Abstract
Prion diseases, including scrapie, are incurable neurodegenerative disorders. Some compounds can delay disease after a peripheral scrapie inoculation, but few are effective against advanced disease. Here, we tested multiple related porphyrins, but only Fe(III)meso-tetra(4-sulfonatophenyl)porphine injected into mouse brains after intracerebral scrapie inoculation substantially increased survival times.
The transmissible spongiform encephalopathies (TSEs or prion diseases) are neurodegenerative diseases that include Creutzfeldt-Jakob disease (CJD) of humans, bovine spongiform encephalopathy, chronic wasting disease of deer and elk, and scrapie of sheep. The infectious agent of TSEs is not fully characterized, but there is evidence that an abnormal, protease-resistant form of prion protein is involved (10). Over 160 cases of variant CJD, caused by the consumption of bovine spongiform encephalopathy-infected beef, have increased concern about the impact of TSEs on human health. While TSEs are incurable, various compounds dosed at or near the time of infection have delayed the onset of scrapie in animals after inoculation with high peripheral doses of infectant or even prevented disease after low peripheral doses (reviewed in references 1 and 4). Compounds that have delayed the onset of clinical scrapie after intracerebral (i.c.) inoculation include amphotericin B (7), pentosan polysulfate (PPS) (3), and, to a lesser extent, Congo red (6).
Most compounds active against scrapie, including cyclic tetrapyrroles, also inhibit protease-resistant prion protein formation in cell cultures (2), which may explain their in vivo activity. A metal-free phthalocyanine and two iron porphyrins, types of cyclic tetrapyrroles, have been shown to delay scrapie onset after peripheral but not i.c. inoculation (8, 9). In the search for more effective anti-TSE compounds, we evaluated two types of previously untested porphyrins with or without central metals (Fig. 1).
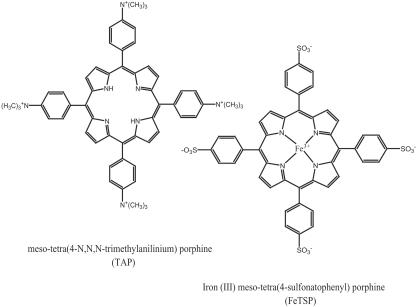
FIG. 1.
Structures of the two types of porphyrins tested. Metal-free TAP is shown on the left. The central metal ion of these porphyrins is coordinated with the nitrogen atoms as is shown for FeTSP on the right.
meso-tetra(4-sulfonatophenyl)porphine (TSP), iron(III)TSP (FeTSP), meso-tetra(4-N,N,N-trimethylanilinium)porphine (TAP), and iron(III)TAP (FeTAP) were tested for the ability to delay scrapie in transgenic mice (Tg7) that are very susceptible to hamster scrapie strain 263K (9, 11). (All animal use was approved by the appropriate institution’s animal care and use committee.) All four porphyrins injected intraperitoneally (i.p.) prior to and for 4 or 5 weeks after i.p. scrapie inoculation significantly increased survival times (Table 1). FeTAP was most effective, increasing survival times more than fourfold. In a further test, FeTAP administered i.p. beginning 50 days after i.p. scrapie challenge and continuing three times per week until near death was ineffective (average survival time ± standard deviation of 85.0 ± 13.2 days versus 83.1 ± 7.5 days for the control). This is not surprising as TAP and TSP compounds may have little blood-brain barrier (BBB) permeability. Since these four porphyrins demonstrated prophylactic activity after i.p. scrapie inoculation in a test where infectant and compound can interact without crossing the BBB, they were further tested against scrapie via i.c. injections to bypass the BBB.
TABLE 1.
Porphyrins as prophylactic compounds against 263K scrapie infection
| Compound | i.p. dose [mg/kg (mmol/kg)]a | Dosing regimen | i.p. scrapie inoculation (day 0)b | Survival times (days)c | Mean survival time ± SD |
|---|---|---|---|---|---|
| None | 50 μl 1% BH | 78, 82, 91, 91, 92, 92, 92, 92, 100 | 90.0 ± 6.4 | ||
| FeTSP | 12.5 (0.012) | 3 doses/wk for 6 wks starting 2 wks prior to inoculation | 50 μl 1% BH | 124, 143, 145, 147, 163, 171, 196, 203 | 161.5 ± 27.3d |
| TSP | 25 (0.025) | Dosing on days −2, −1, and 0, then 3 doses/wk for 5 wks | 50 μl 1% BH | 119, 122, 122, 126, 129, 136, 141, 161 | 132.0 ± 13.9d |
| FeTAP | 12.5 (0.012) | 3 doses/wk for 6 wks starting 2 wks prior to inoculation | 50 μl 1% BH | 295, 299, 376, 388, 581, 686 | 437.5 ± 160.0d |
| TAP | 6.25 (0.006)e | 3 doses/wk for 6 wks starting 2 wks prior to inoculation | 50 μl 1% BH | 100, 127, 142, 156, 182, 183, 205, 233 | 166.0 ± 43.3d |
In phosphate-buffered saline.
BH, 263K-infected brain homogenate in phosphate-buffered saline.
Tg7 mice dying from nonscrapie causes were removed from the data set.
P < 0.0001 versus control group by unpaired t test.
The dose of 12.5 mg/kg was toxic.
In one type of antiscrapie assay, the test compound and infected brain homogenate are mixed prior to i.c. inoculation. Some compounds in such tests have produced increased survival times, presumably due to either direct inactivation of the infectant or the presence of the compound in the brain at the time of infection (5). As FeTAP was the most effective prophylactic compound, FeTAP and other metal TAPs were tested in this manner. The toxicity of i.c.-administered TAP compounds varied greatly, and 50 μl of 0.5 mM TAP, ZnTAP, CrTAP, InTAP, or CdTAP was not tolerated (data not shown). The results from FeTAP and other tolerated TSP and TAP compounds are shown in Table 2. A dilution series of untreated infected brain homogenate was also included to allow estimation of the apparent reduction in scrapie titer. NiTAP and FeTAP, the most active compounds in this “inactivation” test, produced survival times that correlated with a reduction of between 3 and 4 logs of infectivity. When the metal was changed to Cu(II), the activity was greatly reduced, indicating the importance of the metal ion.
TABLE 2.
Infectivity of scrapie-infected brain homogenate incubated with TAP or TSP compounds
| Inoculum (50 μl)a | Survival timesb (days) | Mean survival time ± SD |
|---|---|---|
| 1% BH | 50, 50, 50, 51, 51, 52, 52, 56 | 51.5 ± 2.0 |
| 0.1% BH | 50, 51, 52, 56, 56, 56, 56 | 53.9 ± 2.7 |
| 0.01% BH | 56, 56, 58, 58, 61, 61, 62, 62 | 59.3 ± 2.5 |
| 0.001% BH | 61, 61, 62, 62, 67, 68, 70 | 64.4 ± 3.8 |
| 0.0001% BHc | 69, 74, 87, 89, 97, 98 | 85.7 ± 11.9 |
| 0.5 mM CuTAP + 1% BH | 52, 52, 53, 56, 56, 56, 56, 62 | 55.4 ± 3.2 |
| 0.5 mM NiTAP + 1% BH | 65, 70, 70, 70, 71, 71, 73, 77 | 70.9 ± 3.4d |
| 0.5 mM FeTAP + 1% BH | 62, 67, 68, 68, 71, 73, 76, 79 | 70.5 ± 5.4d |
| 0.5 mM PdTAP + 1% BH | 58, 61, 65, 66, 69, 76 | 65.8 ± 6.3d |
| 0.5 mM TSP + 1% BH | 55, 55, 56, 56, 56, 56, 57, 57 | 56.0 ± 0.8d |
| 0.5 mM CuTSP + 1% BH | 52, 52, 54, 54, 54, 54, 57, 59 | 54.5 ± 2.4 |
| 0.5 mM FeTSP + 1% BH | 56, 56, 57, 58, 58, 60, 63, 65 | 59.1 ± 3.3d |
BH, 263K-infected brain homogenate in phosphate-buffered saline. BH was incubated for 1 hour at 37°C with different metal-substituted TAP or TSP compounds prior to i.c. inoculation into Tg7 mice.
Mice dying from nonscrapie causes were removed from the data set.
Not done at the same time as that of other controls, but data are typical.
P < 0.0001 versus 1% BH group by unpaired t test.
While this inactivation test can help rank compounds' abilities to slow the effects of scrapie inocula, it does not measure activity against late-stage TSE infection. To test therapeutic potential, a number of the more effective TAP and TSP scrapie inactivation compounds were dosed once a week for 5 weeks starting ∼2 weeks after i.c. scrapie inoculation (Table 3). Compounds were injected i.c. to overcome suspected low BBB permeability. PPS, which has antiscrapie activity when it is continuously infused into an infected brain (3), was injected directly to the brain as a positive control (Table 3). Other than a small but statistically significant increase in survival time with FeTAP, only FeTSP was effective as a therapeutic treatment, with activity comparable to that of a 10-fold-lower dose of PPS. The reason that FeTAP was the most active prophylactic compound but had little activity as a treatment after i.c. scrapie inoculation is not known. FeTSP was then further tested using six weekly i.c. doses of 50 μl of 0.5, 0.16, or 0.05 mM FeTSP (25, 8, or 2.5 nanomoles/mouse) (Table 3). The average survival time increased between the 8- and 25-nanomole doses but changed little between the 8- and 2.5-nanomole doses. ZnTSP and InTSP, injected at the same dose and frequency as that of FeTSP, gave no benefit, further demonstrating the importance of the central metal ion. It is also curious that NiTAP, which was quite effective in the inactivation test, was ineffective when dosed i.c. weekly starting 2 weeks after i.c. scrapie inoculation. Thus, differences in the central metal may affect not only porphyrin stereochemistries and reactivities but also, as shown here, antiscrapie potential. Understanding the reason for the differences in activity due to metal substitutions may be instructive in designing therapies for TSEs.
TABLE 3.
Effect of compounds injected into the brain of Tg7 micea
| Treatment (50 μl in PBS) | Days of dose (postinoculation) | Survival timesb (days) | Mean survival time ± SD | Control mean survival time ± SD |
|---|---|---|---|---|
| 0.5 mM FeTAP | 14, 16, 18, 21c | 45, 51, 51, 54, 54, 60, 60, 60 | 54.4 ± 5.4d | 46.1 ± 1.2 |
| 0.5 mM FeTAP | 14, 21, 28, 35, 42 | 46, 47, 47, 47, 47, 48, 56, 57 | 49.4 ± 4.4 | 48.3 ± 3.0 |
| 0.5 mM NiTAP | 13, 20, 27, 34, 41 | 44, 44, 49, 49, 49, 61, 61 | 50.1 ± 7.2 | 48.7 ± 6.8 |
| 0.25 mM PdTAP | 13, 20, 27, 34, 41 | 43, 49, 51, 56 | 49.8 ± 5.4 | 48.7 ± 6.8 |
| 0.1 mM ZnTAP | 13, 20, 27, 34, 41 | 49, 49, 50, 50, 51, 51, 53, 63 | 52.0 ± 4.6 | 48.7 ± 6.8 |
| 0.1 mM TAP | 13, 20, 27, 34, 41 | 43, 44, 49, 49, 49, 51, 54, 54, 54 | 49.7 ± 4.1 | 48.7 ± 6.8 |
| 0.5 mM ZnTSP | 13, 20, 27, 34, 41 | 47, 49, 49, 50, 51, 53, 53, 54, 57, 65 | 52.8 ± 5.2 | 48.7 ± 6.8 |
| 0.5 mM InTSP | 13, 20, 27, 34, 41 | 44, 49, 51, 53, 54, 54, 56, 57, 65 | 53.7 ± 5.8 | 48.7 ± 6.8 |
| 0.5 mM FeTSP | 13, 20, 27, 34, 41 | 57, 68, 70, 70, 72, 73, 83, 83, 85 | 73.4 ± 9.0e | 48.7 ± 6.8 |
| 0.5 mM FeTSP | 14, 21, 28, 35, 42, 49 | 54, 68, 68, 74, 76, 76, 80 | 70.9 ± 8.6e | 50.6 ± 3.0 |
| 0.16 mM FeTSP | 14, 21, 28, 35, 42, 49 | 54, 56, 56, 61, 64, 66, 66, 67 | 61.3 ± 5.3d | 50.6 ± 3.0 |
| 0.05 mM FeTSP | 14, 21, 28, 35, 42, 49 | 52, 54, 57, 60, 62, 62, 64, 67 | 59.8 ± 5.1d | 50.6 ± 3.0 |
| ∼0.05 mM PPSf | 14, 21, 28, 35, 42 | 67, 68, 70, 72, 73, 73, 77, 78 | 72.3 ± 3.9e | 48.3 ± 3.0 |
Compounds were dosed after i.c. inoculation with 50 μl of 1% 263K-infected brain homogenate. A control group of eight Tg7 mice dosed i.c. with 50 μl of phosphate-buffered saline (PBS) at the same interval as that of treated mice was included with each experiment.
Mice dying from nonscrapie causes were removed from the data set.
Dosing was halted due to observed toxicity.
P < 0.001 versus corresponding control group by unpaired t test.
P < 0.0001 versus corresponding control group by unpaired t test.
This concentration was based on the average molecular weight of ∼5,000.
Based on its antiscrapie activity in mice, PPS is currently being infused into the brains of CJD patients as an experimental treatment (first patient described in reference 12). As there is no known effective CJD therapy, experimental treatment will likely start as soon as a diagnosis is made and will continue as long as possible. It is not known whether neurodegeneration can be stopped or reversed, but an important first goal is to slow disease progression. The discovery reported here that FeTSP has activity similar to that of PPS suggests that the use of cyclic tetrapyrroles as a CJD treatment is worth pursuing. With that goal in mind, testing of FeTSP by continuous brain infusion in mice to increase efficacy is ongoing. Until this brain infusion test is completed, it is impossible to know just how effective FeTSP treatment might be. Depending on these results and additional toxicology testing, a more informed decision on human clinical trials can be made. Finally, the demonstrated benefit of FeTSP against i.c.-inoculated scrapie suggests that other cyclic tetrapyrroles with even greater activity may yet be discovered.
Acknowledgments
This work was funded in part by the Intramural Research Program of the NIH, NIAID, U.S. Department of Defense prion interagency transfer NP020114, and contract N01-AI-15435 from the Virology Branch, NIAID, NIH.
We also thank Suzette A. Priola for helpful discussions and Biopharm Australia for a gift of pentosan polysulfate.
REFERENCES
- 1.Cashman, N. R., and B. Caughey. 2004. Prion diseases-close to effective therapy? Nat. Rev. Drug Discov. 3:874-884. [DOI] [PubMed] [Google Scholar]
- 2.Caughey, W. S., L. D. Raymond, M. Horiuchi, and B. Caughey. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 95:12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doh-ura, K., K. Ishikawa, I. Murakami-Kubo, K. Sasaki, S. Mohri, R. Race, and T. Iwaki. 2004. Treatment of transmissible spongiform encephalopathy by intraventricular drug infusion in animal models. J. Virol. 78:4999-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dormont, D. 2003. Approaches to prophylaxis and therapy. Br. Med. Bull. 66:281-292. [DOI] [PubMed] [Google Scholar]
- 5.Forloni, G., S. Iussich, T. Awan, L. Colombo, N. Angeretti, L. Girola, I. Bertani, G. Poli, M. Caramelli, B. M. Grazia, L. Farina, L. Limido, G. Rossi, G. Giaccone, J. W. Ironside, O. Bugiani, M. Salmona, and F. Tagliavini. 2002. Tetracyclines affect prion infectivity. Proc. Natl. Acad. Sci. USA 99:10849-10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingrosso, L., A. Ladogana, and M. Pocchiari. 1995. Congo red prolongs the incubation period in scrapie-infected hamsters. J. Virol. 69:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pocchiari, M., S. Schmittinger, and C. Masullo. 1987. Amphotericin B delays the incubation period of scrapie in intracerebrally inoculated hamsters. J. Gen. Virol. 68:219-223. [DOI] [PubMed] [Google Scholar]
- 8.Priola, S. A., A. Raines, and W. Caughey. 2003. Prophylactic and therapeutic effects of phthalocyanine tetrasulfonate in scrapie-infected mice. J. Infect. Dis. 188:699-705. [DOI] [PubMed] [Google Scholar]
- 9.Priola, S. A., A. Raines, and W. S. Caughey. 2000. Porphyrin and phthalocyanine antiscrapie compounds. Science 287:1503-1506. [DOI] [PubMed] [Google Scholar]
- 10.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Race, R., M. Oldstone, and B. Chesebro. 2000. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todd, N. V., J. Morrow, K. Doh-ura, S. Dealler, S. O'Hare, P. Farling, M. Duddy, and N. G. Rainov. 2005. Cerebroventricular infusion of pentosan polysulphate in human variant Creutzfeldt-Jakob disease. J. Infect. 50:394-396. [DOI] [PubMed] [Google Scholar]