Abstract
The calcium-dependent lectin, DC-SIGN, binds to human immunodeficiency virus (HIV) (and simian immunodeficiency virus) gp120 and mediates the binding and transfer of HIV from monocyte-derived dendritic cells (MDDCs) to permissive T cells. However, it has been recently reported that DC-SIGN binding to HIV gp120 may be carbohydrate independent. Here, we formally demonstrate that gp120 binding to DC-SIGN and MDDCs is largely if not wholly carbohydrate dependent. Endo-β-N-glucosaminidase H (EndoH) treatment of gp120-Fc under conditions that maintained wild-type CD4 binding—and the full complement of complex glycans—significantly decreased (>90%) binding to DC-SIGN expressing cell lines, as well as to MDDCs. Any residual binding of EndoH-treated gp120-Fc to DC-SIGN was completely competed off with mannan. Mutational analysis indicated that no single glycosylation site affected the ability of gp120-Fc to bind DC-SIGN. To further guide our efforts in mapping the DC-SIGN binding sites on gp120, we used two well-characterized HIV inhibitory agents (2G12 monoclonal antibody and cyanovirin) that bind to high-mannose sugars on gp120. We showed that 2G12 and DC-SIGN bound to nonoverlapping sites in gp120 because (i) 2G12 did not block soluble gp120 or virion binding to DC-SIGN, (ii) 2G12 bound to gp120-Fc that was prebound to cell surface DC-SIGN, and (iii) gp120-Fc mutants that lack glycosylation sites involved in 2G12's epitope were also fully capable of binding DC-SIGN. These data were substantiated by the inability of cyanovirin to block gp120-Fc binding to DC-SIGN. Cyanovirin has been shown to effectively compete for 2G12 binding to gp120. Indeed, high concentrations of cyanovirin dramatically enhanced gp120-Fc binding to cell surfaces in the presence or absence of DC-SIGN. We provide evidence that this enhancement may be due to cyanovirin's ability to bridge gp120 to mannosylated cell surface proteins. These results have implications for antiviral therapeutics and for ongoing efforts to finely map the glycan structures on gp120 responsible for DC-SIGN binding.
Human immunodeficiency virus type 1 (HIV-1) envelope (Env), which is responsible for both receptor binding and membrane fusion, consists of a gp120 surface subunit noncovalently associated with a gp41 membrane-bound subunit. It is the gp120 subunit that binds and interacts with CD4 and a coreceptor prior to entrance of the virus into a cell (4, 8, 16). In addition to the primary receptor and coreceptor required for viral entry, viral attachment molecules have been described that could modulate the efficiency of the viral entry process (20, 39). DC-SIGN, a C-type lectin predominantly expressed on dendritic cells (DCs), can bind to the HIV envelope gp120 and transfer the virus to other permissive cell types (12, 28). The relatively specific expression of DC-SIGN on DCs (12, 34), and the ability of DCs to facilitate infection in trans via DC-SIGN, has led to the proposed role for DC-SIGN in the transfer of virus from the submucosa to secondary lymphoid organs (12, 35). In addition, DC-SIGN can also facilitate infection in cis. That is, when present on cells that express CD4 and coreceptor, DC-SIGN can markedly enhance the efficiency of viral entry, especially when CD4 and coreceptor levels are limiting (20). In effect, the expression of DC-SIGN can expand the tropism of HIV by allowing the virus to infect cells with levels of CD4 and/or coreceptor that are normally below the threshold required for viral entry. Thus, DC-SIGN can facilitate viral infection in trans and in cis, and the interaction between HIV Env- and DC-SIGN-expressing cells may have implications for viral transmission and pathogenesis (27).
Viral attachment to DCs is considered to be the crucial step in the establishment of a primary viral infection and DC-SIGN is clearly involved in this initial attachment step. Although it remains to be shown if DC-SIGN is solely responsible for viral attachment to DCs (38, 43), designing immunogens to elicit antibodies that might block gp120/DC-SIGN or HIV-DC interactions may be a novel addition to the traditional targets of HIV vaccinology. Understanding the structural basis for DC-SIGN-gp120 interactions will inform efforts to design the appropriate immunogens that can elicit antibodies to thwart DC attachment.
gp120 can bind to monocyte-derived DCs (MDDCs) in a CD4-independent fashion (12, 38). This binding appears to be mediated by a variety of lectins, including DC-SIGN. However, it has not been formally shown what kind of glycan structures on gp120 are responsible for binding to DC-SIGN or MDDCs. DC-SIGN binds to high-mannose moieties (25), and the structural data indicate that DC-SIGN binds to an internal trimannose structure found only in high-mannose oligosaccharides and not in complex glycans (10). Since DC-SIGN binds with high affinity to HIV-1/HIV-2 and simian immunodeficiency virus (SIV) Envs (28), it is likely that a conserved set of N-linked glycosylation sites giving rise to high-mannose residues is responsible for the glycan structures involved in DC-SIGN binding. However, it is not known whether complex carbohydrates also contribute to gp120's ability to bind DCs, which express a variety of C-type lectins in addition to DC-SIGN (11). Nevertheless, Envs missing the cognate glycan structures required for DC-SIGN binding or DC binding in general, might “unmask” the underlying polypeptide backbone and elicit antibodies that can interfere with DC-SIGN or DC attachment. As in the case of the selectively deglycosylated SIV Envs which elicited neutralizing antibodies against even the parental Env (29), antibodies generated to Envs deficient in DC-SIGN or DC binding might then access the parental Env in a manner which hinder DC-SIGN or DC binding. Thus, delineating the envelope determinants of DC-SIGN binding has implications for the design of novel immunogens that might elicit antibodies that can neutralize gp120/DC-SIGN binding.
We present here our initial attempts to finely map the N-linked sites on gp120 carrying the glycan structures responsible for DC-SIGN binding. Since it was reported recently that gp120 could bind to DC-SIGN in a carbohydrate-independent manner (13), we first provide direct evidence formally demonstrating that high-mannose moieties on gp120 were responsible for binding not only to DC-SIGN but also to MDDCs. We report our initial efforts to map the N-linked glycosylation sites in gp120 contributing to DC-SIGN binding by using two HIV inhibitory reagents that bind to high-mannose residues (monoclonal antibody [MAb] 2G12 and cyanovirin) to guide our efforts. These results have implications in helping to finely map the glycan structures responsible for DC-SIGN binding.
MATERIALS AND METHODS
Viruses and cells.
Construction of the DC-SIGN-IRES-EGFP MIGR1 retroviral vector was previously described (20). DC-SIGN/GFP-positive cell lines were established by retroviral transduction and single cell cloning for high expressing (green fluorescent protein [GFP]bright) clones. GFP-positive cell lines were confirmed to be DC-SIGN positive by costaining with a MAb against DC-SIGN (DC028) (1, 20). Pseudotyped GFP HIV-reporter viruses were made as previously described (31). SupT1 cell lines were originally obtained from Jim Hoxie at the University of Pennsylvania. The BC7 cell line is a CD4− clone of SupT1 cells used previously to obtain CD4-independent viruses (also a kind gift from Jim Hoxie). Monocyte-derived DCs (MDDCs) were made by first isolating monocytes from leukopheresed peripheral blood mononuclear cells (PBMCs) by using the RosetteSep monocyte enrichment cocktail (Stem Cell Technologies, Vancouver, British Columbia, Canada). Purified monocytes (>95% CD14+) were cultured in Aim-V serum-free medium (Life Technologies, Rockville, Md.), supplemented with granulocyte-macrophage colony-stimulating factor (50 ng/ml) and interleukin-4 (100 ng/ml) (Preprotech, Rocky Hill, N.J.). On day 7, the MDDCs were >95% BDCA-4+, CD11c+, and CD83−.
Plasmids and proteins.
A NotI-XhoI gp120-Fc fragment (gp120 from the JRCSF clone) was subcloned from pRSC-GS-Rev-gp120FC/RRE into pCR3 (Invitrogen, Carlsbad, Calif.). This maintained the natural signal leader sequence present in gp120 and placed gp120-Fc downstream of a T7 promoter. All glycosylation mutants (Asn-X-Ser/Thr to Ala/Glu-X-Ser/Thr) were made in the context of the pCR3-gp120-Fc plasmid by using site-directed mutagenesis (QuickChange; Stratagene, La Jolla, Calif.). Mutations were confirmed by sequencing. Recombinant gp120-Fc was made by the vaccinia-driven T7 polymerase system. Briefly, 293T cells were infected with vTF1.1 (recombinant vaccinia virus expressing T7 polymerase) at a multiplicity of infection (MOI) of 5 for 1 h. Subsequently, 10 μg of pCR3-gp120-Fc was transfected into vaccinia virus-infected cells by calcium phosphate precipitation. At 4 h posttransfection, cells were washed twice with 1× phosphate-buffered saline (PBS) and replaced with serum-free medium (Dulbecco modified Eagle medium [DMEM]). At 48 h posttransfection, the supernatant was harvested, filtered through a 0.22-μm (pore-size) filter and stored at 4°C in the presence of a protease inhibitor cocktail (Complete, EDTA-free; Roche, Indianapolis, Ind.) until further use. Preliminary experiments indicate no decrease in DC-SIGN binding activity when stored under these conditions for 2 months. The gp120-Fc thus produced was dimeric as judged by its migration (∼300 kDa) by native sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). 2G12 was obtained from the AIDS Reference Reagent Program, and cyanovirin was a kind gift from Paul Parren and Dennis Burton (The Scripps Research Institute).
gp120-Fc binding assay.
Most gp120-Fc glycosylation mutants were produced at similar levels as gauged by Western blotting with horseradish peroxidase-conjugated goat anti-human Fc antibodies (data not shown). Blots were developed by using the ECLplus chemiluminesence kit (Amersham Bioscience). A total of 5 × 105 DC-SIGN-expressing cells or parental untransduced cells were resuspended in a 1:2 dilution of gp120-Fc supernatant for 20 min and washed twice with DC-SIGN binding buffer (1× Tris-buffered saline, 2 mM CaCl2, 2.5% calf serum, 0.05% sodium azide) before subsequent detection of gp120-Fc binding by goat phycoerythrin (PE)-conjugated anti-human Fc (Caltag, Burlingame, Calif.). Mannan and EGTA competitions were performed by preincubating the cells with the indicated amounts of reagents 15 min before addition of gp120-Fc. 2G12 and cyanovirin competitions were performed by preincubating the gp120-Fc supernatant with the reagents 15 min before the addition of the supernatant to the cells. Endo-β-N-glucosaminidase H (EndoH; New England Biolabs, Cambridge, Mass.) digestion of gp120-Fc was performed by incubating gp120-Fc supernatant with 20,000 U of EndoH/ml overnight. The extent of deglycosylation was confirmed by SDS-PAGE analysis of untreated and EndoH-treated gp120-Fc. The conformational integrity of EndoH-treated gp120-Fc was determined by CD4 staining on SupT1 cells. For the “bead-based” adhesion assay, paramagnetic Miltenyi microbeads (Miltenyi Biotech, Inc., Auburn, Calif.) covalently linked to anti-PE MAb were used. gp120-Fc was linked to anti-PE microbeads via PE-conjugated goat anti-human Fc antibodies (see Fig. 1C for schematic). Approximately 500 ng of gp120-Fc (100 μl of supernatant) per 2.5 μl of PE-conjugated anti-human Fc (Caltag) per 4 μl of anti-PE microbeads per 2 × 106 cells were used. A 200-fold excess of parental (DC-SIGN-negative) BC7 cells was added to 104 BC7-DC-SIGN-positive cells before the cell mixture was subjected to gp120-Fc-mediated isolation. Magnetic isolation of the cognate cells were performed according to the manufacturer's protocol by using the VarioMacs manifold.
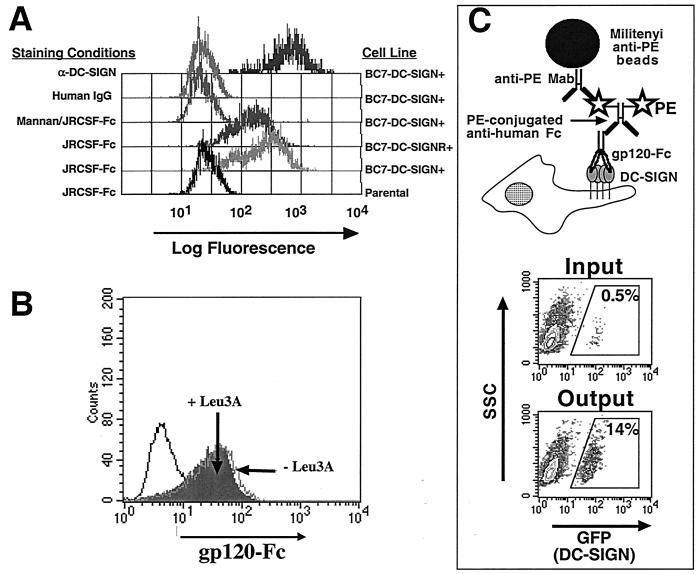
FIG. 1.
gp120-Fc binding assay. A CD4− subclone of SupT1 cells, BC7, was transduced with a MSCV vector that expresses DC-SIGN in tandem with GFP via an internal ribosome entry site linker. GFP-positive cells were sorted and expanded. All GFP-positive cells were confirmed to be DC-SIGN positive by using a MAb against DC-SIGN (20). (A) gp120-Fc (0.5 μg/ml) binding to BC7-DC-SIGN+ cells was detected by secondary PE-conjugated anti-human Fc antibodies. Binding to DC-SIGN was specific as it was competed off by mannan, and there was no binding to parental untransduced BC7 cells. (B) gp120-Fc binding to MDDCs was not affected by the presence of 10 μg of Leu3A/ml, indicating CD4-independent binding of gp120-Fc. Human IgG was used as negative control (open histogram, bold line). (C) gp120-Fc binding to DC-SIGN in a “bead-based” adhesion assay. A schematic of the gp120-Fc bead-binding assay is shown. Paramagnetic Miltenyi microbeads covalently linked with anti-PE MAb was used to bind to and enrich for DC-SIGN-positive cells via a gp120-Fc and PE-conjugated anti-human Fc complex. A representative assay showing that Miltenyi microbeads linked to gp120-Fc can effectively bind to and enrich for DC-SIGN-positive cells (BC7-DC-SIGN) diluted in a 200-fold excess of DC-SIGN-negative cells (parental BC7). GFP-positive cells are necessarily DC-SIGN positive (see above).
Lectin-gp120-Fc binding assay.
Reacti-Bind streptavidin-coated clear strip plates with blocker bovine serum albumin (BSA; Pierce, Rockford, Ill.) were washed three times with wash buffer (TBS, 0.1% BSA, 0.05% Tween 20). Then, 0.2 μg of biotinylated lectins (DSA, SNA, PNA, GNA, and PHA-E from Vector Laboratories, Burlingame, Calif.) were added to each well and incubated for 1 h at room temperature. Each well was rinsed three times with wash buffer. A total of 100 μl of EndoH, PNGase F-treated gp120-Fc, or wild-type gp120-Fc (∼500 ng of protein) was added to the appropriate wells and incubated at room temperature for 30 min, followed by three washes with wash buffer. Horseradish peroxidase-conjugated α-human-Fc antibodies (Caltag) was added to each well at a 1:10,000 dilution and incubated at room temperature for 30 min. Again, each well was rinsed three times with wash buffer. A total of 100 μl of 1-Step UltraTMB enzyme-linked immunosorbent assay (ELISA) substrate (Pierce) was used for colormetric detection. After 10 min of incubation at room temperature, 100 μl of Stop solution (8 M acetic acid, 1 M H2SO4) was added to each well. The binding specificities of the lectins used are as follows (only DSA and GNA bound appreciably to gp120-Fc): Datura stramonium agglutinin recognizes β1-4 GlcNAc in complex and hybrid N-glycans; Sambucus nigra agglutinin recognizes terminal sialic acid-linked α2-6 to galactose; peanut agglutinin recognizes the core disaccharide Gal β1-3 to GalNAc which is in O-link glycans; Galanthus nivalis agglutinin recognizes terminal mannose α1-3, α1-6, α1-2 linked to mannose; and Phaseolus vulgaris erythroagglutinin, with which lectin binding was inhibited by Gal β1-4 GlcNAc β1-2 mannose.
Tertiary 2G12 binding assay.
To determine whether 2G12 can bind to gp120-Fc bound to DC-SIGN, we performed gp120-Fc binding to DC-SIGN as indicated above. After primary gp120-Fc binding, cells were washed twice in DC-SIGN binding buffer, and then 10 μg of 2G12/ml was added for an additional 20-min incubation on ice. Cells were washed once in DC-SIGN binding buffer and then a PE-conjugated MAb against the human kappa light chain (Caltag) was added to detect any 2G12 bound to gp120-Fc. After another 20-min incubation, cells were washed twice in ice-cold DC-SIGN binding buffer and fixed in 2% paraformaldehyde before fluorescence-activated cell sorting (FACS) analysis by using the FACSCalibur machine for acquisition and the CellQuest software for analysis (Becton Dickinson, San Jose, Calif.).
Virion binding assay.
293T human embryonic kidney cells were transiently transfected with DC-SIGN in a T25 flask by using calcium phosphate. After 48 h, the cells were seeded into a 48-well tissue culture plate and allowed to grow for an additional 24 h. Blocking agents (cyanovirin or 2G12) were preincubated with 5 ng of p24 equivalents of virus (as determined by a commercially available p24 ELISA kit (Perkin-Elmer, Boston, Mass.) in a total volume of 50 μl for 30 min before the addition to the wells. The plate was spun at 2,000 rpm at 37°C for 3 h, at which point the cells were washed three times with DMEM. The cells were then lysed and assayed for p24 content by using the p24 ELISA kit. For mannan or EGTA blocking, reagents were added to the cells at the indicated concentrations before addition of the virus.
Virus infections.
SupT1 cells were seeded into a 24-well tissue culture plate in a concentration of ca. 5 × 105 per well. Then, 100 μl of GFP-reporter virus was added in the presence or absence of 2G12 or cyanovirin and the plate was spun at 2,000 rpm and 37°C for 2 h. The infection was allowed to proceed for 48 h at which point the cells were spun down and fixed with 2% paraformaldehyde. The extent of infection as indicated by the percentage of GFP+ cells was quantified by flow cytometry.
2G12/gp120 binding ELISA.
One hundred microliters of neat gp120-Fc supernatant was added to Dynex Immulon 2 plates (Fisher Scientific, Tustin, Calif.) and allowed to dry overnight at 37°C. The plate was blocked with 300 μl of blocking buffer (PBS, 0.1% Tween, 0.25% BSA) at room temperature for 1 h then washed three times with PBS-Tween plus 0.1% BSA. Next, 100 μl of 2G12 at various concentrations ranging from 100 to 0 ng was added followed by incubation at room temperature for 1 h. The plates were washed three times and 100 μl of goat α-human κ conjugated to horseradish peroxidase was added in a dilution of 1:1,000 and incubated for 30 min at room temperature. The plates were again washed three times and developed with 1-Step UltraTMB substrate (Pierce). The reaction was stopped by the addition of 2 M H2SO4 and read at 450 nm in a microtiter plate reader (Dynex Technologies, Chantilly, Va.).
RESULTS
DC-SIGN binding assay using gp120-Fc.
In order to facilitate our binding assay, we used a HIV-1 gp120 construct fused to the Fc region of human immunoglobulin G1 (IgG1) as previously reported for feline immunodeficiency virus gp95 (6). Secondary reagents against the human Fc region were used to detect binding of this gp120-Fc fusion in a FACS-based assay. This strategy avoids modifying gp120 itself (e.g., via biotinylation) or the use of anti-Env reagents that might themselves interfere with DC-SIGN/Env binding. Figure 1A shows that gp120-Fc bound specifically to DC-SIGN or DC-SIGNR/L-SIGN expressing cell lines in a mannan-inhibitable fashion. This gp120-Fc also bound to MDDCs (Fig. 1B). There was no significant difference in gp120-Fc binding in the presence or absence of saturating amounts of a neutralizing anti-CD4 antibody (Leu3A), indicating that gp120-binding to MDDCs was independent of CD4. To determine whether gp120-Fc can also bind to DC-SIGN in a “bead-based” adhesion assay, a tertiary binding assay with gp120-Fc was used. Figure 1C shows that gp120-Fc linked to Miltenyi anti-PE beads via PE-conjugated goat anti-human Fc antibodies can bind to and enrich for DC-SIGN-positive cells in a 200-fold excess of DC-SIGN-negative cells. The homologous orienting of oligomeric Env on Miltenyi beads is arguably a more native presentation of gp120 than is random cross-linking of monomeric gp120 on carboxylate-modified TransFluorSpheres beads used by Geijtenbeek et al. (13). In addition, Miltenyi beads (∼50 nm) are much closer in size to virion particles (∼100 nm) than are the TransFluorSpheres beads (∼1,000 nm).
gp120 binding to DC-SIGN and MDDCs is carbohydrate dependent.
Although gp120 binding to DC-SIGN can be competed off with mannan and related sugars (5, 12, 28), it has recently been reported that DC-SIGN can bind to gp120 in a carbohydrate-independent fashion (13). Competition with carbohydrate ligands that target DC-SIGN provide only indirect evidence that gp120 binding itself is carbohydrate dependent. Therefore, we sought to determine whether removal of high-mannose structures on gp120 under conditions that maintained its conformational integrity would affect its ability to bind DC-SIGN. Indeed, we found that EndoH treatment of recombinant gp120-Fc resulted in a significant decrease in DC-SIGN binding activity. EndoH removes the chitobiose core of high-mannose structures while leaving most hybrid or complex glycans intact (23). Figure 2A shows that EndoH treatment of gp120-Fc resulted in a much lower molecular weight species, indicating successful removal of high-mannose N-linked glycans. The same EndoH treated gp120-Fc was conformationally intact as shown by its ability to bind CD4 on SupT1 cells (Fig. 2B, left panel). However, EndoH-treated gp120-Fc was severely compromised in its ability to bind DC-SIGN (Fig. 2B, middle panel). Strikingly, EndoH treatment also abrogated gp120's ability to bind MDDCs, indicating that carbohydrate-lectin interactions were responsible for the CD4-independent binding of gp120 to MDDCs (Fig. 2B, right panel). We also show that the residual amount of DC-SIGN binding shown by EndoH-treated gp120-Fc was effectively competed off with mannan but with an 50% effective concentration (EC50) 2 orders of magnitude lower than that for the parental fully glycosylated gp120-Fc (Fig. 2C). Finally, we show that our EndoH treatment only removed high-mannose glycans from gp120, while leaving the complex glycans intact. Figure 2D shows a lectin-binding ELISA assay, indicating that EndoH treated gp120 abrogated its binding to G. nivalis (15, 42), a lectin specific for terminal α(1,3) mannose residues found only in high-mannose glycans, while not affecting its recognition by D. stramonium, a lectin highly specific for β(1,4)-linked GlcNac oligomers (a moiety found only in a subset of complex and hybrid glycans) (47). This underscores the data presented in Fig. 2B, which indicate that CD4-independent binding of gp120 to DC-SIGN and primary DCs is wholly dependent on high-mannose sugars and not on complex carbohydrates also found on gp120. To our knowledge, this salient fact has never been previously reported.
FIG. 2.
gp120 binding to DC-SIGN and MDDCs is carbohydrate dependent. (A) gp120-Fc was treated overnight with 20,000 U of EndoH/ml. An aliquot of the treated gp120-Fc supernatant and the orginal untreated supernatant was subjected to SDS-8% PAGE and Western blotted with polyclonal rabbit anti-gp120 antibodies. (B) EndoH-treated gp120-Fc or untreated gp120-Fc was tested for CD4 binding on SupT1 cells (left panel), DC-SIGN binding on BC7-DC-SIGN+ cells (middle panel), and DC binding on MDDCs in the presence of 10 μg of Leu3A (right panel)/ml. The unfilled histogram in the left panel is the human IgG-negative control. Equivalent amounts of gp120-Fc supernatant was used throughout. EndoH treatment of gp120-Fc decreased MDDC binding by >95%. The percentage of MDDCs positive for gp120-Fc binding is indicated. The region demarcated R1 is based on human IgG staining of MDDCs (<1%). (C) Mannan competition of EndoH-treated gp120-Fc versus untreated gp120-Fc binding to BC7-DC-SIGN+ cells. The mean fluorescence intensity (MFI) of gp120-Fc binding in the presence of increasing amounts of mannan was normalized to the MFI of gp120-Fc binding in the absence of mannan (set at 100%). Nonlinear regression was performed with the Graphpad Prism 3 program, which also calculated the EC50 values. All experiments in this figure were repeated at least twice with similar results. (D) Lectin-binding assay showing specific removal of only high-mannose glycans from gp120. G. nivalis recognizes terminal α(1,3) mannose residues found only in high-mannose glycans, while D. stramonium, specifically binds β(1,4) linked GlcNAc oligomers (a moiety found only in a subset of complex and hybrid glycans). Note that EndoH treatment of gp120 had no effect on binding by D. stramonium, whereas PNGase F treatment abolished recognition by both lectins. gp120-Fc was denatured prior to PNGase F treatment. See Materials and Methods for the experimental details.
Multiple N-linked glycosylation sites in gp120 contribute to DC-SIGN binding.
We next attempted to determine which of the N-linked glycosylation sites in gp120 give rise to the glycan structures responsible for DC-SIGN binding. About half of the glycosylation sites in gp120 give rise to high-mannose residues with the other half giving rise to complex or hybrid glycans (48). We therefore individually mutated N-linked sites previously determined to give rise to high mannose sugars, and determined their ability to bind DC-SIGN (Table 1.) None of the single N-linked glycosylation mutants affected the ability of gp120 to bind DC-SIGN (Fig. 3) or its homolog DC-SIGNR/L-SIGN. This result was not unexpected as high-affinity DC-SIGN binding is thought to be oligomeric (25) and multiple high-mannose structures that fulfill some spatial criteria are likely responsible for DC-SIGN binding (25). gp120-Fc and the indicated glycosylation mutants are produced at similar levels and dimerize appropriately as shown by Western blot of the proteins under nonreducing and reducing conditions (Fig. 3B).
TABLE 1.
N-linked glycosylation sites previously shown to give rise to high-mannose structures in SF2 gp120a
| JRCSF (no. of sites) | No. of sites or structures
|
Mutation
|
||
|---|---|---|---|---|
| SF2 | HXB2 (crystal) | Asn→Ala | Asn→Gln | |
| 195 | 170 | 197 | + | − |
| 228 | 203 | 230 | + | + |
| 239 | 214 | 241 | + | + |
| 260 | 235 | 262 | + | + |
| 293 | 268 | 295 | − | + |
| 299 | 274 | 301 | + | + |
| 328 | 304 | 332 | + | − |
| 336 | 311 | 339 | + | (+)b |
| 382 | 358 | 386 | + | + |
| 388 | 364 | 392 | + | + |
| 393 | 368 | 397 | − | + |
Homologous sites in JRCSF and the HXBc2 crystal structures are indicated. N-linked sites involved in 2G12's epitope are indicated in boldface in the shaded rows. N-linked sites were mutated by changing the Asn residue to either Ala or Glu as indicated. The numbering of residues in SF2 is based on a study by Zhu et al. (48). Residue 1 is the first amino acid after the signal peptide cleavage. The numbering of residues JRCSF and HXBc2 is based on the initiator methionine residue in the signal peptide sequence.
Mutation severely compromised protein production.
FIG. 3.
More than one N-linked glycosylation site in gp120 contributes to DC-SIGN binding. (A) A representative sample of the mutations indicated in Table 1 is shown. Depicted are first-generation Asn-to-Ala mutants. The conformational integrity of these mutants was checked by binding to CD4 on SupT1 cells. Binding was specific as it could be competed away with 10 μg of a neutralizing anti-CD4 antibody (Leu3A)/ml (data not shown). These N-linked mutants bound equally well to DC-SIGN and its related homolog DC-SIGNR/L-SIGN, although the N260A and N239A mutant was compromised in its ability to bind CD4. Asn-to-Glu mutants were subsequently made, and all of them bound to DC-SIGN as well as wild-type gp120-Fc (data not shown and Fig. 6). (B) gp120-Fc (wild type [wt]), and its single glycosylation mutants were produced at equivalent levels and were secreted as native dimers. gp120-Fc and its mutants were produced as described in Materials and Methods. Then, 50 μl of neat supernatant was loaded onto an SDS-8% PAGE gel by using 6× sample buffer with (reducing) or without β-mecaptoethanol (nonreducing). When the nonreducing sample buffer was used, samples were not boiled for 5 min before loading onto the SDS-PAGE gel. gp120-Fc was detected in the Western blot by using rabbit polyclonal anti-Env antibodies. Note that gp120-Fc runs as a more diffuse band under nondenaturing conditions. However, the loss of a single glycosylation site was not manifestly evident in the migration rate of a protein the size of gp120-Fc (∼150 kDa).
2G12 and cyanovirin do not block gp120 binding to DC-SIGN.
In order to guide further mutational analysis, we sought to determine whether 2G12 could block gp120-DC-SIGN binding. 2G12 is a neutralizing human MAb whose epitope involves at least five N-linked glycosylation sites known to give rise to high-mannose residues (Fig. 4) (37). 2G12 was not able to block gp120-Fc binding to DC-SIGN even up to a concentration of 100 μg/ml (Fig. 5A), although it was clearly active in blocking infection via R5 (JRFL), X4 (NL4-3), and R5X4 (89.6) viruses (data not shown). These results were substantiated by using cyanovirin, a cyanobacterial polypeptide that binds to high-mannose structures (3) and competes with 2G12 binding on gp120 (7, 9). Cyanovirin was also not able to inhibit gp120-Fc binding to DC-SIGN even at a concentration of 1 mM (Kd of cyanovirin for gp120 is 2 to 45 nM [26]) (Fig. 5A). On the contrary, increasing amounts of cyanovirin dramatically enhanced gp120-Fc binding to cells expressing DC-SIGN (Fig. 5A). This enhancement was also evident on DC-SIGN-negative cells and was tempered on tunicamycin-treated cells (data not shown and Fig. 5B). Since tunicamycin prevents N-linked glycosylation, these results suggest that cyanovirin may act as a bridge to attach gp120 to other mannosylated proteins on the cell surface. As binding of virion associated gp120 to DC-SIGN may have different spatial constraints compared to gp120-Fc binding, we confirmed the inability of 2G12 and cyanovirin to block DC-SIGN binding by whole virions. Figure 5C shows that neither 2G12 nor cyanovirin were able to block virus binding to DC-SIGN, although virus-DC-SIGN binding was clearly inhibited by mannan and calcium chelation (EGTA).
FIG. 4.
Model of gp120 N-linked glycosylation sites that give rise to high-mannose sugars. A ribbon diagram of the crystal structure of gp120 (gray) complexed with soluble CD4 (D1D2 domain) (red) is shown (19). Homologous N-linked glycosylation sites (Asn residues) in SF2 and JRCSF giving rise to high-mannose structures (Table 1) were projected onto the HxBc2 crystal structure (blue space-filling residues). A subset of these contributes to the 2G12 epitope and is shown as yellow space-filling residues.
FIG. 5.
2G12 and cyanovirin do not block gp120 binding to DC-SIGN. (A) Either 2G12 or cyanovirin (CVN) was preincubated with gp120-Fc at the indicated final concentrations before the gp120-Fc was placed onto BC7-DC-SIGN+ cells. The MFI obtained by gp120-Fc binding in the presence of 2G12 or cyanovirin was normalized to the MFI obtained in the absence of these agents. In all cases, the background was substracted by using the MFI obtained from human IgG staining alone. 2G12 and cyanovirin inhibition was repeated four and three times, respectively. The results are shown as means (± the standard deviation). (B) gp120-Fc binding (with or without cyanovirin) was performed on untreated BC7-DC-SIGN+ cells or on cells treated overnight with 10 μg of tunicamycin/ml. Note that cyanovirin enhanced gp120-Fc binding was reduced after tunicamycin treatment. (C) DC-SIGN-transfected 293T cells were pulsed with 5 ng of the indicated virus in the presence or absence of cyanovirin (0.2 to 20 μg/ml), 2G12 (2 to 200 μg/ml), mannan (500 μg/ml), or EGTA (10 mM). The amount of virus bound was determined by a p24 ELISA and normalized to the amount of p24 bound in the absence of any reagent (UnRx). Virus-binding experiments were performed twice in quadriplicates and once in duplicates with similar results.
2G12 and DC-SIGN binding sites are nonoverlapping.
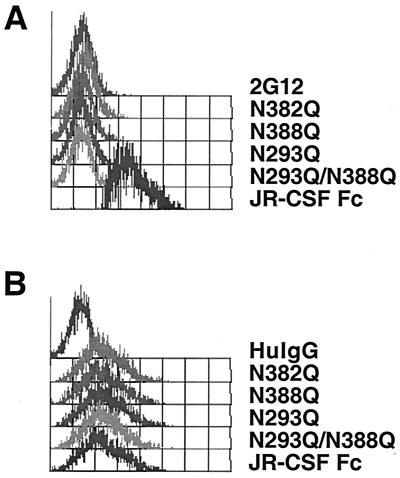
Since 2G12 did not compete for DC-SIGN binding to gp120, it is likely that the N-linked glycosylation sites required for DC-SIGN binding do not involve those in 2G12's epitope. To formally demonstrate that 2G12 and DC-SIGN binding sites on gp120 are nonoverlapping, we developed an assay to show that 2G12 can still bind to gp120-Fc prebound to DC-SIGN expressing cells (Fig. 6A). 2G12 binding to gp120-Fc prebound to cell surface DC-SIGN was detected by a PE-conjugated MAb against the kappa light chain of 2G12 (17). 2G12 itself did not bind to DC-SIGN+ cells in the absence of gp120-Fc (Fig. 6A) and human IgG itself did not show any specific binding to gp120-Fc (Fig. 1A). Since gp120-Fc does not contain the kappa light chain, the positive FACS staining can only be explained by 2G12 binding to gp120-Fc already bound to DC-SIGN. Note that single and double glycosylation mutants deficient in 2G12's epitope did not result in any positive staining when using this tertiary anti-kappa/2G12 binding assay (Fig. 6A), further confirming the specificity of this assay. However, these glycosylation site mutants defective in 2G12 binding still bound to cell surface DC-SIGN (Fig. 6B) at levels comparable to wild-type gp120-Fc (Fig. 6B). We also confirmed that these mutants were no longer able to bind 2G12 via a direct ELISA-based 2G12/gp120 binding assay (see Materials and Methods [data not shown]). In toto, these results suggest that DC-SIGN and 2G12 binding sites on gp120 were nonoverlapping.
FIG. 6.
2G12 and DC-SIGN binding sites on gp120 are nonoverlapping. (A) 2G12 can bind to gp120-Fc prebound to DC-SIGN. gp120-Fc was used to stain BC7-DC-SIGN+ cells. After a washing step, 2G12 was added for 20 min, followed by another washing, and a PE-conjugated monoclonal anti-kappa antibody was used to detect 2G12 binding. Note that 2G12 alone in the absence of gp120-Fc did not show any nonspecific binding. In addition, no positive staining was detected by using gp120-Fc mutants lacking 2G12's epitope. (B) Wild-type gp120-Fc and representative gp120-Fc mutants lacking 2G12's epitope were used to stain BC7-DC-SIGN+ cells. PE-conjugated goat anti-human Fc antibodies were used to detect gp120-Fc binding to DC-SIGN. There was no significant difference in DC-SIGN binding between these glycosylation mutants and wild-type gp120-Fc.
DISCUSSION
The ability of DC-SIGN to facilitate HIV infection in trans and its expression on submucosal DCs have led to the suggestion that it may serve as a conduit for the transfer of HIV from the mucosa to secondary lymphoid organs (12, 35). Although DC-SIGN on MDDCs is clearly involved in the binding and transfer of HIV to permissive cells (12, 18), there is evidence that other lectins are involved as neutralizing anti-DC-SIGN antibodies cannot fully abrogate the binding and transmission of viruses on MDDCs (43, 44). In addition, at least one other trypsin-resistant lectin on DCs has been reported to bind gp120 (38). However, it has not been formally demonstrated that specific glycan structures on gp120 itself are responsible for binding to DC-SIGN or MDDCs.
Given that gp120 has been reported recently to bind DC-SIGN in a carbohydrate-independent manner, we first sought to resolve the issue of whether gp120 binding to DC-SIGN is carbohydrate dependent. Thus, we showed that removal of high mannose sugars on gp120 under conditions that maintained wild-type CD4 binding (as a measure of conformational integrity) severely compromised gp120's ability to bind DC-SIGN. Interestingly, high-mannose deficient gp120 was also unable to bind to MDDCs, indicating that these carbohydrate structures are wholly responsible for gp120 binding to DCs. Importantly, we were also able to show via differential lectin binding (Fig. 2D) that gp120 containing the full complement of complex glycans (but not high-mannose glycans) was not able to bind MDDCs or DC-SIGN. However, we were unable to prove the same for whole virions. This is not unexpected since Desrosiers and coworkers have shown that the cognate sugars on virion-associated Envs are somewhat refractory to EndoH digestion (24). Nevertheless, to our knowledge, this is the first formal demonstration that high-mannose sugars on gp120 were wholly responsible not only for DC-SIGN binding but also for binding to primary monocyte-derived DCs.
The impetus for the current studies is our desire to delineate the N-linked glycosylation sites involved in gp120 or virion binding to DC-SIGN and DCs. We believe this information to be useful for designing immunogens aimed at eliciting antibodies that might block DC-SIGN-gp120 or even DC-gp120 interactions. We have adopted the rationale that targeted deglycosylation of certain N-linked sites in gp120 can result in an immunogen that elicits high titers of broadly neutralizing antibodies in the SIV model (29). These antibodies target the polypeptide backbone underlying the removed glycan structures (29) and are active against the parental, fully glycosylated Env. This supports the hypothesis that glycosylation patterns on primate lentiviral envelopes have evolved to “mask” conserved regions in the polypeptide core (45, 46). Thus, we sought to define the glycan structures that contribute to DC-SIGN as well as DC binding as Envs missing these cognate glycan structures might “unmask” the underlying polypeptide backbone. As in the case of the deglycosylated SIV Envs, antibodies generated to Envs deficient in DC-SIGN binding might then access the parental Env in a manner which hinder DC-SIGN binding.
Despite the fact that high-mannose sugars on gp120 were required for DC-SIGN binding, we showed that no single high-mannose N-linked glycosylation site on gp120 was responsible for its DC-SIGN binding activity. This is likely, since lectins have evolved to achieve high-affinity binding through clustering of its binding sites in oligomeric structures (40), and DC-SIGN has been reported to exist as a tetramer via oligomerization of its repeat neck region (25). The spatial arrangement of each carbohydrate recognition domain (CRD) in the context of the oligomer adds another layer of specificity to a lectins' ligand-binding profile. For example, human mannose-binding protein (MBP) exists as trimers with the carbohydrate-binding site in each lectin domain spaced 45 Å apart in the trimer (33). This is beyond the upper limit of the spacing (20 to 30 Å) found between most vertebrate terminal mannose residues (21, 22). However, terminal mannose residues found in bacterial and fungal surfaces are present in highly repetitive arrays that can span the distance between the CRDs in the oligomeric MBP (41). Thus, it is likely that the spatial arrangement of the CRDs in combination with its carbohydrate specificity determines the particular ligand-binding profile of DC-SIGN. There is probably a limited set of N-linked glycosylation sites in gp120 that give rise to the terminal high-mannose structures that match the spatial constraints required for oligomeric DC-SIGN-gp120 binding. However, the precise stochiometry of DC-SIGN-gp120 is currently unknown and is being actively investigated, although we do have evidence that DC-SIGN on DCs oligomerize as tetramers (unpublished observations).
To facilitate a more rational mutational analysis of N-linked glycosylation sites in gp120 involved in DC-SIGN binding, we used the 2G12 glycosylation-dependent epitope to guide our efforts. We showed that 2G12 had no effect on gp120-DC-SIGN interactions, and further demonstrated that 2G12 bound to gp120 prebound to DC-SIGN. Therefore, it is likely that the DC-SIGN binding site on gp120 involves N-linked glycosylation sites removed from 2G12's epitope (Fig. 4). Indeed, some multiple glycosylation site mutants that do not involve 2G12's epitope have significantly decreased DC-SIGN binding activity (unpublished observations). The immunogenicity of these Env mutants is currently being investigated.
Our 2G12 results were substantiated by the inability of cyanovirin to block DC-SIGN-gp120 interactions. Although cyanovirin can compete for 2G12 binding to gp120, the converse is not true (9). This suggests that cyanovirin can bind to a larger set of high-mannose structures on gp120. Since cyanovirin cannot block DC-SIGN-gp120 interactions, we postulate that the spatial requirements for binding high-mannose structures differ between cyanovirin (which has two high-mannose binding sites) and DC-SIGN. However, we also note that cyanovirin binds preferentially to oligomannose-8 (Man-8) and oligomannose-9 (Man-9) glycan structures (32), whereas DC-SIGN appears to bind equally well to glycan structures with Man-5 to Man-9 moieties (10, 25). Since glycosylation on gp120 is heterogeneous, it is possible that DC-SIGN can bind to the subset of gp120 molecules not already bound by cyanovirin (e.g., Man-5 to Man-7 structures). Thus, it is not necessarily true that different N-linked glycosylation sites per se give rise to the high-mannose structures recognized by these two molecules. Our present study completes the cross-competition profile between the three high-mannose binding agents of gp120 (Table 2, originally summarized by Sanders et al. [30]). Although we believe that 2G12 and DC-SIGN occupy truly distinct and nonoverlapping sites on gp120, the same cannot be said of cyanovirin and DC-SIGN, as the negative competition data may be explained by the greater flexibility of these two ligands for the high-mannose structures on gp120.
TABLE 2.
Competition between reagents that bind mannose residues on gp120a
| Ligand | Competition with inhibitorb
|
||
|---|---|---|---|
| 2G12 | DC-SIGN | CV-N | |
| 2G12 | X | NOc | YESd |
| DC-SIGN | NOe | X | NOf |
| CVN | NOg | (NO)h | X |
This table is reproduced from Sanders et al. (30) with additional data filled in. CVN, cyanovirin.
X, autologous cross-competition; YES, heterologous cross-competition occurs; NO, no competition; ?, no information available.
Derived from reference 7.
Indirect evidence from cyanovirin-mediated enhancement of gp120 binding to DC-SIGN, formerly listed as “?” in reference 30.
We note that high concentrations of cyanovirin markedly enhanced gp120 binding to cell surfaces, probably via a bridging effect with other mannosylated cell surface proteins as cyanovirin is known to have two distinct carbohydrate (mannose) binding sites. This hypothesis was supported by a reduction in the enhancement effect seen when cells were pretreated with tunicamycin, an agent which prevents global N-linked glycosylation. This cross-linking-mediated enhancement of viral attachment is not entirely novel since high concentrations of RANTES have also been shown to enhance HIV attachment to cells via cross-linking of the virion to cell surface glycosoaminoglycans (14, 36). However, we observed no such enhancement when virion binding experiments were performed. This suggests that the glycan structures available for cyanovirin binding may differ between monomeric gp120 and oligomeric gp120 on virions (2). Thus, our future efforts to discern lectin binding sites on gp120 would have to be confirmed in the context of virion associated Env.
In summary, we showed that gp120 binding to DC-SIGN and monocyte-derived DCs was wholly dependent on high-mannose structures. We showed that more than one N-linked glycosylation site in gp120 gave rise to the high-mannose structures involved in DC-SIGN binding. We believe that continuing efforts to identify the glycan structures on gp120 responsible for DC-SIGN binding can concentrate on sites not involved in 2G12's epitope.
These results have immediate implications for ongoing efforts to derive immunogens that can elicit antibodies that thwart gp120-DC interactions.
Acknowledgments
We thank members of the B. Lee lab for technical assistance and review of the manuscript. We thank John Moore for input and for suggesting that we complete the information for Table 2. We also thank Dennis Burton and Paul Parren for the gifts of cyanovirin.
K.B.F. is supported by a training grant from the Department of Education. B.L. is a Charles E. Culpepper Medical Scholar supported by the Rockefeller Brothers Fund and a recipient of the Burroughs-Wellcome Fund Career Development Award and is supported by NIH grants RO1-AI52021 and KO8-HL03923. This research was also supported in part by grant R01AI25825 (J.H.E.) from NIAID. We also acknowledge the support of the UCLA AIDS Institute and the flow cytometry core (UCLA CFAR grant and NIH grant AI-28697) and the Pallotta Teamworks AIDS Vaccine Rides.
REFERENCES
- 1.Baribaud, F., S. Pohlmann, T. Sparwasser, M. T. Kimata, Y. K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-sign. J. Virol. 75:10281-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bewley, C. A. 2001. Solution structure of a cyanovirin-N:Man α1-2Manα complex: structural basis for high-affinity carbohydrate-mediated binding to gp120. Structure 9:931-940. [DOI] [PubMed] [Google Scholar]
- 3.Bolmstedt, A. J., B. R. O'Keefe, S. R. Shenoy, J. B. McMahon, and M. R. Boyd. 2001. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol. Pharmacol 59:949-954. [DOI] [PubMed] [Google Scholar]
- 4.Choe, H., K. A. Martin, M. Farzan, J. Sodroski, N. P. Gerard, and C. Gerard. 1998. Structural interactions between chemokine receptors, gp120 Env and CD4. Semin. Immunol. 10:249-257. [DOI] [PubMed] [Google Scholar]
- 5.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Parseval, A., and J. H. Elder. 2001. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J. Virol. 75:4528-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey, B., D. L. Lerner, P. Lusso, M. R. Boyd, J. H. Elder, and E. A. Berger. 2000. Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J. Virol. 74:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 9.Esser, M. T., T. Mori, I. Mondor, Q. J. Sattentau, B. Dey, E. A. Berger, M. R. Boyd, and J. D. Lifson. 1999. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J. Virol. 73:4360-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 11.Figdor, C. G., Y. van Kooyk, and G. J. Adema. 2002. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2:77-84. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 13.Geijtenbeek, T. B., G. C. van Duijnhoven, S. J. van Vliet, E. Krieger, G. Vriend, C. G. Figdor, and Y. van Kooyk. 2002. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 277:11314-11320. [DOI] [PubMed] [Google Scholar]
- 14.Gordon, C. J., M. A. Muesing, A. E. Proudfoot, C. A. Power, J. P. Moore, and A. Trkola. 1999. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 73:684-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hester, G., H. Kaku, I. J. Goldstein, and C. S. Wright. 1995. Structure of mannose-specific snowdrop (Galanthus nivalis) lectin is representative of a new plant lectin family. Nat. Struct. Biol. 2:472-479. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman, T. L., and R. W. Doms. 1999. HIV-1 envelope determinants for cell tropism and chemokine receptor use. Mol. Membr. Biol. 16:57-65. [DOI] [PubMed] [Google Scholar]
- 17.Kunert, R., F. Ruker, and H. Katinger. 1998. Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res. Hum. Retrovir. 14:1115-1128. [DOI] [PubMed] [Google Scholar]
- 18.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T-cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 19.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, R. T., Y. Ichikawa, T. Kawasaki, K. Drickamer, and Y. C. Lee. 1992. Multivalent ligand binding by serum mannose-binding protein. Arch. Biochem. Biophys. 299:129-136. [DOI] [PubMed] [Google Scholar]
- 22.Lee, Y. C., R. T. Lee, K. Rice, Y. Ichikawa, and T.-C. Wong. 1991. Topography of binding sites of animal lectins: ligands' view. Pure Appl. Chem. 63:499-506. [Google Scholar]
- 23.Maley, F., R. B. Trimble, A. L. Tarentino, and T. H. Plummer, Jr. 1989. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal. Biochem. 180:195-204. [DOI] [PubMed] [Google Scholar]
- 24.Means, R. E., and R. C. Desrosiers. 2000. Resistance of native, oligomeric envelope on simian immunodeficiency virus to digestion by glycosidases. J. Virol. 74:11181-11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell, D. A., A. J. Fadden, and K. Drickamer. 2001. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR: subunit organization and binding to multivalent ligands. J. Biol. Chem. 276:28939-28945. [DOI] [PubMed] [Google Scholar]
- 26.O'Keefe, B. R., S. R. Shenoy, D. Xie, W. Zhang, J. M. Muschik, M. J. Currens, I. Chaiken, and M. R. Boyd. 2000. Analysis of the interaction between the HIV-inactivating protein cyanovirin-N and soluble forms of the envelope glycoproteins gp120 and gp41. Mol. Pharmacol. 58:982-992. [DOI] [PubMed] [Google Scholar]
- 27.Pohlmann, S., F. Baribaud, and R. W. Doms. 2001. DC-SIGN and DC-SIGNR: helping hands for HIV. Trends Immunol. 22:643-646. [DOI] [PubMed] [Google Scholar]
- 28.Pohlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Munch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 30.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharron, M., S. Pohlmann, K. Price, E. Lolis, M. Tsang, F. Kirchhoff, R. W. Doms, and B. Lee. 2000. Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood 96:41-49. [PubMed] [Google Scholar]
- 32.Shenoy, S. R., B. R. O'Keefe, A. J. Bolmstedt, L. K. Cartner, and M. R. Boyd. 2001. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J. Pharmacol. Exp. Ther. 297:704-710. [PubMed] [Google Scholar]
- 33.Sheriff, S., C. Y. Chang, and R. A. Ezekowitz. 1994. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple alpha-helical coiled-coil. Nat. Struct. Biol. 1:789-794. [DOI] [PubMed] [Google Scholar]
- 34.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, Q. Luo, E. Levroney, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71:445-457. [PubMed]
- 35.Steinman, R. M. 2000. DC-SIGN: a guide to some mysteries of dendritic cells. Cell 100:491-494. [DOI] [PubMed] [Google Scholar]
- 36.Trkola, A., C. Gordon, J. Matthews, E. Maxwell, T. Ketas, L. Czaplewski, A. E. Proudfoot, and J. P. Moore. 1999. The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J. Virol. 73:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turville, S. G., J. Arthos, K. M. Donald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 39.Ugolini, S., I. Mondor, and Q. J. Sattentau. 1999. HIV-1 attachment: another look. Trends Microbiol. 7:144-149. [DOI] [PubMed] [Google Scholar]
- 40.Weis, W. I., and K. Drickamer. 1996. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 65:441-473. [DOI] [PubMed] [Google Scholar]
- 41.Weis, W. I., M. E. Taylor, and K. Drickamer. 1998. The C-type lectin superfamily in the immune system. Immunol. Rev. 163:19-34. [DOI] [PubMed] [Google Scholar]
- 42.Wright, C. S., and G. Hester. 1996. The 2.0 Å structure of a cross-linked complex between snowdrop lectin and a branched mannopentaose: evidence for two unique binding modes. Structure 4:1339-1352. [DOI] [PubMed] [Google Scholar]
- 43.Wu, L., A. A. Bashirova, T. D. Martin, L. Villamide, E. Mehlhop, A. O. Chertov, D. Unutmaz, M. Pope, M. Carrington, and V. N. KewalRamani. 2002. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 99:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 46.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita, K., K. Totani, T. Ohkura, S. Takasaki, I. J. Goldstein, and A. Kobata. 1987. Carbohydrate binding properties of complex-type oligosaccharides on immobilized Datura stramonium lectin. J. Biol. Chem. 262:1602-1607. [PubMed] [Google Scholar]
- 48.Zhu, X., C. Borchers, R. J. Bienstock, and K. B. Tomer. 2000. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry 39:11194-11204. [DOI] [PubMed] [Google Scholar]