Abstract
Adhesion of Plasmodium falciparum-infected erythrocytes (IE) to host endothelium has been associated with pathology in malaria. Although the interaction with endothelial cells can be complex due to the relatively large number of host receptors available for binding, specific proteins have been identified that are more commonly used than others. For example, binding to intercellular adhesion molecule 1 (ICAM 1) is found frequently in parasites from pediatric cases of malaria. The binding site for P. falciparum-infected erythrocytes on ICAM 1 has been mapped in some detail and is distinct from the site for lymphocyte function-associated antigen 1 (LFA-1). Part of the ICAM 1 binding site for P. falciparum-infected erythrocytes (the DE loop) was used to screen a library of compounds based on its structure (derived from the crystal structure of human ICAM 1). This resulted in the identification of 36 structural mimeotopes as potential competitive inhibitors of binding. One of these compounds, (+)-epigalloyl-catechin-gallate [(+)-EGCG], was found to inhibit IE adhesion to ICAM 1 in a dose-dependent manner with two variant ICAM 1-binding parasite lines, providing the first example of a potential mimeotope-based anticytoadherence inhibitor for Plasmodium falciparum.
Malaria imposes a huge burden of mortality and morbidity in developing countries (25), particularly in sub-Saharan Africa, where it is responsible for over 1 million deaths per year. Most of these are caused by infection with Plasmodium falciparum, one of four human malaria parasites and the only one to undergo significant adhesion to host endothelium, resulting in the sequestration of mature erythrocytic-stage infected erythrocytes (IE) from the peripheral circulation. The pathology of P. falciparum malaria is complex and comprises a number of syndromes, at least some of which are thought to be related to the ability of IE to adhere to host small-vessel endothelium. Several endothelial receptors have been implicated in this interaction (for a review, see reference 9), including intercellular adhesion molecule 1 (ICAM 1) (5). For IE that use ICAM 1, the receptor appears to be an important component of binding, as inhibition using specific monoclonal antibodies reduces cytoadherence to almost background levels despite the presence of other receptors, such as CD36 (12). Although there is some evidence associating sequestration with severe disease, the identification of specific receptor usage linked with pathology has been difficult, with different studies producing conflicting conclusions. In one large study in Kenya, the ability of patient isolates to bind to ICAM 1 was linked to disease (with a trend toward cerebral malaria, a serious, life-threatening manifestation of disease) (14). However, other studies have shown no effect (18). Despite these difficulties, it seems likely that being able to prevent or reverse adhesion to endothelium will be beneficial in acute disease by providing direct access by host clearance systems to the sequestered mass of parasites and alleviating local metabolic imbalances through the removal of IE from the endothelial surface back into the general circulation.
ICAM 1 has a number of normal functions in the host, such as T-cell maturation and leukocyte recruitment, as well as being subverted as a receptor for IE and human rhinoviruses (13, 26, 27). The binding site for IE has been mapped to the BED side of the N-terminal immunoglobulin-like domain (4, 15) and shows subtle differences in the contact residues used by ICAM 1-binding P. falciparum antigenic variants (28). The DE loop appears to be a common feature of the ICAM 1 binding sites for the three variants tested and so was selected to screen a library of small-molecule structures in silico using a molecular-alignment technique based on the program package 4Scan (22). Thirty-six compounds were identified as having structures similar to that of the DE loop of human ICAM 1, and they were tested for the ability to inhibit adhesion of IE to ICAM 1 under flow conditions. One compound, (+)-epi-2-galloylcatechin-3-gallate [(+)-EGCG], at micromolar concentrations inhibited binding of two variant ICAM 1-binding parasites in a highly specific, dose-dependent manner.
MATERIALS AND METHODS
Screening for ICAM 1 DE loop mimeotopes in silico.
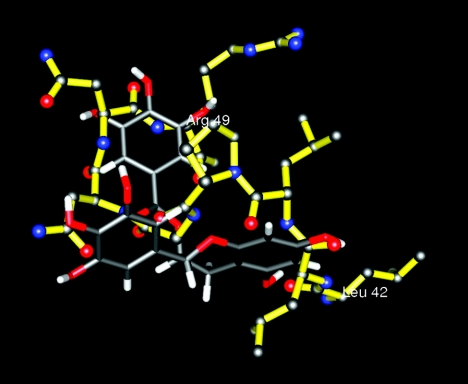
The structures of the two amino-terminal domains of human ICAM 1 published by Bella et al. (Protein Data Bank entry 1IAM) (2) were used to set up a template for a molecular alignment library screening with 4Scan (4SC AG, Martinsried, Germany). The atom coordinates of the residues of the L43 loop (Leu42-Arg49) (see Fig. 6) were extracted and transformed into a target description file for the underlying alignment tool ProPose (20, 21). The proprietary ProPose alignment score was then used to rank the library compounds. This score judges similarities of molecular shape, as well as the match for possible intermolecular interactions. This is facilitated via a pseudo-receptor box reflecting the template molecule. The pseudo-receptor box consists of discrete points modeling excluded volume and typical interactions, like hydrophilic and lipophilic contacts. The screening technology 4Scan, which is performed in silico on virtual molecular libraries of up to 10 million entries, was applied in this study to a diverse library of about 3 million commercially available compound structures.
FIG. 6.
Overlay of the structures of the L43 loop of ICAM 1 (color) and (+)-EGCG (grey).
Parasite culture.
The human ICAM 1-binding laboratory lines ITO4-A4, ITO4-C24 (17), and ItG-ICAM (16) were used in this study. Parasites were cultured in type O erythrocytes in RPMI 1640 medium supplemented with 37.5 mM HEPES, 7 mM d-glucose, 6 mM NaOH, 25 μg/ml gentamicin sulfate, 2 mM l-glutamine, and 10% human serum at pH 7.2 in a gas mixture of 96% nitrogen, 3% carbon dioxide, and 1% oxygen.
The parasites were washed twice in binding buffer (RPMI 1640 medium supplemented with 6 mM glucose, pH 7.2) and resuspended in the same buffer at 3% parasitemia and 1% hematocrit prior to adhesion assays.
Adhesion assays.
Binding assays under flow conditions were carried out as described by Gray et al. (12). This type of assay attempts to mimic the conditions seen in the postcapillary venules by allowing infected erythrocytes to flow over microslides coated with endothelial receptors under carefully controlled conditions, and it more closely reflects physiological conditions during malaria infection. In summary, microslides were coated for 2 h at 37°C with 25 mg/ml human ICAM 1-Fc (10) or 50 mg/ml human CD36 (R&D Systems, Abingdon, United Kingdom) and blocked overnight at 4°C with phosphate-buffered saline-1% bovine serum albumin. The parasite suspensions (see above) were allowed to flow through ICAM 1-coated microslides for a total of 5 min, followed by binding buffer to remove unbound cells (at a wall shear stress of 0.05 Pa). The stationary IE were counted in six separate fields on the slide, and the IE rolling velocity was analyzed from video recordings.
Inhibitor testing.
Fifty millimolar stock solutions were made up in dimethyl sulfoxide (DMSO). They were screened at 50 μM in flow adhesion assays by adding the compound to the parasite suspension 20 min prior to the assay. The compounds were stored at −20°C as solids, although working stock solutions were used over periods not exceeding 5 days.
RESULTS
Identification of ICAM 1 DE loop mimeotopes.
Molecular-alignment screening with the program package 4SCan/ProPose of the DE loop structure, derived from the existing crystal structures of human ICAM 1 (2, 7), resulted in 36 top-scoring commercially available compounds. These compounds were purchased, analyzed for purity using reversed-phase high-performance liquid chromatography, and submitted for biological testing.
Analysis of inhibition of binding to ICAM 1 by candidate compounds.
The flow assay system was chosen as a screen for inhibition of IE binding, as it is more sensitive to changes under these dynamic conditions than static assays. ITO4-A4 was chosen for the same reason, namely, because in other experiments using a natural mutant of ICAM 1 it had been demonstrated to be more sensitive to alterations in binding than higher-avidity strains (1). No significant differences were seen in IE binding in the presence of DMSO at concentrations present in diluted drug preparations (data not shown). This type of assay is known to be relatively variable, and quantitative differences on a single-pass experiment are unlikely to be significant. However, qualitative results are generally reproducible and are more likely to produce false positives rather than to rule out effective inhibitors of adhesion.
Adhesion assays under flow yield two parameters, namely, numbers of stationary cells (moving at <15 μm/s) per mm2 and distributions of rolling velocities. Changes in rolling velocity are more sensitive indicators of potential inhibition of adhesion and so were chosen for preliminary screening.
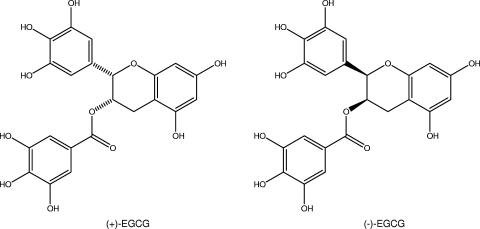
Thirty-six compounds resulting from the in silico panning were selected for the initial screening. Several of these compounds showed a reduction in the number of cells rolling and an increase in rolling velocity (data not shown). Single-pass experiments generally generate robust qualitative data, but assay-to-assay variation makes quantitative analysis more difficult. In later experiments, both rolling- and static-adhesion parameters were used to determine which compounds would proceed to secondary screening. From this, only one compound was selected for further analysis based primarily on a large reduction in stationary adhesion (<50% no drug binding), with supporting information from the number of IE rolling, characterized by a reduction in the number of cells rolling and an increase in the rolling velocity. This lead compound is the polyphenolic natural product (+)-EGCG (Fig. 1).
FIG. 1.
The lead compound (+)-EGCG and its more frequently found stereoisomer, (−)-EGCG.
Further analysis of the antiadhesive properties of (+)-EGCG.
In preliminary experiments, the compound (+)-EGCG produced large reductions in both stationary and rolling components of adhesion under flow. They were repeated several times to demonstrate that the effect was reproducible (Fig. 2).
FIG. 2.
Inhibition of stationary adhesion using compound (+)-EGCG. The number of stationary IE binding to ICAM 1 under flow conditions is shown for the laboratory parasite line ITO4-A4 with and without (+)-EGCG (three independent experiments). The error bars indicate standard deviations.
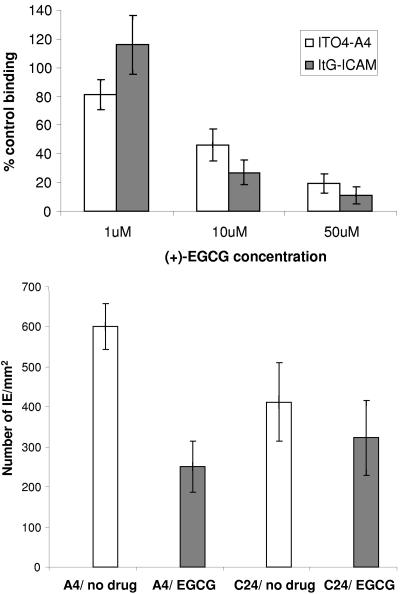
Adhesion in P. falciparum is mainly mediated by the variant surface antigen Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) (see reference 9 for a review). This is a highly variant protein that differs at the primary protein sequence level between parasite isolates despite retaining some functional similarities. A number of ICAM 1-binding isolates have been identified and analyzed in detail (28). These experiments have shown that although ICAM 1-binding variants have different binding sites on ICAM 1, which might preclude the development of a pan-ICAM 1 adhesion-blocking mimeotope, they all involve the L43 (DE) loop of ICAM 1. The preliminary screens were carried out using a single ICAM 1-binding variant, ITO4-A4; therefore, we selected a different parasite isolate to test the effect of (+)-EGCG on binding to ICAM 1. This second isolate, ItG-ICAM, differs from ITO4-A4 in that it shows higher avidity for ICAM 1, as well as being antigenically distinct. The measurement of stationary binding under flow conditions (Fig. 3, top) shows that (+)-EGCG is also able to block ICAM 1 binding by ItG-ICAM and so is not dependent on the parasite variant type.
FIG. 3.
(Top) Dose effect of compound (+)-EGCG on binding to ICAM 1 by ITO4-A4 and ItG-ICAM. Control binding was carried out in the presence of DMSO at the same concentration as (+)-EGCG. (Bottom) Effect of 50 μM (+)-EGCG on CD36 binding by ITO4-A4 and ITO4-C24 (a non-ICAM 1 binder). (Mann-Whitney test: A4-no drug versus A4-EGCG, P = 0.002; C24-no drug versus C24-EGCG, P=0.24.) The error bars indicate standard deviations.
In order to test whether (+)-EGCG is specific for ICAM 1 binding, we tested the effect of the drug on adhesion to another endothelial receptor, CD36. The parasite line ITO4-A4 is able to bind both ICAM 1 and CD36, whereas ITO4-C24 can bind to CD36 but does not adhere to ICAM 1. ITO4-A4 binding to CD36 is inhibited by (+)-EGCG, but ITO4-C24 is not affected (Fig. 3, bottom), suggesting that the inhibition of binding is specific, but also that the interaction between the drug and ITO4-A4 results in the loss of more than one binding activity, possibly through the transmission of a structural change through the PfEMP1 molecule.
Secondary screening using (+)-EGCG as a lead compound.
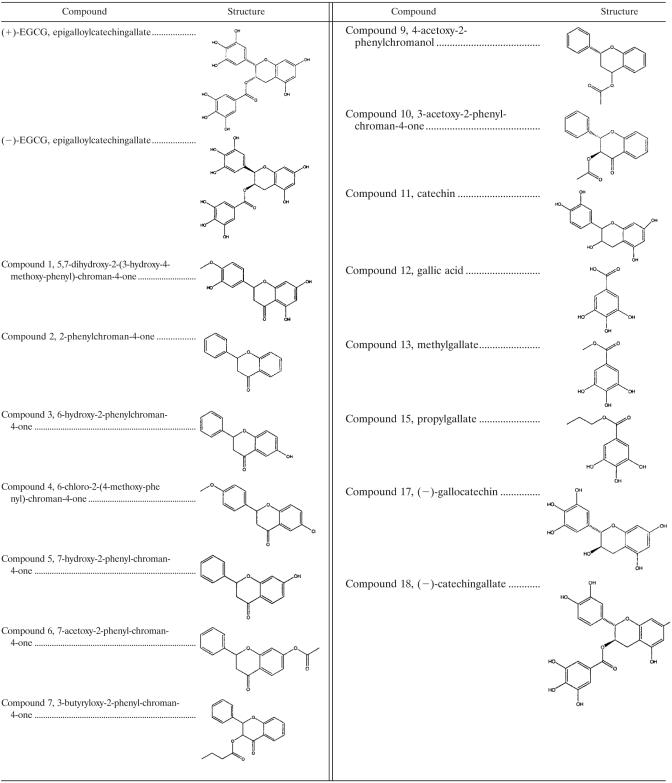
A more focused inhibitory library was derived based on sterioisomers of (+)-EGCG and structurally related small molecules. None of the 15 structural derivatives showed higher inhibitory potential than the lead molecule (Table 1 and Fig. 4). To confirm the initial results, a new batch of (+)-EGCG was screened for stationary adhesion under flow conditions, along with its enantiomer, (−)-EGCG [, i.e., the “mirror image” of (+)-EGCG], and the closely related structure (−)-gallocatechin (compound 17 in Table 1) (Fig. 5). The enantiomer (−)-EGCG showed inhibitory action similar to that of the parent structure, but the other related compounds had no major effect on adhesion to ICAM 1. Taken together with the earlier result showing no significant inhibition by (−)-catechingallate (compound 18) (Fig. 4), this indicates a high degree of specificity in the interaction rather than merely a charge effect.
TABLE 1.
Secondary screen compoundsa
See Fig. 4 for results.
FIG. 4.
Secondary screen of compounds (see Table 1 for details) for rolling (top) and stationary (bottom) adhesion of ITO4-A4 to ICAM 1. Each data point in the upper panel represents one IE. Compounds were screened at 50 μM. The error bars indicate standard deviations.
FIG. 5.
Screening of isomers of EGCG and gallocatechin (compound 17) for inhibition of ICAM 1 static binding. The error bars indicate standard deviations.
DISCUSSION
The pathology of severe disease in P. falciparum malaria is not fully understood, but the ability of infected red blood cells to adhere to the endothelial cells lining the small vessels is thought to be a major factor. One of the receptors used by a significant proportion of patient isolates is ICAM 1, which is a member of the immunoglobulin family of protein structures. In this study, the crystal structure of human ICAM 1 was used as a basis to screen for inhibitors of cytoadherence. The region of ICAM 1 chosen was based on previous work showing that three different ICAM 1-binding P. falciparum isolates all used this part of the ICAM 1 molecule for binding (28). A set of 36 compounds were identified by means of in silico screening and tested in vitro using an assay based on infected-erythrocyte adhesion under flow conditions. Flow adhesion assays provide two forms of information, namely, the number of cells binding statically and the rolling velocity distribution. The combination of a major reduction in both values was used to identify a single compound able to block adhesion to ICAM 1. The only hit resulting from this approach was (+)-EGCG, a polyphenol compound. Its ability to inhibit adhesion by P. falciparum-infected erythrocytes to ICAM 1 was retested and showed consistent and dose-dependent inhibition (Fig. 2 and 3). In addition, it was also able to block binding by two antigenically distinct ICAM 1-binding lines (ITO4-A4 and ItG-ICAM), demonstrating that its activity might not be confined to a single isolate. This is in agreement with earlier studies that showed that mutagenic analysis of the ICAM 1 binding site for ITO4-A4 and ItG-ICAM revealed a common region implicated in binding to both isolates (the DE, or L43, loop) (28). The superposition of (+)-EGCG and the L43 loop of ICAM 1 is shown in Fig. 6.
The (−)-EGCG enantiomer is a particularly interesting molecule. This major constituent of green tea has been implicated as an anticancer, anti-inflammatory, and anti-infective agent through a range of mechanisms, including inhibition of metalloproteinases and antioxidant activity. As both enantiomers have essentially the same activity in terms of antiadhesive properties, it appears warranted not to distinguish between the stereoisomers. Some toxicity has been reported for (−)-EGCG, but this was at relatively high concentrations (200 μM) (19). The inhibitory levels seen in our study are achievable in plasma, although it is unlikely that this would be possible after oral dosing. Unlike previous applications, we assume that the mode of action in this case is through structural mimicry of part of the ICAM 1 binding site for IE (Fig. 6), producing an inhibitor of cytoadherence. The discovery of a specific inhibitor from a list of only 36 candidates provides strong support for the in silico screening approach, and the compound identified is an interesting lead on which to base further work. This would include improvement of the 50% inhibitory-concentration value to reduce it from micromolar to nanomolar levels, potentially through chemical modifications to provide better coverage of the L43 loop, particularly in the area of the crucial residues L42 to L44, which remain largely accessible. The subinhibitory plasma levels of 200 to 300 nM available via oral administration (8, 29) are less important, as the most likely use of this agent would be the acute removal of a sequestered parasite mass in people with severe malaria in a hospital setting where intravenous administration would be an option, although other routes of delivery would be beneficial. Further work will also be needed to assess the potential harmful effects of releasing large numbers of mature infected erythrocytes into the peripheral circulation and, in particular, their effect on the spleen. In addition, we were unable to show reversal of adhesion to ICAM 1 using (+)-EGCG (data not shown), probably due to relative low affinity between the drug and the parasite target, which will also need to be addressed by future developmental work.
Inhibition by (+)-EGCG was highly significant, with a 50% inhibitory concentration of approximately 5 to 10 μM. A series of 17 structural derivatives of the lead compound (+)-EGCG were tested using flow-based adhesion. Unfortunately, none of them was a more potent inhibitor (Fig. 4), although several compounds showed variable inhibition of static or rolling adhesion. A number of these were tested further but did not demonstrate significant or reproducible inhibition (data not shown). However, the inclusion of several closely related compounds revealed some interesting information on the specificity of inhibition by (+)-EGCG (Fig. 6). Despite the obviously narrow structure-activity relationship, both enantiomers, (+)-EGCG and (−)-EGCG, show similar activities in good accordance with the in silico predictions. There is only a small variation in ITO4-A4 binding inhibition, suggesting that the effect is independent of stereochemistry, although a very closely related compound, (−)-catechingallate (compound 18), has little antiadhesive effect (Fig. 4), and equally, removal of the 2-galloyl moiety (compound 17; gallocatechin) completely abolishes any inhibitory activity (Fig. 5).
There are a number of problems associated with EGCG as a potential drug. Lack of stability in DMSO solution at 4°C (data not shown) manifested itself in early experiments as decreasing inhibitory activity when working concentrations were prepared from a stock solution over 2 to 3 weeks, and this would need to be monitored in aqueous solution. Its range of activity in a number of processes, including inhibition of metallo- and serine proteases (3), reduction of oxygen-derived free radicals (6), proteasome inhibition (24), and antibacterial action (30), while useful, do pose some problems in terms of potential adverse effects.
Inhibition of cell adhesion processes is becoming increasingly interesting in the discovery of novel therapeutics (23), including compounds based on the structure of ICAM 1 (11). In this study, we were able to demonstrate the power of combining structural biology and in silico drug design with our knowledge of parasite biology to design a series of compounds, one of which is the first nonpeptide molecule to demonstrate direct antiadhesion effects in malaria. This rational design process has shown the potential of small molecules to inhibit cytoadherence and potentially to contribute to the reversal of pathogenesis in severe malaria.
Acknowledgments
We thank other members of the ADMALI network (European Union) for their support and encouragement and Steve Ward for his comments on the manuscript.
This work was funded by the European Union, Framework 5 (QLCK-CT-2000-00109), and The Wellcome Trust (A.C.).
REFERENCES
- 1.Adams, S., G. D. Turner, G. B. Nash, K. Micklem, C. I. Newbold, and A. G. Craig. 2000. Differential binding of clonal variants of Plasmodium falciparum to allelic forms of intracellular adhesion molecule 1 determined by flow adhesion assay. Infect. Immun. 68:264-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bella, J., P. R. Kolatkar, C. W. Marlor, J. M. Greve, and M. G. Rossmann. 1998. The structure of the two amino-terminal domains of human ICAM-1 suggests how it functions as a rhinovirus receptor and as an LFA-1 integrin ligand. Proc. Natl. Acad. Sci. USA 95:4140-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benelli, R., R. Vene, D. Bisacchi, S. Garbisa, and A. Albini. 2002. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol. Chem. 383:101-105. [DOI] [PubMed] [Google Scholar]
- 4.Berendt, A. R., A. McDowall, A. G. Craig, P. A. Bates, M. J. Sternberg, K. Marsh, C. I. Newbold, and N. Hogg. 1992. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell 68:71-81. [DOI] [PubMed] [Google Scholar]
- 5.Berendt, A. R., D. L. Simmons, J. Tansey, C. I. Newbold, and K. Marsh. 1989. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 341:57-59. [DOI] [PubMed] [Google Scholar]
- 6.Buttemeyer, R., A. W. Philipp, L. Schlenzka, J. W. Mall, M. Beissenhirtz, and F. Lisdat. 2003. Epigallocatechin gallate can significantly decrease free oxygen radicals in the reperfusion injury in vivo. Transplant Proc. 35:3116-3120. [DOI] [PubMed] [Google Scholar]
- 7.Casasnovas, J. M., T. Stehle, J. H. Liu, J. H. Wang, and T. A. Springer. 1998. A dimeric crystal structure for the N-terminal two domains of intercellular adhesion molecule-1. Proc. Natl. Acad. Sci. USA 95:4134-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow, H. H., Y. Cai, I. A. Hakim, J. A. Crowell, F. Shahi, C. A. Brooks, R. T. Dorr, Y. Hara, and D. S. Alberts. 2003. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 9:3312-3319. [PubMed] [Google Scholar]
- 9.Craig, A., and A. Scherf. 2001. Molecules on the surface of the Plasmodium falciparum infected erythrocyte and their role in malaria pathogenesis and immune evasion. Mol. Biochem. Parasitol. 115:129-143. [DOI] [PubMed] [Google Scholar]
- 10.Craig, A. G., R. Pinches, S. Khan, D. J. Roberts, G. D. Turner, C. I. Newbold, and A. R. Berendt. 1997. Failure to block adhesion of Plasmodium falciparum-infected erythrocytes to ICAM-1 with soluble ICAM-1. Infect. Immun. 65:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadek, T. R., D. J. Burdick, R. S. McDowell, M. S. Stanley, J. C. Marsters, Jr., K. J. Paris, D. A. Oare, M. E. Reynolds, C. Ladner, K. A. Zioncheck, W. P. Lee, P. Gribling, M. S. Dennis, N. J. Skelton, D. B. Tumas, K. R. Clark, S. M. Keating, M. H. Beresini, J. W. Tilley, L. G. Presta, and S. C. Bodary. 2002. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science 295:1086-1089. [DOI] [PubMed] [Google Scholar]
- 12.Gray, C., C. McCormick, G. Turner, and A. Craig. 2003. ICAM-1 can play a major role in mediating P. falciparum adhesion to endothelium under flow. Mol. Biochem. Parasitol. 128:187-193. [DOI] [PubMed] [Google Scholar]
- 13.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. McClelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 14.Newbold, C., P. Warn, G. Black, A. Berendt, A. Craig, B. Snow, M. Msobo, N. Peshu, and K. Marsh. 1997. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 57:389-398. [DOI] [PubMed] [Google Scholar]
- 15.Ockenhouse, C. F., R. Betageri, T. A. Springer, and D. E. Staunton. 1992. Plasmodium falciparum-infected erythrocytes bind ICAM-1 at a site distinct from LFA-1, Mac-1, and human rhinovirus. Cell 68:63-69. [DOI] [PubMed] [Google Scholar]
- 16.Ockenhouse, C. F., M. Ho, N. N. Tandon, G. A. Van Seventer, S. Shaw, N. J. White, G. A. Jamieson, J. D. Chulay, and H. K. Webster. 1991. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J. Infect. Dis. 164:163-169. [DOI] [PubMed] [Google Scholar]
- 17.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogerson, S. J., R. Tembenu, C. Dobano, S. Plitt, T. E. Taylor, and M. E. Molyneux. 1999. Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am. J. Trop. Med. Hyg. 61:467-472. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt, M., H. J. Schmitz, A. Baumgart, D. Guedon, M. I. Netsch, M. H. Kreuter, C. B. Schmidlin, and D. Schrenk. 2005. Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture. Food Chem. Toxicol. 43:307-314. [DOI] [PubMed] [Google Scholar]
- 20.Seifert, M. H. J. 2005. ProPose: steered virtual screening by simultaeneous protein-ligand docking and ligand-ligand alignment. J. Chem. Infect. Model. 45:449-460. [DOI] [PubMed] [Google Scholar]
- 21.Seifert, M. H. J., F. Schmitt, T. Herz, and B. Kramer. 2004. ProPose: a docking engine based on a fully configurable protein-ligand interaction model. J. Mol. Model. 10:342-357. [DOI] [PubMed] [Google Scholar]
- 22.Seifert, M. H. J., K. Wolf, and D. Vitt. 2003. Virtual high-throughput in silico screening. Biosilico 1:143-149. [Google Scholar]
- 23.Simmons, D. L. 2005. Anti-adhesion therapies. Curr. Opin. Pharmacol. 5:398-404. [DOI] [PubMed] [Google Scholar]
- 24.Smith, D. M., K. G. Daniel, Z. Wang, W. C. Guida, T. H. Chan, and Q. P. Dou. 2004. Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design. Proteins 54:58-70. [DOI] [PubMed] [Google Scholar]
- 25.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staunton, D. E., V. J. Merluzzi, R. Rothlein, R. Barton, S. D. Marlin, and T. A. Springer. 1989. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 56:849-853. [DOI] [PubMed] [Google Scholar]
- 27.Tomassini, J. E., D. Graham, C. M. DeWitt, D. W. Lineberger, J. A. Rodkey, and R. J. Colonno. 1989. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 86:4907-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tse, M. T., K. Chakrabarti, C. Gray, C. E. Chitnis, and A. Craig. 2004. Divergent binding sites on intercellular adhesion molecule-1 (ICAM-1) for variant Plasmodium falciparum isolates. Mol. Microbiol. 51:1039-1049. [DOI] [PubMed] [Google Scholar]
- 29.Ullmann, U., J. Haller, J. D. Decourt, J. Girault, V. Spitzer, and P. Weber. 2004. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int. J. Vitam. Nutr. Res. 74:269-278. [DOI] [PubMed] [Google Scholar]
- 30.Yoda, Y., Z. Q. Hu, W. H. Zhao, and T. Shimamura. 2004. Different susceptibilities of Staphylococcus and gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 10:55-58. [DOI] [PubMed] [Google Scholar]