Abstract
(−)−Epicatechin gallate (ECg) and (−)−epigallocatechin gallate (EGCg) reduce oxacillin resistance in mecA-containing strains of Staphylococcus aureus. Their binding to staphylococcal cells is enhanced by the nongalloyl analogues (−)−epicatechin (EC) and (−)−epigallocatechin (EGC). EC and EGC significantly increased the capacity of ECg and EGCg to reduce levels of staphylococcal oxacillin resistance.
Extracts of leaves from the tea plant Camellia sinensis have only weak antibacterial properties, although some polyphenolic constituents of tea extracts have the capacity to sensitize strains of methicillin-resistant Staphylococcus aureus to methicillin, oxacillin, and other β-lactam antibiotics (5, 12, 13). For example, moderate concentrations (6.25 to 25 μg/ml) of galloyl catechins such as (−)−epicatechin gallate (ECg), (−)−epigallocatechin gallate (EGCg), and (−)−catechin gallate (Cg) are able to reduce the MIC of β-lactams from high levels of resistance (>256 μg/ml) to below the antibiotic breakpoint (12, 14). Nongalloylated catechins such as (−)−epicatechin (EC) and (−)−epigallocatechin (EGC) are unable to effect this transition (12). The mechanism of catechin gallate-mediated β-lactam resistance modulation is at present unclear, although there is some evidence that EGCg may bind to peptidoglycan and induce β-lactam susceptibility by interference with cell wall integrity (14). Structures of key catechins are shown in Fig. 1.
FIG. 1.
Structures of green tea-derived catechins.
The concentrations of ECg and EGCg needed to produce these in vitro effects are relatively high. EGCg has successfully undergone phase I (human safety) trials (4), and studies are under way to evaluate its efficacy in chronic lymphocytic leukemia. Although these activities are creating a precedent for therapeutic interventions with naturally occurring polyphenols, it is clear that the potential of catechins as antibiotic resistance-modifying agents would be significantly increased if their potency could be improved through synthesis by increasing stability (1) or by enhancing interactions with cellular targets (2). Evidence that nanomole concentrations of catechins are able to modulate the structure and function of model membranes through their capacity to partition into the phospholipid palisade is emerging (6). Galloylated catechins are able to penetrate deeper into phosphatidylcholine and phosphatidylethanolamine bilayers than their nongalloylated analogues; ECg occupies a deeper location than EGCg, and EC and EGC localize at a shallow location close to the phospholipid-water interface (3). These differences in membrane penetration parallel the extent to which these molecules modify staphylococcal β-lactam resistance. Interestingly, the quantities of ECg and EGCg that are incorporated into lipid bilayers are markedly increased in the presence of EC (8), raising the possibility that the reduction in the level of staphylococcal β-lactam resistance induced by catechin gallates could be potentiated by nongalloylated catechins.
Phospholipid composition and polar head group charge density have a significant impact on catechin binding (8), and S. aureus membranes are uniquely composed of phosphatidylglycerol (63 to 74%), lysylphosphatidylglycerol (17 to 22%), and cardiolipin (5 to 15%) (10, 11). We therefore determined the extent to which nongalloylated catechins enhance ECg binding to S. aureus by using the high-performance liquid chromatography assay described previously by Kajiya et al. (7). EC, EGC, ECg, and EGCg were provided by the Tokyo Food Techno Co. Ltd., Tokyo, Japan; these compounds were purified from green tea extracts. S. aureus BB568 is a constitutive PBP2a producer (provided by B. Berger-Bächi, University of Zürich), and EMRSA-15 and EMRSA-16 are clinical isolates from the Royal Free Hospital, London, United Kingdom. Binding of ECg to mid-exponential-phase EMRSA-16 cells was enhanced by EC; at concentrations of 25 μg/ml, 13% of the EC pool bound after 20 min of incubation at 35°C, and 22% of ECg was associated with the cells. In the presence of EC, ECg binding rose to 41%. EC binding was also enhanced by the presence of ECg (35.5%). EC appears, therefore, to facilitate ECg binding to staphylococcal cells as well as to phosphatidylcholine and phosphatidylethanolamine liposomes.
To investigate whether this cooperative binding elicited enhanced biological activity, the capacity of EC and EGC to increase the degree of sensitization of S. aureus to oxacillin was determined. Checkerboard MIC assays were performed in 96-well microtiter trays with an inoculum of about 104 CFU in 200 μl of Mueller-Hinton broth (Oxoid, Basingstoke, United Kingdom) supplemented with 2% NaCl. MICs were obtained after incubation at 35°C for 24 h. S. aureus ATCC 29213 was used as the standard susceptible strain. Fractional inhibitory concentration (FIC) indices for triple combinations were calculated as follows: ΣFIC = FICOXA + FICB + FICC = [CcombOXA/MICOXA] + [CcombB/MICB] + [CcombC/MICC], where CcombB and CcombC are the concentrations of catechins tested, CcombOXA is the lowest concentration of oxacillin in the combination that inhibited growth, and MICOXA, MICB, and MICC are the MICs of the compounds when used alone. For combinations of two compounds, the term CcombC/MICC was omitted. An FIC index of ≤0.5 indicates synergy.
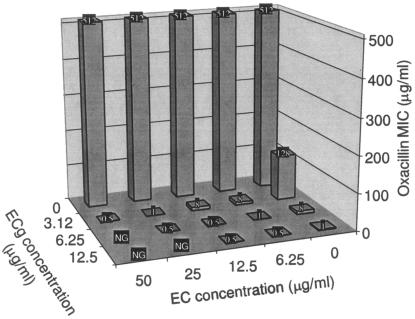
We determined the effect of EC, EGC, and EGCg on the sensitization by ECg of EMRSA-16, EMRSA-15, and BB568 to oxacillin (Table 1). The MICs of oxacillin for BB568, EMRSA-16, and EMRSA-15 were 256, 512, and 16 μg/ml, respectively. The catechin compounds had little or no intrinsic antistaphylococcal activity, with MICs ranging from 64 to 512 μg/ml. An ECg concentration of 12.5 μg/ml reduced the oxacillin MIC for S. aureus BB568 and EMRSA-16 to below the oxacillin breakpoint; a similar effect was achieved for EMRSA-15 with the lower concentration of 3.12 μg/ml (Table 1). The nongalloylated catechins EC and EGC were unable to reduce the MIC of oxacillin for these isolates. However, these compounds markedly enhanced the reduction of oxacillin resistance by ECg. A concentration of 3.12 μg/ml of ECg reduced the MICs to 64 and 128 μg/ml for BB568 and EMRSA-16, respectively. In combination with 6.25 μg/ml of EC, oxacillin susceptibility increased to 8 μg/ml for these isolates; increasing the EC concentration to 25 μg/ml produced values of 2 μg/ml in the presence of 3.12 μg/ml of ECg (Table 1). Similar reductions were observed when EGC was used in combination with ECg. FIC indices indicated strong synergy between oxacillin, ECg, and either EC or EGC against all three S. aureus isolates (Table 1). At high concentrations, a combination of ECg (≥12.5 μg/ml) with both EC and EGC (≥50 μg/ml) inhibited the growth of EMRSA-16 in the absence of oxacillin. The enhancement by EC and EGC of ECg-mediated sensitization to oxacillin was clearly concentration dependent, as illustrated by the three-dimensional representation shown in Fig. 2, for the effect of ECg and EC on oxacillin MICs for EMRSA-16. The galloylated catechin EGCg was significantly less effective at reducing the MIC for oxacillin in the presence of ECg. For example, with 3.12 μg/ml of ECg, 25 μg/ml of EGCg reduced the MIC only twofold (Table 1). At the higher ECg concentration of 12.5 μg/ml, EGCg compromised the capacity of ECg to reduce the MICs for strains BB568 and EMRSA-16. EGCg was also able to sensitize BB568, EMRSA-16, and EMRSA-15 to oxacillin (Table 2), but the effect was much less pronounced than that associated with ECg. Both EC and EGC could enhance EGCg-mediated sensitization (Table 2), but the effect was correspondingly less in comparison to EC/EGC-ECG-oxacillin combinations. ECg elicited only a small (one dilution step) reduction in the oxacillin susceptibility of methicillin-sensitive S. aureus strain ATCC 29213.
TABLE 1.
Effect of EC, EGC, and EGCg on the capacity of ECg to sensitize S. aureus strains to oxacillin
| Compound (μg/ml) | Oxacillin MIC (μg/ml) determined in the presence of ECg (μg/ml) for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. aureus BB568
|
S. aureus EMRSA-16
|
S. aureus EMRSA-15
|
||||||||||
| 0 | 3.12 | 6.25 | 12.5 | 0 | 3.12 | 6.25 | 12.5 | 0 | 3.12 | 6.25 | 12.5 | |
| No compound | ||||||||||||
| 0 | 256 | 64 | 8 | 1 | 512 | 128 | 8 | 1 | 16 | 1 | 0.5 | 0.25 |
| EC | ||||||||||||
| 6.25 | 256c | 8 | 1 | 0.5 | 512 | 8 | 1 | 0.5 | 16 | 0.5 | 0.25 | 0.25 |
| 12.5 | 256 | 4 | 0.5 | 0.5 | 512 | 8 | 0.5 | 0.5 | 16 | 0.5 | 0.25 | 0.25 |
| 25 | 256 | 2 | 0.5 | 0.5 | 512 | 1 | 0.5 | NCb | 16 | 0.5 | 0.25 | 0.12 |
| 50 | NDa | NDa | NDa | NDa | 512 | 0.5 | NGb | NGb | NDa | NDa | NDa | NDa |
| EGC | ||||||||||||
| 6.25 | 256 | 32 | 1 | 0.5 | 512 | 32 | 1 | 0.5 | 16 | 0.5 | 0.25 | 0.25 |
| 12.5 | 256 | 8 | 1 | 0.5 | 512 | 16 | 1 | 0.5 | 16 | 0.5 | 0.25 | 0.12 |
| 25 | 128 | 4 | 0.5 | 0.5 | 512 | 8 | 0.5 | NGb | 8 | 0.25 | 0.12 | 0.12 |
| 50 | NDa | NDa | NDa | NDa | 512 | 2 | NGb | NGb | NDa | NDa | NDa | NDa |
| EGCg | ||||||||||||
| 6.25 | 128 | 32 | 16 | 8 | 256 | 128 | 32 | 16 | 4 | 1 | 1 | 0.5 |
| 12.5 | 64 | 32 | 32 | 8 | 128 | 128 | 32 | 32 | 1 | 1 | 1 | 1 |
| 25 | 32 | 16 | 16 | 8 | 64 | 32 | 8 | 4 | 1 | 1 | 1 | 0.5 |
ND, not determined.
NG, no growth observed for the catechin combination in the absence of oxacillin.
Combinations producing FIC indices of >0.5 are shown in boldface type (see the supplemental material). All MIC determinations were performed in duplicate.
FIG. 2.
Effect of the combination of EC and ECg on the MIC of oxacillin for EMRSA-16. NG, no growth.
TABLE 2.
Effect of EC and EGC on the capacity of EGCg to sensitize S. aureus strains to oxacillin
| Compound (μg/ml) | Oxacillin MIC (μg/ml) determined in the presence of EGCg (μg/ml) for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. aureus BB568
|
S. aureus EMRSA-16
|
S. aureus EMRSA-15
|
||||||||||
| 0 | 6.25 | 12.5 | 25 | 0 | 6.25 | 12.5 | 25 | 0 | 6.25 | 12.5 | 25 | |
| No compound | ||||||||||||
| 0 | 256 | 128 | 64 | 32 | 512 | 256 | 128 | 64 | 8 | 4 | 1 | 1 |
| EC | ||||||||||||
| 6.25 | 256 | 64 | 64 | 16 | 512a | 256 | 256 | 128 | 16 | 4 | 2 | 0.5 |
| 12.5 | 256 | 64 | 32 | 16 | 512 | 256 | 256 | 128 | 16 | 4 | 2 | 0.5 |
| 25 | 256 | 64 | 32 | 8 | 512 | 256 | 256 | 128 | 16 | 2 | 1 | 0.5 |
| EGC | ||||||||||||
| 6.25 | 256 | 64 | 32 | 16 | 512 | 256 | 128 | 64 | 16 | 2 | 1 | 0.50 |
| 12.5 | 256 | 64 | 32 | 8 | 512 | 128 | 64 | 16 | 16 | 2 | 0.5 | 0.25 |
| 25 | 128 | 32 | 16 | 1 | 512 | 64 | 8 | 2 | 8 | 2 | 0.5 | 0.125 |
Combinations producing FIC indices of >0.5 are shown in boldface type (see the supplemental material). All MIC determinations were performed in duplicate.
The capacity of nongalloylated catechins such as EC and EGC to enhance the oxacillin susceptibility of methicillin-resistant S. aureus strains by the galloyl catechins ECg and EGCg supports the notion that the staphylococcal cytoplasmic membrane is the primary bacterial target for these bioactive molecules. We predicted that increased ECg membrane binding in the presence of EC or EGC would be reflected in a higher degree of sensitization to oxacillin, and this was found to be the case. As catechins do not enter cells (3, 7), it is likely that they modulate β-lactam resistance in S. aureus by alteration of the biophysical properties of the membrane, compromising the function of proteins associated with the bilayer and affecting transport of materials across the membrane.
This study highlights the cooperative nature of the interactions between galloylated and nongalloylated catechins with regard to their membrane-associated biological effects. The capacity of structurally related compounds to act synergistically on bacterial cells is established, and the combination of amoxicillin and clavulanate (as well as quinupristin and dalfopristin) provides an example of the therapeutic potential of such combinations. This pairing is effective because clavulanate inhibits the β-lactamase that would otherwise inactivate amoxicillin. Previous studies have shown that the gallate group is essential for epicatechin gallate activity (12); it is attached to the catechin moiety via an ester linkage that has the potential for cleavage by bacterial esterases (9). However, as EC and EGC lack the gallate group, it is unlikely that they act as esterase inhibitors. In addition, esterase-stable derivatives of ECg have similar activities when used in combination with oxacillin against S. aureus, suggesting that esterase activity does not lead to the inactivation of the compounds in vitro (1). Other forms of inactivation, particularly modification to the catechin moiety, where EC and EGC could act as potential inhibitors, cannot be ruled out.
Supplementary Material
Acknowledgments
This research was funded through Strategic Grant G0000996 from the Medical Research Council.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Anderson, J. C., C. Headley, P. D. Stapleton, and P. W. Taylor. 2005. Synthesis and antibacterial activity of a hydrolytically stable (−)-epicatechin gallate analogue for the modulation of β-lactam resistance in Staphylococcus aureus. Bioorg. Med. Chem. Lett. 15:2633-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. C., C. Headley, P. D. Stapleton, and P. W. Taylor. 2005. Asymmetric total synthesis of B-ring modified (−)-epicatechin gallate analogues and their modulation of β-lactam resistance in Staphylococcus aureus. Tetrahedron 61:7703-7711. [DOI] [PubMed] [Google Scholar]
- 3.Caturla, N., E. Vera-Samper, J. Villalaín, C. R. Mateo, and V. Micol. 2003. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 34:648-662. [DOI] [PubMed] [Google Scholar]
- 4.Chow, H.-H. S., Y. Cai, I. A. Hakim, J. A. Crowell, F. Shahi, C. A. Brooks, R. T. Dorr, Y. Hara, and D. S. Alberts. 2003. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 9:3312-3319. [PubMed] [Google Scholar]
- 5.Hamilton-Miller, J. M. T., and S. Shah. 2000. Activity of the tea component epicatechin gallate and analogues against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 46:852-853. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto, T., S. Kumazawa, F. Nanjo, Y. Hara, and T. Nakayama. 1999. Interaction of tea catechins with lipid bilayers investigated with liposome systems. Biosci. Biotechnol. Biochem. 63:2252-2255. [DOI] [PubMed] [Google Scholar]
- 7.Kajiya, K., S. Kumazawa, and T. Nakayama. 2001. Steric effects on the interaction of tea catechins with lipid bilayers. Biosci. Biotechnol. Biochem. 65:2638-2643. [DOI] [PubMed] [Google Scholar]
- 8.Kajiya, K., S. Kumazawa, and T. Nakayama. 2002. Effects of external factors on the interaction of tea catechins with lipid bilayers. Biosci. Biotechnol. Biochem. 66:2330-2335. [DOI] [PubMed] [Google Scholar]
- 9.Kohri, T., N. Matsumoto, M. Yamakawa, M. Suzuki, F. Nanjo, Y. Hara, and N. Oku. 2001. Metabolic fate of (−)-[4-3H]epigallocatechin gallate in rats after oral administration. J. Agric. Food Chem. 49:4102-4112. [DOI] [PubMed] [Google Scholar]
- 10.Koprivnjak, T., A. Pesche, M. H. Gelb, N. S. Liang, and J. P. Weiss. 2002. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 277:47636-47644. [DOI] [PubMed] [Google Scholar]
- 11.Raychaudhuri, D., and A. N. Chatterjee. 1985. Use of resistant mutants to study the interaction of Triton X-100 with Staphylococcus aureus. J. Bacteriol. 164:1337-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stapleton, P. D., S. Shah, J. C. Anderson, Y. Hara, J. M. T. Hamilton-Miller, and P. W. Taylor. 2004. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents 23:462-467. [DOI] [PubMed] [Google Scholar]
- 13.Yam, T. S., S. Shah, and J. M. T. Hamilton-Miller. 1997. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol. Lett. 152:169-174. [DOI] [PubMed] [Google Scholar]
- 14.Zhao, W.-H., Z.-Q. Hu, S. Okubo, Y. Hara, and T. Shimamura. 2001. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.