Abstract
It is generally accepted that the lipid formulations of amphotericin B (AMB) are not as potent as conventional AMB on a milligram-per-kilogram basis. We used a neutropenic murine disseminated candidiasis model to compare the in vivo potencies of AMB, liposomal AMB (L-AMB), and AMB lipid complex (ABLC) pharmacodynamically. The pharmacokinetics of the antifungals were examined in serum and in three organs commonly seeded in disseminated candidiasis (kidneys, liver, and lung). Both single-dose time-kill studies and multiple-dosing-regimen studies were used with each of the compounds. Determinations of the numbers of CFU in the kidneys were performed following the administration of three escalating single doses of the polyenes at various times over 48 h. The areas under the time-kill curves (AUTKs) for each dose level of the drugs were compared by analysis of variance (ANOVA). In the multiple-dosing-regimen studies with five Candida isolates, AMB, L-AMB, and ABLC were administered daily for 72 h. The organism burdens in the mouse kidneys were similarly used as the treatment end point. Additional multiple regimen-dosing-studies were performed with a single Candida albicans isolate, and the microbiologic outcomes in four internal organs (kidneys, liver, spleen, and lung) were examined at the end of therapy (48 h). The relationship between the dose and the drug exposure expressed by the pharmacokinetics of the dosing regimens in serum and organ tissue were analyzed by using a maximum-effect model. ANOVA was used to compare the drug exposures necessary to achieve the 25% effective dose (ED25), ED50, ED75, and 1 log10 killing. Comparison of AUTKs suggested that AMB was 4.3- to 5.9-fold more potent than either ABLC or L-AMB. The time-kill curves for both lipid formulations were very similar. In the multiple-dosing-regimen studies, AMB was 5.0- to 8.0-fold more potent than each of the lipid formulations against five Candida isolates in the kidneys. Similar differences in potency (5.1- to 7.2-fold) were observed in the other end organs. The difference in pharmacokinetics in serum accounted for much of the difference in potency between AMB and ABLC (ratio of serum ABLC area under the curve of effective doses to serum AMB area under the curve of effective doses, 1.2). The differences in the kinetics in the various end organs between AMB and L-AMB were better at explaining the disparate potencies at these infection sites (ratio of organ L-AMB area under the curve of effective doses to organ AMB area under the curve of effective doses, 1.1).
Amphotericin B (AMB) remains the most broad spectrum and potent antifungal agent. Until the recent development of several new antifungal agents, the deoxycholate formulation of amphotericin B was the first-line choice of treatment for nearly all life-threatening fungal infections. The major factor limiting the use of this compound has been dose-limiting nephrotoxicity. The development of lipid formulations of amphotericin B resulted in reduced toxicity (15, 27). However, it is generally accepted that the lipid formulations of AMB are not as potent as conventional AMB on an mg/kg basis. Most clinical trials and current dosing conventions with each of the lipid preparations use doses 4- to 10-fold in excess of those for amphotericin B deoxycholate (1, 8, 9, 10, 18, 24, 25). However, few studies have performed systematic analyses to define the relative in vivo potencies of these compounds to discern if these dose escalations are optimal.
Each of the lipid formulations is complexed to a different lipid. The three approved formulations exhibit unique pharmacokinetic characteristics (4, 5, 15, 20, 22, 23). For example, the liposomal formulation of amphotericin B (L-AMB) achieves extraordinarily high serum concentrations relative to those achieved by the other formulations. Conversely, following administration of the lipid complex formulation of amphotericin B (ABLC), serum levels are quite low, yet the distribution to certain organs, such as the lungs, are reported to exceed those of the other formulations. The relevance of these pharmacokinetic differences in serum and tissues has not yet been shown.
The goals of the current investigations were to (i) systematically examine the differences in potencies among the conventional amphotericin B formulation and two of the lipid preparations and (ii) determine if the pharmacokinetics in serum and various end organ tissues may explain the differences in potencies in a disseminated candidiasis model.
MATERIALS AND METHODS
Antifungal.
AMB, L-AMB, and ABLC were obtained as pharmaceutical-grade powders. The drug solutions were prepared on the day of study by dissolving them in sterile H2O. Subsequent drug dilutions were made with 5% dextrose.
Organisms.
Three clinical isolates of Candida albicans (isolates K-1, 98-17, and 98-234) and single clinical isolates of Candida krusei (isolate 5810) and Candida dubliniensis (isolate 3588) were used in the experiments. Each Candida strain with the exception of C. dubliniensis was a bloodstream isolate. C. dubliniensis was an oropharyngeal isolate. The organisms were grown, subcultured, and quantified on Sabouraud dextrose agar (SDA) slants (Difco Laboratories, Detroit, MI).
In vitro susceptibility testing.
MICs were determined by a broth microdilution modification of the Clinical Laboratory Standards Institute (formerly the NCCLS) M27-A method (17). Determinations were performed in duplicate on at least two separate occasions. The final results are expressed as the means of these results.
Animals.
Six-week-old ICR/Swiss specific-pathogen-free female mice (Harlan Sprague-Dawley, Indianapolis, IN) weighing 23 to 27 g were used for all studies. The animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial Veterans Affairs Hospital.
Infection models.
(i) Disseminated infection. The mice were rendered neutropenic (<100 per mm3) by injection of cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) intraperitoneally 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before infection. Neutropenia (<100 polymorphonuclear leukocytes per mm3) was confirmed throughout the entire study (2, 3). The organisms were subcultured on SDA 24 h prior to infection. The fungal counts of the inoculum were determined with a hemacytometer; and the viable counts, confirmed by plating on SDA, were 105.0 to 105.30 CFU/ml. Disseminated infection with the Candida organisms was achieved by injection of 0.1 ml of inoculum via the lateral tail vein 2 h prior to the start of drug therapy. At the treatment end points, the animals were euthanized by CO2 asphyxiation. After the mice were killed, the kidneys, spleen, and liver of each mouse were immediately removed and placed in sterile 0.9% saline at 4°C. The homogenate was then serially diluted 1:10, and aliquots were plated on SDA for determination of viable fungal colony counts after incubation for 24 h at 35°C. The lower limit of detection was 100 CFU/ml. The results are expressed as the mean log10 CFU per organ(s) for two mice.
(ii) Lung infection.
Although there was hematogenous spread to the mouse lungs in the disseminated model described above, the burden of organisms was relatively low. The inhalation pneumonitis model produces an invasive infection (histopathologic data not shown) and provides a larger organism burden but is less clinically relevant. However, the intent of this experiment was to allow examination of the relationship between lung tissue drug exposure and microbiologic effect. In order to appropriately compare the various organ end points, we believed that it was important to use the same organism (C. albicans). Thus, in order to provide a larger dynamic range for determination of the differences between effective and ineffective therapies, we used the direct (nasal) inoculation model. Mice were similarly rendered neutropenic. The Candida albicans inoculum was similarly prepared to a concentration of 105.9 CFU/ml. The inoculum was administered in a 0.2-ml volume via the nares of mice anesthetized with halothane. Antifungal therapy was started 2 h after infection, at which time the organism burden in the lungs of mice was 104.77 CFU/lungs. At the end of therapy, the lungs were removed and the organism burden was enumerated as described above for the other organs.
Pharmacokinetics.
Single-dose pharmacokinetics of AMB, L-AMB, and ABLC were determined in neutropenic ICR/Swiss mice. Two dose levels of each compound were examined. For AMB, doses of 5 and 20 mg/kg of body weight were administered intraperitoneally in a 0.2-ml volume, and for both of the lipid-associated preparations, doses of 20 and 80 mg/kg were administered intraperitoneally in a 0.2-ml volume. The choice of drug doses for both the pharmacokinetic and the treatment efficacy studies was based upon prior experience with AMB in this model and previously reported estimates of potency differences between AMB and the lipid preparations (3). The intraperitoneal route instead of the intravenous route was chosen due to the difficulty with repeated intravenous dosing in mice and our prior experience with this method (3). Drug concentrations were measured in serum and organ homogenates, including homogenates of the kidney, liver, and lung, by using a microbiologic assay. In choosing an assay method, we considered three factors: (i) the sensitivity of the assay or the assay's limit of detection, (ii) reproducibility, and (iii) the ability to estimate concentrations available for drug activity (16, 19, 26). A direct physiochemical measurement method such as high-pressure liquid chromatography (HPLC) provides a more sensitive assay than a microbiologic assay. Both methods are reproducible. The HPLC method provides an accurate estimate of the total tissue drug concentration. Our eventual choice of a microbiologic assay was based upon the ability of the assay to estimate the drug concentrations available for treatment effect (the microbiologically active concentrations). Attempts were made to include measurements in splenic tissue; however, the results were not reproducible and are not reported. For each dose examined in the serum studies, groups of three mice each were sampled three or four times by retro-orbital puncture. Blood (100 μl) was collected in heparinized capillary tubes (Fisher Scientific, Pittsburgh, PA) at 0.5- to 12-h intervals (eight time points) over a 48-h period. The maximal volume of blood collected from any single animal was less than 5% of the total blood volume. The tubes were centrifuged (model MB; International Equipment Co.) at 10,000 × g for 5 min. The serum was subsequently removed, and drug levels were determined by a drug diffusion bioassay by using Paecilomyces variotii as the assay organism in antibiotic medium 12 (26). Assay of serum samples and the preparation of standard curves for mouse serum were performed on the same day. The intraday coefficients of variation ranged from 4.2 to 7.5%. The lower level of detection for this assay was 0.15 μg/ml. Pharmacokinetic constants, including the elimination half-life and the concentration of drug in serum at time zero, were calculated by the nonlinear least-squares techniques. The area under the concentration-time curve (AUC) was calculated by use of the trapezoidal rule. For doses for which no kinetics were determined, the pharmacokinetic parameters were extrapolated from the measured values.
Assay of the tissue homogenate concentrations of each of the compounds was performed as described above. Tissue homogenates were produced with a mechanical tissue grinder as described previously (3). The same drug doses and sampling schedule were used. For each time point examined, three mice were euthanized; the organs were aseptically removed, placed in 0.85% NaCl, and homogenized for 15 s. The drug concentrations in the tissue homogenate supernatants were measured by the same microbiologic assay. Standard curves were prepared for each of the drugs both as described above and by using 10% organ homogenates spiked with three concentrations of each compound (5, 20, and 80 μg/ml) (Table 1). Homogenates from each organ (kidney, liver, and lung) were used in an attempt to estimate tissue binding, which may affect measurement of microbiologically active drug in the assay. Reporting of tissue values in the Results section is based upon correction for tissue binding (i.e., total organ drug levels). The limit of detection and variation of the assay were the same as those reported above.
TABLE 1.
Polyene tissue homogenate binding assay
| Tissue | % of spiked drug concn recovered in homogenate supernatant
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B
|
L-AMB
|
ABLC
|
|||||||
| 80 μg/ml | 20 μg/ml | 5 μg/ml | 80 μg/ml | 20 μg/ml | 5 μg/ml | 80 μg/ml | 20 μg/ml | 5 μg/ml | |
| Kidney | 25 | 23 | 30 | 90 | 98 | 93 | 2 | 5 | 6 |
| Liver | 25 | 21 | 32 | 100 | 100 | 100 | 1 | 2 | 1 |
| Lung | 12 | 9 | 19 | 100 | 100 | 100 | 1 | 2 | 2 |
Single-dose time-kill study.
C. albicans K1 was used for the time-kill studies. The growth of the organisms in saline-treated control animals was determined at four sampling points over 36 h. Treatment (a single intraperitoneal dose) with either AMB (0.25, 1.0, and 4 mg/kg), L-AMB (1.25, 5, and 20 mg/kg), or ABLC (1.25, 5, and 20 mg/kg) was initiated 2 h after infection. The three fourfold increasing doses were chosen based upon efficacy in prior published studies. Groups of two mice were euthanized at six time points (every 1 to 12 h) over 48 h to determine log10 CFU/kidneys. We observed the rate and the extent of organism killing for each of the compounds at the doses studied. To compare the time course activities among the three drugs, we calculated the area under the time-kill curve (AUTK) for each drug dose by use of the trapezoidal rule and by using the log10 CFU/kidneys and the time of sampling. Smaller AUTK values are indicative of more extensive in vivo antifungal activity over time. The results of the AUTK studies with AMB, L-AMB, and ABLC were compared relative to the doses used (the doses of the lipid-associated compounds were five times higher). The statistical significance of the differences was analyzed by analysis of variance (P values <0.05 were considered statistically significant).
Multiple-dose treatment studies.
All five Candida isolates were used in the multiple-dose treatment studies, where the organism burden in mouse kidneys was the end point. C. albicans K1 was used in similar studies in which the organism burdens in the kidneys, liver, spleen, and lung were the end points examined. Groups of two animals were treated for 48 to 72 h (48 h for the lung-only end point and 72 h for the all-organ end point) with fourfold increasing doses administered every 24 h. The total doses of AMB ranged from 0.08 to 20 mg/kg/24 h, and the total doses of L-AMB and ABLC ranged from 0.312 to 80 mg/kg/24 h. Mice were euthanized at 48 or 72 h, and their organs were removed for CFU determination. Saline-treated control mice were euthanized just before treatment and at the end of the experiment. Efficacy was defined as the change in log10 CFU/organ over the treatment period. A sigmoid dose-effect (maximum-effect [Emax]) model was used to analyze the results. The model is derived from the Hill equation: E = (Emax × DN)/(ED25, ED50, ED75N + DN), where E is the observed effect (change in log10 CFU/organ compared with the number of CFU in the organs of the saline-treated controls); D is the cumulative dose; ED25, ED50, and ED75 are the doses required to achieve 25, 50, and 75% of the maximum effect, respectively; and N is the slope of the dose-response relationship. The significance of differences among values for ED25, ED50, ED75, and 1-log10-CFU/kidney killing for AMB, L-AMB, and ABLC were determined by analysis of variance.
Pharmacodynamic analysis.
Pharmacodynamic analysis was undertaken to determine if the differences in potencies among the three compounds on an mg/kg basis could be explained on the basis of pharmacokinetics in serum or tissue. The drug exposure for each of the end organ dose-response relationships was considered relative to the serum AUC and specific end organ AUC. The same sigmoid dose-effect (Emax) model was used to analyze the relationships between these pharmacokinetic values and the outcomes.
RESULTS
In vitro susceptibility.
The AMB MIC for the C. dubliniensis isolate was 0.5 μg/ml. The AMB MIC for the four other isolates was 0.25 μg/ml.
Pharmacokinetics.
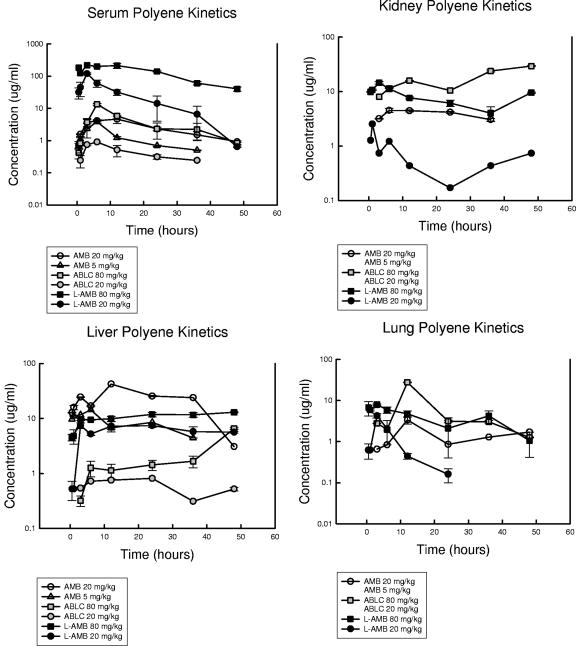
The pharmacokinetic profiles of AMB, ABLC, and L-AMB at the two dose levels examined in serum and tissue are shown in Table 2 and Fig. 1. The 20-mg/kg dose was studied with each of the three drugs. At this dose level, the maximum concentration (Cmax) was markedly higher (26-fold) for L-AMB than AMB, and the Cmax for ABLC was lower than that for AMB (5-fold). Conversely, the elimination half-life was 2.4 to 4 h shorter for the L-AMB preparation than for the other compounds, which exhibited similar serum elimination profiles. The AUC in serum was not surprisingly larger for L-AMB (eightfold larger than that that of AMB) and was smaller for ABLC (fivefold lower than that of AMB).
TABLE 2.
Polyene pharmacokinetics in serum and tissue homogenates
| Tissue | Pharmacokinetic parameter | AMB (20)a | AMB (5) | L-AMB (80) | L-AMB (20) | ABLC (80) | ABLC (20) |
|---|---|---|---|---|---|---|---|
| Serum | Cmax (μg/ml) | 4.58 ± 1.20 | 218 ± 39.0 | 119 ± 7.00 | 13.3 ± 0.82 | 13.3 ± 0.82 | 0.90 ± 0.00 |
| t1/2b (h) | 16 | 14.3 | 10.1 | 12.5 | 12.5 | 16 | |
| AUC0-∞c (mg · h/liter) | 140 | 7,006 | 1,181 | 195 | 195 | 22 | |
| Kidney | Cmax (μg/ml) | 4.48 ± 0.40 | NDd | 14.4 ± 0.66 | 2.51 ± 0.00 | 29.1 ± 0.68 | ND |
| Mean ± SD Cmax (μg/ml) | 3.84 ± 0.70 | ND | 9.20 ± 3.22 | 0.93 ± 0.74 | 16.4 ± 8.32 | ND | |
| AUC0-48e (mg · h/liter) | 137 | ND | 351 | 26.5 | 761 | ND | |
| Liver | Cmax (μg/ml) | 42.1 ± 2.0 | 14.3 ± 0.52 | 12.9 ± 0.20 | 7.40 ± 0.50 | 46 ± 18 | 22 ± 2.8 |
| Mean ± SD Cmax (μg/ml) | 20.5 ± 11.4 | 9.47 ± 301 | 9.30 ± 3.11 | 4.95 ± 2.90 | 32 ± 14 | 16 ± 5.4 | |
| AUC0-48 (mg · h/liter) | 1,150 | 301 | 521 | 297 | 1,760 | 778 | |
| Lung | Cmax (μg/ml) | 3.33 ± 0.66 | ND | 7.90 ± 0.23 | 4.23 ± 0.54 | 218 ± 26 | ND |
| Mean ± SD Cmax (μg/ml) | 1.44 ± 1.00 | ND | 4.53 ± 2.20 | 1.35 ± 0.54 | 52 ± 81 | ND | |
| AUC0-48 (mg · h/liter) | 72 | ND | 181 | 33.1 | 2,739 | ND |
Values in parentheses are doses (milligram per kilogram).
t1/2, half-life.
AUC0-∞, AUC from time zero to infinity.
ND, not detected.
AUC0-48, AUC from time zero to 48 h.
FIG. 1.
Concentrations of AMB, ABLC, and L-AMB in serum, kidneys, liver, and lung of neutropenic mice after administration of intraperitoneal doses of 5 and 20 mg/kg of AMB and 20 and 80 mg/kg of ABLC and L-AMB. Each symbol represents the geometric mean ± standard deviation of concentrations from three measurements.
The tissue binding assay produced remarkably reproducible results in three replicate studies (Table 1). The degrees of tissue homogenate binding were relatively similar across the three organs and over the 16-fold range of drug concentrations. ABLC exhibited the highest degree of tissue binding, ranging from 94 to 99%. The tissue binding of AMB (range, 75 to 89%) was intermediate among the three compounds, and L-AMB was the least bound (range 1 to 10%) in the assay. Tissue binding was taken into consideration in the reporting of the organ pharmacokinetic results. For example, the reported tissue concentration for AMB in the kidney was a calculation of the measured value multiplied by a factor to account for the drug that the assay did not detect due to tissue binding. Thus, the estimated values represent total tissue concentrations. At the 20-mg/kg dose level, the AMB exposures in the kidney were fivefold higher than those for L-AMB based upon the tissue AUC. However, for the lower doses of AMB (5 mg/kg) and ABLC (20 mg/kg), tissue binding precluded the detection of measurable levels in the kidneys and lungs; thus, it was not possible to correct and report the values. Liver tissue concentrations were relatively similar for AMB and ABLC, and the concentrations of both were nearly fourfold greater than those of L-AMB. The site of the greatest apparent distribution for ABLC was the lung. The lung tissue AUC for ABLC was more than 15-fold higher than that for L-AMB. The concentrations of ABLC in liver tissue were slightly higher than those in the kidney tissue, which in turn were higher than the serum exposures. AMB exposures were the highest in the liver, were relatively similar in the kidney and serum, and were the lowest in the lung. L-AMB concentrations in the serum far exceeded those in the tissue sites examined, which were relatively similar.
Single-dose time-kill study.
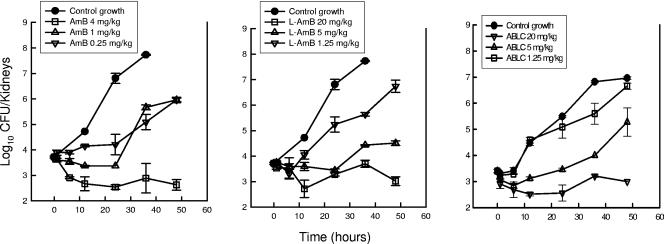
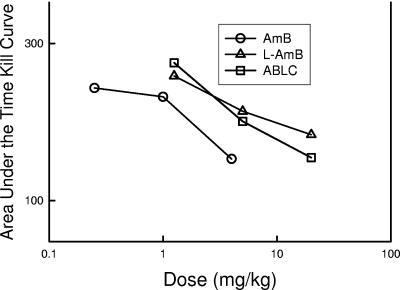
The time courses of antifungal activity for the three doses of AMB, L-AMB, and ABLC are shown in Fig. 2. The control growth in the saline-treated animals over the 36-h sampling period increased more than 4 log10 CFU/kidneys. Only the highest doses of each of the three compounds resulted in significant organism reductions (killing) relative to the counts in the control animals at the start of drug therapy. The 4-mg/kg dose of AMB resulted in a 101.1-CFU/kidney reduction at the 12-h time point. The 20-mg/kg doses of both L-AMB and ABLC similarly resulted in 101.0-CFU/kidney and 100.90-CFU/kidney reductions, respectively, at the 12-h time point. Organism regrowth from the lowest kidney burden after exposure did not occur for each of the highest dose levels of the polyenes during the study period. Following the administration of two lower dose levels of AMB, the organisms did not begin to regrow markedly until 24 h following the single-dose administration. Similarly, after exposure to the middle dose level of each of the lipid-associated polyenes, marked organism recovery did not begin until 24 h. In mice treated with the lowest doses of L-AMB and ABLC, however, regrowth began as early as 6 h after treatment. The relationship between drug dose and AUTK for AMB, L-AMB, and ABLC, shown in Fig. 3, was linear over the dose range studied. Comparison of the AUTKs at each of the three dose levels (the low, middle, and high doses for each compound) suggests that AMB was 4.3- to 5.9-fold more active than the lipid preparations of AMB (ratio of the L-AMB and ABLC AUTK to the AMB AUTK times a dose difference factor of 5). The difference between the AMB curves and each of the lipid-associated AMB curves was statistically significant (P = 0.01).
FIG. 2.
In vivo time-kill activity against C. albicans in neutropenic mice following the administration of single doses of AMB, L-AMB, and ABLC at doses of 0.25, 1, and 4 mg/kg of AMB and 1.25, 5, and 20 mg/kg of L-AMB and ABLC. Each symbol represents the mean ± standard deviation for two mice. The solid symbols represent the growth of organisms in saline-treated mice.
FIG. 3.
Calculated area under the time-kill curve for activity against C. albicans in neutropenic mice following the administration of AMB, L-AMB, and ABLC at single doses of 0.25, 1, and 4 mg/kg of AMB and 1.25, 5, and 20 mg/kg of L-AMB and ABLC. Each symbol represents the area under the time-kill curve for a dose level calculated by using the trapezoidal rule.
Multiple-dose treatment studies.
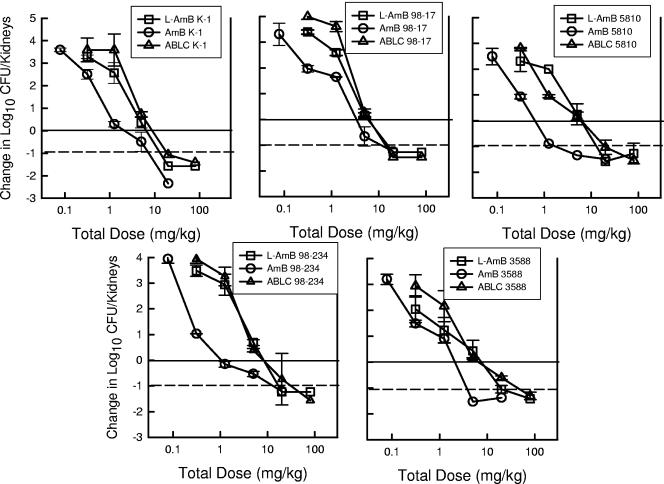
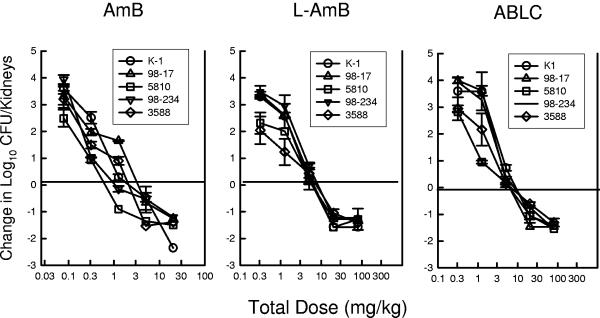
All five Candida isolates grew well in the mice. The kidney burden at the start of therapy ranged from 103.4 ± 100.30 to 103.5 ± 100.24 CFU/kidneys. The organism burden after 48 h in control mice increased from 103.7 ± 100.32 to 104.2 ± 100.27 CFU/kidneys. The dose-response relationship for each of the five strains is shown in Fig. 4 and 5. The slopes of the dose-response relationships were relatively similar among the five strains. The higher doses of each of the polyene compounds resulted in at least a 1-log10 CFU/kidney reduction (dashed horizontal lines) relative to the organism burden at the start of therapy. For all of the strains, the L-AMB and ABLC dose-response curves were shifted to the right relative to the AMB curves, suggesting less activity in vivo. The ED25, ED50, ED75, and 1-log10-CFU/kidney killing (and 95% confidence interval [CI]) for each drug and strain are shown in Table 3. AMB was 4.9- to 8.0-fold (median fold difference) more potent than the lipid preparations at these treatment end points. The fold differences between AMB and either of the lipid preparations were statistically significant for nearly all of the end points and organisms. There was not an appreciable difference in potency between the two lipid preparations (ratio of end point dose of ABLC to end point dose of L-AMB, 1.4 ± 2.2). The amount of drug on an mg/kg basis associated with the various end points was not statistically different when the results for the five Candida isolates were compared. Similarly, the fold differences among the treatment end points were also not markedly different.
FIG. 4.
Relationship between the 24-h total dose (mg/kg/24 h) and the change in the log10 CFU/kidneys relative to the organism burden at the start of therapy for AMB, L-AMB, and ABLC against five Candida isolates in neutropenic mice. Each symbol represents data from two mice. The solid horizontal line represents the burden of organisms at the start of therapy. The dashed horizontal line represents a 1-log10 reduction in organism burden in kidneys.
FIG. 5.
Relationship between the 24-h total dose (mg/kg/24 h) and the change in the log10 CFU/kidneys relative to the organism burden at the start of therapy for AMB, L-AMB, and ABLC against five Candida isolates in neutropenic mice. Each symbol represents data from two mice.
TABLE 3.
Comparative in vivo efficacies of AMB, L-AMB, and ABLC against five C. albicans isolates
| C. albicans strain | Efficacy (mg/kg/24 h [95% CI])
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB
|
L-AMB
|
ABLC
|
||||||||||
| ED25 | ED50 | ED75 | ED for 1-log killing | ED25 | ED50 | ED75 | ED for 1-log killing | ED25 | ED50 | ED75 | ED for 1-log killing | |
| K1 | 0.34 (0.33-0.35) | 1.49 (1.45-1.53) | 6.35 (6.17-6.52) | 5.06 (4.91-5.21) | 2.05 (1.95-2.15) | 3.94 (3.74-4.14) | 7.59 (7.21-7.97) | 11.40 (10.8-12.0) | 2.42 (2.05-2.78) | 4.11 (3.49-4.73) | 7.00 (6.00-8.00) | 14.8 (12.6-17.0) |
| 98-17 | 0.29 (0.18-0.40) | 1.91 (1.19-2.63) | 12.5 (7.7-17.3) | 11.8 (6.87-16.7) | 1.16 (1.11-1.21) | 2.63 (2.52-2.64) | 6.03 (5.79-6.27) | 15.8 (15.2-16.4) | 2.46 (2.36-2.56) | 3.97 (3.80-4.15) | 6.43 (6.15-6.71) | 6.02 (5.78-6.26) |
| 98-234 | 0.14 (0.10-0.18) | 0.25 (0.17-0.33) | 0.42 (0.29-0.55) | 10.84 (7.44-14.2) | 1.03 (0.53-1.54) | 3.01 (1.34-4.67) | 8.79 (4.40-12.3) | 23.5 (21.8-25.2) | 2.08 (0.65-3.49) | 3.31 (1.03-5.59) | 5.26 (1.68-8.84) | 45 (15-60) |
| 3588 | 0.17 (0.14-0.20) | 0.70 (0.59-0.81) | 2.92 (2.42-3.42) | 5.31 (4.42-6.20) | 0.46 (0.38-0.54) | 3.78 (3.10-4.46) | 31.5 (25.8-37.2) | 28.5 (23.3-33.7) | 0.85 (0.21-1.49) | 2.22 (0.46-3.98) | 5.81 (1.40-10.2) | 14.7 (5.60-25.9) |
| 5810 | 0.04 (0.03-0.05) | 0.15 (0.12-0.18) | 0.57 (0.46-0.68) | 2.09 (1.68-2.51) | 1.27 (1.09-1.44) | 3.23 (2.78-3.68) | 8.21 (7.06-9.36) | 15.0 (12.9-17.1) | 0.30 (0.23-0.37) | 0.58 (0.45-0.71) | 1.08 (0.84-1.32) | 3.45 (2.69-4.21) |
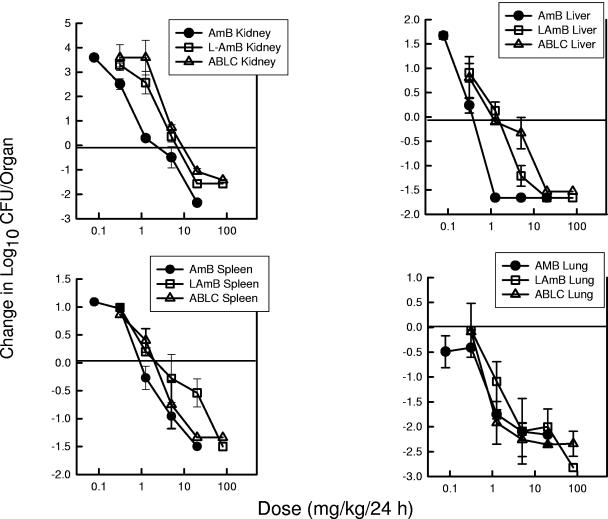
The next goal of the studies was to determine the impact of the internal organ end point. Candida albicans K1 grew relatively well in each of the other organs assayed. The organ burdens at the start of therapy were 103.5 ± 100.30 CFU/liver, 103.4 ± 100.17 CFU/spleen, and 104.8 ± 100.10 CFU/lungs. The organism grew to a higher burden in the kidneys than the other organs, as previously shown in other Candida animal model investigations (3a). The organism burdens in the liver, spleen, and lungs of untreated control mice increased 101.4 ± 100.33 CFU/liver, 101.2 ± 100.40 CFU/spleen, and 101.0 ± 100.10 CFU/lungs, respectively. The dose-response relationship based on dose level (mg/kg/24 h) for each of the three end organs is shown in Fig. 6. The slopes of the dose-response relationships were relatively similar among the four infection sites. The higher doses of each of the polyene compounds resulted in a reduction of at least 1 log10 CFU/organ relative to the organism burden at the start of therapy. For all of the end organs, the L-AMB and ABLC dose-response curves are shifted to the right relative to the AMB curves, suggesting less in vivo activity. The difference in potency was most marked in the liver. The ED25, ED50, ED75, and 1-log10-CFU/kidney killing (and 95% CI) for each drug and organ are shown in Table 4. AMB was 3- to 3.5-fold (mean fold difference) more potent in the kidneys, 6- to 19-fold more potent in the liver, 3- to 6.2-fold more potent in the spleen, and 2.5- to 4.1-fold more potent in the lungs than either of the lipid-associated preparations. The fold differences for AMB compared to results for the lipid preparations were statistically significant for the kidney and liver at each of the end points. In the lung and spleen, only the higher-level end points (ED75 and 1-log10 killing) were statistically significant.
FIG. 6.
Relationship between the 24-h total dose (mg/kg/24 h) and the change in the log10 CFU/kidneys, spleen, liver, and lung relative to the organism burden at the start of therapy for AMB, L-AMB, and ABLC against a strain of C. albicans in neutropenic mice. Each symbol represents data from two mice.
TABLE 4.
Comparative in vivo efficacies of AMB, L-AMB, and ABLC against C. albicans K1 in four organ systems
| Parameter | Efficacy (mg/kg/24 h [95% CI])
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB
|
L-AMB
|
ABLC
|
||||||||||
| Kidney | Liver | Spleen | Lung | Kidney | Liver | Spleen | Lung | Kidney | Liver | Spleen | Lung | |
| ED25 | 0.34 (0.14-0.54) | 0.29 (0.28-0.30) | 0.36 (0.43-1.13) | 0.04 (0.02-0.05) | 2.05 (1.85-2.35) | 0.46 (0.29-0.63) | 0.41 (−2.6-2.7) | 0.30 (0.06-0.54) | 2.42 (2.08-2.76) | 1.28 (1.08-1.48) | 1.36 (1.23-1.49) | 0.27 (0.26-0.28) |
| ED50 | 1.49 (0.58-2.4) | 0.32 (0.31-0.33) | 0.78 (0.43-1.13) | 0.30 (0.20-0.40) | 3.94 (3.37-4.51) | 1.31 (0.82-1.80) | 2.98 (−16.2-22.6) | 1.08 (0.28-1.94) | 4.11 (3.53-4.69) | 4.10 (3.45-4.75) | 2.68 (2.43-2.93) | 0.47 (0.45-0.49) |
| ED75 | 6.30 (2.45-10.1) | 0.39 (0.38-0.40) | 1.72 (0.96-2.48) | 2.27 (2.18-2.36) | 7.59 (6.59-8.59) | 3.78 (2.38-5.18) | 21.7 (2.1-41.3) | 3.93 (3.06-4.80) | 7.00 (6.0-8.0) | 13.1 (11.1-15.1) | 5.28 (5.23-5.53) | 0.83 (0.81-0.85) |
| ED for 1-log killing | 5.06 (3.28-6.84) | 0.34 (0.33-0.35) | 7.13 (3.96-10.3) | 0.41 (0.27-0.55) | 11.4 (10.8-12.0) | 4.17 (2.61-5.73) | 51.9 (32.3-71.5) | 1.48 (1.28-1.68) | 14.8 (12.7-16.9) | 10.1 (8.5-11.7) | 7.35 (6.69-8.01) | 0.57 (0.54-0.60) |
Pharmacodynamic analysis.
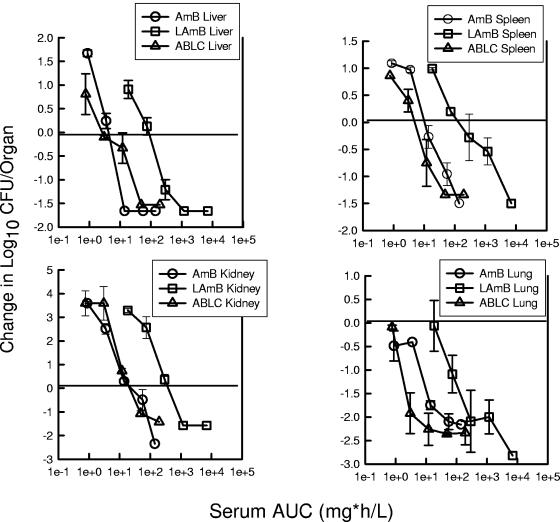
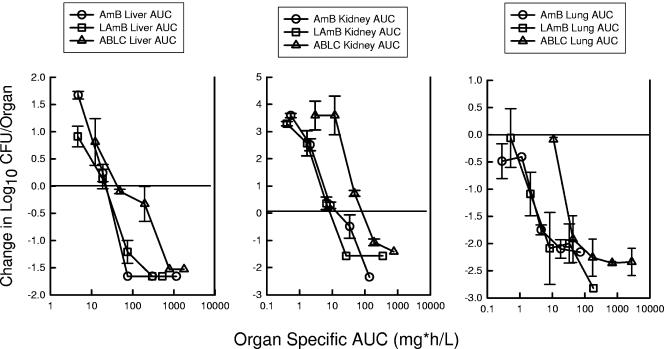
The final data analyses were intended to discern if the pharmacokinetics in serum and in the organ tissues may explain the apparent differences in potency on an mg/kg basis. The slopes of the exposure-response relationships with the pharmacodynamic values (serum AUC and organ AUC) remained similar to the original dose (mg/kg)-effect relationship. However, consideration of serum values moved the exposure response curve for ABLC to the left for each of the end organs (Fig. 7). Serum pharmacokinetic considerations eliminated apparent differences in potencies between AMB and ABLC. The ratio of serum AUC of ABLC to serum AUC of AMB required for each of the end points (ED25, ED50, and ED75) was only 1.2 ± 1.9. Similar consideration of the serum pharmacokinetic relationship for L-AMB shifted the dose-response curve even farther to the right, suggesting less potency. The ratio of the serum AUC of L-AMB to the serum AUC of AMB for the same end points was 21 ± 16. The organ-specific pharmacokinetic response relationship, however, moved the L-AMB curve to the left, very similar to that for AMB (Fig. 8). The ratio of the organ-specific AUC of L-AMB to the organ-specific AUC of AMB for the treatment end points was only 1.1 ± 0.8. The ABLC organ AUC-response curve was shifted to the right, with an organ AUC exposure ratio (organ-specific AUC of ABLC to organ-specific AUC of AMB) of 10 ± 12.
FIG. 7.
Relationship between the 24-h serum AUC (mg · h/liter) and the change in the log10 CFU/kidneys, liver, and lung relative to the organism burden at the start of therapy for AMB, L-AMB, and ABLC against a strain of C. albicans in neutropenic mice. Each symbol represents data from two mice.
FIG. 8.
Relationship between the 24-h organ AUC (mg · h/liter) and the change in the log10 CFU/kidneys, liver, and lung relative to the organism burden at the start of therapy for AMB, L-AMB, and ABLC against a strain of C. albicans in neutropenic mice. Each symbol represents data from two mice.
DISCUSSION
AMB was the first systemic antifungal drug to be made available. The deoxycholate formulation has been in use since the mid-1950s and remained the primary systemic antifungal until the development of the triazoles in the late 1980s (6). AMB was the first-line treatment for most life-threatening systemic antifungal infections because it was the only choice and because of its potency and broad spectrum of activity. The predominant factor which has made use of this compound difficult is the associated dose-limiting nephrotoxicity (6, 13). The more recent development of less toxic lipid formulations of amphotericin B has significantly improved the therapeutic drug window for this drug class (4, 15, 20, 27).
Three lipid formulations were developed and approved for use in the 1990s. While each of the formulations involves complexing of AMB to a lipid entity, the specific molecules vary markedly. The active compound in AMB colloidal dispersion is complexed to cholesterol sulfate, resulting in the formation of 0.12- to 0.14-μm disk-like structures. ABLC forms ribbon-like particles. The lipid components of ABLC include dimyristoyl phosphatidylcholine and dimyristoyl phosphatidylglycerol in a 7:3 ratio. The lipid moiety in liposomal AMB is a mixture of phosphatidylcholine-distearoyl phosphatidylglycerol-cholesterol in a ratio of 2:0.8:1:0.4 that forms a unilamellar liposome. Conventional AMB is solubilized with deoxycholate as a micellar suspension.
Depending on the composition of the lipid moiety, electrical charge, particle size, and configuration, each of the AMB formulations possesses unique pharmacokinetic characteristics. Following the intravenous infusion of AMB, the amphotericin B molecule is released from the deoxycholate carrier molecule, distributed with lipoproteins, and subsequently taken up by organs of the mononuclear phagocytic system. Each of the lipid formulations also distributes preferentially to organs of the mononuclear phagocytic system and functionally spares the kidney. However, there are extreme differences in serum kinetics and other tissue distribution profiles. Compared with AMB deoxycholate, ABLC has a lower serum Cmax, a shorter circulating half-life, a smaller AUC, and a larger volume of distribution, consistent with uptake into tissues. In contrast, the small unilamellar formulation, liposomal AMB, is more slowly cleared from the bloodstream and achieves much higher Cmax and AUC values but has a smaller volume of distribution than AMB. Whether these distinct kinetic features translate into different pharmacodynamic properties and clinical effectiveness is largely unknown.
The current pharmacokinetic studies with each of the compounds demonstrated profiles similar to those described previously (7, 14, 21). The serum exposure observed with L-AMB exceeded that observed with either AMB or ABLC. Conversely, the serum profile following administration of ABLC was marked by low Cmax and AUC values. In the tissue distribution studies, the AMB kidney exposure exceeded that of either of the lipid preparations. Among the lipid formulations, the tissue exposure of L-AMB was much less than the serum exposure. ABLC exposures in tissues were much greater than those in serum. The ABLC exposure in the lung exceeded that of L-AMB by a factor of 15. The distribution of ABLC in the liver was also greater than that of L-AMB.
The reduced nephrotoxicity of lipid formulations allows the delivery of doses higher than those of the deoxycholate preparation that can be delivered. However, a number of animal model investigations have demonstrated that higher doses of a liposomal preparation of AMB on an mg/kg basis are necessary to obtain the same antifungal effect achieved with AMB desoxycholate (1, 8, 9, 10, 13). Studies with animal models of cryptococcosis and aspergillosis compared the efficacies of three lipid amphotericin B formulations and amphotericin B deoxycholate. Comparable microbiologic efficacies and rates of survival were observed with lipid formulation dose levels 5- to 10-fold higher than that of the conventional formulation of this polyene. The present investigations examined the relative in vivo potencies of AMB, ABLC, and L-AMB against five Candida isolates in four end organs by using both single-dose time-kill regimen and multiple-dose-regimen studies. Over a 16-fold dose range in the time-kill studies and a more than 250-fold dose range in the multiple-dose-regimen studies, AMB was 4.3- to 8.0-fold more potent on an mg/kg basis. In the single-dose time-kill studies, the differences in potencies between conventional AMB and the lipid preparations ranged from 4.3- to 5.9-fold. The time-kill curves obtained with the lipid formulations were nearly identical. Comparison of the potencies in the multiple-does-regimen studies similarly identified a 5.0- to 8.0-fold difference between the deoxycholate formulation and each of the lipid products. The mean differences were similar among the Candida isolates and the various end organs.
The use of pharmacodynamic analyses to correlate antimicrobial pharmacokinetics with treatment outcome has been critical for adequate comparison of the in vivo potencies of antimicrobials (2, 3, 11). Comparative pharmacokinetic studies with this group of antifungal compounds have demonstrated disparate serum and tissue profiles. Yet, few studies have been able to demonstrate a relationship between these unique kinetic profiles and antifungal effect. Groll et al. examined the relationship between the pharmacokinetics of these compounds in serum, cerebrospinal fluid, and brain and the organism burden in the brain and cerebrospinal fluid using a Candida meningitis model (12). The concentrations of AMB deoxycholate and liposomal AMB were significantly higher in the brain tissue, and treatment resulted in a reduction of the organism burden to below the limit of detection. Conversely, treatment with ABLC and AMB colloidal dispersion regimens, which achieved lower concentrations in the brain tissue, resulted in residual meningitis in this rabbit model. The current studies similarly examined the relationship between the pharmacokinetics of the three polyene preparations and in vivo microbiologic activity. Expression of the polyene drug exposure in pharmacokinetic terms in place of the dose level (mg/kg) helped to explain many of the differences in potencies between the conventional AMB formulation and each of the lipid formulations. The ABLC and AMB serum pharmacokinetics accounted for the most of the apparent disparity in potencies between these two compounds. However, the much higher serum levels achieved with L-AMB did not appear to be entirely available for microbiologic activity compared to the availabilities of the other two preparations. Conversely, consideration of tissue pharmacokinetics explained the disparate potencies for AMB and L-AMB. However, the estimated tissue pharmacokinetics for ABLC suggested that it has less potency than the other compounds. It is possible that the current assay and the organ concentration correction for ABLC tissue binding may have markedly affected these relationships. Sensitivity would be enhanced by the use a physiochemical assay, such as HPLC. However, these types of assays measure total tissue content and do not differentiate between drug that is microbiologically available and that for which tissue or protein binding may preclude activity against the target organism. It would be interesting to compare the results from HPLC and microbiologic assays in future studies.
In sum, the pharmacokinetics of these AMB preparations are disparate. On an mg/kg basis, AMB is four- to eightfold more potent than either of the lipid-associated drugs. On the same basis, both of the lipid-associated compounds are similarly potent in vivo. Consideration of the differences in pharmacokinetics in serum and tissues helps to explain these differences. These investigations underscore the importance of considering drug concentrations in both serum and tissue in attempting to further our understanding of the antifungal drug-fungus interaction in the host.
REFERENCES
- 1.Adler-Moore, J. P., J. A. Olson, and R. T. Proffitt. 2004. Alternative dosing regimens of liposomal amphotericin B effective in treating murine systemic candidiasis. J. Antimicrob. Chemother. 54:1096-1102. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., T. Stamstad, and R. Conklin. 2001. Pharmacodynamics of amphotericin B in a neutropenic mouse disseminated candidiasis model. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Andes, D., and W. A. Craig. 2002. A critical review of animal model pharmacokinetics and pharmacodynamics. Int. J. Antimicrob. Agents 19:261-268. [DOI] [PubMed] [Google Scholar]
- 4.Bakker-Woudenberg, I. A., R. M. Schiffeirs, G. Strorm, M. J. Becker, and L. Guo. 2005. Long circulating sterically stabilized liposomes in the treatment of infections. Methods Enzymol. 391:228-260. [DOI] [PubMed] [Google Scholar]
- 5.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bindschadler, D. D., and J. E. Bennett. 1969. A pharmacologic guide to the clinical use of amphotericin B. J. Infect. Dis. 120:427-436. [DOI] [PubMed] [Google Scholar]
- 7.Boswell, G. W., I. Bekersky, D. Buell, R. Hiles, and T. J. Walsh. 1998. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob. Agents Chemother. 42:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemons, K. V., and D. A. Stevens. 1998. Comparison of Fungizone, Amphotec, Ambisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob. Agents Chemother. 42:899-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons, K. V., and D. A. Stevens. 2004. Comparative efficacies of four amphotericin B formulations—Fungizone, Amphotec, Ambisome, and Abelcet—against systemic murine aspergillosis. Antimicrob. Agents Chemother. 48:1047-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemons, K. V., R. A. Sobel, P. L. Williams, D. Pappagianis, and D. A. Stevens. 2002. Efficacy of intravenous liposomal amphotericin B against coccidioidal meningitis in rabbits. Antimicrob. Agents Chemother. 46:2420-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 12.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 13.Groll, A. H., J. C. Gea-Banacloche, A. Glasmacher, G. Just-Nuebing, G. Mashmeyer, and T. J. Walsh. 2003. Clinical pharmacology of antifungal compounds. Infect. Dis. Clin. N. Am. 17:159-191. [DOI] [PubMed] [Google Scholar]
- 14.Groll, A. H., D. Mickiene, V. Petraitis, R. Petraitiene, R. M. Alfaro, C. King, S. C. Piscitelli, and T. J. Walsh. 2003. Comparative drug disposition, urinary pharmacokinetics, and renal effects of multilamellar liposomal nystatin and amphotericin B deoxycholate in rabbits. Antimicrob. Agents Chemother. 47:3917-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janknegt, R., S. de Marie, I. A. Bakker Woudenberg, and D. J. Crommelin. 1992. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. Clin. Pharmacokinet. 23:279-291. [DOI] [PubMed] [Google Scholar]
- 16.Klein, R. D., and S. C. Edberg. 2005. Applications, significance of, and methods for the measurement of antimicrobial concentrations in human body fluids, p. 290-364. In V. Lorian (ed.), Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 17.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Pahls, S., and A. Schaffner. 1994. Comparison of the activity of free and liposomal amphotericin B in vitro and in a model of systemic and localized murine candidiasis. J. Infect. Dis. 169:1057-1061. [DOI] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., D. J. Diekema, and M. G. Rinaldi. 2005. Antifungal drugs: mechanisms of action, drug resistance, susceptibility testing, and assays of activity in biological fluids, p. 226-265. In V. Lorian (ed.), Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 20.Proffitt, R. T., A. Satorius, S. M. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B in rodents. J. Antimicrob. Chemother. 28(Suppl. B):49-61. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy, M., K. D. Peteherych, A. L. Kennedy, and K. M. Wasan. 2001. Amphotericin B lipid complex or amphotericin B multiple-dose administration to rabbits with elevated plasma cholesterol levels: pharmacokinetics in plasma and blood, plasma lipoprotein levels, distribution in tissues, and renal toxicities. Antimicrob. Agents Chemother. 45:1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Etten, E. W., M. Otte-Lambillion, W. van Vianen, M. T. ten Kate, and A. J. Bakker-Woudenberg. 1995. Biodistribution of liposomal amphotericin B and amphotericin B desoxycholate in uninfected immunocompetent and leukopenic mice with C. albicans. J. Antimicrob. Agents 35:509-519. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, T. J., A. J. Jackson, J. W. Lee, M Amantea, T. Sein, J. Bacher, and L. Zech. 2000. Dose-dependent pharmacokinetics of amphotericin B lipid complex in rabbits. Antimicrob. Agents Chemother. 44:2068-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh, T. J., V. Yeldandi, M. McEvoy, C. Gonzalez, et al. 1998. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B in neutropenic patients. Antimicrob. Agents Chemother. 42:2191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warnock, D. W., E. M. Johnson, and D. A. White. 1999. Antifungal drug measurements, p. 221-233. In D. S. Reeves, R. Wise, J. M. Andrews, L. O. White, and D. Speller (ed.), Clinical antimicrobial assays. Oxford University Press, Oxford, United Kingdom.
- 27.Wong-Beringer, A., R. A. Jacobs, and B. J. Guglielmo. 1998. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin. Infect. Dis. 27:603-618. [DOI] [PubMed] [Google Scholar]