Abstract
Among the Candida species, Candida parapsilosis has a unique mitochondrial respiratory network. The addition of inhibitors of the respiratory pathways in three clinical isolates of C. parapsilosis with high (≥2- μg/ml) MICs of caspofungin significantly (fivefold) decreased caspofungin MICs but did not change fluconazole MICs.
The echinocandins are a new class of antifungal agents that target the fungal cell wall by inhibiting 1,3-β-d-glucan synthetase (3). Caspofungin (CAS), the first licensed echinocandin, possesses broad-spectrum activity against Candida and Aspergillus species. Because CAS is fungicidal against Candida species, including most azole-resistant non-Candida albicans species, it has become a preferred drug for invasive candidiasis (11).
Candida parapsilosis is an important cause of neonatal and device-related infections (2, 11). In contrast to other Candida spp., C. parapsilosis often exhibits reduced susceptibility to CAS in vitro (8, 12). In addition, failure of CAS in the treatment of C. parapsilosis infections has been reported previously (8). The mechanisms of the suboptimal efficacy of CAS against this species are unknown.
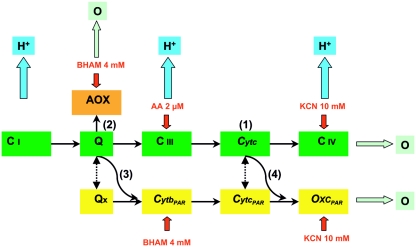
Interestingly, C. parapsilosis displays natural resistance to a wide range of toxic agents (e.g., oligomycin and paromomycin), which has been largely attributed to its unique electron flux pathways (1, 7). Specifically, in contrast with the other Candida spp., which have a classical respiratory chain (CRC), C. parapsilosis also has two alternative respiratory electron flux pathways (1, 7): a cyanide-resistant alternative oxidase (AOX) branched with the CRC at the level of ubiquinone (Q) and a parallel respiratory chain (PAR) secondary to the CRC (Fig. 1). These two alternative respiratory pathways are capable of driving electrons to O2 from both Krebs cycle and cytosolic NADH with cross talk between these pathways and the CRC (7). Notably, both AOX and PAR are insensitive to classical mitochondrial inhibitors such as antimycin A (AA) but can be inhibited by benzohydroxamate (BHAM) and cyanide at high concentrations (7).
FIG. 1.
Simplified figure (adapted from the work of Milani et al. [7]) of the respiratory network in C. parapsilosis mitochondria, which includes three terminal oxidases and four electron transport pathways. The CRC consists of Q, complex I (C i), complex III (C iii), and complex IV (C iv; cytochrome c [Cytc] oxidase), as shown in the green boxes. A secondary PAR comprises alternative ubiquinone (Qx), cytochrome b (CytbPAR), cytochrome c (CytcPAR), and secondary terminal oxidase (OxcPAR) insensitive to AA but inhibited by BHAM and cyanide, as shown in the yellow boxes. A cyanide-resistant AOX branches at the Q level, as shown in the orange box. The numbers in parentheses show the four electron transport pathways, the blue arrows indicate sites of H+ pumping, the red arrows indicate targets of the inhibitors (AA, BHAM, and cyanide), and the dotted lines depict putative interactions.
We hypothesized that the C. parapsilosis complex respiratory network may account for its decreased susceptibility to CAS. To that end, we inhibited the classical and alternative respiratory pathways of different clinical isolates of C. parapsilosis and examined the effects on CAS and fluconazole (FLC; control drug) MICs. We observed a profound decrease in CAS MICs after simultaneous inhibition of all respiratory pathways in all isolates tested, whereas FLC MICs remained unchanged.
We tested three clinical isolates of C. parapsilosis with high CAS MICs (≥2 μg/ml) collected from patients with cancer and hematogenous candidiasis. We obtained FLC (Pfizer Inc., New York, N.Y.), CAS (Merck, Rahway, N.J.), AA (Sigma Chemical Co., St. Louis, Mo.), and BHAM (Sigma Chemical Co.) powder. We prepared drug stock solutions in distilled water (CAS and FLC, 1.28 mg/ml) or 100% methanol (AA, 10 mM; BHAM, 1 M) and stored them at −80°C until use.
We determined the CAS and FLC MICs for each C. parapsilosis isolate according to Clinical and Laboratory Standards Institute (CLSI)-approved document M27-A2 (9). We used standard RPMI 1640 medium as well as yeast-peptone-dextrose and yeast nitrogen base media (Difco, Detroit, MI). We performed susceptibility testing with microtitration plates (Corning, New York) containing serial twofold dilutions of FLC (0.06 to 64.00 μg/ml) and CAS (0.03 to 32.00 μg/ml) and a final inoculum of 1 × 103 to 5 × 103 CFU/ml of each isolate. We determined the CAS and FLC MICs visually and spectrophotometrically 24 and 48 h after a shaking incubation at 35°C as the lowest concentrations that resulted in a prominent (50%) decrease in turbidity for both drugs (9, 10). We next prepared microtitration plates for CAS and FLC as described above and added a standard concentration of either AA (2 μM) or BHAM (4 mM) to each well to selectively inhibit the classical and alternative respiratory pathways, respectively, of C. parapsilosis (7). We added standard concentrations of AA (2 μM) plus BHAM (4 mM) to each well to simultaneously inhibit the respiratory pathways.
We also performed 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT)-based microdilution studies as described previously (6). For the XTT colorimetric assay, we prepared CLSI microtitration plates containing serial twofold dilutions of CAS, of FLC, and of both drugs in combination with either AA (2 μM), BHAM (4 mM), or AA (2 μM) plus BHAM (4 mM) at a standard concentration as described above. In pilot experiments, the combination of AA plus BHAM at higher concentrations did not alter the ability of XTT to be reduced into its colorimetric formazan derivatives (data not shown). We assessed formazan absorbance at 492 and 690 nm (plate absorbance) by using a microplate spectrophotometer (Powerwave X; Biotech Instruments, Winooski, Vt.) and determined the change in optical density at 492 nm (6).
Additionally, we performed disk diffusion susceptibility testing of CAS against each C. parapsilosis isolate in RPMI agar plates and RPMI plates containing a standard noninhibitory concentration of AA or BHAM (1 μM or 2 mM, respectively) defined in pilot experiments. Briefly, we plated 200 μl of 106- CFU/ml inoculum of each isolate, allowed the plates to dry, and then placed a sterile 1/4-inch paper disk (Schleicher and Schuell, Keene, N.H.) on the agar surface and inoculated it with 25 μl of CAS (from a stock solution of 1 mg/ml), producing a final concentration of 2 μg/ml in each plate. We incubated plates at 30°C and measured the radii of the zones of inhibition with a micrometer at 48 h. We accordingly assessed the effect of mitochondrial inhibitors on CAS activity against C. parapsilosis isolates by statistically comparing the changes in the CAS radii of the zones of inhibition.
We performed all experiments in triplicate on different days using C. parapsilosis strain ATCC 20199 as a quality control. For statistical comparisons, we used the Mann-Whitney U test where appropriate. We fitted a four-parameter logistic regression model (Hill equation) to XTT reduction data and calculated 50% effective doses by using a curve-fitting software program (Prism 4; GraphPad Software, Inc., San Diego, Calif.). We considered P values of less than 0.05 to be statistically significant.
All C. parapsilosis isolates exhibited significantly elevated CAS MICs (mean MIC50, 2 μg/ml; range, 1 to 2 μg/ml) in all media. All C. parapsilosis isolates but one were susceptible to FLC (mean MIC50, 0.5 μg/ml; range, 0.5 to 64.0 μg/ml). There were no significant differences in CAS or FLC MICs among the media; MICs were equal at 24 and 48 h (data not shown).
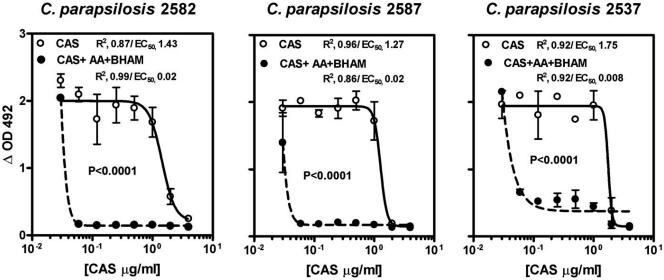
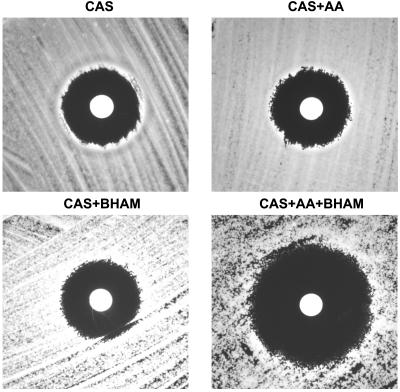
Importantly, mitochondrial inhibitors (AA and BHAM) had no effect on the growth of test isolates at the concentrations used. We found that inhibition of either the classical (with AA) or the alternative (with BHAM) mitochondrial pathway of C. parapsilosis isolates did not significantly affect CAS or FLC MICs (Table 1). However, simultaneous inhibition of all mitochondrial pathways by BHAM plus AA resulted in a profound fivefold drop in CAS MICs for all isolates (MIC50, 0.06 μg/ml; P < 0.0001) but did not change FLC MICs; similar decreases in CAS MICs were observed by using an MIC endpoint of 100% inhibition of growth (data not shown). The effects of the mitochondrial inhibitors on CAS MICs were medium independent (data not shown) and further confirmed in XTT-based microdilution studies (Fig. 2). Additionally, the mean ± standard deviation radius of the zone of inhibition for CAS (8.286 ± 1.069 mm) increased significantly in the presence of both mitochondrial inhibitors (16.4300 ± 0.9376 mm; P = 0.002) (Fig. 3).
TABLE 1.
Effects of the specific mitochondrial inhibitors AA and BHAM at inhibitory concentrations on CAS and FLC MICs against three clinical isolates of C. parapsilosis in RPMI mediuma
| Isolate | Median MICb (μg/ml) of the following drug with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No inhibitor
|
AA (2 μM)
|
BHAM (4 mM)
|
AA + BHAM (2 μM + 4 mM)
|
|||||
| CAS | FLC | CAS | FLC | CAS | FLC | CAS | FLC | |
| C. parapsilosis 2537 | 2 | >64.0 | 2 | 32.0 | 2 | >64.0 | 0.06 | 32.00 |
| C. parapsilosis 2582 | 2 | 0.5 | 2 | 0.5 | 1 | 0.5 | 0.06 | 0.25 |
| C. parapsilosis 2587 | 2 | 0.5 | 1 | 0.5 | 2 | 0.5 | 0.06 | 0.50 |
CLSI broth microdilution method M27-A2.
The range of MICs was less than 1 dilution in all experiments.
FIG. 2.
XTT-based analysis of in vitro activities of CAS alone (solid lines) and of CAS in combination with the mitochondrial inhibitors AA and BHAM at standard concentrations (2 μM and 4 mM, respectively; dotted lines) in RPMI medium against three clinical isolates of C. parapsilosis. Sigmoid concentration inhibitory-effect curves were generated by fitting data to a four-parameter logistic regression model (Hill equation). The symbols represent the means ± standard deviations from experiments performed in triplicate in each case. ΔOD492, optical density at 492 mm.
FIG. 3.
Effects of the mitochondrial inhibitors AA (1 μM), BHAM (2 mM), and AA plus BHAM (1 μM and 2 mM, respectively) on CAS activity against a representative clinical isolate of C. parapsilosis (2537) as seen in disk diffusion susceptibility testing. Each disk contained 25 μl of CAS, resulting in a final CAS concentration in each plate of 2 μg/ml from a stock solution of 1 mg/ml.
Although susceptibility breakpoints for CAS have not been established, a provisional MIC of ≥1 μg/ml was proposed recently (3). We demonstrated that whereas inhibition of each of the mitochondrial pathways of C. parapsilosis had no effect on susceptibility to CAS, simultaneous inhibition of all mitochondrial pathways dramatically lowered CAS MICs but not FLC MICs. These results were also shown by the XTT assay, a viability staining method that precisely assesses the fungal biomass and is a marker for mitochondrial activity, as it is based on the reduction of XTT by mitochondrial dehydrogenase (6). Finally, our findings were confirmed by another independent disk diffusion susceptibility method.
Although rare, reports of CAS-resistant Candida have begun to emerge (4). The mechanisms of Candida resistance to echinocandins remain obscure. In vitro resistance in laboratory-selected Candida albicans mutants has been associated with reduced glucan synthetase activity (5). Of interest is the fact that mitochondria have not been shown to be involved in resistance of Candida or other pathogenic fungi to echinocandins. Nevertheless, the ability of C. parapsilosis respiratory pathways to resist oxidative stress has long been encountered with resistance to a variety of antimicrobial agents (1). Our work implies that the unique respiratory network of C. parapsilosis partially accounts for its reduced resistance to CAS. It is tempting to speculate that the inhibition of cell wall synthesis might be associated with increased susceptibility to oxidative stress more than the inhibition of other important cellular components (ergosterol synthesis) is. However, other mechanisms of resistance (e.g., altered glucan synthetase activity) might also be involved in the decreased susceptibility of C. parapsilosis to CAS. To that end, our preliminary observations must be explored further in studies measuring specific aspects of oxidative burst associated with sequential or simultaneous inhibition of the different respiratory pathways of C. parapsilosis in response to CAS.
Acknowledgments
We thank Nathaniel D. Albert for excellent technical assistance and Don Norwood for editorial assistance.
This study was supported by The University of Texas M. D. Anderson Cancer Center Faculty E. N. Cobb Scholar Award Research Endowment (D.P.K.).
REFERENCES
- 1.Camougrand, N., G. Velnous, and M. Guerin. 1986. Resistance of Candida parapsilosis to drugs. Biol. Cell 58:71-78. [DOI] [PubMed] [Google Scholar]
- 2.Chapman, R. L. 2003. Candida infections in the neonate. Curr. Opin. Pediatr. 15:97-102. [DOI] [PubMed] [Google Scholar]
- 3.Deresinski, S. C., and D. A. Stevens. 2003. Caspofungin. Clin. Infect. Dis. 36:1445-1457. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez, S., J. L. Lopez-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtz, M. B., G. Abruzzo, A. Flattery, K. Bartizal, J. A. Marrinan, W. Li, J. Milligan, K. Nollstadt, and C. M. Douglas. 1996. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect. Immun. 64:3244-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, J. P. Donnelly, P. E. Verweij, and Eurofung Network. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milani, G., W. Jarmuszkiewicz, C. M. Sluse-Goffart, A. Z. Schreiber, A. E. Vercesi, and F. E. Sluse. 2001. Respiratory chain network in mitochondria of Candida parapsilosis: ADP/O appraisal of the multiple electron pathways. FEBS Lett. 508:231-235. [DOI] [PubMed] [Google Scholar]
- 8.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas, G. P., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, and T. J. Walsh. 2004. Guidelines for the treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., D. J. Diekema, R. N. Jones, S. A. Messer, and R. J. Hollis. 2002. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. J. Clin. Microbiol. 40:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]