Abstract
The pharmacokinetic disposition of telavancin administered 7.5 mg/kg of body weight every 24 h was determined in plasma and skin blister fluid. The mean penetration of telavancin into blister fluid was 40%. This study reveals that adequate concentrations are achieved in both plasma and blister fluid for pathogens frequently implicated in skin and soft tissue infections.
Telavancin is a semisynthetic glycopeptide antibiotic currently undergoing development for the treatment of infections caused by clinically important gram-positive bacteria (5). Due to the concern of rising resistance rates among gram-positive pathogens, the development of new antibiotics with the ability to overcome current resistance mechanisms is essential. Telavancin displays rapid, bactericidal, concentration-dependent activity against clinically important gram-positive aerobic pathogens, such as Staphylococcus aureus (methicillin susceptible and methicillin resistant) (3, 8, 10). Telavancin exhibits its killing effects via multiple mechanisms of action, including inhibition of peptidoglycan synthesis and disruption of cell membrane integrity (7, 10). Telavancin has also been shown to have a long postantibiotic effect (PAE) (≥4 h) against S. aureus (3, 10).
Currently, there are limited pharmacokinetic data available for telavancin, and no studies regarding the ability for telavancin to penetrate into skin structures have been published. The evaluation of drug concentrations by using the cantharidin-induced skin blister model attempts to mimic situations within an infected tissue compartment. Previous studies using this model have been successfully performed at our institution (9, 13). The primary objective of this study was to determine the steady-state pharmacokinetic profile of telavancin in plasma and blister fluid when telavancin is administered intravenously over one hour as 7.5 mg/kg of body weight every 24 h for a total of three doses. Safety was also assessed.
This study was approved by Hartford Hospital's Institutional Review Board. All subjects were given a detailed description of the study, and all provided written informed consent. Nine healthy subjects (eight males and one female) were enrolled in this single-center, open-label study. One male subject was withdrawn due to elevated serum transaminases and LDH levels, which were unrelated to study drug. Thus, only eight subjects completed the study. The subjects were 21 to 46 years of age (mean age, 23.9 years) and 134 to 252 lb in weight (mean weight, 176.7 lb). Participation included a screening evaluation within 3 weeks of drug administration on day 1 and a 5-day (4-night) inpatient period, with a follow-up visit 72 h after the last dose of study drug. Subjects were enrolled after the screening evaluation and laboratory evaluations (including hematologies, blood chemistries, and urinalyses) revealed no clinically significant abnormalities.
Each subject received a one-hour infusion of telavancin (Theravance, Inc., South San Fransisco, CA), at a dose of 7.5 mg/kg (based on subjects' weights on study day 0), every 24 h for a total of three doses. Subjects were allowed to consume a light meal approximately one hour prior to the start of the infusion. Water and fruit juice were allowed after the completion of the infusion, and food was allowed approximately 4 h following the completion of drug administration. No tobacco, alcohol, grapefruit juice, caffeinated tea, chocolate, or caffeinated coffee, or any other caffeine-containing product was permitted during the study.
Approximately 14 h prior to the start of the final dose of telavancin, six drops (each drop measuring 0.2 ml) of 0.25% cantharidin ointment made from cantharidin powder (Sigma Laboratories, St. Louis, MO) and standard ointment base were applied to the anterior forearms of the subjects to produce six skin blisters per subject. The seventh and final blister was induced using the same methodology as described above approximately 14 h prior to the final sample collection on study day 4.
Blood samples were collected from an indwelling catheter into blood collection tubes containing sodium heparin. Blood sampling was performed just prior to administration of the third and final dose of drug (0 h) and at 2, 4, 6, 8, 12, and 24 h. Blood samples were centrifuged at 4°C at 1,000 × g for 10 min. Plasma samples were then collected and stored at −80°C until they were shipped for analysis. Skin blister fluid samples (100 to 200 μl in volume) were collected using a 28-gauge needle at simultaneous time points and were stored at −80°C until they were shipped for analysis.
All samples were analyzed by validated reverse phase, high-performance liquid chromatography-tandem mass spectrometry methodology. Due to the limited availability of the blister matrix, an artificial matrix (70% human plasma in normal saline) was used to mimic the study sample composition. As a result, a cross-matrix validation between this proxy matrix and blank blister fluid was undertaken; the recovery of study drug was >90% in both. The lower limits of quantitation for telavancin in human plasma and blister fluid were 250 and 10 ng/ml, respectively. Mean interassay precision (≤4.9% coefficient of variation [CV], n = 18) and accuracy (≤±2.5% deviation of the mean from the theoretical mean [DMT], n = 18) of quality control samples were obtained for plasma over the linear range of 250 to 100,000 ng/ml. Mean interassay precision (≤5.7% CV, n = 18) and accuracy (≤±5.5% DMT, n = 18) of quality control samples were obtained for skin blister fluid over the linear range of 10 to 5,000 ng/ml. The overall precision and accuracy of the quality control samples and standards analyzed during the course of the study were better than 15% for all concentrations.
The following pharmacokinetic parameters were estimated by noncompartmental analysis using WinNonlin software (version 3.2; Pharsight Corportation, Mountain View, CA): the maximum concentration of drug in serum and blister fluid (Cmax), the time at which the maximum concentration was reached (Tmax), the area under the curve at steady state (AUCss), the elimination half-life, the clearance, mean residence time, the volume of distribution at steady state (for plasma only), and the Cmin. The penetration of telavancin into blister fluid was determined by comparing the AUC in blister fluid with that in plasma.
Telavancin was well tolerated among the subjects. The most commonly reported adverse events were the presence of dysgeusia (n = 8) and foamy urine (n = 7). The etiology of the foamy urine was most likely due to the excretion of cyclodextrin, a solubilizing agent that is incorporated into the intravenous formulation of telavancin. Both events were mild in intensity and resolved within the study period without any interventions or discontinuations from the study.
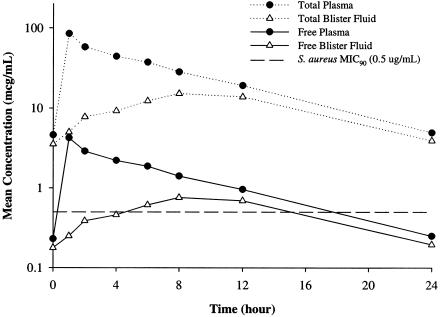
The mean concentration-versus-time profile of plasma and blister fluid for the eight subjects who completed the study is illustrated in Fig. 1. The mean pharmacokinetic parameters ± standard deviations found for telavancin in plasma and blister fluid are displayed in Table 1. The peak telavancin level ± standard deviation in plasma was 84.8 ± 5.3 μg/ml, the mean elimination half-life in plasma was 6.26 ± 0.78 h, and the mean AUCss in plasma was 604 ± 83 μg · h/ml. The Tmax in skin blister fluid occurred at 9.3 ± 2.4 h. The mean Cmax of telavancin in blister fluid was 16.0 ± 2.0 μg/ml, the mean elimination half-life in blister fluid was 6.91 ± 0.53 h, and the mean AUCss in blister fluid was 241 ± 33 μg · h/ml. Peak telavancin concentrations in skin blister fluid lagged behind peak concentrations in plasma, but the elimination half-lives remained similar. Telavancin levels were detectable in both plasma and skin blister fluid at all time points. Over the dosing interval, the mean (± standard deviation) ratio of AUCss obtained in blister fluid compared to that in plasma was 40.3% (± 5.8%).
FIG. 1.
Mean total and estimated free plasma and blister fluid concentrations of telavancin.
TABLE 1.
Pharmacokinetic parameters of telavancin in plasma and blister fluid
| Parametera | Mean ± SD inb:
|
|
|---|---|---|
| Plasma | Blister fluid | |
| Cmax (μg/ml) | 84.8 ± 5.3 | 16.0 ± 2.0 |
| Tmax (h) | 1.0 ± 0.0 | 9.3 ± 2.4 |
| AUCss (μg · h/ml) | 604 ± 83 | 241 ± 33 |
| Elimination t1/2 (h) | 6.26 ± 0.78 | 6.91 ± 0.53 |
| CL (ml/h/kg) | 11.8 ± 2.1 | |
| MRT (h) | 6.57 ± 0.42 | 10.3 ± 0.6 |
| Vss (ml/kg) | 98.0 ± 14.8 | |
t1/2, half-life; CL, clearance; MRT, mean residence time; Vss, volume of distribution at steady state.
Pharmacokinetics was determined after the administration of 7.5 mg/kg every 24 hours to healthy subjects (n = 8).
At a time when antimicrobial resistance rates are continually rising (5, 11), it is essential for the development of new antimicrobial agents with the ability to combat current resistance mechanisms. Telavancin is a novel glycopeptide antibiotic currently under clinical development. It displays potent in vitro activity against clinically important gram-positive organisms, with MIC90s of ≤1 μg/ml for Staphylococcus aureus (including methicillin-resistant S. aureus), ≤0.125 μg/ml for Streptococcus spp., ≤1 μg/ml for vancomycin-susceptible Enterococcus spp., and 4 μg/ml for vancomycin-resistant Enterococcus spp. (8, 10). Skin and soft tissue infections are often primarily caused by gram-positive pathogens such as S. aureus, Streptococcus pyogenes, Streptococcus agalactiae, and Enterococcus spp. (1, 2, 6, 11). Telavancin exhibits excellent in vitro activity against these agents.
Telavancin is highly protein bound, at approximately 95% (4). Although only total drug concentrations were determined in the current study, Fig. 1 displays both total and estimated free telavancin concentrations in plasma and blister fluid. Estimated free concentrations represent 5% of the total drug concentrations. This is a conservative approach for blister fluid, since protein content and binding ability in this matrix has not been clearly elucidated. Using a S. aureus MIC of 0.5 μg/ml, mean free concentrations of telavancin are above the MICs for approximately 70% and 40% of the dosing intervals for plasma and blister fluid, respectively. In a study by Hedge and colleagues, in which the pharmacodynamics of telavancin was determined in a neutropenic murine thigh infection model, the AUC/MIC ratio was found to be the most predictive parameter describing telavancin's bactericidal activity (4). These authors found that AUC/MIC ratios of approximately 100 for total and 7 for free telavancin concentrations resulted in maximal kill at 24 h. From our current investigation using a S. aureus MIC of 0.5 μg/ml, the derived total and free AUC/MIC ratios are 1,208 and 60.4, respectively, in plasma and 482 and 24.1, respectively, in blister fluid.
This was the first study investigating the penetration of telavancin into skin blister fluid and televancin's pharmacokinetics. Treatment for skin and soft tissue infections can be problematic if the antibiotic does not achieve adequate concentrations at the site of infection. Our study has shown that in both plasma and blister fluid, adequate drug concentrations are achieved that are above the MIC for those pathogens often implicated in skin and soft tissue infections such as S. aureus (including methicillin-resistant S. aureus) and Streptococcus spp. Moreover, AUC/MIC exposures for both total and estimated free concentrations in plasma and blister fluid appear more than adequate to ensure microbiologic eradication. This study is consistent with results from phase II studies indicating telavancin's potential role as a treatment alternative for complicated skin and skin structure infections caused by resistant gram-positive pathogens (12).
Acknowledgments
This study was supported through a research grant from Theravance, Inc. (South San Francisco, CA).
We thank Dana Maglio for her assistance in completing this study.
REFERENCES
- 1.Eron, L. J., B. A. Lipsky, D. E. Low, D. Nathwani, A. D. Tice, and G. A. Volturo. 2003. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J. Antimicrob. Chemother. 52:i3-i17. [DOI] [PubMed] [Google Scholar]
- 2.File, T. M., Jr., and J. S. Tan. 1995. Treatment of skin and soft-tissue infections. Am. J. Surg. 169:27S-33S. [PubMed] [Google Scholar]
- 3.Goldstein, E. J., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrrell, and H. T. Fernandez. 2004. In vitro activities of the new semisynthetic glycopeptide telavancin (TD-6424), vancomycin, daptomycin, linezolid, and four comparator agents against anaerobic gram-positive species and Corynebacterium spp. Antimicrob. Agents Chemother. 48:2149-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedge, S. S., N. Reyes, T. Wiens, N. Vanasse, R. Skinner, J. McCullough, K. Kaniga, J. Pace, R. Thomas, J. Shaw, G. Obedencio, and J. K. Judice. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoban, D., K. Waites, and D. Felmingham. 2003. Antimicrobial susceptibility of community-acquired respiratory tract pathogens in North America in 1999-2000: findings of the PROTEKT surveillance study. Diagn. Microbiol. Infect. Dis. 45:251-259. [DOI] [PubMed] [Google Scholar]
- 6.Jones, M. E., J. A. Karlowsky, D. C. Draghi, C. Thornsberry, D. F. Sahm, and D. Nathwani. 2003. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int. J. Antimicrob. Agents 22:406-419. [DOI] [PubMed] [Google Scholar]
- 7.Judice, J. K., and J. L. Pace. 2003. Semi-synthetic glycopeptide antibacterials. Bioorg. Med. Chem. Lett. 12:4165-4168. [DOI] [PubMed] [Google Scholar]
- 8.King, A., I. Phillips, and K. Kaniga. 2004. Comparative in vitro activity of telavancin (TD-6424), a rapidly bactericidal, concentration-dependent anti-infective with multiple mechanisms of action against gram-positive bacteria. J. Antimicrob. Chemother. 53:797-803. [DOI] [PubMed] [Google Scholar]
- 9.Maglio, D., R. Teng, P. T. Thyrum, C. H. Nightingale, and D. P. Nicolau. 2003. Pharmacokinetic profile of meropenem, administered at 500 milligrams every 8 hours, in plasma and cantharidin-induced skin blister fluid. Antimicrob. Agents Chemother. 47:1771-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pace, J. L., K. Krause, D. Johnston, D. Debabov, T. Wu, L. Farrington, C. Lane, D. L. Higgins, B. Christensen, J. K. Judice, and K. Kaniga. 2003. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3602-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennie, R. P., R. N. Jones, A. H. Mutnick, and the SENTRY Program Study Group (North America). 2003. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY antimicrobial surveillance program (United States and Canada, 2000). Diagn. Microbiol. Infect. Dis. 45:287-293. [DOI] [PubMed] [Google Scholar]
- 12.Styjewski, M. E., W. D. O'Riordan, W. K. Lau, F. D. Pien, L. M. Dunbar, M. Vallee, V. G. Fowler, Jr., V. H. Chu, E. Spencer, S. L. Barriere, M. M. Kitt, C. H. Cabell, and G. R. Corey. 2005. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 40:1601-1607. [DOI] [PubMed] [Google Scholar]
- 13.Sun, H. K., C. T. Ong, A. Umer, D. Harper, S. Troy, C. H. Nightingale, and D. P. Nicolau. 2005. Pharmacokinetic profile of tigecycline in serum and skin blister fluid of healthy subjects after multiple intravenous administrations. Antimicrob. Agents Chemother. 49:1629-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]