Abstract
HIV-specific CD8+ T cells are critical in controlling human immunodeficiency virus (HIV) replication. We present the evaluation of a gamma-interferon (IFN-γ)-based enzyme linked immunospot (ELISPOT) assay for the quantification of HIV-specific CD8+ T cells from HIV-infected children. We studied 20 HLA-A∗0201-positive HIV-infected children. The IFN-γ production in response to stimulation with two HLA-A∗0201-restricted immunodominant CD8 epitopes (SLYNTVATL [SL9] in Gag and ILKEPVHGV [IV9] in Pol) was tested using the ELISPOT assay. The results were compared to labeling with the corresponding tetramers. Among the 20 children, 18 had detectable responses against the SL9 and/or the IV9 epitope using the ELISPOT assay (medians, 351 and 134 spot-forming cells/106 peripheral blood mononuclear cells, respectively). Comparison of results from the tetramer and ELISPOT assays suggests that only a fraction of HIV-specific CD8+ T cells were able to produce IFN-γ. Most importantly, we found that the frequencies of IFN-γ-producing CD8+ T cells were positively correlated with the viral load whereas the frequencies of tetramer-binding CD8+ T cells were not. The high sensitivity of the ELISPOT assay and the fact that this functional assay provided information different from that of tetramer labeling support its use for measurement of HIV-specific CD8+ T cells. In conclusion, our results show that the ex vivo-activated IFN-γ-producing HIV-specific CD8+-T-cell subset is dependent upon continuous antigenic stimulation.
Human immunodeficiency virus (HIV)-specific CD8+-T-cell responses are critical in restricting viral replication and altering the course of HIV infection (38, 48). Ex vivo cytotoxic T lymphocytes (CTL) are much less frequent in vertically infected children than in adults (9, 10, 41, 42, 46, 60). In contrast, after in vitro culture, memory CTL can be readily detected in HIV-infected children, with magnitude, breadth, and specificity similar to those observed in adults (9, 10, 41, 46). These memory CTL can be detected during the first weeks or months of life (10, 41, 56, 75), and the presence of HIV-specific CTL is associated with slower evolution toward disease (10).
Complete suppression of viral replication following combined therapy is less frequent in children than in adults (40, 51, 52), and the kinetics of viral decrease is slower (49). However, children have a more active thymus function than adults (19, 44), which allows better lymphocyte regeneration following combined therapy (13, 69). In children treated with combined therapy before 3 months of age, and with undetectable viral load, neither HIV-specific antibodies nor HIV-specific CD4 or CD8 cells were detected (43). This contrasts with the beneficial effect of early treatment on preservation of HIV-specific T-cell immunity in adults (8, 50). New immunotherapeutic interventions are being developed for adults (8, 50). These treatments are of great interest for children due to observance problems and because complete viral suppression is more difficult to obtain in children than in adults. Therefore, it is crucial to characterize the dynamics of HIV-specific T cells in pediatric HIV infection.
On encountering viral antigen, naive CD8+ T cells proliferate and differentiate into effector cells able to lyse infected cells and to secrete cytokines. As virus is cleared, most activated effector cells undergo apoptosis, but some survive and enter the memory pool that persists for long periods (76). Most memory cells are in a resting state, unable to secrete cytokine or to lyse infected cells, until reactivation on reexposure to viral antigen (61). During persistent infection, continuous stimulation of T cells may lead to dysfunction, anergy, or clonal exhaustion. In the absence of CD4+ T cells, exhaustion and anergization of CD8+ T cells is more rapid (78). The complex interplay between the antigen load, virus-specific CD8+-T-cell dynamics and function, and the immune status of the infected host has been illustrated by a number of studies of HIV-infected adults. In untreated chronically HIV-infected patients, CTL numbers are inversely correlated with the plasma viral load (5, 24, 25, 34, 54, 58). On the other hand, reduction of HIV replication by potent antiretroviral therapy is associated with a decline in HIV-specific CD8+ T cells (11, 55). Evidence that a fraction of HIV-specific CD8+ T cells are not functional was recently obtained (22, 31). Furthermore, loss of gamma-interferon (IFN-γ)-producing cells with persistence of tetramer binding cells was observed in subjects progressing to AIDS (32).
CTL derived from the memory pool after in vitro expansion are easily detected by the standard 51Cr release assay in most HIV-infected children (9). In contrast, ex vivo-activated effector cytolytic cells are infrequently detected immediately after isolation (9). Cytokine synthesis following short-term ex vivo stimulation with the antigen can be measured using assays that are more sensitive than the chromium release assay and could be used to quantify the effector subset of HIV-specific CD8+ T cells (3, 18). Cytokine production can be measured at the single-cell level using the enzyme-linked immunospot (ELISPOT) technique, allowing direct calculation of T-cell frequencies (15). This technique is very sensitive and has been found to reliably detect CD8+ T cells in various human diseases (35, 63, 64, 74), including HIV infection (16, 36).
The aim of the present work was to evaluate the use of the ELISPOT assay for the ex vivo study of HIV-specific CD8+ T cells from HIV-infected children. We chose to focus our initial effort on two immunodominant HLA-A∗0201-restricted HIV epitopes that are frequently recognized by infected children, as we showed previously using tetramers (66). In this cross-sectional study, HLA-A∗0201-positive HIV-infected children were systematically tested for the presence of HIV-specific CD8+ T cells using the ELISPOT assay. The frequencies of HIV-specific IFN-γ-producing CD8+ T cells were compared to the frequencies of HIV-specific CD8+ T cells measured by tetramer labeling, and relationships with biological parameters of HIV infection were investigated. The results from the ELISPOT and the tetramer assays were well correlated, but a comparison of the results from both assays suggests that a significant fraction of CD8+ T cells were unable to produce IFN-γ. Most importantly, the frequencies of ex vivo-activated HIV-specific CD8+-T-cell-mediated IFN-γ production were positively correlated with plasma HIV RNA, showing that this subset of antiviral CD8+ T cells is dependent upon continuous antigenic stimulation.
MATERIALS AND METHODS
Patients.
All patients were prospectively followed at Hôpital Necker, Paris, France. Twenty HLA-A∗0201-positive children included in the longitudinal follow-up of their HIV-specific immune responses (9, 10, 66) were systematically tested for their IFN-γ production in response to HIV peptides during a 6-month period. Four HLA-A∗0201-negative HIV-infected children were included as negative controls. All of the children were infected by HIV type 1 (HIV-1). Twenty-one patients were born to HIV-1-infected mothers, and three patients (EM50, EM67, and EM76) were infected by blood transfusion during their first 2 years of life. Among the 20 HLA-A∗0201-positive patients, 10 had received three or four antiretroviral drugs from at least two different classes and 10 were either untreated or had received a combination of two inhibitors of reverse transcriptase. For each patient, biological data, the Centers for Disease Control and Prevention (CDC) disease stage designation (12), and the antiretroviral treatment at the time of evaluation are shown in Table 1. For the 24 patients, median values were as follows: age, 12.7 years (range, 3.0 to 17.7 years); CD4+ T cells, 26% (range, 7 to 46%); CD8+ T cells, 50% (range, 35 to 71%); and log10 HIV RNA copies/ml, 3.8 (range, <1.7 to 5.7).
TABLE 1.
Characteristics of HIV-1-infected children at time of evaluation
| Patient | Age (yr) | CD4 (%) | CD8 (%) | NbCD4a | NbCD8a | Viral loadb | CDC stagec | Treatment |
|---|---|---|---|---|---|---|---|---|
| HLA-A*0201-negative (n = 4) | ||||||||
| EM60 | 7.3 | 28 | 39 | 4.95 | B | Combined therapyd (12) | ||
| EM106 | 7.4 | 27 | 48 | 837 | 1,488 | 2.9 | A | Combined therapy (33) |
| EM93 | 12.3 | 28 | 44 | 476 | 748 | 3.6 | A | None |
| EM68 | 14.3 | 19 | 53 | 209 | 583 | 5 | B | None |
| HLA-A*0201-positive (n = 20) | ||||||||
| EM102 | 3.0 | 35 | 50 | 2,135 | 3,050 | 5 | C | Nonee |
| EM89 | 4.5 | 21 | 63 | 1,386 | 4,158 | 4.8 | N | None |
| EM78 | 9.2 | 24 | 53 | 840 | 1,855 | 4.5 | B | None |
| EM76 | 12.7 | 19 | 61 | 342 | 1,098 | 5.7 | B | None |
| EM28 | 12.9 | 33 | 51 | 545 | 842 | <1.7 | B | None |
| EM40 | 14.8 | 40 | 45 | 480 | 540 | 3.2 | N | Nonee |
| EM31 | 17.7 | 7 | 66 | 77 | 726 | 4.2 | N | None |
| EM45 | 9.9 | 19 | 56 | 627 | 1,848 | 4.2 | N | 2 NRTIf (46) |
| EM86 | 14.4 | 42 | 42 | 504 | 504 | 4.1 | A | 2 NRTI (59) |
| EM67 | 16.8 | 46 | 40 | 506 | 440 | 3.8 | N | 2 NRTI (38) |
| EM63 | 8.2 | 26 | 52 | 468 | 936 | 5 | C | Combined therapy (13) |
| EM42 | 9.9 | 20 | 56 | 900 | 2,520 | 3.8 | A | Combined therapy (26) |
| EM83 | 12.0 | 19 | 35 | 437 | 805 | 3 | A | Combined therapy (1) |
| EM18 | 12.7 | 23 | 45 | 874 | 1,710 | <2.6 | B | Combined therapy (34) |
| EM71 | 12.4 | 40 | 36 | 1,200 | 1,080 | <1.7 | C | Combined therapy (43) |
| EM3 | 13.4 | 28 | 54 | 840 | 1,620 | 3.6 | B | Combined therapy (39) |
| EM30 | 13.8 | 23 | 48 | 391 | 816 | 3.5 | A | Combined therapy (8) |
| EM23 | 14.2 | 20 | 40 | 500 | 1,000 | 2.9 | B | Combined therapy (23) |
| EM20 | 17.1 | 32 | 47 | 512 | 752 | 2.7 | C | Combined therapy (39) |
| EM50 | 17.7 | 14 | 71 | 217 | 1,100.5 | 5.2 | B | Combined therapy (18) |
Absolute CD4 and CD8 T-cell numbers per cubic millimeter of blood.
log10 HIV-RNA copies per milliliter of plasma.
Stage of HIV disease, as defined by the CDC (12). N, asymptomatic infection; A, paucisymptomatic infection; B, moderately symptomatic infection; C, severely symptomatic infection.
Combined therapy is defined as treatment with at least three antiretroviral drugs from two different classes. The total duration of treatment, expressed in months, is indicated in parentheses.
These children were previously under combined therapy.
Children were treated with a combination of two nucleoside inhibitors of the HIV reverse transcriptase (NRTI). The total duration of treatment, expressed in months, is indicated in parentheses.
Plasma HIV-1 RNA monitoring.
The Amplicor HIV Monitor test (Roche, Neuilly, France) was used to quantitate HIV RNA in plasma. The cutoff value was 2.6 log unit copies/ml for the classical assay and 1.7 copies/ml for the ultrasensitive assay. When results were below detection level, half of the assay cutoff was taken as the value for calculation.
HLA class I typing.
HLA genotyping was performed in the Department of Immunology at the Chelsea and Westminster Hospital, London, United Kingdom, or in the Service d'Immunologie Clinique, Hôpital Necker, Paris, France, using amplification refractory mutation system PCR (33).
ELISPOT assay.
The ELISPOT assay for analysis of IFN-γ production was adapted from its original description (15). Nitrocellulose-bottom 96-well plates (MAHA N45; Millipore, Molsheim, France) were coated overnight at 4°C with 50 μl of the anti-IFN-γ monoclonal antibody 1-D1K at 15 μg/ml (Mabtech, Stockholm, Sweden) in carbonate buffer (15 mM NaCO3, 35 mM NaHCO3, 0.2% NaN3, pH 9.6). The antibody-coated plates were washed three times with phosphate-buffered saline (PBS) and blocked with PBS containing 5% fetal calf serum (Dutscher, Brumath, France) for at least 2 h at room temperature. The plates were washed three times with PBS before the addition of peripheral blood mononuclear cells (PBMC) in RPMI 1640 (Whittaker, Gagny, France) supplemented with 10% fetal calf serum, 2 mM l-glutamine (Gibco-BRL, Cergy-Pontoise, France), penicillin, and streptomycin (Gibco-BRL). PBMC were isolated from whole heparinized blood by density centrifugation on Ficoll-Paque (Pharmacia, Les Ulis, France) and tested immediately after isolation. Triplicates of two cell concentrations (5 × 105 and 1 × 105/well) were stimulated with peptides corresponding to two HIV immunodominant epitopes, one in the p17gag protein, (SLYNTVATL [SL9]) and the other in the reverse transcriptase (ILKEPVHGV [IV9]), at a final concentration of 1 μg/ml. Negative controls consisted of cells incubated in medium. Phorbol myristate acetate and ionomycin (25 and 100 ng/ml, respectively; Sigma-Aldrich Chimie, Saint-Quentin Fallavier, France) were used as positive controls. For some experiments, CD8+ cells were depleted using beads coupled to anti-CD8 monoclonal antibodies (MACS system; Miltenyi Biotec Gmbh, Bergisch Gladbach, Germany). The efficiency of the depletion was analyzed by flow cytometry. For tetramer stimulation, 1 μg of HLA-A∗0201 tetramer/ml coupled to SL9 or IV9 peptide was added to the wells together with the PBMC. We found that 1 μg of tetramer/ml was the optimal dose to induce IFN-γ production using a CD8+ CTL line specific for the SL9 peptide (data not shown). The cells were incubated overnight at 37°C in 5% CO2. The wells were washed three times in PBS with 0.05% Tween 20 (Sigma) and three times with PBS, followed by a 3- to 4-h incubation at room temperature with the biotinylated anti-IFN-γ monoclonal antibody 7-B6-1 in PBS (1 μg/ml). The wells were washed as previously described, and Streptavidin-bound alkaline phosphatase (0.5 U/ml; Boehringer Mannheim, Meylan, France) diluted in PBS was added for 90 min at room temperature. The wells were washed, and 5-bromo-4-chloro-3-indolylphosphate-Nitro Blue Tetrazolium color substrate (Promega, Lyon, France) diluted in 100 mM Tris, 150 mM NaCl, and 5 mM MgCl2 (pH 9.5) was added for 1 h at room temperature before the reaction was stopped with tap water. The number of spot-forming cells (SFC) was determined with computer-assisted image analysis software (KS-ELISPOT; Zeiss, Munich, Germany). A minimum of 10 spots/well were retained to calculate the frequencies of IFN-γ-producing cells. Responses were considered positive if the number of spots exceeded the mean plus 3 standard deviations of cells incubated with medium alone and was at least twice the mean of cells incubated with medium. The threshold for positivity depends on spontaneous secretion. Its median value in this study was 60/106 PBMC. Results were expressed as follows: SFC/106 PBMC = (number of SFC per well) × 106/(number of cells per well) or percent SFC among CD8+ T cells = (number of SFC per well) × 100/(number of CD8+ T cells per well). The number of CD8+ T cells per well was calculated as follows: [(number of cells per well) × (number of CD8+ T cells per cubic millimeter of blood)/(number of lymphocytes and monocytes/cubic millimeter of blood)]. The numbers of cells per well were defined after Ficoll isolation, whereas the numbers of lymphocytes and monocytes were obtained by standard techniques performed on whole blood. Isolation of PBMC by the Ficoll procedure may slightly modify the percentages of PBMC subpopulations. Nevertheless, it is unlikely that this technical bias will profoundly modify the results.
Tetrameric peptide-MHC complexes.
the HLA-A∗0201 tetrameric complexes used in this study were synthesized as previously described (7). The major histocompatibility complex (MHC)-peptide complexes were formed with two HIV immunodominant epitopes, one in the p17gag protein (SL9) and the other in the reverse transcriptase (IV9).
Flow cytometry analyses.
Absolute CD4 and CD8 counts were performed on fresh whole-blood samples by four-color flow cytometry analysis of cells positive for CD45, CD3, CD4, or CD8, with fluorescent beads as an internal standard (flow count beads; Coulter, Paris, France) as previously described (66). After lysis, the cells were washed one time in PBS, adjusted to 500 μl in PBS, and stored at 4°C until they were analyzed. The following associations were used: (i) CD45-fluorescein isothiocyanate, CD3-phycoerythrin-cyanin 5.1 (PCy5), CD4-phycoerythrin (RD1), and CD8-phycoerythrin-Texas Red (ECD); (ii) CD4-ECD, CD8-PCy5, Tetra-phycoerythrin, and CD16-fluorescein isothiocyanate. Labeled cells were analyzed on an EPICS-XL MCL flow cytometer (Beckman-Coulter Electronics Inc., Hialeah, Fla.). Event accumulation was followed up until 50,000 living cells were counted on the lymphocyte gate, which was set up using both forward and right angle scatters. All monoclonal antibodies were purchased from Beckman-Coulter-Immunotech (Paris, France). Results were expressed as the percentage of CD8+ tetramer-positive cells among total CD8+ T cells. The threshold of positivity was 0.1% of CD8+ T cells.
Statistical analysis.
The correlations between the frequencies of IFN-γ-producing cells, tetramer-binding cells, plasma viral load, and CD4+ and CD8+ T cells were determined using the Spearman rank test. Statistical significance was defined as a P value of <0.05.
RESULTS
Most HLA-A∗0201-positive HIV-1-infected children had CD8+ T cells specific for the SL9 and IV9 epitopes detectable by the IFN-γ ELISPOT assay.
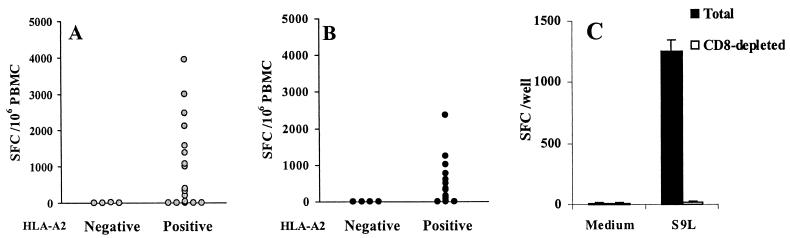
The two optimally defined, immunodominant, and HLA-A∗0201-restricted HIV CTL epitopes from the p17gag protein (SL9) and the reverse transcriptase (IV9) were added to freshly isolated PBMC from HIV-infected children, and the frequency of peptide-specific cells was determined by the IFN-γ ELISPOT assay. None of the four HLA-A∗0201-negative HIV-infected children secreted IFN-γ in response to the peptides (SL9 median, 2 SFC/106 PBMC [range, 0 to 10]; IV9 median, 0 SFC/106 PBMC [range, 0 to 0]) (Fig. 1A and B). In contrast, 12 out of 20 and 13 out of 20 HLA-A∗0201-positive HIV-infected children had a significant response to SL9 and IV9 peptides, respectively. Eighteen out of 20 patients recognized at least one peptide, and 7 out of 20 recognized both epitopes. The frequency of SL9-specific cells was higher than the frequency of IV9-specific cells (SL9 median, 351 SFC/106 PBMC [range, 0 to 3,939]; IV9 median, 134 SFC/106 PBMC [range, 0 to 2,373]) (Fig. 1A and B). Depletion of CD8+ T cells led to undetectable IFN-γ production in response to peptide stimulation, as shown in a representative experiment (Fig. 1C). Thus, the majority of HIV-infected children bearing the HLA-A∗0201 molecule had CD8+ T cells specific for the immunodominant SL9 and/or IV9 epitope.
FIG. 1.
(A and B) IFN-γ production by HIV-1-specific CD8+ T cells of HLA-A∗0201-negative and HLA-A∗0201-positive children stimulated with peptides SL9 and IV9. Fresh PBMC were stimulated overnight with either peptide SL9 (A) or peptide IV9 (B) at 1 μg/ml. SFC were enumerated in a 16-h ELISPOT assay. Mean values from triplicate wells are shown. (C) Peptide SL9 induces IFN-γ production by CD8+ T lymphocytes. Total PBMC and CD8-depleted PBMC from the HIV-infected patient EM78 were stimulated overnight with peptide SL9 at 1 μg/ml. SFC were enumerated in a 16-h ELISPOT assay. Mean values (and standard deviations) from triplicate wells are shown. CD8+ CD3+ T cells were 54 and 0.8% in total and CD8-depleted PBMC, respectively.
Free peptides are more efficient than peptides bound to tetrameric HLA molecules in stimulating IFN-γ production by HIV-specific CD8+ T cells.
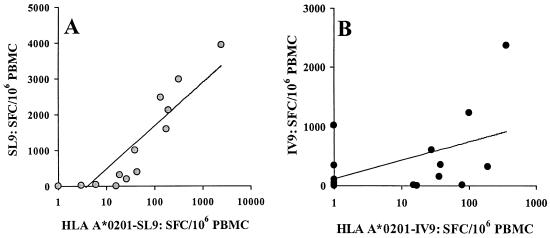
As HLA-peptide tetrameric complexes may induce T-cell receptor (TCR) oligomerization and activate the cells (2, 45), the abilities of HLA-A∗0201 tetramers bound to peptides SL9 and IV9 to stimulate HIV-specific CD8+ T cells were investigated in the ELISPOT assay. Fresh PBMC from 14 patients were therefore tested for IFN-γ production using the HLA-A∗0201 tetramers complexed to peptides SL9 and IV9 as stimuli (Fig. 2). The tetramers induced IFN-γ production by HIV-specific CD8+ T cells, but the frequencies of SFC were 10 times lower than those obtained with free peptide (SL9 peptide median, 357 SFC/106 PBMC [range, 0 to 3,939]; SL9 tetramer median, 33 SFC/106 PBMC [range, 0 to 2,439]; IV9 peptide median, 241 SFC/106 PBMC [range, 0 to 2,373]; IV9 tetramer median, 23 SFC/106 PBMC [range, 0 to 359]). The IFN-γ production induced by peptide bound to tetramer was specific, as it was undetectable if peptide-specific CD8+ T cells were undetectable using peptide-based ELISPOT and/or tetramer labeling. The numbers of HIV-specific CD8+ T cells detected with free or HLA-bound peptide were strongly correlated for the SL9 peptide (ρ = 0.965; P < 0.0005), and a positive trend was observed for the IV9 peptide (ρ = 0.437; P < 0.13) (Fig. 2A and B). In conclusion, tetrameric HLA-A∗0201-peptide complexes stimulated IFN-γ production by epitope-specific CD8+ T cells but less efficiently than the exogenous peptide presented by living cells.
FIG. 2.
IFN-γ production by HIV-1-specific CD8+ T cells of HLA-A∗0201-positive patients following stimulation with free peptide and peptide bound to HLA-tetramers. (A) Fresh PBMC were stimulated overnight with either peptide SL9 at 1 μg/ml or HLA-A∗0201-SL9 tetramer at 1 μg/ml. (B) Fresh PBMC were stimulated overnight with peptide IV9 at 1 μg/ml or HLA-A∗0201-IV9 tetramer at 1 μg/ml. SFC were enumerated in a 16-h ELISPOT assay, and results are expressed as SFC per 106 PBMC. Mean values from triplicate wells and the linear regression curves are shown.
IFN-γ-producing HIV-specific CD8+ T cells are less numerous than total HIV-specific CD8+ T cells binding tetramers.
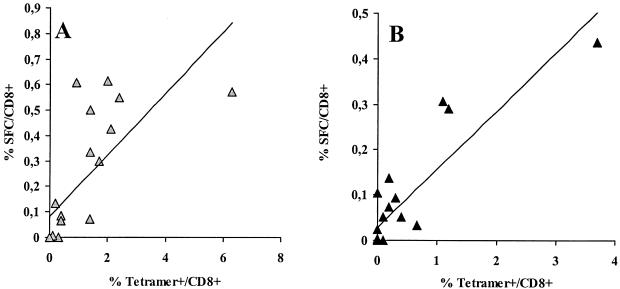
We next compared the frequencies of IFN-γ-producing cells determined by the ELISPOT assay with the frequencies of CD8+ T cells binding the corresponding tetramer. PBMC from 17 patients tested in the ELISPOT assay have also been tested simultaneously for tetramer binding (66). When the results were expressed as negative-positive, the results from both assays were concordant in all but five cases. In three cases, the tetramer assay was positive and the ELISPOT assay was negative (two for the SL9 epitope; one for IV9). In two cases, the ELISPOT assay was positive and the tetramer assay was negative (both for the IV9 epitope).
As shown in Fig. 3, results from both assays were strongly correlated for the SL9 epitope (ρ = 0.822; P < 0.002), as well as for the IV9 epitope (ρ = 0.746; P < 0.004). For the 12 patients positive in both assays, the ratios of IFN-γ-producing CD8+ T cells to tetramer binding CD8+ T cells were as follows: SL9 median, 0.25 (range, 0.05 to 0.68); IV9 median, 0.28 (range, 0.05 to 0.69). In conclusion, epitope-specific CD8+ T cells that produce IFN-γ are less numerous than total epitope-specific CD8+ T cells that bind the tetramer, and the ratio of frequencies measured by the ELISPOT assay to frequencies measured by the tetramer assay varied widely among patients. This result suggests that a significant fraction of HIV-specific CD8+ T cells are not functional.
FIG. 3.
Frequency of HIV-1-specific CD8+ T cells determined by ELISPOT and tetramer assays. Results from 17 HLA-A∗0201-positive patients are shown for the SL9 (A) and the IV9 (B) epitopes. SFC numbers and tetramer binding cells are expressed as percentages of CD8+ T cells. The linear regression curves are shown.
Correlations between HIV-specific CD8+ T cells and biological parameters.
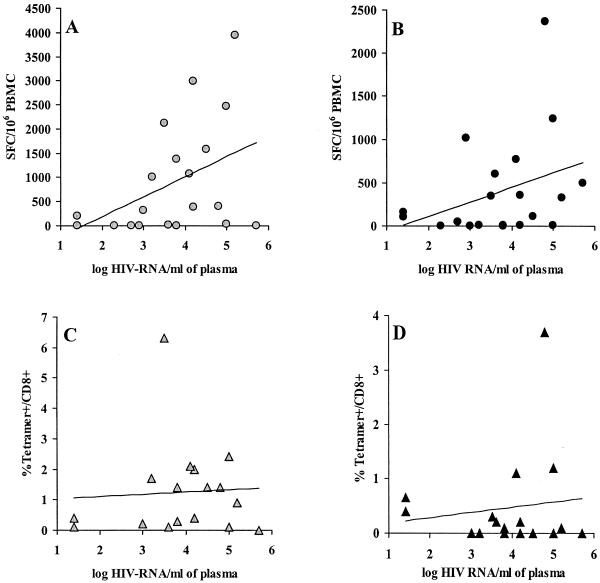
Neither the CD4+- nor the CD8+-T-lymphocyte subset (expressed as percents or as absolute numbers) was correlated with the frequencies of epitope-specific CD8+ T cells determined by the ELISPOT or the tetramer assay (data not shown). In contrast, SL9-specific CD8+ T cells detected by the ELISPOT assay were positively correlated with the plasma viral load (ρ = 0.463; P < 0.05) (Fig. 4A), and a positive trend was observed for IV9-specific CD8+ T cells (ρ = 0.370; P < 0.11) (Fig. 4B). However, when SL9- and IV9-specific CD8+ T cells were detected by using tetramers, their frequencies were not correlated with the plasma viral load (SL9, ρ = 0.015, P < 0.97 [Fig. 4C]; IV9, ρ = −0.063, P < 0.68 [Fig. 4D]). As the quantity of HIV-RNA present in plasma is a hallmark of both viral replication and antigenic stimulation, our data strongly suggest that among HIV-specific CD8+ T cells, the subset of functionally active effector cells detected by the ELISPOT assay was stimulated by viral production in vivo, whereas the frequencies of total HIV-specific CD8+ T cells detected by tetramers were not linked to the viral load.
FIG. 4.
Correlations between frequencies of HIV-1-specific CD8+ T cells and plasma viral load. The frequencies of SL9-specific CD8+ T cells (A and C) and IV9-specific CD8+ T cells (B and D) are represented as function of the plasma viral load. (A and B) ELISPOT assay; (C and D) tetramer assay. The linear regression curves are shown.
DISCUSSION
In this study, we found that 90% of HLA-A∗0201-positive HIV-1-infected children had CD8+ T cells specific for the SL9 and/or the IV9 epitope. This T-cell response to HLA-A∗0201-restricted epitopes correlated strictly with the expression of the HLA-A∗0201 molecule and was restricted to the CD8+-T-cell subset, showing the specificity of the ELISPOT assay. Although the percentages of responders to the SL9 and IV9 epitopes were similar, the CD8+ T cells specific for the SL9 epitope were found at higher frequencies than the CD8+ T cells specific for the IV9 epitope (351 versus 134 SFC/106 PBMC), as we described previously for the tetramer assay (66).
We observed that addition of peptide-HLA-A∗0201 tetrameric complexes to PBMC stimulates IFN-γ production. Usually, T cells are activated upon encountering another cell that carries on its surface appropriate complexes of MHC proteins and peptide antigens. A single peptide-MHC complex per target cell can trigger activation of the CD8+-T-cell cytolytic response (73). Monomers of soluble peptide-MHC trigger Ca2+ responses in CD8+ T cells (17). Finally, in vivo injection of tetramers has been shown to efficiently prime naive CD8+ T cells (45). Even if IFN-γ production induced by free peptide and peptide bound to tetramers of HLA molecules was correlated (Fig. 2), we observed that HLA-peptide tetramers were less efficient than peptide-pulsed PBMC in stimulating IFN-γ production, as previously reported for infected adults (2). It should be noted that the molarity of peptide combined with tetramers is lower than the molarity of free peptide in the experiments described. However, the low molarity of peptide combined with a tetramer was found to be optimal, as increasing the peptide-tetramer concentration led to lower numbers of spots. These results are concordant with the observation that epitope density requirements for induction of response are determined by the affinity of the TCR-peptide-MHC reaction (73). In addition, at 37°C, only high-affinity TCRs were labeled with tetramers and internalized them (77). Therefore, one might expect that tetramers of HLA-peptide complexes will be unable to stimulate CD8+ T cells with low-affinity TCRs, whereas peptide-pulsed cells will activate IFN-γ production of these low-affinity CD8+ T cells, leading to lower numbers of spots in response to peptide complexed to tetramers than in response to free peptide.
The frequencies of HIV-specific CD8+ T cells observed in this study of infected children are very similar to those reported in chronically infected adults tested with a similar peptide-based ELISPOT assay (1, 16, 22). We previously showed that chronically infected children had frequencies of tetramer binding CD8+ T cells (66) and memory CTL (9, 10) similar to those of chronically infected adults. These data are now extended with the detection of IFN-γ production by ex vivo-activated T cells. However, during the first months or years of infection, ex vivo IFN-γ production by HIV-specific CD8+ T cells appeared to be significantly lower than that in older children or adults (65, 71). Our study group includes only children >3 years old, and the median age is 12.7 years. Thus, our data showed that the relative deficiency of HIV-specific CD8+-T-cell response during the first months of life does not persist later in life in children with the slow pattern of disease progression (6).
Our study group is very diverse regarding viral load, CD4+-T-cell numbers, and antiretroviral treatment. Children receiving combined therapy had lower viral loads and higher CD4+-T-cell numbers at the time of the study than the baseline levels (data not shown). However, the viral load and CD4+-T-cell numbers from the group receiving combined therapy were not significantly different from those of untreated children, most of whom were slow progressors. The frequencies of HIV-specific CD8+ T cells were similar in both groups (data not shown). Antiretroviral therapy is usually associated with reduced numbers of virus-specific CD8+ T cells when viral replication is completely suppressed. Nonetheless, we observed large numbers of HIV-specific CD8+ T cells in children under combined therapy, but most of the children had detectable viral loads. In adults, high frequencies of virus-specific CD8+ T cells in patients under combined therapy without suppression of viral replication were similar to those of untreated patients (21). The frequency of HIV-specific CD8+ T cells increased in children with incomplete viral suppression but with increasing CD4+-T-cell counts (72). Further longitudinal study of treated children is required to determine the impact of complete viral suppression by combined therapy on the HIV-specific CD8+-T-cell response.
Tetramer staining provides quantitative information about a T-cell population based on its specificity but not on its function. The IFN-γ secretion in response to short-term restimulation with the antigen is a functional assay for the detection of the effector memory, but not central memory, CD8+ T cells (3, 61). Using a rank test, we found that results from the tetramer and the ELISPOT assays are closely correlated, as shown by other studies of virus-specific CD8+ T cells in humans (22, 59, 74). However, all tetramer-positive CD8+ T cells are not functionally equivalent, as we observed that frequencies of epitope-specific T cells measured by ELISPOT assays are lower than those measured with the tetramer assay. The presence of nonfunctional HIV-specific CD8+ T cells is not specific to HIV-infected children, as it was observed in adults infected by HIV or other viruses (22, 57, 70, 74). A combination of tetramer staining and detection of cytokine production using intracellular cytokine staining directly showed that not all tetramer-positive cells produced cytokine in response to stimulation with the epitope (2, 21, 23, 27, 30, 31, 68). Discrepancies between the tetramer binding assay and the IFN-γ secretion assay can be explained by at least three mechanisms. The tetramer binding cells that do not secrete IFN-γ may be central memory CD8+ T cells, as described by Sallusto and coworkers (61); they may be anergic (38); or they may secrete other cytokines or chemokines, such as tumor necrosis factor alpha (2, 28, 62, 67), MIP-1β (2, 29), interleukin-2 (47, 62), transforming growth factor β (20), and interleukin-4 (26). In conclusion, our comparison of tetramer and ELISPOT assays showed that the fraction of functional CD8+ T cells among total CD8+ T cells was low and varied widely among patients. A combination of tetramer-labeling and intracellular cytokine cytometry assays is being performed on PBMC from HIV-infected children to gain further insight into this phenomenon.
We observed a positive correlation between the frequency of HIV-specific CD8+ T cells and the plasma viral load. Our results are in agreement with data from Betts et al. (4) but contradict previous findings of direct inverse correlation between anti-HIV CD8+-T-cell responses and viremia (5, 14, 24, 25, 34, 37, 39, 53, 54, 58). It is important to note that the latter results were obtained by measurement of tetramer binding CD8+ T cells, the cytolytic activity of freshly isolated PBMC (effector CTL), or the cytolytic activity of in vitro-expanded PBMC (memory CTL), whereas in our study and in that of Betts et al. (4), ex vivo IFN-γ production was used to quantify virus-specific CD8+ T cells. The state of activation of the CD8+ T cells may determine their relation to the antigen load. The numbers of memory CD8+ T cells that can proliferate in response to stimulation, and that are usually measured by the chromium release assay, are correlated with the capacity of these cells to suppress viral replication by direct cytolysis or secretion of soluble factors and thus are inversely correlated with the viral load (5, 14, 24, 37, 39, 53, 58). In contrast, the frequencies of effector memory T cells able to respond to short-term stimulation, but with poor proliferation potential, will be determined by recent encounters with the antigen and thus are positively correlated with viral replication. In addition, cytokine production and perforin expression appear to be differently affected by HIV infection (2).
As discussed above, different assays for detection of virus-specific CD8+ T cells measure different functions and/or subsets of CD8+ T cells, and it is not completely unexpected that these different techniques show different relationships between the antiviral CD8+-T-cell-mediated responses and the antigen load. Indeed, we show here that, in contrast to IFN-γ production detected in response to stimulation with the SL9 and IV9 peptides, the frequency of CD8+ T cells labeled with tetrameric HLA-A∗0201 complexed to the corresponding peptide was not correlated with the viral load. Furthermore, in HIV-infected children, we found that memory CTL responses were inversely correlated with the plasma viral load and that ex vivo IFN-γ-producing CD8+ T cells and memory CTL were not correlated with each other (10a; F. Buseyne and Y. Rivière, unpublished observations). Therefore, we can conclude from the present study that the magnitude of ex vivo-activated HIV-specific CD8+-T-cell-mediated IFN-γ production is dependent upon continuous restimulation with a viral load, but this conclusion may not be true for other subsets and/or functions of HIV-specific CD8+ T cells.
Using the peptide-based ELISPOT assay, we showed that most of the children studied had an HIV-specific CD8+-T-cell response of similar magnitude to the one detected in adults. We found a positive correlation between the frequency of ex vivo-activated HIV-specific IFN-γ-producing T cells and the plasma viral load, suggesting that the frequency of this subset of HIV-specific CD8+ T cells is dependent upon continuous antigenic stimulation. These data support the use of the ELISPOT assay to study ex vivo-activated virus-specific CD8+ T cells because of its high sensitivity and because it provides information different from that provided by tetramer labeling of memory CD8+ T cells.
Acknowledgments
This work was supported by the Institut Pasteur, the Agence Nationale de la Recherche sur le SIDA, and Sidaction. Y.R. was an Elizabeth Glaser scientist.
We thank Frances Gotch (Chelsea and Westminster Hospital, London, United Kingdom) and Sophie Caillat-Zucman (Service d'Immunologie Clinique, Hôpital Necker) for HLA genotyping. We are grateful to A. Catteau for her help in CD8 depletion experiments and to H. Necker (Beckmann-Coulter-Immunotech) for providing the tetramers.
REFERENCES
- 1.Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. R. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. A. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. L. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bercovici, N., M. T. Duffour, S. Agrawal, M. Salcedo, and J. P. Abastado. 2000. New methods for assessing T-cell responses. Clin. Diagn. Lab. Immunol. 7:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 1991. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts, M. R., J. F. Krowka, T. B. Kepler, M. Davidian, C. Christopherson, S. Kwok, L. Louie, J. Eron, H. Sheppard, and J. A. Frelinger. 1999. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res. Hum. Retrovir. 15:1219-1228. [DOI] [PubMed] [Google Scholar]
- 6.Blanche, S., M. L. Newell, M. J. Mayaux, D. T. Dunn, J. P. Teglas, C. Rouzioux, C. S. Peckham, et al. 1997. Morbidity and mortality in European children vertically infected by HIV-1. J. Acquir. Immune Defic. Syndr. 14:442-450. [DOI] [PubMed] [Google Scholar]
- 7.Bodinier, M., M. A. Peyrat, C. Tournay, F. Davodeau, F. Romagne, M. Bonneville, and F. Lang. 2000. Efficient detection and immunomagnetic sorting of specific T cells using multimers of MHC class I and peptide with reduced CD8 binding. Nat. Med. 6:707-710. [DOI] [PubMed] [Google Scholar]
- 8.Bucy, R. P., and J. M. Kilby. 2001. Perspectives on inducing efficient immune control of HIV-1 replication—a new goal for HIV therapeutics? AIDS 15:S36-S42. [DOI] [PubMed] [Google Scholar]
- 9.Buseyne, F., S. Blanche, D. Schmitt, C. Griscelli, and Y. Rivière. 1993. Detection of HIV-specific cell-mediated cytotoxicity in the peripheral blood from infected children. J. Immunol. 150:3569-3581. [PubMed] [Google Scholar]
- 10.Buseyne, F., M. Burgard, J. P. Teglas, E. Bui, C. Rouzioux, M. J. Mayaux, S. Blanche, and Y. Rivière. 1998. Early human immunodeficiency virus (HIV)-specific cytotoxic T lymphocytes (CTL) and disease progression in children born to HIV-infected mothers. AIDS Res. Hum. Retrovir. 14:1435-1444. [DOI] [PubMed] [Google Scholar]
- 10a.Buseyne F., J. Le Chenadec, B. Corre, F. Porrot, M. Burgard, C. Rouzioux, S. Blanche, M. J. Mayaux, and Y. Rivière. Memory Gag-specific CTL are inversely correlated with viral replication in HIV-infected children. J. Infect. Dis., in press. [DOI] [PubMed]
- 11.Casazza, J. P., M. R. Betts, L. J. Picker, and R. A. Koup. 2001. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J. Virol. 75:6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1994. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morb. Mortal. Wkly. Rep. 43(RR-12):1-10. [Google Scholar]
- 13.Cohen Stuart, J. W., W. A. Slieker, G. T. Rijkers, A. Noest, C. A. Boucher, M. H. Suur, R. de Boer, S. P. Geelen, H. J. Scherpbier, N. G. Hartwig, H. Hooijkaas, M. T. Roos, B. de Graeff-Meeder, R. de Groot, et al. 1998. Early recovery of CD4+ T lymphocytes in children on highly active antiretroviral therapy. AIDS 12:2155-2159. [DOI] [PubMed] [Google Scholar]
- 14.Connick, E., R. L. Schlichtemeier, M. B. Purner, K. M. Schneider, D. M. Anderson, S. MaWhinney, T. B. Campbell, D. R. Kuritzkes, J. M. Douglas, F. N. Judson, and R. T. Schooley. 2001. Relationship between human immunodeficiency virus type 1 (HIV-1)-specific memory cytotoxic T lymphocytes and virus load after recent HIV-1 seroconversion. J. Infect. Dis. 184:1465-1469. [DOI] [PubMed] [Google Scholar]
- 15.Czerkinsky, C., G. Andersson, H. P. Ekre, L. A. Nilsson, L. Klareskog, and O. Ouchterlony. 1988. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J. Immunol. Methods 110:29-36. [DOI] [PubMed] [Google Scholar]
- 16.Dalod, M., M. Dupuis, J. G. Deschemin, C. Goujard, C. Deveau, L. Meyer, N. Ngo, C. Rouzioux, J. G. Guillet, J. F. Delfraissy, M. Sinet, and A. Venet. 1999. Weak anti-HIV CD8+ T-cell effector activity in HIV primary infection. J. Clin. Investig. 104:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delon, J., C. Gregoire, B. Malissen, S. Darche, F. Lemaitre, P. Kourilsky, J. P. Abastado, and A. Trautmann. 1998. CD8 expression allows T cell signaling by monomeric peptide-MHC complexes. Immunity 9:467-473. [DOI] [PubMed] [Google Scholar]
- 18.Doherty, P. C., and J. P. Christensen. 2000. Accessing complexity: the dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 18:561-592. [DOI] [PubMed] [Google Scholar]
- 19.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. B. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690-695. [DOI] [PubMed] [Google Scholar]
- 20.Garba, M. L., C. D. Pilcher, A. L. Bingham, J. Eron, and J. A. Frelinger. 2002. HIV antigens can induce TGF-beta1-producing immunoregulatory CD8+ T cells. J. Immunol. 168:2247-2254. [DOI] [PubMed] [Google Scholar]
- 21.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. de Quiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 22.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulder, P. J. R., Y. H. Tang, C. Brander, M. R. Betts, M. Altfeld, K. Annamalai, A. Trocha, S. Q. He, E. S. Rosenberg, G. Ogg, C. A. O'Callaghan, S. A. Kalams, R. E. McKinney, K. Mayer, R. A. Koup, S. I. Pelton, S. K. Burchett, K. McIntosh, and B. D. Walker. 2000. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med. 192:1819-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenough, T. C., D. B. Brettler, F. Kirchhoff, L. Alexander, R. C. Desrosiers, S. J. O'Brien, M. Somasundaran, K. Luzuriaga, and J. L. Sullivan. 1999. Long-term nonprogressive infection with human immunodeficiency virus type 1 in a hemophilia cohort. J. Infect. Dis. 180:1790-1802. [DOI] [PubMed] [Google Scholar]
- 25.Greenough, T. C., D. B. Brettler, M. Somasundaran, D. L. Panicali, and J. L. Sullivan. 1997. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes (CTL), virus load, and CD4 T cell loss: evidence supporting a protective role for CTL in vivo. J. Infect. Dis. 176:118-125. [DOI] [PubMed] [Google Scholar]
- 26.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hel, Z., J. Nacsa, B. Kelsall, W. P. Tsai, N. Letvin, R. W. Parks, E. Tryniszewska, L. Picker, M. G. Lewis, Y. Edghill-Smith, M. Moniuszko, R. Pal, L. Stevceva, J. D. Altman, T. M. Allen, D. Watkins, J. V. Torres, J. A. Berzofsky, I. M. Belyakov, W. Strober, and G. Franchini. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251-and simian-human immunodeficiency virus KU2-infected macaques. J. Virol. 75:11483-11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herr, W., U. Protzer, A. W. Lohse, G. Gerken, K. H. Meyer zum Buschenfelde, and T. Wolfel. 1998. Quantification of CD8+ T lymphocytes responsive to human immunodeficiency virus (HIV) peptide antigens in HIV-infected patients and seronegative persons at high risk for recent HIV exposure. J. Infect. Dis. 178:260-265. [DOI] [PubMed] [Google Scholar]
- 29.Kamin-Lewis, R., S. F. Abdelwahab, C. Trang, A. Baker, A. L. DeVico, R. C. Gallo, and G. K. Lewis. 2001. Perforin-low memory CD8+ cells are the predominant T cells in normal humans that synthesize the beta-chemokine macrophage inflammatory protein-1 beta. Proc. Natl. Acad. Sci. USA 98:9283-9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kern, F., E. Khatamzas, I. Surel, C. Frommel, P. Reinke, S. L. Waldrop, L. J. Picker, and H. D. Volk. 1999. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 29:2908-2915. [DOI] [PubMed] [Google Scholar]
- 31.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31:677-686. [DOI] [PubMed] [Google Scholar]
- 32.Kostense, S., K. Vandenberghe, J. Joling, D. van Baarle, N. Nanlohy, E. H. Manting, and F. Miedema. 2002. Persistent numbers of tetramer+ CD8+ T cells, but loss of interferon-γ+ HIV-specific T cells during progression to AIDS. Blood 99:2505-2511. [DOI] [PubMed] [Google Scholar]
- 33.Krausa, P., M. Brywka III, D. Savage, K. M. Hui, M. Bunce, J. L. Ngai, D. L. Teo, Y. W. Ong, D. Barouch, C. E. Allsop, et al. 1995. Genetic polymorphism within HLA-A∗02: significant allelic variation revealed in different populations. Tissue Antigens 45:223-231. [DOI] [PubMed] [Google Scholar]
- 34.Kundu, S. K., and T. C. Merigan. 1994. Relationship of HIV-1 provirus load, CD8+ CD11+ T cells and HIV-1 envelope-specific cytotoxic T lymphocytes in HIV-infected asymptomatic patients. Immunology 83:81-85. [PMC free article] [PubMed] [Google Scholar]
- 35.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson, M., X. Jin, B. Ramratnam, G. S. Ogg, J. Engelmayer, M. A. Demoitie, A. J. McMichael, W. I. Cox, R. M. Steinman, D. Nixon, and N. Bhardwaj. 1999. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of Pol-specific CD8 T cells in HIV-1-positive individuals. AIDS 13:767-777. [DOI] [PubMed] [Google Scholar]
- 37.Legrand, E., I. Pellegrin, D. Neau, J. L. Pellegrin, J. M. Ragnaud, M. Dupon, B. Guillemain, and H. J. Fleury. 1997. Course of specific T lymphocyte cytotoxicity, plasma and cellular viral loads, and neutralizing antibody titers in 17 recently seroconverted HIV type 1-infected patients. AIDS Res. Hum. Retrovir. 13:1383-1394. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98:1667-1677. [DOI] [PubMed] [Google Scholar]
- 39.Lubaki, N. M., M. E. Shepherd, R. S. Brookmeyer, H. Hon, T. C. Quinn, M. Kashamuka, M. Johnson, R. Gottle, J. Devers, H. M. Lederman, and R. C. Bollinger. 1999. HIV-1-specific cytolytic T-lymphocyte activity correlates with lower viral load, higher CD4 count, and CD8+CD38−DR− phenotype: comparison of statistical methods for measurement. J. Acquir. Immune Defic. Syndr. 22:19-30. [DOI] [PubMed] [Google Scholar]
- 40.Luzuriaga, K., Y. Bryson, P. Krogstad, J. Robinson, B. Stechenberg, M. Lamson, S. Cort, and J. L. Sullivan. 1997. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N. Engl. J. Med. 336:1343-1349. [DOI] [PubMed] [Google Scholar]
- 41.Luzuriaga, K., D. Holmes, A. Hereema, J. Wong, D. L. Panicali, and J. L. Sullivan. 1995. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J. Immunol. 154:433-443. [PubMed] [Google Scholar]
- 42.Luzuriaga, K., R. A. Koup, C. A. Pikora, D. B. Brettler, and J. L. Sullivan. 1991. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J. Pediatr. 119:230-236. [DOI] [PubMed] [Google Scholar]
- 43.Luzuriaga, K., M. McManus, M. Catalina, S. Mayack, M. Sharkey, M. Stevenson, and J. L. Sullivan. 2000. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J. Virol. 74:6984-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackall, C. L., T. A. Fleisher, M. R. Brown, M. P. Andrich, C. C. Chen, I. M. Feuerstein, M. E. Horowitz, I. T. Magrath, A. T. Shad, S. M. Steinberg, et al. 1995. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N. Engl. J. Med. 332:143-149. [DOI] [PubMed] [Google Scholar]
- 45.Maile, R., B. Wang, W. Schooler, A. Meyer, E. J. Collins, and J. A. Frelinger. 2001. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J. Immunol. 167:3708-3714. [DOI] [PubMed] [Google Scholar]
- 46.McFarland, E. J., P. A. Harding, D. Luckey, B. Conway, R. K. Young, and D. R. Kuritzkes. 1994. High frequency of Gag- and envelope-specific cytotoxic T lymphocyte precursors in children with vertically acquired human immunodeficiency virus type 1 infection. J. Infect. Dis. 170:766-774. [DOI] [PubMed] [Google Scholar]
- 47.McKay, P. F., J. E. Schmitz, D. H. Barouch, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, D. A. Gorgone, and N. L. Letvin. 2002. Vaccine protection against functional CTL abnormalities in simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 168:332-337. [DOI] [PubMed] [Google Scholar]
- 48.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 49.Melvin, A. J., A. G. Rodrigo, K. M. Mohan, P. A. Lewis, L. Manns-Arcuino, R. W. Coombs, J. I. Mullins, and L. M. Frenkel. 1999. HIV-1 dynamics in children. J. Acquir. Immune Defic. Syndr. 20:468-473. [DOI] [PubMed] [Google Scholar]
- 50.Montaner, L. J. 2001. Structured treatment interruptions to control HIV-1 and limit drug exposure. Trends Immunol. 22:92-96. [DOI] [PubMed] [Google Scholar]
- 51.Mueller, B. U., R. P. Nelson, J. Sleasman, J. Zuckerman, M. Heathchiozzi, S. M. Steinberg, F. M. Balis, P. Brouwers, A. Hsu, R. Saulis, S. Sei, L. V. Wood, S. Zeichner, T. T. K. Katz, C. Higham, D. Aker, M. Edgerly, P. Jarosinski, L. Serchuck, S. M. Whitcup, D. Pizzuti, and P. A. Pizzo. 1998. A phase I/II study of the protease inhibitor ritonavir in children with human immunodeficiency virus infection. Pediatrics 101:335-343. [DOI] [PubMed] [Google Scholar]
- 52.Mueller, B. U., J. Sleasman, R. P. Nelson, S. Smith, P. J. Deutsch, W. Ju, S. M. Steinberg, F. M. Balis, P. F. Jarosinski, P. Brouwers, G. Mistry, G. Winchell, S. Zwerski, S. Z. Sei, L. V. Wood, S. Zeichner, and P. A. Pizzo. 1998. A phase I/II study of the protease inhibitor indinavir in children with HIV infection. Pediatrics 102:101-109. [DOI] [PubMed] [Google Scholar]
- 53.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 54.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 55.Ogg, G. S., S. Kostense, M. R. Klein, S. Jurriaans, D. Hamann, A. J. McMichael, and F. Miedema. 1999. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J. Virol. 73:9153-9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pikora, C. A., J. L. Sullivan, D. Panicali, and K. Luzuriaga. 1997. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J. Exp. Med. 185:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pittet, M. J., D. Valmori, P. R. Dunbar, D. E. Speiser, D. Lienard, F. Lejeune, K. Fleischhauer, V. Cerundolo, J. C. Cerottini, and P. Romero. 1999. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J. Exp. Med. 190:705-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pontesilli, O., M. R. Klein, S. R. Kerkhof-Garde, N. G. Pakker, F. de Wolf, H. Schuitemaker, and F. Miedema. 1998. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J. Infect. Dis. 178:1008-1018. [DOI] [PubMed] [Google Scholar]
- 59.Propato, A., E. Schiaffella, E. Vicenzi, V. Francavilla, L. Baloni, M. Paroli, L. Finocchi, N. Tanigaki, S. Ghezzi, R. Ferrara, R. Chesnut, B. Livingston, A. Sette, R. Paganelli, F. Aiuti, G. Poli, and V. Barnaba. 2001. Spreading of HIV-specific CD8+ T-cell repertoire in long-term nonprogressors and its role in the control of viral load and disease activity. Hum. Immunol. 62:561-576. [DOI] [PubMed] [Google Scholar]
- 60.Rivière, Y., and F. Buseyne. 1998. Cytotoxic T lymphocytes generation capacity in early life with particular reference to HIV. Vaccine 16:1420-1422. [DOI] [PubMed] [Google Scholar]
- 61.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 62.Sandberg, J. K., N. M. Fast, and D. F. Nixon. 2001. Functional heterogeneity of cytokines and cytolytic effector molecules in human CD8+ T lymphocytes. J. Immunol. 167:181-187. [DOI] [PubMed] [Google Scholar]
- 63.Scheibenbogen, C., K. H. Lee, S. Mayer, S. Stevanovic, U. Moebius, W. Herr, H. G. Rammensee, and U. Keilholz. 1997. A sensitive ELISPOT assay for detection of CD8+ T lymphocytes specific for HLA class I-binding peptide epitopes derived from influenza proteins in the blood of healthy donors and melanoma patients. Clin. Cancer Res. 3:221-226. [PubMed] [Google Scholar]
- 64.Scheibenbogen, C., P. Romero, L. Rivoltini, W. Herr, A. Schmittel, J. C. Cerottini, T. Woelfel, A. M. Eggermont, and U. Keilholz. 2000. Quantitation of antigen-reactive T cells in peripheral blood by IFN gamma-ELISPOT assay and chromium-release assay: a four-centre comparative trial. J. Immunol. Methods 244:81-89. [DOI] [PubMed] [Google Scholar]
- 65.Scott, Z. A., E. G. Chadwick, L. L. Gibson, M. D. Catalina, M. M. McManus, R. Yogev, P. Palumbo, J. L. Sullivan, P. Britto, H. Gay, and K. Luzuriaga. 2001. Infrequent detection of HIV-1-specific, but not cytomegalovirus-specific, CD8+ T cell responses in young HIV-1-infected infants. J. Immunol. 167:7134-7140. [DOI] [PubMed] [Google Scholar]
- 66.Scott-Algara, D., F. Buseyne, S. Blanche, C. Rouzioux, C. Jouanne, F. Romagne, and Y. Riviere. 2001. Frequency and phenotyping of human immunodeficiency virus (HIV)-specific CD8+ T cells in HIV-infected children, using major histocompatibility complex class I peptide tetramers. J. Infect. Dis. 183:1565-1573. [DOI] [PubMed] [Google Scholar]
- 67.Sester, M., U. Sester, H. Kohler, T. Schneider, L. Deml, R. Wagner, N. Mueller-Lantzsch, H. W. Pees, and A. Meyerhans. 2000. Rapid whole blood analysis of virus-specific CD4 and CD8 T cell responses in persistent HIV infection. AIDS 14:2653-2660. [DOI] [PubMed] [Google Scholar]
- 68.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 69.Sleasman, J. W., R. P. Nelson, M. M. Goodenow, D. Wilfret, A. Hutson, M. Baseler, J. Zuckerman, P. A. Pizzo, and B. U. Mueller. 1999. Immunoreconstitution after ritonavir therapy in children with human immunodeficiency virus infection involves multiple lymphocyte lineages. J. Pediatr. 134:597-606. [DOI] [PubMed] [Google Scholar]
- 70.Smith, S. M., R. Brookes, M. R. Klein, A. S. Malin, P. T. Lukey, A. S. King, G. S. Ogg, A. V. S. Hill, and H. M. Dockrell. 2000. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J. Immunol. 165:7088-7095. [DOI] [PubMed] [Google Scholar]
- 71.Spiegel, H. M., R. Chandwani, M. E. Sheehy, J. Dobroszycki, G. Fennelly, A. Wiznia, J. Radding, M. Rigaud, H. Pollack, W. Borkowsky, M. Rosenberg, and D. F. Nixon. 2000. The impact of early initiation of highly active antiretroviral therapy on the human immunodeficiency virus type 1-specific CD8 T cell response in children. J. Infect. Dis. 182:88-95. [DOI] [PubMed] [Google Scholar]
- 72.Spiegel, H. M. L., E. DeFalcon, G. S. Ogg, M. Larsson, T. J. Beadle, P. Tao, A. J. McMichael, N. Bhardwaj, C. O'Callaghan, W. I. Cox, K. Krasinski, H. Pollack, W. Borkowsky, and D. E. Nixon. 1999. Changes in frequency of HIV-1-specific cytotoxic T cell precursors and circulating effectors after combination antiretroviral therapy in children. J. Infect. Dis. 180:359-368. [DOI] [PubMed] [Google Scholar]
- 73.Sykulev, Y., M. Joo, I. Vturina, T. J. Tsomides, and H. N. Eisen. 1996. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4:565-571. [DOI] [PubMed] [Google Scholar]
- 74.Tan, L. C., N. Gudgeon, N. E. Annels, P. Hansasuta, C. A. O'Callaghan, S. Rowland-Jones, A. J. McMichael, A. B. Rickinson, and M. F. Callan. 1999. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J. Immunol. 162:1827-1835. [PubMed] [Google Scholar]
- 75.Wasik, T. J., J. Bratosiewicz, A. Wierzbicki, V. E. Whiteman, R. R. Rutstein, S. E. Starr, S. D. Douglas, D. Kaufman, A. V. Sison, M. Polansky, H. W. Lischner, and D. Kozbor. 1999. Protective role of beta-chemokines associated with HIV-specific Th responses against perinatal HIV transmission. J. Immunol. 162:4355-4364. [PubMed] [Google Scholar]
- 76.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:F19-F22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whelan, J. A., P. R. Dunbar, D. A. Price, M. A. Purbhoo, F. Lechner, G. S. Ogg, G. Griffiths, R. E. Phillips, V. Cerundolo, and A. K. Sewell. 1999. Specificity of CTL interactions with peptide-MHC class I tetrameric complexes is temperature dependent. J. Immunol. 163:4342-4348. [PubMed] [Google Scholar]
- 78.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]