Abstract
The physiological function of gap junction channels goes well beyond their initially discovered role in electrical synchronization of excitable cells. In most tissues, gap junction cells facilitate the exchange of second messengers and metabolites between cells. To test which parts of the channels formed by connexins determine the exclusion limit for the transit of molecules in the size range of second messengers and metabolites a domain exchange approach was used in combination with an accessibility assay for nonelectrolytes and flux measurements. The experimental results suggest that two open hemichannel forming connexins, Cx46 and Cx32E143, differ in accessibility and permeability. Sucrose is at the exclusion limit for Cx46 channels whereas sorbitol is at the exclusion limit for Cx32E143 channels. In chimeras between these connexins, where the first transmembrane segment M1 is exchanged, the exclusion limits correlate with those of the M1 donor. The same segregation was found in a separate study for the unitary conductance of the channels. Thus, conductance and permeability/accessibility of the channels cosegregate with M1.
INTRODUCTION
The function of gap junction channels was first established for the electrical synchronization of cells (1–4). Subsequently it was found, that with very few exceptions, cells in most tissues are connected to each other by gap junctions (5,6). Because the majority of these cells are not electrically excitable the main function of gap junctions is metabolic coupling involving the flux of large permeants from cell to cell (7). The cutoff limit for intercellular molecule flux through gap junction channels is typically at ∼1 kD. This allows the transit of many metabolites and second messengers. Gap junctions are formed by the connexin family of proteins. The human genome contains at least 21 connexins that are expressed with some tissue specificity (8). The existence of a variety of connexin specific diseases suggests that the various members of the connexin family exert discrete functions although allowing for some overlap. Accordingly, replacement of connexins for each other by “knock-in” shows that they only partially can substitute for each other (9). Recently, a second family with three members, the pannexins have been discovered in vertebrates, however, their physiological role remains to be elucidated (10–12).
Gap junction channels formed by different connexins exhibit distinct channel characteristics such as conductance, permeability, and gating properties (13–15). The unitary conductance varies widely from 15 pS for Cx36 to 300 pS for Cx37 (16,17). Transfer of fluorescent tracer molecules as well as endogenous metabolites and second messengers may vary greatly among different connexin isoforms. It was shown that Cx43 channels were as much as 120–160-fold more permeable to ADP and ATP, and 30–45-fold more permeable to glutathione and glutamate than Cx32 channels (18).
In a cylindrical channel with exclusively steric constraints, channel conductance and permeability for tracer molecules should correlate. However, comparisons of various connexin channels revealed a poor correlation between these factors (19–21). Typically, tests for gap junction permeability involve charged fluorescent tracer molecules whose flux from cell to cell is analyzed. Interpretation of such data as relating to channel dimensions is difficult as macroscopic data and single-channel data need to be compared. Moreover, the transit of molecules through a channel is not only a question of channel geometry but electrostatic factors need to be considered also, in particular when charged tracers are involved (22–24).
To circumvent such problems in this study we studied the effect of molecules free of a net charge on channels. Nonelectrolytes have been used before to probe other channel dimensions as well as gap junction channels (25–34). This approach is based on the observation that nonelectrolytes affect channel conductance in a size-dependent way. The conductance is expected to remain unaffected if the nonelectrolyte is excluded from the channel lumen, whereas the conductance is reduced if the hydrodynamic radius of the nonelectrolyte is sufficiently small for it to enter the pore. Although often referred to as a channel transit test this approach does not allow the discrimination between permeability and accessibility. As long as the nonelectrolyte enters the channel it will exert its effect with or without passing through. We, therefore, used the same nonelectrolytes to perform influx and efflux measurements for corroboration.
MATERIALS AND METHODS
The same connexins and mutants were used as described in the accompanying manuscript. Xenopus oocytes were used for expression of injected mRNA.
Patch-clamp recording
The vitelline membrane of the oocytes was removed. If not specified, both bath and pipette solutions were potassium gluconate solution (140 mM KGlu, 10 mM KCl, and 5.0 mM TES, pH 7.5). Electrode pipettes (OD, 1.5 mm, ID, 0.86 mm, Warner Instruments, Hamden, CT) were pulled by Flaming-Brown micropipette puller (Sutter Instrument, Novato, CA) and polished by microforge (Narishige Scientific Instrument, Tokyo, Japan) to 0.5–1 μm with a resistance of 10–20 MΩ in KGlu solution. Single connexin hemichannels were studied by patch-clamp technique (23,34) using an Axopatch-1B amplifier (Axon Instruments, Inverurie, Scotland, UK). Excised inside-out and outside-out patches were used. All recordings were filtered at 5 kHz, digitized using a VR-10B digital data recorder and stored on videotape. The recordings were transferred to a Power Macintosh (Apple, Cupertino, CA) and data were analyzed by TAC software (Bruxton, Seattle, WA).
After an inside-out or outside-out patch was excised from the membrane and a connexin hemichannel was identified, the patch was transferred into a microperfusion chamber, which was continuously perfused with solution. The perfusion system was driven by gravity at a flow rate of 100 μl/s.
Accessibility test
The sugar to be tested was dissolved in KGlu solution to a sugar concentration of 100 mM and applied to the patch through a microchamber perfusion system. Inside-out patches were excised from connexin expressing oocytes. After a connexin channel was identified, the patch was held negative potentials, and 100 mM sugar was applied from the intracellular side of the channel for at least 5 min before washout with KGlu.
Channel activity was analyzed only for patches containing single channels and with data records exceeding 75 s both before and after the application of sugars. For assessment of the effects of the sugars on channel conductance, open probability (Po) and mean open time (MOT), paired t-tests were performed and the p-values are indicated in the figures. For determination of Po and MOT a threshold of 1/3 full amplitude was used, i.e., any event below this value was considered as a closed event.
3H-labeled sugar flux
3H-D-sorbitol (15 Ci/mM, American Radiolabeled Chemicals, St. Louis, MO) and 3H-sucrose (7.5 Ci/mM, Perkin Elmer, Wellesley, MA) were used for uptake experiment. The expression of connexin channels in oocytes was determined by whole-cell voltage clamp. In theory, the number of channels should be calculated using the following formula:
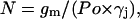 |
where gm is the membrane conductance; Po is the open probability; and γj is the single-channel conductance. However, in this article the Po was set to 1 because only open channels were of interest. Oocytes injected with the respective mRNA were incubated with 16 μCi/ml radioisotope-labeled sugars in high K+ solution (same as single-channel pipette solution) to open hemichannels. Another three groups were performed at the same time for control purposes. These control groups were: a), uninjected oocytes incubated with radioisotope-labeled sugars in 5 mM Ca2+ OR2 solution; b), oocytes injected with respective connexin mRNA incubated with radioisotope-labeled sugars in 5 mM Ca2+ OR2 solution to keep channel closed; and c), uninjected oocytes in high K+ solution with radioisotope-labeled sugars. After 15 min incubation at 20°C, the oocytes were taken out and washed extensively in 5 mM Ca2+ OR2 solution. Then each oocyte was transferred to a vial (minivial 6 ml, SARSTEDT, Nümbrecht, Germany) and broken by vortexing; 3 ml scintillation fluid (Ultra Gold, Packard Instrument, Boston, MA) was added to the vial and counts were measured in a scintillation counter (model 1212, ELS Life Sciences Technologies, Gaithersburg, MD). 3H-sucrose efflux was also measured. Oocytes without connexin mRNA and with Cx46 were injected with 10 μl 3H-sucrose and incubated in 5 mM OR2 for 30 min. Then oocytes were put into 50 μl of 5 mM Ca OR2 or high K+ Ringer solution for 15 min. An aliquot (25 μl) of the supernatant was collected for counting. Efficiency of the scintillation counter was determined by counting a standard sample under conditions identical to the experiment; the efficiency was ∼40%.
RESULTS
Accessibility of sugars to channels formed by Cx46 and Cx32E143
The accompanying article demonstrated that the unitary conductance could be reciprocally exchanged between two connexin channels by swapping the first transmembrane segment (M1) between them. To test whether the transit of larger permeants than the standard charge carriers, sodium and chloride, is affected in the chimeras we used nonelectrolytes to test their accessibility to the channel and their flux through it. Sugars with different molecular weight and diameter, were chosen to test if their access differs for the two parent connexins and if so if the accessibility limits are changed in the chimeras.
Uncharged molecules have been used before to size the pore in a number of channels including gap junction channels (25–35). Uncharged molecules change channel conductance in a concentration and size-dependent manner. Molecules too large to enter the channel do not change channel conductance. Molecules able to enter the channel decrease channel conductance and increase open channel noise. In theory the reduction of conductance initially will increase with increased molecule size, then the reduction will gradually diminish as the molecule size continues to rise so that their ability to enter the channel is gradually impaired (36). However, in alamethicin channels as well as connexin channels only the inverse relationship, i.e., reduction of conductance diminishes with increased molecule size, was observed (26,33). In Cx46 channels the addition of sugars not only decreased channel conductance but also decreased channel open probability and mean open time. In addition, the sugars induced long-lasting subconductance states. Whether this represents steric block by molecules in the pore lumen as described for cyclodextrins in Cx26 and Cx32 channels and for maltose in maltoporin channels remains to be determined (31,34). In this study we used sorbitol (182 mol wt), sucrose (343 mol wt), and stachyose (666 mol wt) with a size (abaxial diameter) of 5.8 Å, 8.9 Å, and 12 Å, respectively. The sugars were used at 100-mM concentration, which is lower than the typical usage of nonelectrolytes on other channels (26–30,35,36). At this concentration, effects could still be discerned while the osmotic stress imposed by a one-sided application was minimized. Because sugars too large to enter the channel did not change channel properties, osmotic effects are unlikely to cause the changes induced by the smaller sugars.
Typically, the sugars were applied to the cytoplasmic aspect of the channel. However, similar results were obtained with extracellular application for wt Cx46 channels (supplemental Fig. 1). Thus, regardless of the mechanism by which sugars affect channel properties (block or gating), they have to be accessible to at least a portion of the channel to exert an effect.
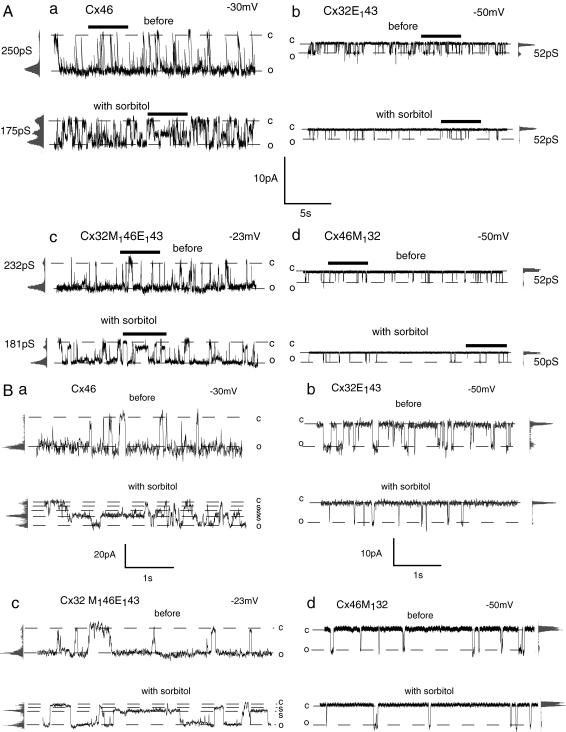
We first tested whether channel accessibility differed between Cx46 and Cx32E143. As shown in Fig. 1 A, the single-channel conductance was 250 pS for Cx46 and 52 pS for Cx32E143. In response to sorbitol (5.8 Å) perfusion the single-channel conductance of Cx46 was reduced from 250 to 175 pS. The mean open time and open probability were also reduced (Fig. 1 A (a)). In addition, at least two subconductance states were observed in Cx46 channels in the presence of sorbitol, similar as described previously (33) (Fig. 1 B (a)). In contrast, the single-channel conductance of Cx32E143 was not changed with sorbitol perfusion (Fig. 1 A (b)). But the mean open time and open probability of Cx32E143 were reduced by this sugar. In Cx32E143 no obvious subconductance state attributable to the sugar was observed (Fig. 1 B (b)).
FIGURE 1.
Effect of sorbitol on single-channel currents of wild-type (Cx46 and Cx32E143) and chimeric channels (Cx32M146E143 and Cx46M132). Representative recordings of the same channel contained in an inside-out membrane patch before (top trace) and with (bottom trace) 100 mM sorbitol perfusion are shown. Open (o) and closed (c) states are indicated by dotted lines. All-point amplitude histograms and single-channel conductance are shown on the side of each record. To facilitate recognition of the subconductance state (s), part of the recordings in Fig. 1 A (bars) are shown in Fig. 1 B at extended scale.
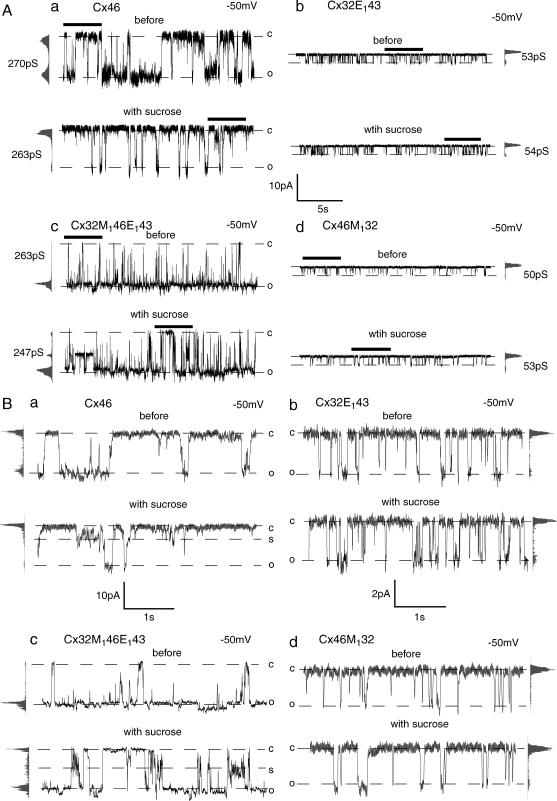
With sucrose (8.9 Å), no significant change in single-channel conductance of Cx46 was observed (Figs. 2 A (a) and Fig. 3). The mean open time and open probability of Cx46 were, however, reduced and at least one subconductance state was seen in response to sucrose perfusion (Fig. 2, A (a) and B (a)). On the other hand, the conductance, mean open time, and open probability were not changed in Cx32E143 channels in response to sucrose perfusion (Fig. 2, A (b) and B (b)).
FIGURE 2.
Effect of sucrose on single-channel currents of wild-type (Cx46 and Cx32E143) and chimeric channels (Cx32M146E143 and Cx46M132). Representative recordings of the same channel contained in an inside-out membrane patch before (top trace) and with (bottom trace) 100 mM sucrose perfusion are shown. Open (o) and closed (c) states are indicated by dotted lines. All-point amplitude histograms and single-channel conductance are shown on the side of each record. To facilitate recognition of the subconductance state (s), part of the recordings in Fig. 2 A (bars) are shown in Fig. 2 B at extended scale.
FIGURE 3.
Quantitative analysis of sorbitol (a, c, e) and sucrose (b, d, f) effects on single-channel conductance (γ), open probability (Po), and mean open time (MOT) of parental (Cx46 and Cx32E143) and chimeric channels (Cx32M146E143 and Cx46M132). Mean ± SE is plotted. Numbers of records analyzed are given above the bars. Statistical significance of difference between before- and after-sugar application based on one-tail paired t-test is indicated as * for p < 0.05 and ** for p < 0.01.
These observations suggest that Cx46 and Cx32E143 have different exclusion limits. Both sorbitol and sucrose were accessible to Cx46. Cx32E143 was accessible to sorbitol (5.8 Å) but excluded sucrose (8.9 Å). In Cx46 sorbitol caused a larger inhibition of channel conductance than sucrose but sucrose caused a larger reduction of channel open probability and mean open time (Fig. 3). These data suggest that the size of sucrose (8.9 Å) is close to the exclusion limit of Cx46 channels. Sucrose molecules apparently cause intermittent complete block of Cx46 channel as indicated by reduced channel open probability and mean open time without a detectable effect on unitary conductance. A similar phenomenon was seen in Cx32E143 channels in the presence of sorbitol. Sorbitol did not change conductance of Cx32E143 but significantly reduced its mean open time and open probability, suggesting sorbitol (5.8 Å) was close to the exclusion limit of Cx32E143. Thus, the accessibility of Cx46 channels for nonionic probes is larger than that of Cx32E143 channels. For these channels conductance and accessibility are correlated.
Accessibility of sugars to chimeric channels
To test whether M1 replacement changes the accessibility of sugars to the channel, the same procedure was applied to the two chimeras, Cx32M146E143 and Cx46M132. Before sugar perfusion the single-channel conductance of Cx32M146E143 was 232 pS, similar to its M1 donor, Cx46 (Fig. 1 A (c)). The single-channel conductance Cx46M132, with M1 derived from Cx32, was 52 pS (Fig. 1 A (d)). In response to sorbitol perfusion the single-channel conductance of Cx32M146E143 was reduced from 232 to 181pS. The mean open time and open probability were also reduced and there were at least two subconductance states observed in Cx32M146E143 (Fig. 1 B (d)). Sorbitol applied to Cx46M132 did not change the single-channel conductance, but the open probability and mean open time were reduced (Fig. 1 A (d)).
With sucrose perfusion the single-channel conductance of Cx32M146E143 remained essentially unaltered, whereas the open probability and mean open time were reduced. Furthermore, at least one subconductance state could be discerned (Fig. 2). In Cx46M132 channels, unit conductance, mean open time, and open probability were not affected by sucrose (Fig. 2 A (d)). No obvious subconductance state was observed in Cx46M132 in response to either sorbitol or sucrose perfusion (Figs. 1 B (d) and 2 B (d)).
Fig. 3 shows the quantitative analysis of changes in channel conductance, open probability, and mean open time in response to sorbitol and sucrose perfusion of wild-type (wt) and chimeric connexin channels. The data indicate that sucrose accessibilty to Cx32M146 E143 and Cx46 is similar. The effects of sorbitol on these channels were also similar. On the other hand, just like Cx32E143, Cx46M132 was accessible to sorbitol but excluded sucrose. Thus, like conductance, the accessibility of connexin channel also segregated with the first transmembrane segments.
Accessibility of sugars to Cx46M137 and Cx46L35G as compared to Cx46
The previous experiments demonstrated accessibility could be exchanged between Cx46 and Cx32E143 by M1 exchange between these two connexins. To test whether the role of M1 in determining channel accessibility also applied to other connexins, we tested the accessibility of Cx46M137, a chimera connexin with M1 of Cx46 replaced by that of Cx37. Cx37 form gap junction channels with the largest known conductance (17). As shown in the accompanying article, Cx46M137 exhibited single-channel conductance of ∼500 pS, which was much larger than that of wild-type Cx46. This chimera also was shown to acquire spermine ion permeability, which was not detectable in wild-type Cx46. Because the increased permeability of Cx46M137 to spermine ion could be the result from either steric constraints or electrostatic interaction, we again used uncharged molecules, in this case sucrose (8.9 Å) and stachyose (12 Å), to probe the size of the pore as described before.
Previous SCAM studies identified position 35 (in M1) in Cx46 as an important pore lining residue because cysteine introduced in this position leads to a large inhibition of channel conductance by thiol reagents (37). Point mutation with leucine replaced by glycine at this position, Cx46L35G, exhibited increased conductance over wild-type Cx46. Like Cx46M137, Cx46L35G was also permeable to spermine ion (accompanying article). Together these data suggest that the leucine residue at position 35 in Cx46 might form an important constraint for both conductance and permeability inside the pore. To test this notion, we probed the accessibility of nonionic probes to this mutant channel.
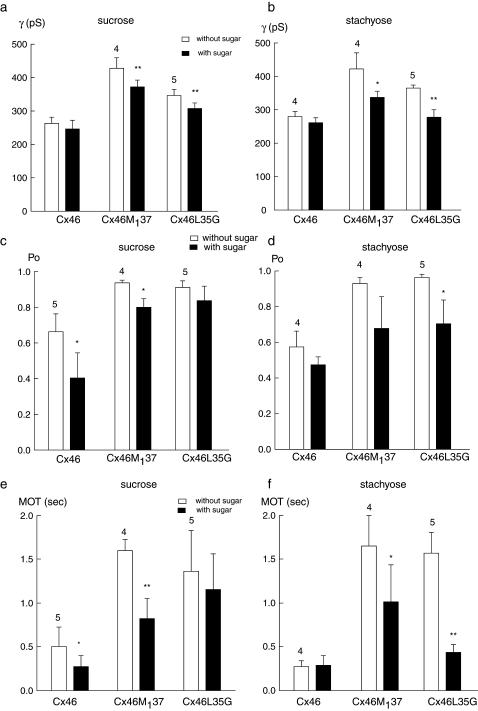
Fig. 4 shows the effect of sucrose on channel parameters of wt Cx46, Cx46M137, and Cx46L35G channels. As shown before, in the presence of sucrose the single-channel conductance of wt Cx46 was not significantly changed, but the open probability and mean open time were reduced. In Cx46M137 channels, the single-channel conductance was reduced from 426 to 363 pS. The mean open time and open probability were also reduced in Cx46M137 in response to sucrose perfusion (Fig. 4 b). In Cx46L35G channels the single-channel conductance was reduced from 380 to 336 pS; the open probability and mean open time were slightly reduced with sucrose perfusion. There was at least one subconductance state observed in Cx46L35G channels in the presence of sucrose.
FIGURE 4.
Effect of sucrose on single-channel currents of Cx46 (a), Cx46M137 (b), and Cx46L35G (c) channels. Representative recordings of the same channels contained in excised membrane patches before (top trace) and with (bottom trace) 100 mM sucrose perfusion are shown. Open (o) and closed (c) states are indicated by dotted lines. All-point amplitude histograms and single-channel conductance are shown on the side of each record.
Fig. 5 shows the effect of stachyose on these channels. This sugar was excluded from wt Cx46 channel. There was no detectable change in channel conductance, open probability, and mean open time in Cx46 in the presence of stachyose. In contrast, single-channel conductance of Cx46M137 channels was reduced from 465 to 317 pS by the sugar (Fig. 5 b). The mean open time of Cx46M137 was also reduced. In Cx46L35G all three parameters (conductance, open probability, and mean open time) were substantially reduced by stachyose perfusion (Fig. 5 c).
FIGURE 5.
Effect of stachyose on single-channel currents of Cx46 (a), Cx46M137 (b), and Cx46L35G (c) channels. Representative recordings of the same channel contained in an excised membrane patch before (top trace) and with (bottom trace) 100 mM stachyose perfusion are shown. Open (o) and closed (c) states are indicated by dotted lines. All-point amplitude histograms and single-channel conductance are shown on the side of each record.
A quantitative analysis of the change of channel property changes by sucrose (8.9 Å) and stachyose (12 Å) on wt Cx46, Cx46M137, and Cx46L35G is shown in Fig. 6. The data indicate that sucrose was accessible to all three types of channels, but was close to the exclusion limit for wild-type Cx46. Although Cx46 excluded stachyose, Cx46M137 and Cx46L35G were accessible to this sugar. The reduction of conductance in Cx46M137 and Cx46L35G was even larger with stachyose than with sucrose, suggesting the accessibility of these two connexin channels were greatly enlarged as compared to wild-type Cx46.
FIGURE 6.
Quantitative analysis of sucrose (a, c, e) and stachyose (b, d, f) effects on single-channel conductance (γ), open probability (Po), and mean open time (MOT) of wild-type Cx46, Cx46M137, and Cx46L35G channels. Mean ± SE is plotted. Numbers of records analyzed are given above the bars. Statistical significance of difference between before- and after-sugar application based on one-tail paired t-test are indicated as * for p < 0.05 and ** for p < 0.01.
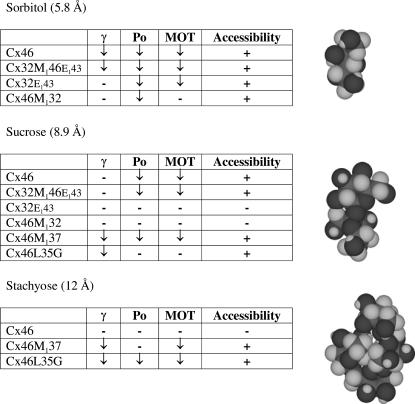
Table 1 summarizes the data obtained with the accessibility tests. The test molecule was deemed inaccessible to the channel if none of the measured channel parameters (γ, Po, and MOT) was affected by it. If one uses a more restrictive interpretation and only considers effects on channel conductance as indicative for channel accessibility, the limits are shifted by one test molecule. Sorbitol, but not sucrose, would be accessible to Cx46 whereas Cx32E143 channels would exclude all tested sugars. This, however, does not impinge on the conclusion that channel properties can be transferred by M1 exchange.
TABLE 1.
Summary of accessibility tests
Flux of 3H-sugars
The previous experiments only tested channel accessibility but not permeability. Molecules that access the channel lumen do not necessarily pass through the channel. To complement the previous experiments, the sugar molecules used in single-channel studies labeled with tritium were used to measure flux of the labeled sugars. Because data on single channel suggested sorbitol was near the exclusion limit for Cx32E143 and sucrose was near the exclusion limit for Cx46, we used the 3H-labeled versions of these sugars to determine flux.
The influx of 3H-sugar in oocytes expressing Cx46 or Cx32E143 was compared first. Table 2 shows the raw data for 3H-sorbitol influx in uninjected control oocytes and in oocytes expressing Cx32E143. The flux was significantly (p < .001) higher in oocytes with the channels opened by depolarization than in oocytes with the channels closed by elevated calcium and in control oocytes.
TABLE 2.
Influx of 3H-sorbitol in oocytes expressing Cx32E143 and control oocytes
| 5 mM Ca2+ OR2 | High K+ OR2 | |
|---|---|---|
| Control | 43 ± 16 | 38 ± 8 |
| Cx32E143 | 26 ± 6 | 472 ± 107* |
Mean ± SD of counts per minute (cpm) is given for 10 oocytes, counted individually. Oocytes were incubated in OR2 with elevated calcium to keep the channels closed, or in high K+ solution to open the channels.
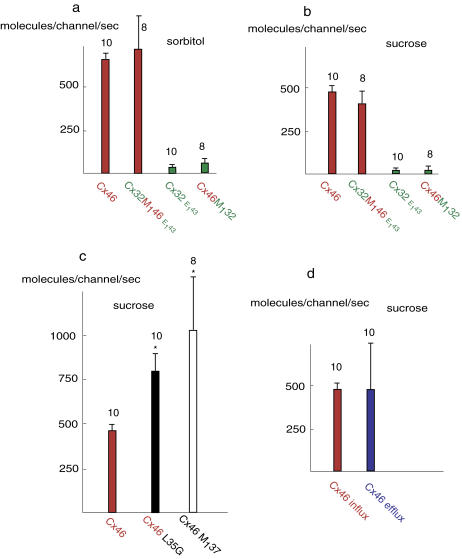
As shown in Fig. 7, the influx of 3H-sugars for Cx46 was much larger than for Cx32E143. The influx of 3H-sorbitol was 655.0 ± 32.2 molecules per second for each Cx46 channel and 36.8 ± 8.3 for each Cx32E143 channel. The mean 3H-sucrose influx per second was 476.8 ± 33.3 molecules for each Cx46 channel and 19.3 ± 10.6 molecules for each Cx32E143 channel.
FIGURE 7.
Quantitative analysis of 3H-sorbitol and 3H-sucrose flux through wild-type and chimeric connexin channels. Data are presented as average influx (a–c) or efflux (d) of 3H-sugar molecules per second through each open connexin hemichannel. Ten oocytes for each condition were averaged and mean ± SD is plotted.
Flux measurements were also performed in the chimeras. The uptake of 3H-sorbitol and 3H-sucrose through Cx32M146E143 channels was similar to that through Cx46 channels, whereas the uptake through Cx46M132 channels was similar to that through Cx32E143 channels (Fig. 7, a and b).
The single-channel data suggested that sucrose was near the exclusion limit for wild-type Cx46, but far below the exclusion limit for Cx46M137 and Cx46L35G. Accordingly, flux of 3H-sucrose through Cx46M137 and Cx46L35G channels was significantly higher than through wild-type Cx46 channels (Fig. 7 c).
Although sucrose was near the exclusion limit for Cx46 channels, the influx of 3H-sucrose in Cx46 was substantial. To exclude the possibility that sucrose molecules were bound to the extracellular portion of the pore rather than passing through the channel, efflux of 3H-sucrose in Cx46 was also evaluated. As shown in Fig. 7 d, the 3H-sucrose efflux was matched with its influx, suggesting sucrose was indeed passing through the Cx46 channel. Both efflux and influx measurements were performed with similar intracellular and extracellular 3H-sucrose concentrations, respectively.
DISCUSSION
The results show that both accessibility and permeability of connexin channels can be transferred between connexins by exchange of the first transmembrane segment, M1. The exchange in either direction, from Cx46 to Cx32 and from Cx32 to Cx46, yielded consistent results indicating that the observations probably are not merely fortuitous. The same type of phenomenon was observed in the accompanying article for single-channel conductance. Thus, conductance and permeability/accessibility are well correlated. This appears to contradict the findings on gap junction channels made by different connexins where a poor correlation between unit conductance and permeability for tracer molecules was observed. In particular, the connexin channel with the largest unit conductance of 300 pS, Cx37, was found to be highly restrictive for the transit of test molecules (21). Yet, insertion of the M1 segment of Cx37 into Cx46 yielded a larger conductance and an accessibility/permeability for stachyose and sucrose exceeding that of wt Cx46 considerably.
It is of note that the experimental approaches taken here differ from those used in the comparison of the various connexin channels substantially. Most studies of the permeability of gap junction channels involve charged test molecules in the form of fluorescent tracers of various molecular sizes and the resulting macroscopic observations are compared with single-channel measurements of conductance. Alternatively, the permeability ratios for various sized ions have been calculated using the Goldman-Hodgkin-Katz equation on the basis of reversal potential determinations with salt gradients across the membrane patch. In both approaches the flux of the test molecules is a function of steric constraints as well as electrostatic factors.
With the use of nonionic probes we minimized the effect of electrostatic effects and mainly tested steric constraints. However, even in the absence of a net charge, dipole moments cannot be completely excluded. Surface charges on the sugars could still influence their transit through the channel.
Another potential reason why these results appear at odds with the findings in the comparison of wild-type connexins could be the limitation of the analysis to the first transmembrane segment. Despite the unqualified correlation between conductance and permeability in the channels tested here, it is feasible that in different connexins additional domains come into play in determining permeability. For example, whereas conductance may be determined by M1, the transit of larger molecules could be limited by an additional constriction provided by cytoplasmic moieties. As long as an additional constriction is short in length it may not significantly contribute to the channel's electrical resistance while representing an effective filter for the flux of larger molecules.
As observed in a previous study (33) the effect of sugars on the channel was complex. The sugars small enough to enter the channel not only reduced the unit conductance (γmax) inversely related to sugar size, but also affected open probability (Po) and mean open time (MOT). In addition, the sugars induced substates characteristic for the respective sugar.
The duration of the sugar-induced substates indicates that the molecules can be retained/trapped in the channel for extended periods of time. This raises the question whether they actually pass through the channel. Flux measurement with the same sugars show that both influx and efflux rates are matched and thus the molecules indeed permeate the membrane rather than merely partitioning into it. The flux measurements yielded transit rates for sugars through hemichannels similar to those reported for fluorescent tracers and 100,000 times lower than the diffusion rates for ions (15,33). In part this discrepancy can be explained by the different concentrations of permeants used in the different measurements. In this study only micromolar concentrations of the H3-labeled sugars were used. Nicholson and co-workers (39,40) published transit rates orders of magnitude higher based on studies of tracer fluxes from cell to cell using fluorescence microscopy on intact oocytes. The authors apparently assumed that the fluorescence seen on the surface is representative for the whole oocyte. Confocal microscopy, however, reveals that the optical properties of oocytes do not permit the visualization of cytoplasmic fluorescence except of a thin rim at the cell surface even with direct injection of the tracer molecule into the cell (Y. Qu and G. Dahl, unpublished data). This suggests that the oocyte's compacted yolk acts as a “black hole” for the exciting and/or the emitted light and only a small fraction of the cytoplasm under the membrane actually can be observed by this method. Thus, fluorescence microscopy on intact oocytes leads to a gross underestimate of the fluorescence in the (injected) donor cell and yields an overestimate of junctional permeabilities.
In conclusion, the following structural and functional aspects of connexin membrane channels and in analogy, gap junction channels, emerge. The channel pore is formed by the carboxy-terminal half of the first transmembrane segment and the amino-terminal part of the first extracellular loop. What segment forms the remainder of the channel wall is as yet undetermined although M3 remains a candidate (37,41,42). The channel probably has an asymmetric hourglass shape with a small vestibulum at the extracellular surface, a pore restriction within the extracellular half of the membrane, and a wide vestibulum at the intracellular half. Some connexins may have additional constraints at the cytoplasmic entry that may be steric or electrostatic in nature. The arguments for such a structural organization are as follows. Single-channel SCAM revealed that the extracellular part of the channel can accept six methanethiosulfonate molecules yet leaving a 40% residual unit conductance (43). A single maleimidobutyryl-biocytin molecule binding at the 35 position in M1, on the other hand leaves a 20% residual conductance (44). Although MTS reagents are smaller than MBB, six of these molecules will occupy a considerably larger space than a single MBB molecule. The observation, that the accessibility and permeability for nonelectrolytes segregate with the M1 segment in connexin chimeras as described here, reemphasize the steric constraint imparted by this segment. Reactivity of M3 positions with thiol reagents is minimal (37,41,43) suggesting that the portion of the channel lined by M3 (if at all) forms a wide vestibulum.
Although the extracellular portion may be less restrictive sterically than the M1 portion, concerning ion flux it may be equally important because of its electrostatic effects (23). Although this effect is well pronounced in hemichannels, where this segment faces the external milieu, it should be of lesser impact on permeation through complete cell to cell gap junction channels. In the latter channels, this segment is located in the center of the channel and thus cannot increase the availability of ions for passage, a mechanism demonstrated for the enhanced transit of potassium ions through the maxi K-channel (45).
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
We thank Drs. C. Grewer and W. Nonner for valuable discussions and for critically reading the manuscript.
This work was supported by the National Institutes of Health (grant GM48610).
References
- 1.Barr, L. 1963. Propagation in vertebrate visceral smooth muscle. J. Theor. Biol. 4:73–85. [DOI] [PubMed] [Google Scholar]
- 2.Barr, L., and W. Berger. 1964. The role of current flow in the propagation of cardiac muscle action potentials. Pflugers Arch. 279:192–194. [DOI] [PubMed] [Google Scholar]
- 3.Barr, L., W. Berger, and M. M. Dewey. 1968. Electrical transmission at the nexus between smooth muscle cells. J. Gen. Physiol. 51:347–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furshpan, E. J., and D. D. Potter. 1959. Transmission of the giant motor synapses of the crayfish. J. Physiol. 145:289–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loewenstein, W. R. 1981. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol. Rev. 61:829–912. [DOI] [PubMed] [Google Scholar]
- 6.Loewenstein, W. R. 1966. Permeability of membrane junctions. Ann. N. Y. Acad. Sci. 137:441–472. [DOI] [PubMed] [Google Scholar]
- 7.Harris, A. L. 2001. Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 34:325–472. [DOI] [PubMed] [Google Scholar]
- 8.Sohl, G., and K. Willecke. 2004. Gap junctions and the connexin protein family. Cardiovasc. Res. 62:228–232. [DOI] [PubMed] [Google Scholar]
- 9.Plum, A., G. Hallas, T. Magin, F. Dombrowski, A. Hagendorff, B. Schumacher, C. Wolpert, J. Kim, W. H. Lamers, M. Evert, P. Meda, O. Traub, et al. 2000. Unique and shared functions of different connexins in mice. Curr. Biol. 10:1083–1091. [DOI] [PubMed] [Google Scholar]
- 10.Panchin, Y., I. Kelmanson, M. Matz, K. Lukyanov, N. Usman, and S. Lukyanov. 2000. A ubiquitous family of putative gap junction molecules. Curr. Biol. 10:R473–R474. [DOI] [PubMed] [Google Scholar]
- 11.Bruzzone, R., S. G. Hormuzdi, M. T. Barbe, A. Herb, and H. Monyer. 2003. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA. 100:13644–13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao, L., S. Locovei, and G. Dahl. 2004. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572:65–68. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg, G. S., V. Valiunas, and P. R. Brink. 2004. Selective permeability of gap junction channels. Biochim. Biophys. Acta. 1662:96–101. [DOI] [PubMed] [Google Scholar]
- 14.Moreno, A. P. 2004. Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc. Res. 62:276–286. [DOI] [PubMed] [Google Scholar]
- 15.Valiunas, V. 2002. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J. Gen. Physiol. 119:147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivas, M., R. Rozental, T. Kojima, R. Dermietzel, M. Mehler, D. F. Condorelli, J. A. Kessler, and D. C. Spray. 1999. Functional properties of channels formed by the neuronal gap junction protein connexin36. J. Neurosci. 19:9848–9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veenstra, R. D., H. Z. Wang, E. C. Beyer, S. V. Ramanan, and P. R. Brink. 1994. Connexin37 forms high conductance gap junction channels with subconductance state activity and selective dye and ionic permeabilities. Biophys. J. 66:1915–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg, G. S., P. D. Lampe, and B. J. Nicholson. 1999. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat. Cell Biol. 1:457–459. [DOI] [PubMed] [Google Scholar]
- 19.Veenstra, R. D., H. Z. Wang, D. A. Beblo, M. G. Chilton, A. L. Harris, E. C. Beyer, and P. R. Brink. 1995. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ. Res. 77:1156–1165. [DOI] [PubMed] [Google Scholar]
- 20.Elfgang, C., R. Eckert, H. Lichtenberg-Frate, A. Butterweck, O. Traub, R. A. Klein, D. F. Hulser, and K. Willecke. 1995. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 129:805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong, X. Q., and B. J. Nicholson. 2001. Size selectivity between gap junction channels composed of different connexins. Cell Commun. Adhes. 8:187–192. [DOI] [PubMed] [Google Scholar]
- 22.Oh, S., J. B. Rubin, M. V. Bennett, V. K. Verselis, and T. A. Bargiello. 1999. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J. Gen. Physiol. 114:339–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trexler, E. B., F. F. Bukauskas, J. Kronengold, T. A. Bargiello, and V. K. Verselis. 2000. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys. J. 79:3036–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholson, B. J., P. A. Weber, F. Cao, H. Chang, P. Lampe, and G. Goldberg. 2000. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz. J. Med. Biol. Res. 33:369–378. [DOI] [PubMed] [Google Scholar]
- 25.Krasilnikov, O. V., R. Z. Sabirov, V. I. Ternovsky, P. G. Merzliak, and J. N. Muratkhodjaev. 1992. A simple method for the determination of the pore radius of ion channels in planar lipid bilayer membranes. FEMS Microbiol. Immunol. 5:93–100. [DOI] [PubMed] [Google Scholar]
- 26.Bezrukov, S. M., and I. Vodyanoy. 1993. Probing alamethicin channels with water-soluble polymers. Effect on conductance of channel states. Biophys. J. 64:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezrukov, S. M., I. Vodyanoy, and V. A. Parsegian. 1994. Counting polymers moving through a single ion channel. Nature. 370:279–281. [DOI] [PubMed] [Google Scholar]
- 28.Bezrukov, S. M., L. Kullman, and M. Winterhalter. 2000. Probing sugar translocation through maltoporin at the single channel level. FEBS Lett. 476:224–228. [DOI] [PubMed] [Google Scholar]
- 29.Bezrukov, S. M. 2000. Ion channels as molecular coulter counters to probe metabolite transport. J. Membr. Biol. 174:1–13. [DOI] [PubMed] [Google Scholar]
- 30.Merzlyak, P. G., L. N. Yuldasheva, C. G. Rodrigues, C. M. Carneiro, O. V. Krasilnikov, and S. M. Bezrukov. 1999. Polymeric nonelectrolytes to probe pore geometry: application to the alpha-toxin transmembrane channel. Biophys. J. 77:3023–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kullman, L., M. Winterhalter, and S. M. Bezrukov. 2002. Transport of maltodextrins through maltoporin: a single-channel study. Biophys. J. 82:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh, S., Y. Ri, M. V. Bennett, E. B. Trexler, V. K. Verselis, and T. A. Bargiello. 1997. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 19:927–938. [DOI] [PubMed] [Google Scholar]
- 33.Qu, Y., and G. Dahl. 2004. Accessibility of Cx46 hemichannels for uncharged molecules and its modulation by voltage. Biophys. J. 86:1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locke, D., I. V. Koreen, J. Y. Liu, and A. L. Harris. 2004. Reversible pore block of connexin channels by cyclodextrins. J. Biol. Chem. 279:22883–22892. [DOI] [PubMed] [Google Scholar]
- 35.Sabirov, R. Z., O. V. Krasilnikov, V. I. Ternovsky, and P. G. Merzliak. 1993. Relation between ionic channel conductance and conductivity of media containing different nonelectrolytes. A novel method of pore size determination. Gen. Physiol. Biophys. 12:95–111. [PubMed] [Google Scholar]
- 36.Krasilnikov, O. V., J. B. Da Cruz, L. N. Yuldasheva, W. A. Varanda, and R. A. Nogueira. 1998. A novel approach to study the geometry of the water lumen of ion channels: colicin Ia channels in planar lipid bilayers. J. Membr. Biol. 161:83–92. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, X. W., A. Pfahnl, R. Werner, A. Hudder, A. Llanes, A. Luebke, and G. Dahl. 1997. Identification of a pore lining segment in gap junction hemichannels. Biophys. J. 72:1946–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deleted in proof.
- 39.Nitsche, J. M., H. C. Chang, P. A. Weber, and B. J. Nicholson. 2004. A transient diffusion model yields unitary gap junctional permeabilities from images of cell-to-cell fluorescent dye transfer between Xenopus oocytes. Biophys. J. 86:2058–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber, P. A., H. C. Chang, K. E. Spaeth, J. M. Nitsche, and B. J. Nicholson. 2004. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 87:958–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skerrett, I. M., J. Aronowitz, J. H. Shin, G. Cymes, E. Kasperek, F. L. Cao, and B. J. Nicholson. 2002. Identification of amino acid residues lining the pore of a gap junction channel. J. Cell Biol. 159:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleishman, S. J., V. M. Unger, M. Yeager, and N. Ben-Tal. 2004. A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Mol. Cell. 15:879–888. [DOI] [PubMed] [Google Scholar]
- 43.Kronengold, J., E. B. Trexler, F. F. Bukauskas, T. A. Bargiello, and V. K. Verselis. 2003. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J. Gen. Physiol. 122:389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfahnl, A., and G. Dahl. 1998. Localization of a voltage gate in connexin46 gap junction hemichannels. Biophys. J. 75:2323–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brelidze, T. I., X. Niu, and K. L. Magleby. 2003. A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc. Natl. Acad. Sci. USA. 100:9017–9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.