a time-dependent manner. In contrast, pretreatment with ACHP reduced this TNF-α-stimulated phosphorylation of p65 (third gel, third row), whereas the total protein level of p65, like that of IκBα, was maintained at basal levels in the absence or presence of TNF-α (fourth row of each gel). β-Actin levels were unchanged (bottom panels), indicating equal loading of proteins in the gel. These results indicate that ACHP promoted a substantial defect in the TNF-α-induction of NF-κB activation.
We then examined the effect of ACHP on HIV-1 gene expression using a transient luciferase assay. As shown in Fig. 1C, TNF-α stimulated the gene expression from CD12wt containing the wild type HIV-1 LTR (47) by approximately threefold. When cells were pretreated with ACHP, the gene expression was inhibited in a dose-dependent manner (Fig. 1C, left). However, when the NF-κB sites were mutated (CD12mt), no such activation by TNF-α or inhibitory effects of ACHP were observed ((Fig. 1C), right).
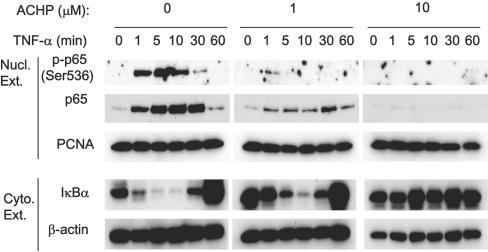
ACHP repressed TNF-α-induced nuclear translocation and phosphorylation of p65.
Because IκBα phosphorylation and its degradation were inhibited by treatment with ACHP, and since both are prerequisite steps for the release and transport of NF-κB to the nucleus, we also examined whether ACHP modulated this event. Following TNF-α stimulation at 1, 5, 10, 30, and 60 min, in either the absence or the presence of ACHP, nuclear extracts from OM10.1 cells were isolated and NF-κB (p65) was examined by Western blotting analysis. TNF-α treatment resulted in a rapid nuclear accumulation of p65 as early as 1 min, followed by increase until 30 min, and then drastically decreased at 60 min (Fig. 2, “Nucl. Ext.,” first gel, second row). Concurrently, IκBα appeared, indicating its nuclear-export function (data not shown). Consistent with previous studies using OM10.1 cells (3, 58, 64), a low level of NF-κB activation, i.e., nuclear localization of p65, was observed in unstimulated cells, which was notably reduced by the presence of ACHP alone (Fig. 2, lanes at 0 min, second row). Whereas TNF-α induced the accumulation of p65 in the nucleus, ACHP almost totally abolished this effect (Fig. 2, “Nucl. Ext.,” third gel, second row), suggesting that the nuclear transport of NF-κB was inhibited. Immunoblots of the cytoplasmic extracts also revealed that 10 μM ACHP maintained IκBα protein levels at basal levels, reaffirming the persistence of latent NF-κB-IκB complexes (Fig. 2, “Cyto. Ext.,” first row of each gel). In addition to nuclear translocation, p65 phosphorylation is also essential for its maximum transcriptional activity (28, 66, 72). Concomitant with the appearance of p65 in the nucleus, rapid phosphorylation of p65 at Ser536 was detected (Fig. 2, “Nucl. Ext.,” first row). This phosphorylation, however, occurred only transiently, followed by a marked reduction after 10 min of TNF-α stimulation, suggesting dephosphorylation of p65. In contrast, no phosphorylation was observed in the presence of 10 μM ACHP, implying that ACHP efficiently blocked the phosphorylation of p65 in the nucleus (Fig. 2, “Nucl. Ext.,” third gel, first row). These observations further strengthen the view that ACHP impairs the IKK activity that enables p65 to translocate to the nucleus and phosphorylates p65.
FIG. 2.
Effect of ACHP on TNF-α-induced p65 translocation and phosphorylation in the nucleus. OM10.1 cells (1 × 106/ml) were pretreated with or without ACHP for an hour and then stimulated with TNF-α (1 ng/ml) for the indicated time periods. Nuclear extracts (Nucl. Ext.) were fractionated by 10% SDS-polyacrylamide gel electrophoresis and immunoblotted with the anti-phospho-p65 (Ser536) antibody (top). The membrane was reprobed with anti-p65 (upper middle) and anti-PCNA (lower middle) antibodies. Cytoplasmic extracts (Cyto. Ext.) were immunoblotted with the anti-IκBα and β-actin antibodies (bottom). The purity of the nuclear and cytoplasmic extracts was confirmed with antibodies specific for PCNA, a known nuclear protein (lower middle) and β-actin, a cytoplasmic protein (bottom), respectively.
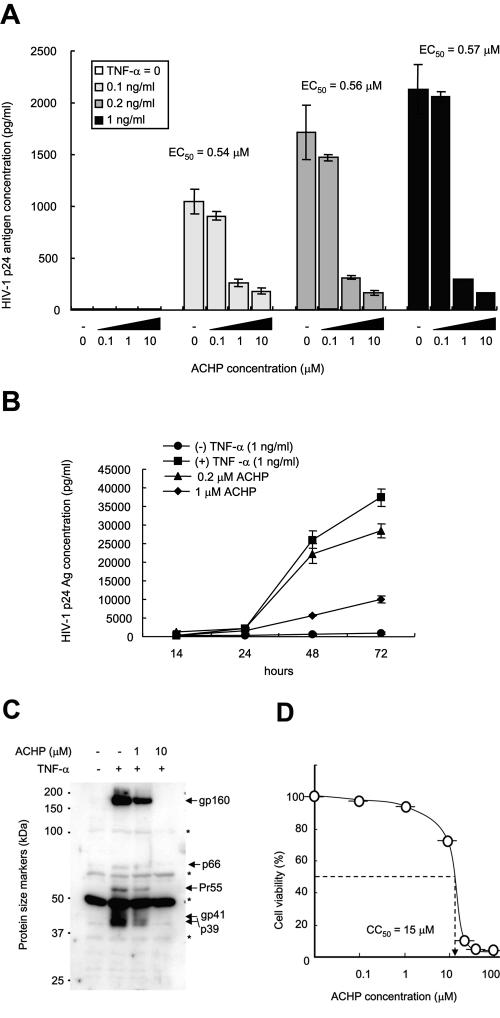
Inhibition of TNF-α-induced HIV-1 production by ACHP from OM10.1 cells.
To examine the effect of ACHP on TNF-α-induced HIV replication in latently infected OM10.1 cells, the cells were treated with various concentrations of TNF-α in the absence or presence of ACHP. After 24 h of incubation, the culture supernatants were collected and assayed for HIV-1 p24 antigen levels. In agreement with previous studies (3, 58, 64), HIV-1 production was dose-dependently induced by TNF-α (Fig. 3A). However, in the presence of ACHP, a dose-dependent inhibition of virus production was observed. A similar effect was also observed in the time course experiment (Fig. 3B). This strongly suggests that ACHP compelled a defect in the TNF-α-induced NF-κB activation in HIV. No significant induction was observed with ACHP alone. An effective concentration for 50% reduction of HIV production was estimated to be approximately 0.56 μM. Meanwhile, the 50% cytotoxic concentration (CC50) was approximately 15 μM, and thus, the estimated therapeutic window of ACHP was approximately 27 (Fig. 3D). Moreover, immunoblotting of whole-cell extracts (Fig. 3C) with the pooled sera from individuals infected with HIV-1 revealed inhibition of the envelope (env) gene products gp160 and gp41 and the gag gene products p66, Pr55, and p39 in the presence of 10 μM ACHP, thus confirming the effect of ACHP on the viral-production step.
FIG. 3.
Effect of ACHP on TNF-α-induced HIV-1 production in latently infected cells. (A) OM10.1 cells (2 × 105/ml) were pretreated with or without ACHP for 1 hour and then stimulated with TNF-α (1 ng/ml) for 24 h. The cell supernatants were then collected and analyzed for HIV-1 p24 antigen levels using a commercial ELISA kit. The data are mean values ± standard deviations of triplicate experiments. EC50, 50% effective concentration. (B) Time course experiment showing the effect of ACHP in OM10.1 cells. Cells (2 × 105/ml) were pretreated in the absence or presence of ACHP for 1 hour and then stimulated with or without TNF-α (1 ng/ml) for 24 h. The cell supernatants were then collected and analyzed for HIV-1 p24 antigen levels using a commercial ELISA kit. The data are mean values ± standard deviations of triplicate experiments. (C) Whole-cell extracts were prepared and immunoblotted with human HIV-1-infected serum for the analysis of HIV-1 viral proteins (whole-cell extracts). The location of each viral product is indicated by an arrow. *, nonspecific bands. (D) Cytotoxicity of ACHP on OM10.1 cells. Cell viability was determined by the WST-1 method, and the CC50 value was extrapolated from this measurement.
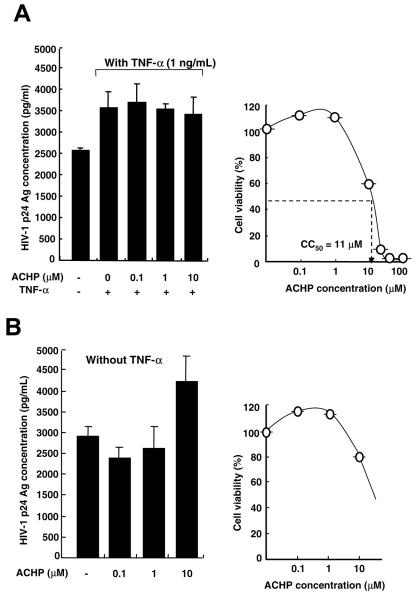
We also explored the effect of ACHP on viral production from MOLT4/IIIB cells chronically infected with HIV-1 (Fig. 4). Whereas ACHP efficiently suppressed viral production in latently infected cells, no significant inhibition was observed in chronically infected cells. A slight decrease in HIV production at 10 μM ACHP, although not statistically significant, was presumably due to a nonspecific cytotoxic effect of ACHP (Fig. 4A, left). The presence of constitutive viral production in the absence of TNF-α suggests the involvement of multiple mechanisms in the regulation of HIV-1 replication (24). From the cytotoxicity profile of ACHP, a CC50 value of 11 μM was obtained for these cells (Fig. 4A, right). There was no effect of ACHP on the level of HIV production without TNF-α (Fig. 4B). These results also imply that the anti-HIV-1 activity of ACHP in chronic infection is cell type specific, as previously reported by others (46).
FIG. 4.
Effect of ACHP on chronically HIV-1 infected cells. (A and B, left) Effect of ACHP on HIV-1 production. MOLT4/IIIB cells (2 × 105/ml) were pretreated with or without ACHP for 1 hour and then stimulated with or without TNF-α for 24 h. Cell supernatants were then collected and analyzed for HIV-1 p24 antigen levels by ELISA. (A and B, right) Cytotoxicity of ACHP on MOLT4/IIIB cells. Cell viability was determined by the WST-1 assay. The CC50 value was extrapolated from this measurement. The data are mean values plus standard deviations of triplicate experiments.
ACHP suppressed NF-κB binding to HIV-1 LTR.
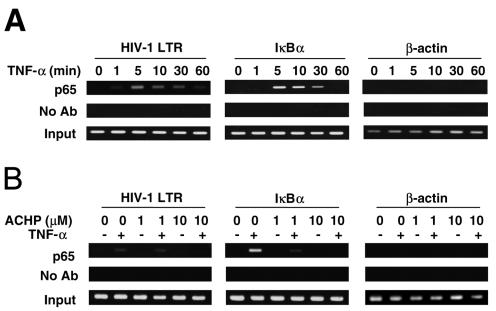
HIV-1 replication, particularly in latently infected cells, such as OM10.1, is promoted by NF-κB binding to the HIV-1 LTR upon induction with TNF-α (37, 44, 55). To assess whether NF-κB binding to the HIV-1 LTR is inhibited by ACHP, ChIP assays were performed. OM10.1 cells were preincubated with ACHP and stimulated with TNF-α for 10 min, the time at which maximum NF-κB binding was observed in repeated experiments. Following stimulation, the cells were cross-linked and lysed, and DNA was sheared by sonication. The lysates were immunoprecipitated with anti-p65 antibody and agarose beads, and DNA-protein cross-links were reversed. The p65-immunoprecipitated samples and controls were probed by PCR for HIV-1 LTR sequence containing NF-κB binding sites. In agreement with our Western blotting results, the kinetics of NF-κB binding to the HIV-1 LTR showed a rapid recruitment of NF-κB (p65) to the promoter in response to TNF-α (Fig. 5A, first gel, first row). A remarkable association was observed until 30 min and thereafter decreased to an almost undetectable level, suggesting the cytoplasmic export of p65. The presence of constitutive, almost undetectable NF-κB (p65) binding in the HIV-1 LTR in unstimulated cells again correlated with our Western blot analysis of nuclear p65, thus conforming to previous studies (19, 26). Similarly, NF-κB (p65) binding in the IκBα promoter was also detected, although with a slower onset of association (Fig. 5A, second gel, first row).
FIG. 5.
ChIP assays for NF-κB (p65) binding to promoters. (A) Kinetics of TNF-α-induced NF-κB binding to the HIV-1 LTR. Ab, antibody. (B) ACHP-mediated inhibition of TNF-α-induced NF-κB binding to the HIV-1 LTR. OM10.1 cells (1 × 105/ml) were treated with TNF-α (1 ng/ml) for the indicated time periods, and ChIP assays were performed with anti-p65 antibody or no antibody (negative control). The detection of the immunoprecipitated DNAs in the HIV-1 LTR (first gel), IκBα (second gel), or β-actin (third gel) promoter was analyzed by PCR with promoter-specific primers. Input DNA represents total input chromatin (1%).
In contrast, in the presence of ACHP, both basal and TNF-α-induced p65 recruitment in both promoters was abolished in a dose-dependent manner (Fig. 5B, first and second gels, first row). No amplification was detected with the β-actin promoter (internal control) or in the absence of anti-p65 antibody, confirming the specificity of the DNA immunoprecipitation (Fig. 5B, second row of each gel). Collectively, these findings suggest that ACHP can efficiently block the binding of NF-κB to the HIV-1 LTR promoter.
DISCUSSION
In this study, we have addressed the question of whether a novel IKK inhibitor, ACHP, can inhibit HIV-1 replication in a macrophage/monocyte cell line latently infected with the virus. We observed that TNF-α-mediated NF-κB signaling could efficiently induce HIV-1 replication, which was subsequently blocked by ACHP. IκBα phosphorylation and degradation, p65 nuclear translocation, and p65 phosphorylation at Ser536 were effectively inhibited by ACHP. Moreover, ACHP suppressed HIV-1 LTR-driven gene expression through the inhibition of the NF-κB activation pathway. We also found, using a ChIP assay, that TNF-α could activate NF-κB (p65)-DNA binding to the HIV-1 LTR in OM10.1 cells and that treatment with ACHP could abolish its binding.
Although NF-κB plays a central role in mediating inducible HIV-1 gene expression (44, 48, 52), the coordinated HIV-1 replication with the cellular activation is partially ascribed to the ability of HIV to assimilate host signaling pathways to activate viral transcription (24, 55). An essential step in the stimulus-induced activation of the canonical NF-κB pathway is the phosphorylation of IκB proteins by the IKK complex (69). In addition, Asin et al. (2) demonstrated that HIV infection itself could induce NF-κB activation through the canonical pathway involving activation, that is, the Ser32 and Ser36 phosphorylation of IκBα by IKK-β, thus highlighting the role of NF-κB in HIV latent infection (11). In fact, transdominant mutants of IκBα that block NF-κB induction inhibited de novo HIV-1 infection in T cells by interfering with viral transcription (32, 54). Furthermore, CD4 engagement with gp120 selectively enhanced IKK activity and mediated the phosphorylation of IκBα, while dominant-negative forms of IKKs inhibited gp120-induced NF-κB activation (11). Moreover, recruitment of NF-κB to the HIV promoter is considered essential for the action of Tat and efficient transcriptional elongation (9, 67). Recent studies using ChIP assays have indicated the importance of IKK-α for induction of NF-κB-mediated gene expression by forming a complex with p65 or CBP in these promoter regions and thus regulating histone H3 phosphorylation, followed by acetylation of CBP (1, 70).
Whereas IKK-β is largely responsible for cytokine-induced IκBα phosphorylation and NF-κB activation (22, 35, 36), IKK-α was initially implicated in more specified biological actions, such as formation of secondary lymphoid tissues (39, 62). However, recent studies have revealed an essential role of IKK-α in the noncanonical/alternative pathway of NF-κB activation, such as lymphotoxin β receptor signaling (28) and NIK-induced p100 processing (16, 17, 18, 22, 60). Moreover, IKK-α has been implicated in the phosphorylation of p65 at Ser536 (7, 28, 66, 72). Of note, the point mutation of Ser536 eventually resulted in the failure of nuclear translocation of NF-κB (40). In addition, since Ser536 is located in the carboxyl-terminal transactivation domain of p65, it is postulated that the phosphorylated NF-κB (p65 at Ser536), once bound in the target DNA, might further recruit basal transcription factors and transcriptional coactivators, thereby increasing the transcriptional competence of NF-κB (p65) (28).
In spite of a robust effect in latently infected cells, ACHP did not show a significant inhibitory effect in chronically infected cells in which HIV-1 was actively replicating. The reason for the unresponsiveness of these cells to the compound is unclear. A previous study of the effects of an NF-κB inhibitor (4, 46) against chronic HIV infection reported it to be ineffective in suppressing constitutive HIV-1 production, which is consistent with what we observed in this study. Hence, it is also possible that the cellular uptake or intracellular metabolism of ACHP is different in OM10.1 and MOLT4/IIIB cells. Another plausible explanation is that the constitutive activation of NF-κB in chronically infected T cells (24, 31, 55) perpetuates the production of other viral proteins, such as Tat and Nef, yielding more important roles for such proteins in activating and regulating the expression of HIV-1 than for NF-κB in these cells. Thus, NF-κB-independent mechanisms appear to be operating in chronic HIV-1 infection.
Although it is evident that ACHP is not potent in actively replicating cells, considering the presence of latent reservoirs that are sources of viral rebounds and its contribution to disease progression (24), a rationale exists for the use of the compound in HIV-1 infection. Apparently, given its mechanism, use of ACHP might cause aberrant regulation of inflammatory cytokines (5, 10, 43). Therefore, further studies are needed to further evaluate its feasibility as a potential drug candidate for novel anti-HIV therapy.
Acknowledgments
We thank T. Murata and T. Hamano for providing ACHP and pooled sera from HIV-infected individuals, respectively. We also thank T. Sanda for his helpful technical comments throughout this study.
This work was supported in part by grants-in-aid from the Ministry of Health, Labor and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Anest, V., J. L. Hanson, P. C. Cogswell, K. A. Steinbrecher, B. D. Strahl, and A. S. Baldwin. 2003. A nucleosomal function of IκB kinase-α in NF-κB-dependent gene expression. Nature 423:659-663. [DOI] [PubMed] [Google Scholar]
- 2.Asin, S., J. A. Taylor, S. Trushin, G. Bren, and C. V. Paya. 1999. IKK mediates NF-κB activation in human immunodeficiency virus-infected cells. J. Virol. 73:3893-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, M., M. Okamoto, and T. Okamoto. 1999. Inhibitor of human immunodeficiency virus type 1 replication in acutely and chronically infected cells by EM2487, a novel substance produced by a Streptomyces species. Antimicrob. Agents Chemother. 43:2350-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba, M., M. Okamoto, N. Kashiwaba, and M. Ono. 2001. Anti-HIV-1 activity and structure-activity relationship of cepharanoline derivatives in chronically infected cells. Antivir. Chem. Chemother. 12:307-312. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 6.Baeuerle, P. A., and D. Baltimore. 1988. I-κB: a specific inhibitor of the NF-κB transcription factor. Science 242:540. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin, A. S. J. 2001. The transcription factor NF-κB and human disease. J. Clin. Investig. 107:3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbeau, B., B. Bernice, N. Dumais, G. Briand, M. Olivier, R. Faure, B. Posner, and M. Tremblay. 1997. Activation of HIV-1 long terminal repeat transcription and virus replication via NF-κB-dependent and -independent pathways by potent phosphotyrosine phosphatase inhibitors, the peroxovanadium compounds. J. Biol. Chem. 272:12968-12977. [DOI] [PubMed] [Google Scholar]
- 9.Barboric, M., R. M. Nissen, S. Kanazawa, M. Jabrane-Ferrat, and B. M. Peterlin. 2001. NF-κB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol. Cell 8:327-337. [DOI] [PubMed] [Google Scholar]
- 10.Bonizzi, G., and M. Karin. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. [DOI] [PubMed] [Google Scholar]
- 11.Bossis, G., S. Salinas, C. Cartier, C. Devaux, and L. Briant. 2002. NF-κB activation upon interaction of HIV-1 envelope glycoproteins with cell surface CD4 involves IκB kinases. FEBS Lett. 516:257-264. [DOI] [PubMed] [Google Scholar]
- 12.Brown, K., S. Park, T. Kanno, G. Franzoso, and U. Siebenlist. 1993. Mutual regulation of the transcriptional activator NF-κB and its inhibitor, IκBα. Proc. Natl. Acad. Sci. USA 91:2532-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butera, S. T., V. L. Rerez, B. Y. Wu, G. J. Nabel, and T. M. Folks. 1991. Oscillation of the human immunodeficiency virus surface receptor is regulated by the state of activation in a CD4+ cell model of chronic infection. J. Virol. 65:4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiao, P. J., S. Miyamoto, and I. Verma. 1994. Autoregulation of IκBα activity. Proc. Natl. Acad. Sci. USA 91:22-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun, T. W., R. T. Davey Jr., M. Ostrowski, J. S. Justemente, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 16.Claudio, E., K. Brown, S. Park, H. Wang, and U. Siebenlist. 2002. BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat. Immunol. 3:958-965. [DOI] [PubMed] [Google Scholar]
- 17.Coope, H. J., P. G. P. Atkinson, B. Huhse, M. Belich, J. Janzen, M. J. Holman, G. G. B. Klaus, L. H. Johnston, and S. C. Ley. 2002. CD40 regulates the processing of NF-κB2 p100 to p52. EMBO J. 21:5375-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejardin, E., N. M. Droin, M. Delhase, E. Haas, Y. Cao, C. Makris, Z.-W. Li, M. Karin, C. F. Ware, and D. R. Green. 2002. The lymphotoxin β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 17:525-535. [DOI] [PubMed] [Google Scholar]
- 19.DeLuca, C., A. Roulston, A. Koromilas, M. A. Wainberg, and J. Hiscott. 1996. Chronic human immunodeficiency virus type 1 infection of myeloid cells disrupts the autoregulatory control of the NF-κB/Rel pathway via enhanced IκBα degradation. J. Virol. 70:5183-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devadas, K., N. J. Hardegen, L. M. Wahl, I. K. Hewlett, K. A. Clouse, K. M. Yamada, and S. Dhawan. 2004. Mechanisms for macrophage-mediated HIV-1 induction. J. Immunol. 173:6735-6744. [DOI] [PubMed] [Google Scholar]
- 21.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh, S., and M. Karin. 2002. Missing pieces in NF-κB puzzle. Cell 109:S81-S96. [DOI] [PubMed] [Google Scholar]
- 23.Haefner, B. 2002. NF-κB: arresting a major culprit in cancer. Drug Discov. Today 7:653-663. [DOI] [PubMed] [Google Scholar]
- 24.Hiscott, J., H. Kwon, and P. Genin. 2001. Hostile takeovers: viral appropriation of the NF-κB pathway. J. Immunol. 107:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai, K., K. Nakata, K. Kawai, T. Hamano, N. Mei, H. Kasai, and T. Okamoto. 2005. Induction of OGG1 gene expression by HIV-1 Tat. J. Biol. Chem. 280:26701-26713. [DOI] [PubMed] [Google Scholar]
- 26.Jacque, J. M., B. Fernandez, F. Arenzana-Seisdedos, D. Thomas, F. Baleux, J. L. Virelizier, and F. Bachelerie. 1996. Permanent occupancy of the human immunodeficiency virus type 1 enhancer by NF-κB is needed for persistent viral replication in monocytes. J. Virol. 70:2930-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang, X., N. Takahashi, K. Ando, T. Otsuka, T. Tetsuka, and T. Okamoto. 2003. NF-κB p65 transactivation domain is involved in the NF-κB-inducing kinase pathway. Biochem. Biophys. Res. Commun. 301:583-590. [DOI] [PubMed] [Google Scholar]
- 28.Jiang, X., N. Takahashi, N. Matsui, T. Tetsuka, and T. Okamoto. 2003. The NF-κB activation in lymphotoxin β receptor signaling depends on the phosphorylation of p65 at serine 536. J. Biol. Chem. 278:919-926. [DOI] [PubMed] [Google Scholar]
- 29.Jones, K. A., and B. M. Peterlin. 1994. Control of RNA initiation and elongation at the HIV-1 promoter. Annu. Rev. Biochem. 63:717-743. [DOI] [PubMed] [Google Scholar]
- 30.Karin, M., Y. Yamamoto, and Q. M. Wang. 2004. The IKK NF-κB system: a treasure trove for drug development. Nat. Rev. Drug Discov. 3:17-26. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan, V., and S. L. Zeichner. 2004. Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency. J. Virol. 78:9458-9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon, H., N. Pelletier, C. DeLuca, P. Genin, S. Cisternas, R. Lin, M. A. Wainberg, and J. Hiscott. 1998. Inducible expression of IκBα repressor mutants interferes with NF-κB activity and HIV-1 replication in Jurkat T cells. J. Biol. Chem. 273:7431-7440. [DOI] [PubMed] [Google Scholar]
- 33.Lee, R., P. Beauparlant, H. Elford, P. Ponka, and J. Hiscott. 1997. Selective inhibition of IκBα and HIV-1 LTR directed gene expression by novel antioxidant compounds. Virology 234:277-290. [DOI] [PubMed] [Google Scholar]
- 34.Leonard, J., C. Parrott, A. J. Buckler-White, W. Turner, E. K. Ross, M. A. Martin, and A. B. Rabson. 1989. The NF-κB binding sites in the human immunodeficiency virus type 1 long terminal repeat are not required for virus infectivity. J. Virol. 63:4919-4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Q., D. V. Antwerp, F. Mercurio, K.-F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 36.Li, Z. W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor-κB activation and prevention of apoptosis. J. Exp. Med. 189:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lusic, M., A. Marcello, A. Cereseto, and M. Giacca. 2003. Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter. EMBO J. 22:6550-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallardo, M., E. Dragonetti, F. Baldassarre, C. Ambrosino, G. Scala, and I. Quinto. 1996. An NF-κB site in the 5′-untranslated leader region of the human immunodeficiency virus type 1 enhances the viral expression in response to NF-κB-activating stimuli. J. Biol. Chem. 271:20820-20827. [DOI] [PubMed] [Google Scholar]
- 39.Matsushima, A., T. Kaisho, P. D. Rennert, H. Nakano, K. Kurosawa, D. Uchida, K. Takeda, S. Akira, and M. Matsumoto. 2001. Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J. Exp. Med. 193:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattioli, I., A. Sebald, C. Bucher, R. P. Charles, H. Nakano, T. Doi, M. Kracht, and M. L. Schmitz. 2004. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase β and controls the kinetics of p65 nuclear import. J. Immunol. 172:6336-6344. [DOI] [PubMed] [Google Scholar]
- 41.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 42.Murata, T., M. Shimada, S. Sakakibara, T. Yoshino, H. Kadono, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami, K. Sakai, H. Inbe, K. Takeshita, T. Niki, M. Umeda, K. B. Bacon, K. B. Ziegelbauer, and T. B. Lowinger. 2003. Discovery of novel and selective IKK-β serine-threonine protein kinase inhibitors. Part 1. Bioorg. Med. Chem. Lett. 13:913-918. [DOI] [PubMed] [Google Scholar]
- 43.Murata, T., M. Shimada, S. Sakakibara, T. Yoshino, T. Masuda, T. Shintani, H. Sato, Y. Koriyama, K. Fukushima, N. Nunami, M. Yamauchi, K. Fuchikami, H. Komura, A. Watanabe, K. B. Ziegelbauer, K. B. Bacon, and T. B. Lowinger. 2004. Synthesis and structure-activity relationships of novel IKK-β inhibitors. Part 3. Orally active anti-inflammatory agents. Bioorg. Med. Chem. Lett. 14:4019-4022. [DOI] [PubMed] [Google Scholar]
- 44.Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326:711-713. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto, H., T. P. Cujec, M. Okamoto, B. M. Peterlin, M. Baba, and T. Okamoto. 2000. Inhibition of the RNA-dependent transactivation and replication of human immunodeficiency virus type 1 by a fluoroquinoline derivative K-37. Virology 272:402-408. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto, M., M. Ono, and M. Baba. 1998. Potent inhibition of HIV type 1 replication by an anti-inflammatory alkaloid, cepharantine, in chronically infected monocytic cells. AIDS Res. Hum. Retrovir. 14:1239-1245. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto, T., and F. Wong-Staal. 1986. Demonstration of virus-specific transcriptional activator(s) in cells infected with HTLV-III by an in vitro cell-free system. Cell 47:29-35. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto, T., S. Sakurada, J. P. Yang, and J. P. Merin. 1997. Regulation of NF-κB and disease control: identification of a novel serine kinase and thioredoxin as effectors for signal transduction pathway for NF-κB activation. Curr. Top. Cell. Regul. 35:149-161. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto, T., T. Benter, S. F. Josephs, M. R. Sadie, and F. Wong-Staal. 1990. Transcriptional activation from the long terminal repeat of human immunodeficiency virus in vitro. Virology 177:606-614. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto, T., T. Matsuyama, S. Mori, S. Hamamoto, N. Kobayashi, N. Yamamoto, S. F. Josephs, F. Wong-Staal, and K. Shimotohno. 1989. Augmentation of human immunodeficiency virus type 1 gene expression by tumor necrosis factor-α. AIDS Res. Hum. Retrovir. 5:131-138. [DOI] [PubMed] [Google Scholar]
- 51.Persaud, D., Y. Zhou, J. M. Siliciano, and R. F. Siliciano. 2003. Latency in human immunodeficiency virus type 1 infection: no easy answers. J. Virol. 77:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterlin, B. M., and D. Trono. 2003. Hide, shield and strike back: how human immunodeficiency virus-infected cells avoid immune eradication. Nat. Rev. Immunol. 3:97-107. [DOI] [PubMed] [Google Scholar]
- 53.Pomerantz, R. J. 2002. Reservoirs of human immunodeficiency virus type 1: the main obstacles to viral eradication. Clin. Infect. Dis. 34:91-97. [DOI] [PubMed] [Google Scholar]
- 54.Quinto, I., M. Mallardo, F. Baldassarre, G. Scala, G. Englund, and K. T. Jeang. 1999. Potent and stable attenuation of live-HIV-1 by gain of a proteolysis-resistant inhibitor of NF-κB (IκB-αS32/36A) and the implications for vaccine development. J. Biol. Chem. 274:17567-17572. [DOI] [PubMed] [Google Scholar]
- 55.Roulston, A., R. Lin, P. Beauparlant, M. A. Wainberg, and J. Hiscott. 1995. Regulation of HIV-1 and cytokine gene expression in myeloid cells by NF-κB/Rel transcription factors. Microbiol. Rev. 59:481-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakurai, H., S. Suzuki, N Kawasaki, H. Nakano, T. Okazaki, A. Chino, T. Doi, and I. Saiki. 2003. Tumor necrosis factor-α-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 278:36916-36923. [DOI] [PubMed] [Google Scholar]
- 57.Sanda, T., S. Iida, H. Ogura, K. Asamitsu, T. Murata, K. B. Bacon, R. Ueda, and T. Okamoto. 2005. Growth inhibition of multiple myeloma cells by a novel IκB inhibitor. Clin. Cancer Res. 11:1974-1982. [DOI] [PubMed] [Google Scholar]
- 58.Sarol, L. C., K. Imai, K. Asamitsu, T. Tetsuka, N. G. Barzaga, and T. Okamoto. Inhibitory effects of IFN-γ on HIV-1 replication in latently infected cells. Biochem. Biophys. Res. Commun. 291:890-896. [DOI] [PubMed]
- 59.Sato, T., K. Asamitsu, J. P. Yang, N. Takahashi, T. Tetsuka, A. Yoneyama, A. Kanagawa, and T. Okamoto. 1998. Inhibition of human immunodeficiency virus type 1 replication by a bioavailable serine/threonine kinase inhibitor, fasudil hydrochloride. AIDS Res. Hum. Retrovir. 14:293-298. [DOI] [PubMed] [Google Scholar]
- 60.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S. C. Sun, and M. Karin. 2001. Activation of IKK-α of a second, evolutionary conserved, NF-κB signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 61.Sun, S. C., P. A. Ganchi, D. W. Ballard, and W. C. Greene. 1993. NF-κB controls expression of inhibitor IκBα. Evidence for an inducible autoregulatory pathway. Science 259:1912-1915. [DOI] [PubMed] [Google Scholar]
- 62.Takeda, K., O. Takeuchi, T. Tsujimura, S. Itami, O. Adachi, T. Kawai, H. Sanjo, K. Yoshikawa, N. Terada, and S. Akira. 1999. Limb and skin abnormalities in mice lacking IKK-α. Science 284:313-316. [DOI] [PubMed] [Google Scholar]
- 63.Teranishi, F., Z.-Q. Liu, M. Kunimatsu, K. Imai, H. Takeyama, T. Manabe, M. Sasaki, and T. Okamoto. 2003. Calpain is involved in the HIV replication from the latently infected OM10.1 cells. Biochem. Biophys. Res. Commun. 303:940-946. [DOI] [PubMed] [Google Scholar]
- 64.Traber, K. E., H. Okamoto, C. Kurono, M. Baba, C. Saliou, T. Soji, L. Packer, and T. Okamoto. 1999. Anti-rheumatic compound aurothioglucose inhibits tumor necrosis factor-α-induced HIV-1 replication in latently infected OM10.1 and Ach2 cells. Int. Immunol. 11:143-150. [DOI] [PubMed] [Google Scholar]
- 65.Viatour, P., M. P. Merville, V. Bours, and A. Chariot. 2005. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem. Sci. 30:43-52. [DOI] [PubMed] [Google Scholar]
- 66.Wang, D., and A. S. Baldwin. 1998. Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-α is mediated through phosphorylation of RelA/p65 on Serine 529. J. Biol. Chem. 273:29411-29416. [DOI] [PubMed] [Google Scholar]
- 67.West, M. J., A. D. Lowe, and J. Karn. 2001. Activation of human immunodeficiency virus transcription in T cells revisited: NF-κB p65 stimulates transcriptional elongation. J. Virol. 75:8524-8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao, G., E. W. Harhaj, and S. C. Sun. 2001. NF-κB-inducing kinase regulates processing of NF-κB2 p100. Mol. Cell 7:401-409. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto, Y., and R. B. Gaynor. 2004. IκB kinases: key regulators of the NF-κB pathway. Trends Biochem. Sci. 29:72-79. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto, Y., U. N. Verma, S. Prajapati, Y. T. Kwak, and R. B. Gaynor. 2003. Histone H3 phosphorylation by IKK-α is critical for cytokine-induced gene expression. Nature 423:655-659. [DOI] [PubMed] [Google Scholar]
- 71.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 991:243-252. [DOI] [PubMed] [Google Scholar]
- 72.Zhong, H., H. SuYang, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1997. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413-424. [DOI] [PubMed] [Google Scholar]