Abstract
To improve biodesulfurization rate is a key to industrialize biodesulfurization technology. The biodesulfurization rate is partially affected by transfer rate of substrates from organic phase to microbial cell. In this study, γ-Al2O3 nanosorbents, which had the ability to selectively adsorb dibenzothiophene (DBT) from organic phase, were assembled on the surfaces of Pseudomonas delafieldii R-8 cell, a desulfurization strain. γ-Al2O3 nanosorbents have the ability to adsorb DBT from oil phase, and the rate of adsorption was far higher than that of biodesulfurization. Thus, DBT can be quickly transferred to the biocatalyst surface where nanosorbents were located, which quickened DBT transfer from organic phase to biocatalyst surface and resulted in the increase of biodesulfurization rate. The desulfurization rate of the cells assembled with nanosorbents was approximately twofold higher than that of original cells. The cells assembled with nanosorbents were observed by a transmission electron microscope.
Recently, biodesulfurization of petroleum products has received growing attention as the production of ultra-low-sulfur products (1). However, it is still not a commercial technology because of some problems, such as desulfurization rate, bioreactor design, and the volumetric ratio between the oil and aqueous phases. Among these, desulfurization rate represents the main limiting factor for an industrial application of the biotechnological process. Many progresses have been done in improvement of biodesulfurization rate in the last years by increasing the activity of biocatalysts. Despite considerable progress in improving the expression and copies of the key enzymes (2,3), the flux through the system is still too low for widespread commercial applications (1). Setti et al. (4) and Mehrnia et al. (5) reported that transfer of polycyclic aromatic sulfur heterocycle (such as DBT) from the oil to the water (and then from the water to the cells) can limit the rate of its metabolism.
In this study, a novel biodesulfurization technology was developed by assembling γ-Al2O3 nanosorbent, which can selectively adsorb DBT (a model polycyclic aromatic sulfur heterocycle) from the organic phase, on the surfaces of microbial cell. The approach to increase the rate of biodesulfurization is based on the improvement of transfer rate of DBT.
Pseudomonas delafieldii R-8 (6), was isolated from the sewage pool of Shengli oil field in China, and has the ability to convert DBT to 2-hydroxy-biphenyl (2-HBP) and sulfate.
We synthesized γ-Al2O3 nanosorbent by the following method. We dissolved 25 g Al(NO3)3·9H2O in 100 ml distilled water including 0.1 g of cetyltrimethylammonium bromide (CTAB), and then added dropwise a 10% of NH4HCO3 aqueous solution including 0.1 g of CTAB. The addition was stopped until the sol was formed. The additional amount of NH4HCO3 aqueous solution was ∼50 ml. To continue to stir for 1 h, and then age for 48 h, the products were dried in vacuum for 5 h at 80°C. Finally, γ-A12O3 nanosorbents were obtained by sintering at 600°C.
Gamma-Al2O3 nanosorbents (0.1 g) and 0.5 g of dry cells were added into 50 ml of saline water (8.5% NaCl). The cells assembled with γ-Al2O3 nanosorbents were harvested and vacuum-dried at −4°C. Each cell preparation (the aforementioned γ-Al2O3-cells or 0.5 of dry cells) was suspended in 15 ml phosphate buffer (0.1 M, pH = 7.0), and the suspension was mixed with 15 ml of model oil (5.0 mM DBT in n-dodecane). The reaction was carried out in 100-ml flasks at 30°C on a rotary shaker at 180 rpm.
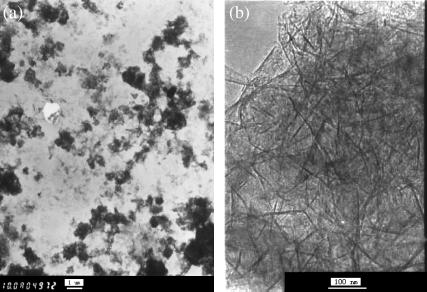
Fig. 1, a and b, are two transmission electron microscope (TEM) images of γ-Al2O3 nanosorbent with different magnifications (×10,000 and ×200,000). It is clearly shown that γ-Al2O3 sorbents prepared are very thin long-fiber shape. Its length is ∼100 nm, and its width is only a few nanometers. Thus, the size of the sorbent is much smaller than that of microbial cell, which is about a few micrometers.
FIGURE 1.
TEM images of γ-Al2O3 nanosorbent (obtained by Hitachi 8100, 200 kV). (a) ×10,000; (b) ×200,000.
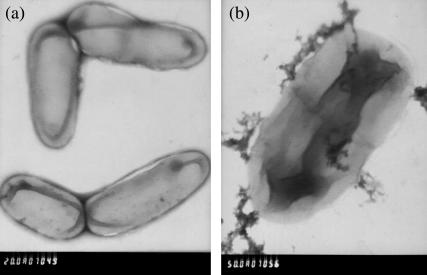
Fig. 2, a and b, are TEM images of free cell and microbial cell assembled with γ-Al2O3 nanosorbents, respectively. The γ-Al2O3 nanosorbents were efficiently assembled on the surfaces of microbial cell as shown in Fig. 2.
FIGURE 2.
TEM images of free cell and cell assembled with γ-Al2O3 nanosorbent. (a) Free cell. (b) γ-Al2O3-cell.
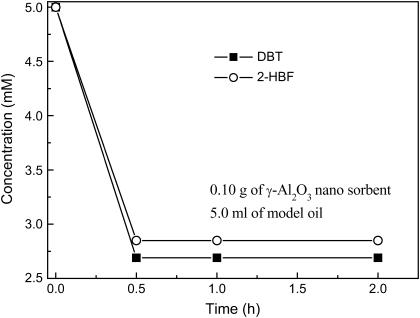
To investigate the adsorptive ability of γ-Al2O3 nanosorbents, an adsorptive process was performed in normal pressure, which 0.1 g sorbents were added into 5 mL model oil containing 5.0 mM of DBT and 5.0 mM of 2-HBP. Fig. 3 shows the adsorptive curve of γ-Al2O3 nanosorbents at 30°C. During the first 0.5 h, DBT and 2-HBP concentrations were, respectively, reduced to 2.69 and 2.85 mM from 5.00 mM. According to Fig. 3, the sorbents have been saturated with DBT and 2-HBP for <0.5 h. So, the adsorptive rate of γ-Al2O3 nanosorbents was >446 mmol·kg−1·h−1.
FIGURE 3.
Adsorption of DBT and 2-HBP using γ-Al2O3 nanosorbents.
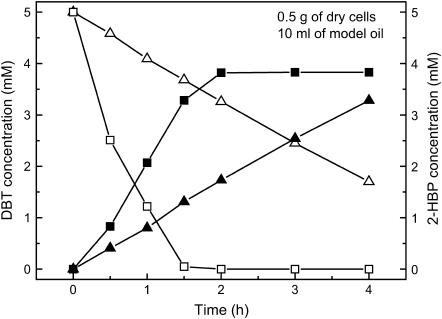
With the purpose of understanding the desulfurizing activity of the γ-Al2O3-cells, we tested the desulfurization rates of free cells and the γ-Al2O3-cells in model oil containing 5.0 mM of DBT, respectively. Fig. 4 shows that the consumption of DBT and the production of 2-HBP using free cells and γ-Al2O3-cells in the model oil. During the first 0.5 h, the rate of DBT consumption by the γ-Al2O3-cells is ∼4.93-fold higher than that by free cells. However, during the same time, the rate of 2-HBP produced by the γ-Al2O3-cells is only 1.02-fold higher than that by free cells. The rate of DBT consumption is not consistent with that of 2-HBP produced in model oil. However, during the subsequent 1.0 h, both the rates of DBT consumption and 2-HBP produced by the γ-Al2O3-cells are the same, which are ∼1.73-fold higher than those by free cells. It is because that the γ-Al2O3 nanosorbents can adsorb DBT from model oil, and the rate of adsorption is far higher than that of BDS (Fig. 2). The adsorption process can increase the rate of DBT transfer. During the first 0.5 h, a part amount of DBT molecules, ∼0.117 mmol (DBT consumed+2-HBP produced)/g(sorbent), was adsorbed into the pores of γ-Al2O3 nanosorbent, which resulted in the reduction of DBT and 2-HBP concentrations in oil phase. However, DBT molecules of these cannot be reacted and converted to 2-HBP by microbial cells. Besides, 2-HBP molecules of these produced were adsorbed into the γ-Al2O3 nanosorbents and could not be detected in oil phase. Therefore, the rate of DBT consumption in oil phase was higher than that of 2-HBP produced, detected in oil phase, during the first time. On the other hand, once the pores of γ-Al2O3 nanosorbents were saturated with DBT and 2-HBP molecules, the consumption of DBT in oil phase can completely be used to convert to 2-HBP, which also was completely transferred to oil phase. Thus, the increase of DBT consumption rate was equivalent to that of 2-HBP production rate after the saturation of sorbents with DBT. The final concentration of 2-HBP produced by γ-Al2O3-cells was ∼3.83 mM, less than the initial concentration of DBT. It may deduce that ∼0.012 mmol of DBT and 2-HBP were adsorbed into the pores of γ-Al2O3 nanosorbent.
FIGURE 4.
Time course of desulfurization of DBT by free cells and γ-Al2O3-cells. Residual DBT concentration after being consumed by free cells (▵); 2-HBP concentration produced by free cells (▴); residual DBT concentration after being consumed by γ-Al2O3-cells (□); 2-HBP concentration produced by γ-Al2O3-cells (▪).
The biodesulfurization rate of R-8 cells has been improved by γ-Al2O3-cell (Fig. 4). It is because that the desulfurization rate was mainly limited by the following two factors, i.e., catalytic activity of dsz enzymes in cells and the rate of mass transfer from oil phase to the cell body through aqueous phase. The adsorption of γ-Al2O3 nanosorbents to DBT can accelerate the DBT transfer from aqueous phase to the cell surfaces, which results in the increase of desulfurization rate. According to Fig. 3, the adsorptive rate of the γ-Al2O3 nanosorbent is >446 mmol·kg−1·h−1, which is far higher than the desulfurization rate of microbial cells. Thus, DBT molecules can be quickly congregated on the cell surface, where nanosorbents were located. Consequently, the DBT molecules can be transported into cell for biodesulfurization reaction. Thus, transfer limitation of the DBT molecules can be eliminated to some extent, which results in the improvement of biodesulfurization rate.
Acknowledgments
We acknowledge the financial support of the State Major Basic Research Development Program (China) (grant No. G2000048004), the National High Technology Research and Development Program (China) (No. 2002AA213041), and the National Natural Science Foundation of China (No. 30370046).
References
- 1.Gray, K. A., G. T. Mrachko, and C. H. Squires. 2003. Biodesulfurization of fossil fuels. Curr. Opin. Microbiol. 6:229–235. [DOI] [PubMed] [Google Scholar]
- 2.Erwin, K. N., S. Nakano, and P. Zuber. 2005. Sulfate-dependent repression of genes that function in organosulfur metabolism in Bacillus subtilis requires Spx. J. Bacteriol. 187:4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka, Y., O. Yoshikawa, and K. Maruhashi. 2002. The cbs mutant strain of Rhodococcus erythropolis KA2–5-1 expresses high levels of Dsz enzymes in the presence of sulfate. Arch. Microbiol. 178:351–357. [DOI] [PubMed] [Google Scholar]
- 4.Setti, L., P. Farinelli, D. S. Martino, S. Frassinetti, G. Lanzarini, and P. Pifferi. 1999. Developments in destructive and non-destructive pathways for selective desulfurizations in oil biorefining processes. Appl. Microbiol. Biotechnol. 52:111–117. [DOI] [PubMed] [Google Scholar]
- 5.Mehrnia, M. R., B. Bonakdarpour, J. Towfighi, and M. M. Akbarnejad. 2004. Design and operational aspects of airlift bioreactors for petroleum biodesulfurization. Environ. Prog. 23:206–214. [Google Scholar]
- 6.Luo, M. F., J. M. Xing, Z. X. Gou, S. Li, H. Z. Liu, and J. Y. Chen. 2003. Desulfurization of dibenzothiophene by lyophilized cells of Pseudomonas delafieldii R-8 in the presence of dodecane. Biochem. Eng. J. 13:1–6. [Google Scholar]