Abstract
Regulated exocytosis of neurotransmitters at synapses is fast and tightly regulated. It is unclear which proteins constitute the “minimal molecular machinery” for this process. Here, we show that a novel technique of capacitance monitoring combined with heterologous protein expression can be used to reconstitute exocytosis that is fast (<0.5 s) and triggered directly by membrane depolarization in Xenopus oocytes. Testing synaptic proteins, voltage-gated Ca2+ channels, and using botulinum and tetanus neurotoxins established that the expression of a Ca2+ channel together with syntaxin 1A, SNAP-25, and synaptotagmin was sufficient and necessary for the reconstitution of depolarization-induced exocytosis. Similar to synaptic exocytosis, the reconstituted release was sensitive to neurotoxins, modulated by divalent cations (Ca2+, Ba2+, and Sr2+) or channel (Lc-, N-type), and depended nonlinearly on divalent cation concentration. Because of its improved speed, native trigger, and great experimental versatility, this reconstitution assay provides a novel, promising tool to study synaptic exocytosis.
INTRODUCTION
Regulated exocytosis is the Ca2+-dependent fusion of cytoplasmic vesicles with the plasma membrane. When coupled to membrane depolarization, this process can achieve extreme speed and efficiency (1).
Understanding neuronal exocytosis requires both the identification of the presumably common “minimal machinery of membrane fusion” (2,3) and the specific molecular additions that knead generic membrane fusion into the rapid, voltage-operated process that eventually afford synaptic transmission (4).
Reconstitution assays of exocytosis offer a powerful experimental approach to study the general and the specifically neuronal aspects of exocytosis (5–8). Various such reconstitution assays of regulated secretion were reported, but the exocytosis process had in all cases lost the characteristic physiological traits of neuronal exocytosis: Reconstituted fusion occurs over minutes or hours instead of a few milliseconds, and it is not triggered by membrane depolarization (5).
To date, direct evidence from reconstituted systems regarding the molecular machinery of neuronal exocytosis is thus missing. On the other hand, simple extrapolation from slow, Ca2+-regulated membrane fusion is discredited by the known complexity and diversity of exocytotic mechanisms as well as by persisting conflicts regarding the interpretation of the available data (2,9–11).
Several groups have used oocytes of the South African clawed frog Xenopus laevis in their attempts to reconstitute regulated exocytosis. For instance, Ca2+-dependent release of ATP could be reconstituted in Xenopus oocytes by injection of total mRNA isolated from the electric lobe of Torpedo marmorata (12) or from rat cerebellum (13), and by injection of membrane vesicles (14,15) or of chromaffin granules (16). These experiments provided proof that exocytosis can be reconstituted in Xenopus oocytes. On the other hand, these assays do not provide an experimental platform that would allow one to study the molecular requirements of synaptic exocytosis: i), the molecular identity of the proteins was unknown and could not be manipulated individually; ii), exocytosis was not triggered by membrane depolarization; and iii), the methods used (e.g., luminometric ATP measurements or lectin secretion assays) had an unfavorable time resolution.
Here, we report a new approach to the use of Xenopus oocytes as a versatile testbed for the reconstitution and functional characterization of fast, depolarization-induced exocytosis, and data regarding the molecular requirements of this reconstituted process. As compared to other reconstitution assays of regulated exocytosis, our approach had the following advantages i), the assay affords selective expression of known proteins whose abundance can be varied and manipulated easily; this versatility allows one to delineate the minimal set of proteins needed to accomplish evoked release; ii), exocytosis is triggered directly via membrane depolarization under voltage-clamped conditions; and iii), exocytosis is detected with great convenience, precision and time resolution by monitoring of membrane capacitance. Our findings constitute the first data on regulated exocytosis that were obtained in a reconstituted system in which exocytosis was directly triggered by depolarization.
MATERIALS AND METHODS
Plasmid cDNAs for full-length BoNT/A-light chain and TetX were generous gifts from H. Gaisano (Toronto Canada) and H. Niemann (Hannover, Germany) and GluR3 (L507Y) from Y. Stern-Bach (Jerusalem, Israel).
Heterologous protein expression in Xenopus oocytes
Xenopus oocytes were injected with a mixture of cRNAs encoding the Ca2+ channel subunits α11.2 (Lc-type; rabbit; 5 ng/oocyte), or α12.2 (N-type; rat; 10 ng/oocyte) together with α2/δ (rabbit; 5 ng/oocyte) and β2A (rat; 10 ng/oocyte); sodium channel Nav1.2 (3 ng/oocyte), or the glutamate receptor GluR3 (3 ng/oocyte), and one day later either with water (for controls) or with a mixture of cRNAs encoding SNAP-25 (0.5 ng/oocyte), syntaxin 1A (0.5 ng/oocyte) and synaptotagmin I (1.0 ng/oocyte). Botulinum toxins cRNAs; Bot-A Bot-C1, and TetX (2 ng/oocyte) were injected at day 3 after channel cRNA injection.
Oocyte preparation, in vitro transcription, and cDNA constructs were as reported previously (17). Depolarization-induced exocytosis was studied after further incubation for 4 or 5 days.
Capacitance monitoring in Xenopus oocytes
Membrane capacitance (Cm) was monitored in the two-electrode voltage-clamp configuration as published elsewhere (18). Briefly, Cm was determined from the current response to a triangular, symmetrical voltage command. The up- and down-ramps (±20 mV in 20 ms each) elicit membrane currents that are the sum of resistive and a capacitive current component. Switching from up- to down-ramp reverses the sign of the capacitive component but not that of the resistive component. Thus, subtraction of the down-ramp current integral from the up-ramp current integral eliminates the resistive component; the resulting pure capacitive charge allows one to compute—together with the known amplitude of the voltage stimulus—membrane capacitance. Continuous monitoring is achieved by applying this stimulus repetitively at a high rate (up to 10/s). Comprehensive tests in an electrical cell model as well as in Xenopus oocytes have demonstrated high precision, accuracy, and robustness of this technique (18). Starting from a holding potential of −80 mV, depolarizing stimuli were applied by clamping the cells to 0 mV for 2 × 500 ms, separated by 100 ms at −80 mV. Capacitance was monitored before and after the stimulus, together with membrane potential (Vm) and current (Im).
Biochemical assay of released ATP
Evoked secretion can be monitored after ATP release since vesicle content is rich in ATP (19). We used the highly sensitive luciferin-luciferase assay (ENLITTEN, Promega, Kibbutz Beit Haemek, Israel) to confirm depolarization-induced ATP release in oocytes. Oocytes were depolarized by incubation in 100 mM KCl for 60 s, and luminescence of the medium was determined directly in a luminometer before and after depolarization to lower basal secretion. Each assay contained 3 oocytes and the assay was repeated 5 times.
Fluorescence measurements
Oocytes were injected as described in the electrophysiology section with GFP-synaptotagmin cRNA (20). Six days after cRNA injection oocytes were fixed in OCT (Miles, Pittsburg, PA) and cut into 50–100 μM slices using a cryostat. Slices were mounted on optical slides, and GFP-synaptotagmin was visualized by a Bio-Rad (Hercules, CA) confocal microscope. Confocal images were generated using the Confocal Assistant program (Bio-Rad).
RESULTS
Expression of a limited set of proteins conveys depolarization-evoked exocytosis to Xenopus oocytes
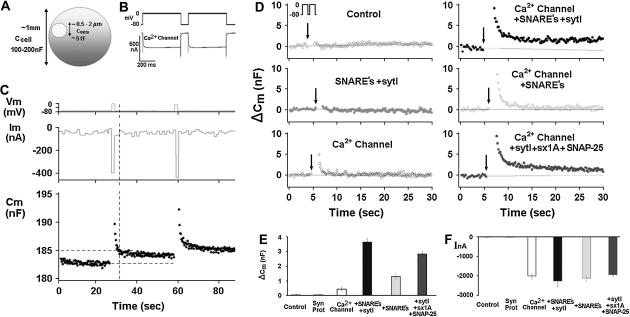
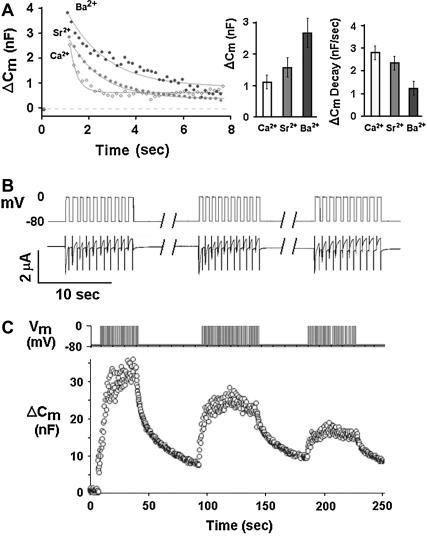
We first attempted to reconstitute depolarization-induced exocytosis in Xenopus oocytes (using membrane capacitance as a readout) by expressing SNARE proteins together with synaptotagmin 1A and a voltage-gated Ca2+ channel. We used Xenopus oocytes expressing the Lc-type channel and the various synaptic proteins as indicated (Fig. 1). Xenopus oocytes (Fig. 1 A) were stimulated from a holding potential of −80 mV to 0 mV by two consecutive 500-ms pulses 100 ms apart (Fig. 1 B). Continuous monitoring of membrane capacitance showed the effect of depolarization on membrane capacitance (Cm) in oocytes expressing tSNARE proteins, syntaxin 1A, SNAP-25, which are key proteins in exocytosis (21,22) and Lc-type channel (Fig. 1 C). Representative original traces of voltage (upper), current (middle), and Cm (lower) are shown (Fig. 1 C). The vertical dashed lines indicates the precise time at which Cm was read for comparison with the baseline Cm before considering depolarization values (Fig. 1 C). Uninjected oocytes did not exhibit depolarization-induced capacitance changes in response to a double 500 ms pulse (Fig. 1, D and E). Upon expression of the synaptic proteins syntaxin 1A, SNAP-25, synaptobrevin, and synaptotagmin I, without the Ca2+ channel, the depolarizing stimuli produced no increases of Cm. Similarly, small Cm changes could be elicited upon expression of a voltage-gated Ca2+ channel alone (subunits α11.2, α 2δ, and β2A) without expression of synaptic proteins (0.44 ± 0.1 nF) (Fig. 1, D and E). However, simultaneous expression of the Ca2+ channel, SNARE proteins and synaptotagmin resulted in much larger depolarization-induced step increases of Cm (ΔCm) (3.5 ± 0.3 nF) (Fig. 1, D and E). These Cm changes correspond to ∼1–2% of an oocyte's total membrane surface area, or to fusion of ∼105–106 cortical granules (assuming a diameter of 0.5–3 μm (23) with an individual capacitance of ∼5 fF; Fig. 1 A). In oocytes expressing Ca2+ channel plus the SNAREs but not synaptotagmin I, depolarization elicited smaller Cm increases (1.3 ± 0.2 nF). In oocytes expressing Ca2+ channel plus synaptotagmin I plus SNARE proteins except for synaptobrevin, depolarization elicited smaller Cm increases (2.8 ± 0.2 nF) than in oocytes expressing the same proteins plus synaptobrevin. Note that the Cm change was completed during the 1.1 s of the depolarizing stimulus. A summary of the average Cm change of oocytes (n = 13) is shown in Fig. 1 E. Amplitude of inward currents was only slightly affected by the presence of the various synaptic proteins (Fig. 1 F).
FIGURE 1.
Reconstitution of depolarization-induced exocytosis in oocytes by expression of exogenous proteins. (A) Diameter and capacitance of plasma membrane and cortical granules in Xenopus oocytes. (B) Effect of depolarization on membrane capacitance in a Xenopus oocyte expressing a voltage-gated Ca2+ channel. (Upper trace) Depolarizing voltage command (see Methods), consisting of depolarization from a holding potential of −80 mV to 0 mV for 2 × 500 ms, separated by 100 ms at −80 mV). (C) Continuous monitoring of membrane capacitance showing the effect of depolarization on membrane capacitance (Cm) in an oocyte expressing, Ca2+ channel, Lc-type Ca2+ channel subunits α11.2, β2A, α2δ; SNAREs: syntaxin 1A, SNAP-25, and synaptotagmin; representative original traces of voltage (upper) current (middle) and Cm (lower). The vertical dashed line indicates the time at which Cm was read for comparison with the baseline Cm before depolarization. (D) Monitoring Cm in oocytes expressing different proteins. Control, uninjected oocytes; other panels, oocytes expressing heterologously synaptic proteins: synaptic proteins, syntaxin 1A, SNAP-25, synaptobrevin, and synaptotagmin, Ca2+ channel, Lc-type Ca2+ channel subunits α11.2, β2A, α2δ; SNAREs: syntaxin 1A, SNAP-25 and synaptobrevin; synaptotagmin I, sytI; Ca2+ channel plus syntaxin 1A, SNAP-25 and syt I. (E) Summary: effect of depolarization on Cm. Groups as in D. ΔCm, depolarization-induced change of membrane capacitance; bars show mean ± SD (n = 13). (F) Effect of depolarization on membrane current. Depolarization-induced membrane current (InA; mean ± SD, n = 13 each). Data show that the Cm changes (C) cannot be explained by membrane current, but rather reflect bona fide exocytosis.
A fast transient component of the depolarization-induced capacitance change was observed in oocytes expressing the Ca2+ channel alone (Fig. 1 C) or in combination with synaptic proteins (panels in the right columns of Fig. 1 D), in addition to a capacitance increase that was stationary or decaying only very slowly (panels in the right columns of Fig. 1 D). The nature of the fast transient is unclear. It is not correlated with the depolarization-induced current changes, which decay considerable faster. Whether this capacitance transient reflects genuine exocytosis is unclear (24). To avoid any confounding effects of current changes on the Cm measurements, and not knowing the nature of the fast capacitance transient (24), we determined the Cm change just after the current had returned to its previous level (see vertical dashed line in Fig. 1 C). Assuming that the permanent Cm changes reflect genuine exocytosis, this approach results in a measure of exocytosis that is specific, but potentially underestimates the extent of exocytosis.
That the observed Cm changes indeed reflect exocytosis was supported by two further observations. Firstly, the amplitude of depolarization-induced current associated with the expression of the voltage-gated Ca2+ channel was not significantly affected by coexpression of other synaptic proteins (Fig. 1 F). This observation rules out depolarization-induced mobilization of gating charges on the voltage-dependent Ca2+ channel as a reason for the greater Cm increases observed upon additional expression of synaptic proteins. Moreover, the lack of effect of the synaptic proteins on the Ca2+ currents excludes improved membrane targeting of the Ca2+ channel as an explanation of the enhanced exocytosis.
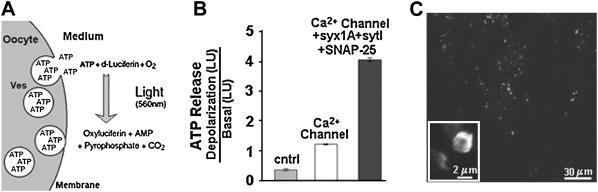
Secondly, similar results were obtained with ATP release as a second, independent approach to detect exocytosis (Fig. 2, A and B). ATP release from oocytes expressing synaptotagmin plus SNAP-25, syntaxin 1A, and Ca2+ channel was >4-fold higher than in oocytes expressing the Ca2+ channel alone, and >10-fold higher than in uninjected oocytes.
FIGURE 2.
Effect of heterologous proteins on depolarization-induced ATP release. (A) A schematic presentation of ATP luminescence assay. (B) Oocytes were depolarized by incubation in high K+-solution, and release of ATP was determined via a luminescence assay (see Methods). ATP release paralleled the Cm changes (cf. Fig. 1, A and B), confirming that Cm changes indeed reflect fusion of cytoplasmic vesicles with the plasma membrane. (C) Heterologously expressed synaptotagmin is localized in cortical granules. Confocal image of oocytes injected with cRNA for a GFP-synaptotagmin 1-fusion protein.
Correct targeting of the heterolgously expressed synaptotagmin, on the other hand, was confirmed by experiments in which we expressed a GFP-synaptotagmin fusion protein in Xenopus oocytes (20). Labeling was localized to vesicles of 0.5–5 μm diameter (Fig. 2) the typical size of cortical granules (23).
Together, these experiments show that expression of synaptotagmin and the Ca2+ channel along with SNAREs conveys depolarization-induced exocytosis to Xenopus oocytes, and this type of exocytosis is fast, robust, and can easily be studied quantitatively. The effect is readily distinguishable against a virtually zero background in native oocytes. Note, however, the small Cm increase in oocytes expressing the Ca2+ channel without the synaptic proteins; this effect could reflect the presence of endogenous homologs to the synaptic proteins that, together with the Ca2+ channel, can mediate depolarization-induced exocytosis of limited magnitude. The contribution of these unknown native proteins is probably rather restricted given their lower level of expression relative to the heterologously expressed proteins. As shown previously, the endogenous level of voltage gated Ca2+ channels is rather low (>100-fold) compared to secreting cells (25).
Role of individual synaptic proteins
We exploited the ability of our assay to establish the contribution of individual proteins to depolarization-induced exocytosis (Fig. 3). In principle, this could be done also with previously available reconstitution assays. Our assay however, has the considerable advantage, that it is sensitive specifically to effects on fast exocytosis, and screens out—by design—exocytotic processes with slower time courses and thus probably different molecular underpinnings than the forms of exocytosis that mediate synaptic transmission.
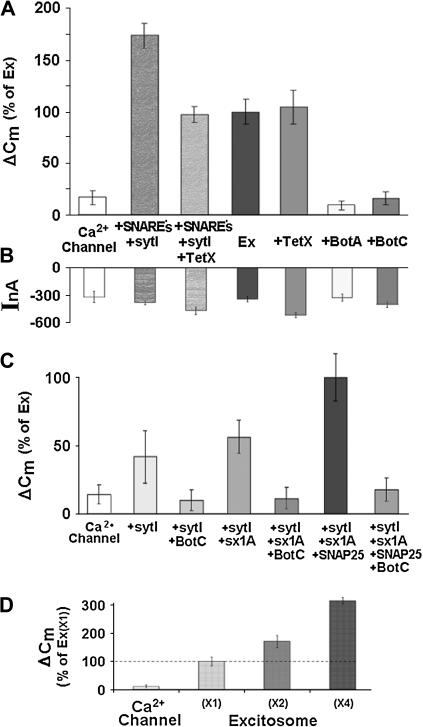
FIGURE 3.
Dissecting out the role of synaptic proteins in reconstituted depolarization-induced exocytosis. (A) Effects of botulinum toxins (BotC and BotA) and tetanus toxin (TetX) on depolarization-induced capacitance changes. Lc-type Ca2+ channel (Cav1.2); SNAREs (syntaxin 1A, synaptobrevin, and SNAP-25) and synaptotagmin I; Ex, Ca2+ channel + syntaxin 1A + SNAP-25, were expressed at a level corresponding to “×1” in Fig. 3 D. Data show that SNAP-25 and syntaxin 1A are absolutely required for depolarization-induced exocytosis. In contrast, synaptobrevin is not required, although it increases exocytosis efficiency. (B) Effects of botulinum toxins and tetanus toxin on depolarization-induced currents. Current amplitudes and Cm changes (A) are not correlated. (C) Stepwise molecular reconstitution of depolarization-induced exocytosis. Synaptic proteins as above, and Lc-type Ca2+ channel (Cav1.2). Data show that syntaxin 1A and SNAP-25 are absolutely required for depolarization-induced exocytosis. The changes in Cm in the presence of the toxins are similar to the step Cm expressed by the channel alone. Data suggest that the synaptic proteins comprised by the excitosome are necessary and sufficient to specifically reconstitute depolarization-induced exocytosis against a very small background in Xenopus oocytes. (D) Quantitative correlation between reconstituted depolarization-induced exocytosis and expression of excitosome proteins. Coexpression of graded amounts of the synaptic proteins syntaxin 1A, synaptotagmin I, and SNAP-25 together with a fixed amount of Lc-type voltage-gated Ca2+-channel subunits that comprise the excitosome (see Methods).
We used a panel of neurotoxins to address the individual contribution of the v-SNARE synaptobrevin and of the tSNAREs syntaxin 1A and SNAP-25, to reconstituted depolarization-induced exocytosis (Fig. 3, A and B).
When we coexpressed synaptobrevin together with the SNARE proteins, depolarization-induced Cm changes were considerably larger than without synaptobrevin (VAMP-2). The tSNAREs, synaptotagmin and the Ca2+ channel without synaptobrevin, were previously shown to form a complex with distinct kinetic properties, named excitosome (26) (Fig. 3 A; see also Fig. 1, C and D). This result is compatible with the notion that synaptobrevin increases either the number or the efficiency of the protein complexes between SNARE proteins, Ca2+ channel, and synaptotagmin, or plays a role in targeting vesicles to the plasma membrane; alternatively, synaptobrevin may somehow modulate exocytosis independently from the excitosome. Coexpression of tetanus toxin (a protease specific for synaptobrevin; TetX) reduced the depolarization-induced Cm increases to the level seen with the excitosome alone (rather than abolishing it completely). Partial inhibition is consistent with the lack of effect of TetX in oocytes expressing the excitosome; also, the inhibition confirms the successful functional expression of this neurotoxin. This finding shows that the reconstitution of depolarization-induced exocytosis in Xenopus oocytes was achieved without the expression of exogenous synaptobrevin, and also without a significant contribution of putative endogenous TetX-sensitive synaptobrevin. Likewise, complete cleavage of synaptobrevin by TetX in pancreatic acini caused only a partial inhibition of exocytosis (27) and in a cell free Ca2+-regulated exocytosis (28).
Coexpression of botulinum neurotoxin A (a protease specific for SNAP-25; BotA) together with the excitosome proteins abolished the depolarization-induced Cm change (Fig. 3 A). A similar inhibition was observed upon coexpression of botulinum toxin C1 light chain (a protease specific for syntaxin 1A; BotC). Both neurotoxins had no significant effect on the depolarization-induced current amplitudes (Fig. 3 B). These results suggest that both syntaxin 1A and SNAP-25 are essential for depolarization-induced exocytosis. In all cases, current amplitudes were marginally affected by coexpression of neurotoxins (Fig. 3 B).
Next, we assembled the synaptic proteins step by step, adding one component after the other (Fig. 3 C). A small depolarization-induced Cm change was seen with the Ca2+ channel alone, and this effect was increased with additional coexpression of the Ca2+ sensor protein synaptotagmin-I. Further coexpression of BotC light chain abolished this effect, suggesting the participation of endogenous syntaxin 1A (or a homolog) in the observed depolarization-induced exocytosis (29). Notably, SNAP-25 and syntaxin 1A together or individually with the channel failed to increase Cm (data not shown), indicating the essential role of synaptotagmin.
Based on these results we chose to continue the Cm studies in oocytes expressing syntaxin 1A, SNAP-25, synaptotagmin and the Ca2+ channel, which appear to be the minimal set of proteins required for secretion.
We then established in a relatively simple yet informative experiment the fundamental fact that the three synaptic proteins comprised by the excitosome (synaptotagmin-I, syntaxin 1A and SNAP-25) are indeed required for and participate in the depolarization-induced exocytosis that we observed in Xenopus oocytes (Fig. 3 A). As demonstrated above (Figs. 1, A and B, and 3 A), only expression of these three proteins, but not expression of Ca2+ channel alone, resulted in large depolarization-induced changes of Cm. Increasing the amount of injected cRNAs for the three synaptic proteins by a factor of two or four (at fixed cRNA amounts of channel subunits) resulted in roughly proportional increases of the Cm changes (Fig. 3 D).
Recall the findings above that suggested the presence of some endogenous synaptic proteins, which might assemble with the heterologous Ca2+ channel into functional exocytotic units (Fig. 1, A and B). Our experiment (Fig. 3 D) shows that with increasing cRNA levels of excitosome one can obtain, with sufficiently strong expression of the exogenous proteins, such high numbers of correctly formed exocytotic units (i.e., comprising exclusively exogenous channel and synaptic proteins) that all other exocytotic units (e.g., comprising exogenous channel and endogenous synaptic proteins) are outnumbered by far. With such a small background, the properties of depolarization-induced exocytosis can be attributed to the well-defined set of exogenous proteins.
Next, we characterized this reconstituted exocytosis in more detail, taking advantage of this assay system to control and vary stimuli, and to gather detailed, quantitative information from the elicited effects.
Reconstituted depolarization-evoked secretion is nonlinear and is affected by the type of charge carriers
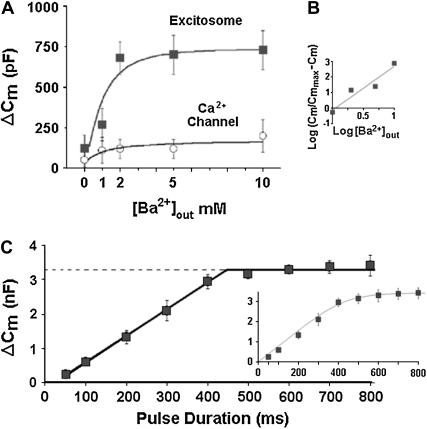
The effect of fixed-duration depolarization on Cm depended strictly on the presence of either extracellular Ca2+ or Ba2+ and was concentration-dependent and saturable (Fig. 4 A). The concentration-dependence of ΔCm was nonlinear with an estimated Hill coefficient of nH ≈ 2.8. This finding is in good agreement with the characteristic cooperativity of transmitter release in neuronal cells (30). Oocytes expressing the channel alone showed a very small change in capacitance, which slightly increased at 10 mM Ba2+. When external Ba2+ concentration was fixed at 5 mM, the effect of depolarization on Cm depended on pulse duration, with a lower threshold at ∼50 ms and saturated at ∼400 ms (Fig. 4 B) (7,8). Thus, reconstituted depolarization-induced exocytosis is by at least 2 orders of magnitude faster than membrane fusion in previous reconstitution assays. The method of varying the depolarizing pulse to obtain the kinetics of exocytosis was first applied to capacitance measurements in nerve terminals by (31). Fitting a straight line to the data points between 50 to 400 ms resulted in a slope of 7.7 nF/s corresponding to initial rate of 1.2 × 106 vesicles/s and a pool of 600,000 vesicles at ∼400 ms (Fig. 4 B). The data could also be fitted although less well, with a single exponential time constant of 101 ± 12 ms corresponding to initial rate of 6 × 106 vesicles/s (inset). The saturation in the capacitance jumps defines a readily releasable pool of ∼106 to 107 vesicles.
FIGURE 4.
Quantitative characterization of reconstituted depolarization-induced exocytosis. (A) Dependence of depolarization-induced ΔCm on extracellular Ba2+ concentration. Depolarization from −80 mV to 0 mV for 2 × 500 ms 100 ms apart in oocytes expressing Lc-type channel without (lower trace) or with (upper trace) the synaptic proteins syntaxin 1A, SNAP-25, and synaptotagmin I, as in Fig. 1. (B) Hill plot. Data (from A) show saturation of the Cm effect >2 mM, and a nonlinear concentration-dependence of ΔCm with an estimated Hill coefficient of nH ≈ 2.8. (C) Dependence of depolarization-induced ΔCm on depolarization duration. Depolarization from −80 mV to 0 mV for indicated times in 5 mM Ba2+. A linear correlation observed with pulse duration for longer depolarization periods, saturation at ∼400 ms and half-maximal capacitance changes are reached at a depolarization time of ∼250 ms. A straight line fitted to the data between 50 and 400 ms had a slope of 7.7 pF ms−1 (R2 = 0.998) corresponding to ∼600,000 vesicles at saturation. The results could be fitted less well with a single exponent (inset) showing a time constant of 101.05 ± 12 ms corresponding to an initial rate of 6 × 106 vesicles/s (inset). The saturation in the capacitance jumps defines a readily releasable pool of ∼106–107 vesicles (see 31).
Both Ca2+, Sr2+, and Ba2+ ions supported depolarization-induced Cm changes, but with quantitative differences (Fig. 5 A). With either ion, a fast initial Cm increase was followed by a slower Cm relaxation; the latter probably reflects compensatory retrieval of the exocytosed membrane surface area. With Ba2+, however, the fast initial Cm increase became greater by a factor of ∼2.5 as compared to Ca2+, whereas the subsequent slow Cm decay became slower by a similar factor. With Sr2+, Cm was similar to Ca2+ showing an intermediate Cm decay (Fig. 5 C). Similar to our findings in this reconstituted form of depolarization-induced exocytosis, the quantitative traits of native neuronal exocytosis are differentially affected by Ca2+ versus Ba2+ (32).
FIGURE 5.
Differential effects of Ca2+, Sr2+, or Ba2+ on depolarization-induced Cm changes, and vesicle depletion by repetitive stimulation (A) Differential effects of Ca2+, Sr2+, or Ba2+ on depolarization-induced exocytosis. Oocytes expressing Cav1.2, syntaxin 1A, syt-1, and SNAP-25 (excitosome). (Left panel) Original traces showing time course of Cm upon depolarization, fitted by simple exponentials; (middle panel) instantaneous depolarization-induced Cm increase in Ca2+, Sr2+, and Ba2+ (mean ± SE, n = 10, each); and (right panel) rate of compensatory Cm decrease (mean ± SE, n = 10, each). As compared to Ca2+, the instantaneous Cm increase was greater in Ba2+ (middle panel), and the compensatory Cm decrease was slower (right panel). In Sr2+, the respective values were between those of Ca2+ and Ba2+. (B) Current amplitude during repeated trains of membrane depolarization. Oocytes were depolarized by three trains composed of 10 depolarizations from −80 to 0 mV 1 s each (upper panel). Depolarization-induced inward current inactivated during trains whereas no run-down of current amplitude was observed from one train to the other (lower panel). (C) Exhaustibility of depolarization-induced capacitance changes. Repeated depolarization pulses from −80 mV to 0 mV for 1 s each (upper trace) and the associated Cm changes (lower trace). Data are consistent with depletion and only partial replenishing of a pool of releasable vesicles.
Interestingly, when the depolarizing stimulus was applied repeatedly, the associated Cm increase became progressively smaller (Fig. 5 C). This exhaustibility is likely to reflect depletion of a pool of releasable vesicles that can be refilled after a period of minutes (33,34). Moreover, this phenomenon provides further evidence against a contribution of gating charges to the observed Cm changes, which should not show exhaustibility.
Role of the Ca2+ channel
Voltage-gated Ca2+ channels (VGCC) are essential components of the molecular machinery that mediates depolarization-induced exocytosis at synapses. One obvious function herein is to transduce the electrical signal, the action potential, into VGCC activation ensued by a rise of cytoplasmic Ca2+. On the other hand, we have shown by various approaches that the Ca2+ channel is also physically associated with synaptic proteins (20,25,26,35), forming a protein complex which we have termed the “excitosome” (26). Such a direct protein-protein interaction (36–41) could allow the Ca2+ channel to affect the process of depolarization-induced exocytosis upstream to Ca2+ entry (42,43). These potential additional roles are barely explored, mainly because this would require an experimental approach that simultaneously affords control of membrane potential and arbitrary variation of the particular Ca2+ channel. Such experiments are a unique strength of reconstitution in Xenopus oocytes.
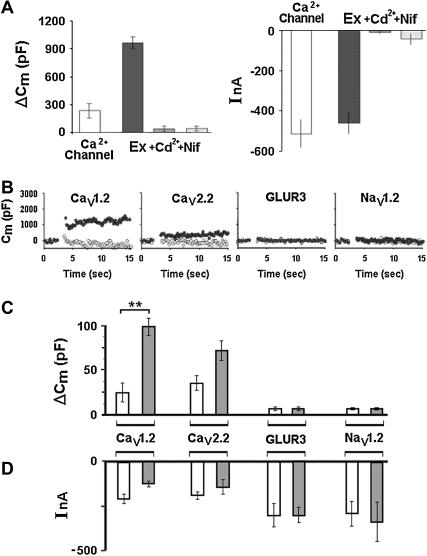
We used two channel blockers to test for the requirement of a voltage-gated Ca2+ channel in depolarization-evoked release (Fig. 6 A). Considerable depolarization-induced inward currents were recorded in oocytes expressing Lc-type Ca2+ channel (Cav1.2; right panel), but these were accompanied by only small Cm changes (left panel). In contrast, coexpression of the entire excitosome resulted in much larger Cm changes, whereas similar current amplitudes were not different from the currents observed with the channel alone (Fig. 6 A; cf. also Fig. 1, D–F). Both in the presence of either the pore blocker Cd2+ (200 μM) or the selective Lc-type channel blocker nifedipine (10 μM), depolarization failed to induce any Cm changes or inward currents.
FIGURE 6.
Dissecting out the role of the Ca2+ channel in reconstituted depolarization-induced exocytosis (A) Effect of Ca2+ channel inhibitors on reconstituted depolarization-induced exocytosis. Oocytes were expressing either the Lc-type Ca2+ channel alone (leftmost bar), or together with the synaptic proteins syntaxin 1A (sx1A), synaptotagmin I (syt I), and SNAP-25 (other three bars). Depolarization-induced changes of capacitance (ΔCm) and current amplitude (I) in 5 mM Ba2+. The effects of the Ca2+ channel blockers Cd2+ (200 μM) and nifedipine (10 μM) show that depolarization-induced exocytosis absolutely requires the presence and functionality of a voltage-gated Ca2+ channel. (B) Effect of channel type on depolarization-induced Cm changes. Time course of Cm in oocytes expressing various voltage-gated channels (L-, N-type Ca2+ channels, GluR3 receptor, or brain-type II Na+ channel; see Methods) without (open circles) or with SNAP-25, syntaxin 1A, and synaptotagmin 1 (solid circles); bath containing 5 mM Ba2+ (with Ca2+ channels and GluR3) or 50 mM Na+ (with Na+ channel). Depolarization from −80 mV to 0 mV for 2 × 500 ms. (C) Effect of channel type on size of depolarization-induced Cm increase. Same protocol as in (A) (open bars) channel only; (solid bars) channel plus SNAP-25, syntaxin 1A, and synaptotagmin 1. Mean ± SD from 10 oocytes, each. Cav1.2 versus Cav1.2 + synaptic proteins (**), P < 0.0015; Cav2.2 versus Cav2.2 + synaptic proteins P* < 0.1 in Student's t-test. (D) Effect of channel type on depolarization-induced current changes. Same protocol as in A and B.
Next, we tested how the particular type of voltage-gated Ca2+ channel affects the extent and the kinetics of depolarization-induced Cm changes (Fig. 6, B–D). Oocytes were injected with a fixed combination of syntaxin 1A, SNAP-25 and synaptotagmin I, and in addition with cRNA species encoding either Lc-type (Cav1.2), N-type (Cav2.2), or the neuronal voltage-gated sodium channel Nav1.2 (Fig. 6 B; see Methods); assembly of these channels into excitosome complexes was shown previously (17,20,26,44) except for the Na+ channel. The effect of depolarization on current and capacitance was tested in 5 mM Ba2+ (Ca2+ channels) or 50 mM Na+ (Na+ channel). In addition, we used the α-amino-3-hydroxy-5-methyl-4-isoxazol propionate (AMPA)-receptor GluR3, a glutamate-gated cation-selective channel that mediates the majority of fast excitatory synaptic transmission in the mammalian brain by transporting Ca2+ into the cell. The ionotropic receptor was expressed in the oocyte and activated to introduce Ca2+ by a hyperpolarizing step in the presence of 1mM glutamate (Fig. 6, B and C).
The particular channel type had a large effect on the magnitude of the depolarization-induced Cm changes (ΔCm; value with versus without synaptic proteins (indicated in % of ΔCm with Cav1.2): Cav1.2, 100 ± 20 vs. 24.5 ± 22; Cav2.2, 72 ± 23 vs. 35 ± 16; GluR3, 6 ± 3 vs. 6 ± 3 and Nav1.2, 5 ± 1 vs. 5 ± 1, respectively. Thus as compared to the Cm changes obtained with the respective channel only, the additional expression of the synaptic proteins enhanced depolarization-induced exocytosis by a factor of 4.16 ± 0.22 (Cav1.2) and 1.3 ± 0.2 (Cav2.2). The corresponding inward currents (Fig. 6 D) showed no correlation with Cm changes. Hence differences in capacitance are due to factors other than the respective channel's ability to mediate Ca2+ influx and maybe depolarization-evoked exocytosis requires the functionality of a voltage-gated Ca2+ channel.
Although the activated ionotropic receptor GluR3 introduced Ca2+ into the cells (hyperpolarization-induced inward currents) with GluR3 alone 300 ± 103nA, and with GluR3 plus syntaxin-1A, SNAP-25, and synaptotagmin, 300 ± 44; (Fig. 6 D) no increase in Cm was detected (Fig. 6 C), consistent with the importance of a physical and functional interaction between channel and the synaptic proteins (25,26,43). Finally, no depolarization-induced capacitance increase was observed when a neuronal Na+ channel was complementing the synaptic proteins. This is in keeping with the requirement for external divalent cations passing through a voltage-gated Ca2+ channel.
DISCUSSION
The principle of our reconstitution assay is to combine heterologous expression of candidate proteins in Xenopus oocytes with monitoring of membrane capacitance as a readout of depolarization-induced exocytosis. Expression of heterologous proteins was accomplished in these large cells by established techniques that involve the injection of cRNA encoding the corresponding proteins (45,46). Control of membrane potential and application of a short depolarizing stimulus, was achieved by the similarly well-established two-electrode voltage-clamp technique (47). Continuous monitoring of membrane capacitance (Cm) by this technique has become feasible by a novel method developed recently by one of us (18). The powerful electrical approach of capacitance monitoring (in the sense of continuous measurements with high time-resolution and precision) was previously applied only in the patch-clamp configuration, precluding its use in large cells such as Xenopus oocytes.
Voltage-gated Ca2+ channels are essential components of the molecular machinery that mediates exocytosis. Besides translating the action potential into a rise of cytoplasmic Ca2+, the Ca2+ channel is also physically associated with synaptic proteins. The physical association with other synaptic proteins provides multiple further points of interaction by which the channel may affect the process of depolarization-induced exocytosis (37–41). We have suggested that vesicle fusion in response to membrane depolarization is initiated by the “excitosome”, a protein complex with distinct kinetic properties that comprises the voltage-gated Ca2+ channel, syntaxin-1A, SNAP-25, and synaptotagmin. Such potential role is barely explored, mainly because this would require an experimental approach that simultaneously affords control of membrane potential and arbitrary variation of the particular synaptic protein and the Ca2+ channel. Such experiments are a unique strength of reconstitution in Xenopus oocytes.
It is important to mention that Xenopus laevis oocytes do not respond to membrane depolarization and are not excitable cells, in contrast to mature eggs (49,50) in which capacitance changes were studied previously (51,52).
Reconstitution of depolarization-induced exocytosis in Xenopus oocytes was achieved by expressing synaptic proteins with CaV1.2, the voltage-gated Ca2+ channel that supports fast release in bipolar cells (53) and neuroendocrine cells (54,55). Exocytosis was not observed when the Ca2+ channel was either absent or blocked (by nifedipine or Cd2+), or when synaptotagmin was not expressed. Similarly, the effect of neurotoxins that selectively cleave either syntaxin 1A (BotC) or SNAP-25 (BotA) showed that syntaxin 1A and SNAP-25 are strictly required for depolarization-induced exocytosis. In contrast, the only partial inhibition caused by TetX demonstrated that synaptobrevin is not absolutely required for depolarization-induced exocytosis although it enhances the process. Our data demonstrate directly for the first time that CaV1.2 together with syntaxin 1A, SNAP-25 and synaptotagmin, but not synaptobrevin, are sufficient and necessary for depolarization-induced secretion. Further studies are needed to explore the fact that the tSNAREs in combination with synaptotagmin can engender a depolarization-evoked secretion in the absence of synaptobrevin. Many further proteins are implied in depolarization-induced exocytosis (e.g., Munc-18 (56), Munc-13 (57), complexins (58), and others). Their exact roles can be assessed using our reconstitution assay, analogously to the approach taken in this study to characterize the Ca2+ channel, syntaxin 1A, SNAP-25, and synaptotagmin I.
The features of the reconstituted process were similar to native depolarization-induced exocytosis: fast, robust, with nonlinear concentration dependence, modulated by the type of charge carrier and the type of Ca2+ channel. The dissociation between the differential effects of these Ca2+ channels on ΔCm on the one hand, and on Ca2+ currents on the other, implies that Ca2+ channels have a role in depolarization-induced exocytosis beyond Ca2+ transport into the cytoplasm. The existence of such additional roles was predicted from the excitosome hypothesis, and their experimental demonstration lends further support to this hypothesis.
Our reconstitution assay appears to mimic fast secretion rather faithfully. When interpreting the data, one should, however, keep in mind the differences compared to the native process at synapses, and to other reconstitution assays. Compared to neurons, the oocyte is clearly not an excitable cell in terms of geometry and organellar or cytoskeletal makeup. These properties may affect the kinetics of depolarization-induced exocytosis.
These shortcomings are seemingly inherent to other ex vivo systems such as cortices of sea urchin eggs, HEK293 cells and to in vitro systems. Compared to reconstitution assays that employ artificial lipid vesicles and purified proteins, the oocytes do provide a background that is neither completely “clean” nor completely defined. Rather, oocytes likely express endogenously multiple proteins that could to some extent affect depolarization-induced exocytosis. However, the contribution of these proteins can easily be unmasked by the significantly higher expression of the other heterologous proteins (which would make the putative contribution of the endogenous proteins rate limiting) or the use of specific neurotoxins (cf. the evidence for a putative endogenous syntaxin in this work provided by the finding that depolarization-induced exocytosis was sensitive to BOT-C even when no heterologous syntaxin was expressed; Fig. 2 C). Interestingly, we found that secretion was more efficient in Ba2+ than in Ca2+ whereas the ensuing compensatory endocytosis was significantly slower. Although this result is in line with certain data on secretion (59,60) it conflicts with reports that Ba2+ ion was the least effective in both bipolar cells and in the squid giant synapse (61,62).
The higher efficacy of Ba2+ with respect to depolarization-induced exocytosis could be explained by the depolarizing effect of extracellular Ba2+, its greater rates of entry through noninactivating Ca2+ channels, by its poor intracellular buffering (largely arising from its weak affinity for plasmalemmal Ca2+ extruders), and—finally—by the possible existence of fast and slow modes of secretion. On the other hand, data from bipolar cells suggested that the influx of Sr2+ or Ba2+ decreased the relative proportion of docked vesicles available for fast exocytosis as compared to Ca2+, and thus Ca2+ was more effective at triggering secretion (62). It appears that the contribution of cation selectivity to the efficacy of exocytosis in secreting cells is not fully resolved to date. Maybe, these aspects of the mechanism of secretion could be easier to investigate in a reconstituted system.
Clear advantages of the oocytes are that they allow one to use full-length proteins expressed with the corresponding signal peptide, targeted to the correct location and operating at the native membrane of a living cell, as opposed to reconstitution assays that employ truncated recombinant proteins and artificial membranes. In these experiments one has to consider that the extent of ΔCm in different batches of oocytes varies, ranging from 1000–4000 pF, and most probably is dependent on the quality of the oocytes in the various batches.
Taken together, the data we could gather with this novel system of reconstituted depolarization-induced exocytosis is consistent with our previous proposed “excitosome” hypothesis. According to this hypothesis, the minimal molecular machinery that is sufficient and necessary for depolarization-induced vesicle fusion with the cell membrane is a protein complex comprising a voltage-gated Ca2+ channel, syntaxin 1A, synaptotagmin, and SNAP-25. Synaptobrevin enhanced release efficiency but was not essential. These findings lend further support to the notion that the outcome of studies that aim to dissect the molecular basis of regulated exocytosis may vary widely depending on which of the many different exocytosis assays was employed. In this respect, our novel reconstitution assay in Xenopus oocytes offers the most direct experimental approach to depolarization-evoked exocytosis and could provide important clues to the further understanding of synaptic transmission.
Acknowledgments
We thank N. Melamed-Book for assistance with confocal imaging.
References
- 1.Katz, B., and R. Miledi. 1969. Spontaneous and evoked activity of motor nerve endings in calcium ringer. J. Physiol. 203:689–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer, A. 2002. Membrane fusion in eukaryotic cells. Annu. Rev. Cell Dev. Biol. 18:289–314. [DOI] [PubMed] [Google Scholar]
- 3.Jahn, R., T. Lang, and T. C. Sudhof. 2003. Membrane fusion. Cell. 112:519–533. [DOI] [PubMed] [Google Scholar]
- 4.Martin, T. F. 2003. Tuning exocytosis for speed: fast and slow modes. Biochim. Biophys. Acta. 1641:157–165. [DOI] [PubMed] [Google Scholar]
- 5.Weber, T., B. V. Zemelman, J. A. McNew, B. Westermann, M. Gmachl, F. Parlati, T. H. Sollner, and J. E. Rothman. 1998. SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772. [DOI] [PubMed] [Google Scholar]
- 6.Avery, J., R. Jahn, and J. M. Edwardson. 1999. Reconstitution of regulated exocytosis in cell-free systems: a critical appraisal. Annu. Rev. Physiol. 61:777–807. [DOI] [PubMed] [Google Scholar]
- 7.Cook, N. R., and H. W. Davidson. 2001. In vitro assays of vesicular transport. Traffic. 2:19–25. [DOI] [PubMed] [Google Scholar]
- 8.Tucker, W. C., T. Weber, and E. R. Chapman. 2004. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 304:435–438. [DOI] [PubMed] [Google Scholar]
- 9.Weimer, R. M., and E. M. Jorgensen. 2003. Controversies in synaptic vesicle exocytosis. J. Cell Sci. 116:3661–3666. [DOI] [PubMed] [Google Scholar]
- 10.Duman, J. G., and J. G. Forte. 2003. What is the role of SNARE proteins in membrane fusion? Am. J. Physiol. Cell Physiol. 285:C237–C249. [DOI] [PubMed] [Google Scholar]
- 11.Szule, J. A., and J. R. Coorssen. 2003. Revisiting the role of SNAREs in exocytosis and membrane fusion. Biochim. Biophys. Acta. 1641:121–135. [DOI] [PubMed] [Google Scholar]
- 12.Cavalli, A., L. Eder-Colli, Y. Dunant, F. Loctin, and N. Morel. 1991. Release of acetylcholine by Xenopus oocytes injected with mRNAs from cholinergic neurons. EMBO J. 10:1671–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alder, J., B. Lu, F. Valtorta, P. Greengard, and M. M. Poo. 1992. Calcium-dependent transmitter secretion reconstituted in Xenopus oocytes: requirement for synaptophysin. Science. 257:657–661. [DOI] [PubMed] [Google Scholar]
- 14.Marsal, J., G. Tigyi, and R. Miledi. 1995. Incorporation of acetylcholine receptors and Cl- channels in Xenopus oocytes injected with Torpedo electroplaque membranes. Proc. Natl. Acad. Sci. USA. 92:5224–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aleu, J., M. Martin-Satue, P. Navarro, I. Perez de Lara, L. Bahima, J. Marsal, and C. Solsona. 2003. Release of ATP induced by hypertonic solutions in Xenopus oocytes. J. Physiol. 547:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheuner, D., C. D. Logsdon, and R. W. Holz. 1992. Bovine chromaffin granule membranes undergo Ca(2+)-regulated exocytosis in frog oocytes. J. Cell Biol. 116:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobi, D., O. Wiser, M. Trus, and D. Atlas. 1998. N-type voltage-sensitive calcium channel interacts with syntaxin, synaptotagmin and SNAP-25 in a multiprotein complex. Receptors Channels. 6:89–98. [PubMed] [Google Scholar]
- 18.Schmitt, B. M., and H. Koepsell. 2002. An improved method for real-time monitoring of membrane capacitance in Xenopus laevis oocytes. Biophys. J. 82:1345–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maroto, R., and O. P. Hamill. 2001. Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. J. Biol. Chem. 276:23867–23872. [DOI] [PubMed] [Google Scholar]
- 20.Cohen, R., and D. Atlas. 2004. R-type voltage-gated Ca(2+) channel interacts with synaptic proteins and recruits synaptotagmin to the plasma membrane of Xenopus oocytes. Neuroscience. 128:831–841. [DOI] [PubMed] [Google Scholar]
- 21.Sollner, T., S. W. Whiteheart, M. Brunner, H. Erdjument-Bromage, S. Geromanos, P. Tempst, and J. E. Rothman. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature. 362:318–324. [DOI] [PubMed] [Google Scholar]
- 22.Sudhof, T. C. 1995. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 375:645–653. [DOI] [PubMed] [Google Scholar]
- 23.Bement, W. M., H. Benink, C. A. Mandato, and B. B. Swelstad. 2000. Evidence for direct membrane retrieval following cortical granule exocytosis in Xenopus oocytes and eggs. J. Exp. Zool. 286:767–775. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita, T., T. Hige, and T. Takahashi. 2005. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 307:124–127. [DOI] [PubMed] [Google Scholar]
- 25.Wiser, O., M. K. Bennett, and D. Atlas. 1996. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. EMBO J. 15:4100–4110. [PMC free article] [PubMed] [Google Scholar]
- 26.Wiser, O., M. Trus, A. Hernandez, E. Renstrom, S. Barg, P. Rorsman, and D. Atlas. 1999. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc. Natl. Acad. Sci. USA. 96:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaisano, H. Y., L. Sheu, J. K. Foskett, and W. S. Trimble. 1994. Tetanus toxin light chain cleaves a vesicle-associated membrane protein (VAMP) isoform 2 in rat pancreatic zymogen granules and inhibits enzyme secretion. J. Biol. Chem. 269:17062–17066. [PubMed] [Google Scholar]
- 28.Edwardson, J. M. 1998. A cell-free system for Ca2+-regulated exocytosis. Methods. 16:209–214. [DOI] [PubMed] [Google Scholar]
- 29.Trus, M., O. Wiser, M. C. Goodnough, and D. Atlas. 2001. The transmembrane domain of syntaxin 1A negatively regulates voltage-sensitive Ca(2+) channels. Neuroscience. 104:599–607. [DOI] [PubMed] [Google Scholar]
- 30.Dodge, F. A., Jr., and R. Rahamimoff. 1967. Cooperative action a calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 193:419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Gersdorff, H., and G. Matthews. 1994. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 367:735–739. [DOI] [PubMed] [Google Scholar]
- 32.Zengel, J. E., and K. L. Magleby. 1977. Transmitter release during repetitive stimulation: selective changes produced by Sr2+ and Ba2+. Science. 197:67–69. [DOI] [PubMed] [Google Scholar]
- 33.Neher, E., and R. S. Zucker. 1993. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 10:21–30. [DOI] [PubMed] [Google Scholar]
- 34.Pieribone, V. A., O. Shupliakov, L. Brodin, S. Hilfiker-Rothenfluh, A. J. Czernik, and P. Greengard. 1995. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 375:493–497. [DOI] [PubMed] [Google Scholar]
- 35.Sheng, Z. H., J. Rettig, M. Takahashi, and W. A. Catterall. 1994. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 13:1303–1313. [DOI] [PubMed] [Google Scholar]
- 36.Xia, F., X. Gao, E. Kwan, P. P. Lam, L. Chan, K. Sy, L. Sheu, M. B. Wheeler, H. Y. Gaisano, and R. G. Tsushima. 2004. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J. Biol. Chem. 279:24685–24691. [DOI] [PubMed] [Google Scholar]
- 37.Mochida, S., Z. H. Sheng, C. Baker, H. Kobayashi, and W. A. Catterall. 1996. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron. 17:781–788. [DOI] [PubMed] [Google Scholar]
- 38.Rettig, J., C. Heinemann, U. Ashery, Z. H. Sheng, C. T. Yokoyama, W. A. Catterall, and E. Neher. 1997. Alteration of Ca2+ dependence of neurotransmitter release by disruption of Ca2+ channel/syntaxin interaction. J. Neurosci. 17:6647–6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bezprozvanny, I., P. Zhong, R. H. Scheller, and R. W. Tsien. 2000. Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating. Proc. Natl. Acad. Sci. USA. 97:13943–13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barg, S., X. Ma, L. Eliasson, J. Galvanovskis, S. O. Gopel, S. Obermuller, J. Platzer, E. Renstrom, M. Trus, D. Atlas, J. Striessnig, and P. Rorsman. 2001. Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys. J. 81:3308–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurley, J. H., A. L. Cahill, M. Wang, and A. P. Fox. 2004. Syntaxin 1A regulation of weakly inactivating N-type Ca2+ channels. J. Physiol. 560:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atlas, D., O. Wiser, and M. Trus. 2001. The voltage-gated Ca2+ channel is the Ca2+ sensor of fast neurotransmitter release. Cell. Mol. Neurobiol. 21:717–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiser, O., R. Cohen, and D. Atlas. 2002. Ionic dependence of Ca2+ channel modulation by syntaxin 1A. Proc. Natl. Acad. Sci. USA. 99:3968–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiser, O., D. Tobi, M. Trus, and D. Atlas. 1997. Synaptotagmin restores kinetic properties of a syntaxin-associated N-type voltage sensitive calcium channel. FEBS Lett. 404:203–207. [DOI] [PubMed] [Google Scholar]
- 45.Barnard, E. A., R. Miledi, and K. Sumikawa. 1982. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc. R. Soc. Lond. B Biol. Sci. 215:241–246. [DOI] [PubMed] [Google Scholar]
- 46.Miledi, R., I. Parker, and K. Sumikawa. 1987. Oscillatory chloride current evoked by temperature jumps during muscarinic and serotonergic activation in Xenopus oocyte. J. Physiol. 383:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stuhmer, W. 1998. Electrophysiologic recordings from Xenopus oocytes. Methods Enzymol. 293:280–300. [DOI] [PubMed] [Google Scholar]
- 48.Atlas, D. 2001. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J. Neurochem. 77:972–985. [DOI] [PubMed] [Google Scholar]
- 49.Scheuner, D., and R. W. Holz. 1994. Evidence that the ability to respond to a calcium stimulus in exocytosis is determined by the secretory granule membrane: comparison of exocytosis of injected bovine chromaffin granule membranes and endogenous cortical granules in Xenopus laevis oocytes. Cell. Mol. Neurobiol. 14:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohan, S. A., and C. B. Gundersen. 2003. Protein synthesis is required for the transition to Ca(2+)-dependent regulated secretion in progesterone-matured Xenopus oocytes. J. Exp. Zoolog. A Comp. Exp. Biol. 300:113–125. [DOI] [PubMed] [Google Scholar]
- 51.Jaffe, L. A., and L. C. Schlichter. 1985. Fertilization-induced ionic conductances in eggs of the frog, Rana pipiens. J. Physiol. 358:299–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaffe, L. A., S. Hagiwara, and R. T. Kado. 1978. The time course of cortical vesicle fusion in sea urchin eggs observed as membrane capacitance changes. Dev. Biol. 67:243–248. [DOI] [PubMed] [Google Scholar]
- 53.Mennerick, S., and G. Matthews. 1996. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 17:1241–1249. [DOI] [PubMed] [Google Scholar]
- 54.Avidor, B., T. Avidor, L. Schwartz, K. S. De Jongh, and D. Atlas. 1994. Cardiac L-type Ca2+ channel triggers transmitter release in PC12 cells. FEBS Lett. 342:209–213. [DOI] [PubMed] [Google Scholar]
- 55.Elhamdani, A., Z. Zhou, and C. R. Artalejo. 1998. Timing of dense-core vesicle exocytosis depends on the facilitation L-type Ca channel in adrenal chromaffin cells. J. Neurosci. 18:6230–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hata, Y., C. A. Slaughter, and T. C. Sudhof. 1993. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 366:347–351. [DOI] [PubMed] [Google Scholar]
- 57.Brose, N., K. Hofmann, Y. Hata, and T. C. Sudhof. 1995. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 270:25273–25280. [DOI] [PubMed] [Google Scholar]
- 58.McMahon, H. T., M. Missler, C. Li, and T. C. Sudhof. 1995. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 83:111–119. [DOI] [PubMed] [Google Scholar]
- 59.Artalejo, C. R., J. R. Henley, M. A. McNiven, and H. C. Palfrey. 1995. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc. Natl. Acad. Sci. USA. 92:8328–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Ruden, L., A. G. Garcia, and M. G. Lopez. 1993. The mechanism of Ba(2+)-induced exocytosis from single chromaffin cells. FEBS Lett. 336:48–52. [DOI] [PubMed] [Google Scholar]
- 61.Augustine, G. J., and R. Eckert. 1984. Divalent cations differentially support transmitter release at the squid giant synapse. J. Physiol. 346:257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neves, G., A. Neef, and L. Lagnado. 2001. The actions of barium and strontium on exocytosis and endocytosis in the synaptic terminal of goldfish bipolar cells. J. Physiol. 535:809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]