Abstract
During productive infection by herpes simplex virus 1 (HSV-1), viral gene expression occurs in a temporally regulated cascade in which transcription of the viral immediate-early (IE) genes is strongly stimulated by the virion protein VP16. We have employed an oligonucleotide microarray to examine the effect of VP16 mutations on the overall pattern of viral gene expression following infection of HeLa cells. This microarray detects essentially all HSV-1 transcripts with relative and absolute levels correlating well with known kinetics of expression. This analysis revealed that deletion of the VP16 activation domain sharply reduced overall viral gene expression; moreover, the pattern of this reduced expression varied greatly from the pattern of a wild-type (wt) infection. However, when this mutant virus was delivered at a high multiplicity of infection or in the presence of the cellular stress inducer hexamethylene bisacetamide, expression was largely restored to the wt levels and pattern. Infection with virions that deliver wt VP16 protein at the start of infection but synthesize only truncated VP16 resulted in a normal kinetic cascade. This suggests that newly synthesized VP16 does not play a significant role in the expression of later classes of transcripts. The VP16 activation domain comprises two subregions. Deletion of the C-terminal subregion resulted in minimal changes in the level and profile of gene expression compared to a normal (wt) cascade. In contrast, deletion of the N-terminal subregion reduced the overall expression levels and skewed the relative levels of IE transcripts but did not significantly alter the kinetic pattern of early and late transcript expression. We conclude that the general activation of IE gene transcription by VP16, but not the specific ratios of IE transcripts, is necessary for the subsequent ordered expression of viral genes. Moreover, this report establishes the feasibility of microarray analysis for globally assessing viral gene expression programs as a function of the conditions of infection.
During productive infection of mammalian cells by herpes simplex virus type 1 (HSV-1), transcription of the viral genes occurs in a coordinated cascade (11, 28; reviewed in references 34 and 37). The five viral immediate-early (IE) genes are transcribed rapidly upon viral entry and uncoating. Synthesis of the IE proteins peaks at approximately 2 to 4 h postinfection (hpi), but they continue to accumulate throughout the infection. The IE proteins stimulate the expression of the early (E) and late (L) genes and also have autoregulatory functions.
The virion protein VP16 activates transcription of the IE genes through specific sequence elements in the IE promoters (reviewed in reference 19). VP16 interacts with the viral DNA template by associating with the cellular proteins Oct-1 and HCF-1 at promoter elements containing the sequence TAATGARAT (where R represents purine) (reviewed in reference 9). Activation by VP16 is also exerted through DNA sequences bound by the cellular protein GABP (15, 25, 32). Transcriptional activation is mediated by interactions of the VP16 activation domain with components of the RNA polymerase II transcription machinery and perhaps any of several cellular coactivators (reviewed in references 7 and 30). Mutations within the VP16 activation domain can result in reduced expression from IE promoters either on reporter plasmids or on viral genomes (for examples, see references 4, 5, 27, 29, 31, and 35). Infection of permissive cells in the absence of expression of IE proteins markedly decreases virus yield and, by inference, the efficiency of initiation of productive infection in any given cell.
Among the open questions pertaining to the kinetic cascade of transcription exhibited in HSV-1 infection is whether specific defects in IE expression result in defects in expression of specific E or L genes or to a more global reduction of all later transcripts. This question has an important bearing on accurate modeling of latency and certain aspects of viral neuropathogenesis. To explore this question, we have used an oligonucleotide-based DNA microarray to determine the steady-state mRNA abundance and thus the level of expression of HSV-1 genes from viruses that harbor specific mutations in the VP16 activation domain (29).
DNA microarrays are powerful tools for analyzing genomewide expression under different biological conditions and have been profitably used to examine both the expression of the viral genes and the effects of viral infection on host gene expression (16, 33). Microarrays can assess steady-state RNA levels of many genes simultaneously from a given sample but, like single-gene assays such as Northern blots, primer extension, and nuclease protection assays, do not distinguish between rates of mRNA synthesis and rates of mRNA decay. Microarray analyses of viral gene expression patterns following infection with cytomegalovirus, human herpesvirus 8, and HSV-1 were used to assign specific viral genes to either the latent or lytic pathway and to define kinetic classes for previously uncharacterized genes (3, 12, 21, 26, 33). Furthermore, despite a general decline in host cell mRNA levels during HSV-1 infection (14, 20), microarray analyses of host gene expression indicated increased expression of several transcription factors, the stress response protein GADD45, and interferon-dependent and interferon-independent transcripts (13). Infection by a viral mutant expressing only one IE protein, ICP0, induced the expression of a small set of host genes, many associated with cell cycle arrest (10). Similar microarray analyses of host gene expression have been performed for infections by other herpesviruses, including human cytomegalovirus (38) and Marek's disease virus (18).
A prototype HSV-1 oligonucleotide microarray was previously used to assess the regulatory impact of a mutation in the IE gene encoding ICP27 (26). In the present report, we describe the use of an expanded HSV-1 array to examine gene expression from viruses that lack part or all of the VP16 activation domain. Complete deletion of the VP16 activation domain resulted in significant disruption of the normal global patterns of regulated HSV gene expression. This disruption could be largely overcome by increasing the number of virion particles or by infecting in the presence of a cellular stress inducer, N′,N′-hexamethylene bisacetamide (HMBA). Deletion of either of two subregions of the activation domain resulted in different changes in the relative levels of IE transcripts but did not markedly alter the overall relative patterns of transcript abundance seen in a normally regulated cascade of expression.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells were obtained from the American Type Culture Collection. 16-8 cells, which are Vero cells bearing an integrated copy of the VP16 gene, were obtained from Steven Weinheimer (36). Cells were maintained at 37°C under 10% CO2 in Dulbecco modified Eagle medium containing 10% fetal bovine serum. Cultures of 3 × 106 HeLa or 16-8 cells in 100-mm2 plates were used for infections. Virus was adsorbed for 1 h prior to the addition of fresh overlay medium consisting of Dulbecco modified Eagle medium containing 2% fetal bovine serum. For cells treated with cycloheximide (Sigma), the inhibitor was added at a concentration of 60 μg/ml for 1 h prior to addition of virus and was present in the virus inoculum and overlay medium. HMBA (Sigma) was added after virus adsorption to a final concentration of 5 mM.
Viral mutants constructed from the KOS strain of HSV-1 have been described (29). Strain RP3 bears a deletion of codons 456 to 490 of the VP16 gene, RP4 lacks codons 413 to 452, and RP5 lacks codons 413 to 490. RP5 grown on the VP16-expressing cell line 16-8 is designated RP5/16-8. The mutation in RP5 was rescued by homologous recombination using a wild-type VP16 gene fragment from strain KOS, yielding strain RP5R, which was used here as the control wt virus.
RNA preparation and generation of fluorescence-tagged cDNA.
Total RNA was harvested at various times after infection by using Tri-Reagent (Molecular Research Center, Inc., Cincinnati, Ohio). Labeling and hybridization procedures have been described in detail (26). Fluorescence-labeled cDNA was prepared from 1-μg aliquots of purified poly(A)-containing RNA by random hexamer-primed polymerization using Superscript II reverse transcriptase (Gibco-BRL). The pool of nucleotides in the labeling reaction consisted of 0.5 mM dGTP, dATP, and dTTP, 0.3 mM dCTP, and fluorescent nucleotides (Cy3dCTP or Cy5dCTP; Amersham) at 0.1 mM. Fluorescence-labeled DNA was purified by chromatography through Microcon YM-20 columns (Amicon), heat denatured for 2 min at 100°C, and incubated for 20 to 30 min at 37°C before use.
Generation of microarrays, hybridization, and scanning.
Oligonucleotide selection, synthesis, and deposition on the chip have been described (26, 33). Microarrays were hybridized for 16 h in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.2% sodium dodecyl sulfate (SDS) at 68°C under coverslips with combined Cy5dCTP and Cy3dCTP-labeled cDNA. The entire assembly was enclosed in a commercial hybridization chamber (GeneMachines, San Carlos, Calif.). After hybridization, the microarray slide assembly was washed for 5 min in 1× SSC-0.2% SDS at room temperature for 5 min, 5 min in 0.1× SSC-0.2% SDS at room temperature, and 1 min in 0.1× SSC and spun dry in a low-speed centrifuge. Microarrays were scanned with a confocal laser ScanArray 4000 system (General Scanning, Inc.). Data were collected at a maximum resolution of 10 μm/pixel with 16 bits of depth by using Quantarray software (General Scanning, Inc.).
The scanning process samples the fluorescence (expressed in arbitrary units) derived from each spotted oligonucleotide. These values were adjusted by subtracting the background fluorescence of an equivalent area within a concentric ring just outside the spotted sample. The data from individual spots were then expressed in a Microsoft Excel spreadsheet, and net values were determined by subtracting the average of values obtained from measuring the fluorescence of a large number (ca. 100) of regions spotted with SSC alone. Typically, and depending on the exact laser power of the scan, this SSC background ranged from 50 to 500 arbitrary units.
Weak fluorescent signals are inherently less reliable than strong ones. Moreover, the ratio of fluorescent signal to actual sample value is linear only to net (minus SSC) values of 40,000 or so. To accommodate these limitations, laser power for scanning was adjusted for optimum signal sensitivity to between 75 and 90, with the photomultiplier set at 5 units less than the laser power. Standardization studies have shown that increasing laser power by 5 units in the range used increases signals in the reciprocity range approximately by a factor of 2. Multiple scans at various laser power settings were used to optimize all signals of interest.
To compare data from the various experimental conditions, the net (minus SSC) hybridization values were compiled in several steps. First, in each separate experiment (hybridization) and for each transcript set, a median value was calculated from the three or six replicate probe spots on a given chip. Each experiment was repeated at least three times, and the median values from those experiments were pooled to calculate the final median values displayed in the tables. To compare data from replicate experiments, the 75th percentile rank for the total viral hybridization was calculated. One experiment was arbitrarily chosen as the reference and the 75th percentile values of all other determinations were adjusted to this value by appropriate factoring. In this way, chips belonging to each experimental group are scaled accordingly. The relative abundance of each transcript was calculated as the quotient of the final median value for that transcript divided by the sum of the median values for all viral transcripts. These relative abundance values are displayed as percentages. To compare expression levels under different conditions, the sets of median values (or, in some cases, the sets of relative abundance values) from all replicates of the conditions being considered were evaluated using Student's two-tailed t test, assuming unequal variance and with the null hypothesis being that the true values under those two conditions are identical.
RESULTS
Lack of VP16 activation of IE transcripts profoundly affects the normal transcription program.
An oligonucleotide microarray specific for HSV-1 has been developed to effectively measure the abundance of a large set of viral RNAs, with results fully consistent with the established kinetics of gene expression (26). The HSV-1 chip used initially contained 60 sets of sense oligonucleotides for the unique detection of HSV-1 transcript families, including 28 probes representing HSV-1 transcripts that are uniquely terminated by a cleavage and polyadenylation signal and 24 additional probes representing partially overlapping transcripts. In the present work, we employed an expanded array that contains 5′-specific as well as 3′-specific probes to resolve nested transcript sets (described in detail in reference 33). This array contains 67 unique sets of HSV-1 probes, of which 43 represent transcripts from single genes. Another 11 probes detect sets of viral transcripts within a single kinetic class that share polyadenylation signals. Thus, a total of 54 probes detect either individual or overlapping transcript sets whose kinetics can be unambiguously assigned, while an additional 13 probes detect either overlapping transcripts of different kinetic classes or transcripts whose kinetics have not been previously assigned. For example, the oligonucleotides corresponding to the IE gene encoding ICP47 also detect transcripts from two E promoters.
To validate the ability of this new microarray to detect viral transcripts of various kinetic classes, we used this array to measure RNAs expressed during infection of HeLa cells by a wt HSV-1 virus. Our nominal wt virus, designated RP5R, is a derivative of strain KOS and was constructed by replacing the truncated VP16 gene of strain RP5 with the intact wt VP16 gene (29). HeLa cells were infected with RP5R virus at a multiplicity of infection (MOI) of 5 PFU/cell. After 2, 4, or 8 h, poly(A)-containing mRNA was isolated. Fluorescent-labeled cDNA was synthesized and hybridized to the HSV-1 chip, and the fluorescence at each oligonucleotide spot was measured by laser scanning. The results derived from four independent hybridizations (two biological replicates) are compiled in Table 1 and displayed in Fig. 1. For ease of comparison, the 54 transcript groups that can be unambiguously assigned to a specific kinetic class are arbitrarily numbered and grouped according to kinetic classes (Table 1). The pattern of gene expression during RP5R infection followed the well-established kinetics of HSV-1 infection, with the IE genes predominating at 2 h, followed by the E genes at 4 h and the L genes at 8 h. After peak expression of the different kinetic classes of transcripts was reached, total amounts of RNA for most representatives of each class did not significantly decrease at later time points; however, the relative abundance of E transcripts declined as later transcripts accumulated. This pattern is typical of the normal E-to-L transition (26, 33).
TABLE 1.
Abundance of HSV-1 transcripts at various times following infection of HeLa cells with RP5R virusa
| Transcript set | Kinetic class and IDb | Abundance atc:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 hpi
|
4 hpi
|
8 hpi
|
||||||||
| Median | SD | % | Median | SD | % | Median | SD | % | ||
| RICP0 | IE-1 | 18,800 | 18,500 | 13.9 | 33,250 | 2,660 | 4.1 | 24,420 | 18,810 | 2.1 |
| U54 | IE-2 | 9,740 | 9,050 | 7.2 | 32,840 | 27,250 | 4.1 | 19,900 | 9,580 | 1.7 |
| RICP4 | IE-3 | 20,200 | 12,800 | 15.0 | 32,150 | 19,350 | 4.0 | 10,500 | 2,320 | 0.9 |
| R/S22 | IE-4 | 15,900 | 14,100 | 11.8 | 38,740 | 17,790 | 4.8 | 41,320 | 5,960 | 3.6 |
| U4-5′ | E-1 | 220 | 160 | 0.2 | 1,160 | 1,330 | 0.1 | 5,060 | 3,030 | 0.4 |
| U4/5 | E-2 | 1,450 | 850 | 1.1 | 15,860 | 7,320 | 2.0 | 24,480 | 7,420 | 2.1 |
| U8/9 | E-3 | 870 | 220 | 0.6 | 10,460 | 5,440 | 1.3 | 22,400 | 5,080 | 1.9 |
| U8-5′ | E-4 | 1,280 | 1,440 | 0.9 | 3,480 | 4,290 | 0.4 | 2,590 | 2,920 | 0.2 |
| U21 | E-5 | 430 | 160 | 0.3 | 5,720 | 3,950 | 0.7 | 15,740 | 2,750 | 1.4 |
| U23 | E-6 | 1,150 | 570 | 0.9 | 24,520 | 7,300 | 3.1 | 16,070 | 6,490 | 1.4 |
| U29 | E-7 | 210 | 110 | 0.2 | 16,340 | 10,820 | 2.0 | 19,730 | 15,650 | 1.7 |
| U30 | E-8 | 880 | 830 | 0.7 | 23,150 | 5,610 | 2.9 | 11,630 | 4,640 | 1.0 |
| U37 | E-9 | 520 | 660 | 0.4 | 6,080 | 5,510 | 0.8 | 17,800 | 9,650 | 1.5 |
| U39-5′ | E-10 | 290 | 110 | 0.2 | 15,590 | 13,660 | 1.9 | 12,870 | 12,020 | 1.1 |
| U39/40 | E-11 | 990 | 730 | 0.7 | 38,680 | 11,210 | 4.8 | 34,720 | 8,190 | 3.0 |
| U42 | E-12 | 1,060 | 550 | 0.8 | 24,590 | 11,770 | 3.1 | 30,930 | 14,020 | 2.7 |
| U43 | E-13 | 1,750 | 460 | 1.3 | 6,180 | 1,090 | 0.8 | 12,340 | 3,110 | 1.1 |
| U50 | E-14 | 670 | 70 | 0.5 | 24,270 | 8,570 | 3.0 | 25,720 | 13,320 | 2.2 |
| U52-5′ | E-15 | 320 | 290 | 0.2 | 1,600 | 1,970 | 0.2 | 620 | 800 | 0.1 |
| U55 | E-16 | 250 | 80 | 0.2 | 5,860 | 2,690 | 0.7 | 19,640 | 18,570 | 1.7 |
| U56 | E-17 | 740 | 620 | 0.5 | 12,650 | 3,900 | 1.6 | 13,220 | 3,290 | 1.1 |
| US2 | E-18 | 920 | 820 | 0.7 | 5,710 | 5,860 | 0.7 | 21,240 | 4,620 | 1.8 |
| U1 | L-1 | 720 | 750 | 0.5 | 20,570 | 4,360 | 2.6 | 23,530 | 9,420 | 2.0 |
| U3 | L-2 | 270 | 620 | 0.2 | 5,010 | 4,190 | 0.6 | 22,020 | 16,390 | 1.9 |
| U10 | L-3 | 1,270 | 370 | 0.9 | 6,670 | 670 | 0.8 | 18,920 | 11,150 | 1.6 |
| U16/17 | L-4 | 780 | 160 | 0.6 | 6,150 | 3,910 | 0.8 | 16,430 | 3,730 | 1.4 |
| U15 | L-5 | 520 | 70 | 0.4 | 2,170 | 710 | 0.3 | 4,140 | 4,500 | 0.4 |
| U18/20 | L-6 | 210 | 170 | 0.2 | 11,890 | 6,700 | 1.5 | 30,790 | 11,990 | 2.7 |
| U19/20 | L-7 | 280 | 290 | 0.2 | 11,750 | 6,630 | 1.5 | 25,330 | 5,480 | 2.2 |
| U19-5′ | L-8 | 1,070 | 1,040 | 0.8 | 2,830 | 3,640 | 0.4 | 5,890 | 6,770 | 0.5 |
| U22 | L-9 | 160 | 190 | 0.1 | 4,800 | 3,270 | 0.6 | 15,000 | 2,020 | 1.3 |
| U24 | L-10 | 920 | 240 | 0.7 | 5,130 | 620 | 0.6 | 18,690 | 9,350 | 1.6 |
| U25 | L-11 | 280 | 140 | 0.2 | 8,000 | 4,150 | 1.0 | 23,210 | 8,880 | 2.0 |
| U27/8 | L-12 | 860 | 50 | 0.6 | 21,300 | 8,140 | 2.7 | 21,500 | 8,330 | 1.9 |
| U27-5′ | L-13 | 310 | 260 | 0.2 | 11,830 | 10,560 | 1.5 | 15,620 | 13,180 | 1.3 |
| U31/34 | L-14 | 890 | 520 | 0.7 | 4,220 | 1,140 | 0.5 | 24,110 | 5,610 | 2.1 |
| U35 | L-15 | 800 | 600 | 0.6 | 13,270 | 8,490 | 1.7 | 27,660 | 15,090 | 2.4 |
| U38 | L-16 | 1,980 | 140 | 1.5 | 3,970 | 1,810 | 0.5 | 22,140 | 21,460 | 1.9 |
| U41 | L-17 | 320 | 400 | 0.2 | 2,050 | 1,040 | 0.3 | 17,340 | 11,000 | 1.5 |
| U44-5′ | L-18 | 290 | 170 | 0.2 | 2,130 | 760 | 0.3 | 14,290 | 9,800 | 1.2 |
| U44/45 | L-19 | 1,920 | 360 | 1.4 | 17,460 | 9,330 | 2.2 | 35,530 | 3,690 | 3.1 |
| U46/47 | L-20 | 3,040 | 1,240 | 2.3 | 15,690 | 1,770 | 2.0 | 23,720 | 7,480 | 2.0 |
| U48 | L-21 | 280 | 140 | 0.2 | 12,820 | 1,890 | 1.6 | 26,470 | 16,710 | 2.3 |
| U51 | L-22 | 1,860 | 990 | 1.4 | 9,310 | 500 | 1.2 | 18,400 | 2,640 | 1.6 |
| RLXY | L-23 | 190 | 340 | 0.1 | 1,830 | 1,670 | 0.2 | 15,620 | 14,630 | 1.3 |
| RLX | L-24 | 2,950 | 1,580 | 2.2 | 5,700 | 2,330 | 0.7 | 12,170 | 2,920 | 1.1 |
| RICP34.5 | L-25 | 1,080 | 400 | 0.8 | 5,280 | 2,100 | 0.7 | 3,670 | 1,440 | 0.3 |
| US8-5′ | L-26 | 2,360 | 2,030 | 1.8 | 4,910 | 6,360 | 0.6 | 420 | 540 | 0.0 |
| US8-5′ | L-27 | 1,740 | 1,340 | 1.3 | 6,650 | 7,620 | 0.8 | 10,150 | 11,240 | 0.9 |
| US8/9 | L-28 | 1,250 | 690 | 0.9 | 33,940 | 22,030 | 4.2 | 39,460 | 10,480 | 3.4 |
| RLAT-5′ | LT-1 | 420 | 380 | 0.3 | 1,220 | 1,450 | 0.2 | 740 | 420 | 0.1 |
| RHA6 | LT-2 | 840 | 640 | 0.6 | 1,330 | 850 | 0.2 | 8,100 | 8,690 | 0.7 |
| RLATX | LT-3 | 620 | 500 | 0.5 | 1,650 | 1,940 | 0.2 | 6,990 | 4,030 | 0.6 |
| RLAT-3′ | LT-4 | 1,660 | 870 | 1.2 | 3,590 | 2,480 | 0.4 | 10,890 | 720 | 0.9 |
| U1X | ? | 280 | 610 | 0.2 | 1,050 | 1,210 | 0.1 | 1,540 | 1,610 | 0.1 |
| U6/7 | L/? | 2,190 | 540 | 1.6 | 11,770 | 5,810 | 1.5 | 25,270 | 13,260 | 2.2 |
| U11/13 | L/E/Ld | 460 | 630 | 0.3 | 16,710 | 9,710 | 2.1 | 27,280 | 2,370 | 2.4 |
| U36 | E/L | 550 | 660 | 0.4 | 3,060 | 3,820 | 0.4 | 14,920 | 13,670 | 1.3 |
| U43.5-5′ | ? | 170 | 90 | 0.1 | 160 | 280 | 0.0 | 110 | 100 | 0.0 |
| U49 | E/L | 1,050 | 520 | 0.8 | 21,930 | 7,640 | 2.7 | 25,580 | 10,320 | 2.2 |
| U52/53 | E/LId | 630 | 150 | 0.5 | 10,740 | 1,190 | 1.3 | 20,840 | 10,770 | 1.8 |
| RLAT-1 | LAT | 470 | 360 | 0.3 | 740 | 870 | 0.1 | 1,590 | 330 | 0.1 |
| ROP | ? | 680 | 240 | 0.5 | 920 | 1,150 | 0.1 | 380 | 50 | 0.0 |
| US3-5′ | L? | 1,760 | 1,380 | 1.3 | 8,250 | 9,350 | 1.0 | 1,030 | 1,250 | 0.1 |
| US3/4 | E/L? | 520 | 840 | 0.4 | 19,170 | 8,100 | 2.4 | 8,290 | 5,890 | 0.7 |
| US5/6/7 | EL/E/Ed | 1,450 | 1,870 | 1.1 | 29,170 | 15,140 | 3.6 | 31,450 | 4,400 | 2.7 |
| US10/11/12 | E/E/IEd | 13,800 | 8,340 | 10.2 | 35,100 | 23,760 | 4.4 | 37,350 | 5,650 | 3.2 |
| Total | 134,900 | 802,760 | 1,157,500 | |||||||
Infection was initiated at an MOI of 5 PFU per cell. Bold entries represent either single transcripts or multiple transcripts of the same kinetic class.
Transcripts are assigned to kinetic classes as described in reference 34. LT, latency associated. Arbitrary identification numbers (ID) are used for simplicity in Fig. 1 and 3.
The median value is based on four separate experiments, calculated as described in Materials and Methods. All signals were measured at a laser power of 80 with a photomultiplier at 75. Relative values (%) are the quotient of the median signal for a given transcript divided by the total viral signal, multiplied by 100.
This probe detects transcripts from several genes of different kinetic classes.
FIG. 1.
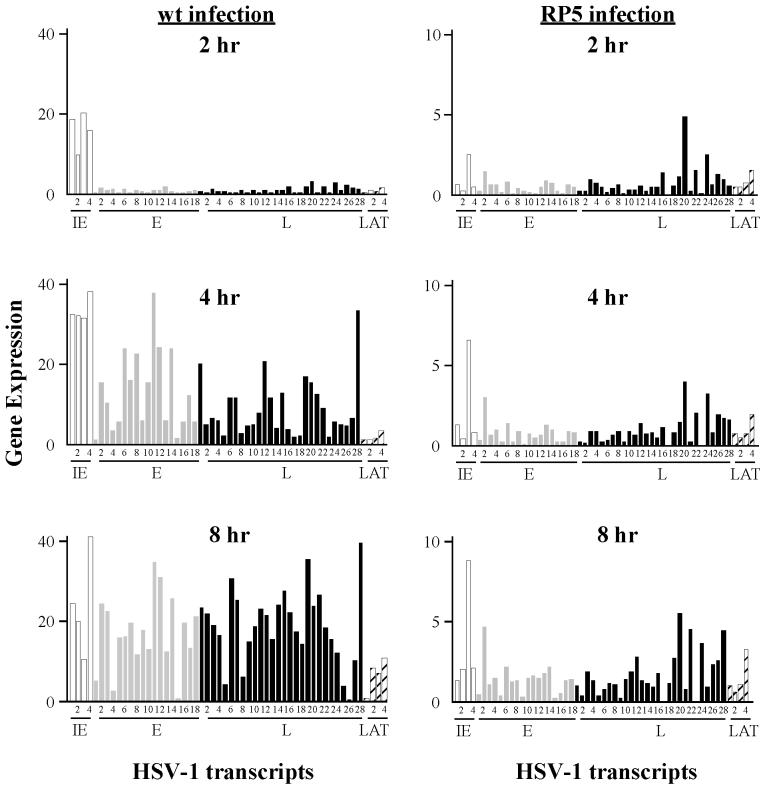
Gene expression levels during infection with wt (RP5R) or VP16 activation domain-deficient (RP5) isolates of HSV-1. Microarray chips were hybridized with probes prepared from RNA harvested from HeLa cells infected with wt or RP5 virus at 2, 4, or 8 hpi. The levels of expression for IE (open), E (shaded), L (solid), and latency-associated (LAT) (hatched) transcripts are in arbitrary units. The numbers corresponding to individual transcripts are defined in Table 1.
To test the effects of the VP16 transcriptional activation domain on the global pattern of viral gene expression in infected cells, we infected cells with RP5, a KOS derivative that lacks the activation domain of VP16 (amino acids 413 to 490). RP5 infections were carried out at a nominal MOI of 0.05 PFU/cell. These conditions result in a particle multiplicity of approximately 50 particles per cell, comparable to that used with the RP5R virus, since the particle/PFU ratio of the RP5 mutant is approximately 103, whereas this ratio is typically 10 for wt viruses such as RP5R (29). Patterns of transcript abundance at 2, 4, and 8 h postinfection by RP5 were assessed using the oligonucleotide array (Table 2 and Fig. 1). At all times, the level of viral transcripts during RP5 infection was markedly reduced compared to the RP5R (wt) infections. Notably, at 2 h postinfection, only one of the five IE genes, ICP4, was expressed at levels higher than twice the SSC background. IE gene transcript levels increased with statistical significance 4 and 8 h after infection, but they still remained well below the wt levels. Interestingly, if IE transcript levels in RP5 infection reflect “basal” activity of the corresponding promoters, then the degree of activation by VP16 is apparently inversely correlated with that basal activity (compare 2- and 4-h time points in Tables 1 and 2). Most transcript sets diagnostic of E and L genes were insignificant at 2 h following infection but showed statistically significant increases in expression at later times. The relatively high levels of some E and L transcripts at 2 hpi (E-2 [UL4/5], L-16 [UL38], L-19 [UL44/45], L-20 [UL46], L-22 [UL51], L-24 [RLX], L-26 [US5], and LT-4 [LAT-3′]) may indicate aberrant expression, since many are also seen in the presence of cycloheximide following RP5R infection (see below). However, since the absolute levels of expression are quite low, we cannot rule out the possibility that some of these elevated values are background artifacts.
TABLE 2.
Abundance of HSV-1 transcripts at various times following infection of HeLa cells with RP5 virusa
| Transcript set | Kinetic class and IDb | Abundance at:
|
t-test value, RP5 vs RP5Rf
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 hpi
|
4 hpi
|
8 hpi
|
|||||||||||
| Medianc | SD | % | Mediand | SD | % | Mediane | SD | % | 2 hpi | 4 hpi | 8 hpi | ||
| RICP0 | IE-1 | 620 | 350 | 1.5 | 1,210 | 1,340 | 2.0 | 1,270 | 830 | 1.2 | 0.04 | 0.01 | 0.28 |
| U54 | IE-2 | 200 | 210 | 0.5 | 350 | 2,000 | 0.6 | 1,950 | 1,380 | 1.8 | 0.01 | 0.20 | 0.48 |
| RICP4 | IE-3 | 2,300 | 3,680 | 5.6 | 6,090 | 4,360 | 9.9 | 8,370 | 6,980 | 7.7 | 0.01 | 0.00 | 0.00 |
| R/S22 | IE-4 | 480 | 310 | 1.2 | 760 | 1,240 | 1.2 | 1,970 | 980 | 1.8 | 0.06 | 0.03 | 0.00 |
| U4-5′ | E-1 | 230 | 210 | 0.6 | 310 | 140 | 0.5 | 450 | 160 | 0.4 | 0.06 | 0.09 | 0.88 |
| U4/5 | E-2 | 1,360 | 1,430 | 3.3 | 2,780 | 2,040 | 4.5 | 4,460 | 3,510 | 4.1 | 0.05 | 0.02 | 0.00 |
| U8/9 | E-3 | 620 | 670 | 1.5 | 570 | 480 | 0.9 | 1,050 | 550 | 1.0 | 0.13 | 0.26 | 0.02 |
| U8-5′ | E-4 | 590 | 940 | 1.4 | 870 | 810 | 1.4 | 1,440 | 690 | 1.3 | 0.42 | 0.02 | 0.00 |
| U21 | E-5 | 170 | 240 | 0.4 | 190 | 610 | 0.3 | 380 | 330 | 0.4 | 0.39 | 0.92 | 0.00 |
| U23 | E-6 | 760 | 980 | 1.8 | 1,280 | 630 | 2.1 | 2,070 | 560 | 1.9 | 0.07 | 0.00 | 0.19 |
| U29 | E-7 | 70 | 210 | 0.2 | 250 | 570 | 0.4 | 1,160 | 810 | 1.1 | 0.37 | 0.17 | 0.48 |
| U30 | E-8 | 340 | 350 | 0.8 | 860 | 540 | 1.4 | 1,260 | 480 | 1.2 | 0.36 | 0.06 | 0.51 |
| U37 | E-9 | 190 | 210 | 0.5 | 100 | 300 | 0.2 | 300 | 290 | 0.3 | 0.49 | 0.25 | 0.07 |
| U39-5′ | E-10 | 180 | 170 | 0.4 | 700 | 620 | 1.1 | 1,370 | 680 | 1.3 | 0.18 | 0.43 | 0.98 |
| U39/40 | E-11 | 100 | 240 | 0.2 | 440 | 770 | 0.7 | 1,570 | 1,090 | 1.4 | 0.16 | 0.00 | 0.01 |
| U42 | E-12 | 430 | 350 | 1.0 | 570 | 530 | 0.9 | 1,390 | 600 | 1.3 | 0.07 | 0.09 | 0.06 |
| U43 | E-13 | 850 | 520 | 2.1 | 1,190 | 500 | 1.9 | 1,690 | 940 | 1.6 | 0.22 | 0.00 | 0.17 |
| U50 | E-14 | 680 | 430 | 1.6 | 880 | 540 | 1.4 | 2,060 | 840 | 1.9 | 0.05 | 0.03 | 0.39 |
| U52-5′ | E-15 | 240 | 150 | 0.6 | 190 | 670 | 0.3 | 200 | 150 | 0.2 | 0.33 | 0.21 | 0.03 |
| U55 | E-16 | 40 | 130 | 0.1 | 230 | 360 | 0.4 | 510 | 410 | 0.5 | 0.82 | 0.42 | 0.19 |
| U56 | E-17 | 560 | 470 | 1.4 | 830 | 570 | 1.3 | 1,290 | 1,370 | 1.2 | 0.36 | 0.59 | 0.22 |
| US2 | E-18 | 480 | 440 | 1.2 | 790 | 680 | 1.3 | 1,360 | 880 | 1.3 | 0.26 | 0.44 | 0.12 |
| U1 | L-1 | 250 | 190 | 0.6 | 220 | 810 | 0.4 | 970 | 860 | 0.9 | 0.60 | 0.02 | 0.10 |
| U3 | L-2 | 220 | 170 | 0.5 | 160 | 350 | 0.3 | 340 | 450 | 0.3 | 0.23 | 0.38 | 0.10 |
| U10 | L-3 | 920 | 760 | 2.2 | 840 | 480 | 1.4 | 1,760 | 530 | 1.6 | 0.02 | 0.02 | 0.45 |
| U16/17 | L-4 | 640 | 210 | 1.5 | 810 | 370 | 1.3 | 1,290 | 440 | 1.2 | 0.01 | 0.63 | 0.24 |
| U15 | L-5 | 470 | 200 | 1.1 | 200 | 250 | 0.3 | 350 | 340 | 0.3 | 0.01 | 0.37 | 0.72 |
| U18/20 | L-6 | 130 | 160 | 0.3 | 290 | 470 | 0.5 | 750 | 700 | 0.7 | 0.19 | 0.16 | 0.02 |
| U19/20 | L-7 | 340 | 230 | 0.8 | 620 | 520 | 1.0 | 1,080 | 850 | 1.0 | 0.02 | 0.44 | 0.02 |
| U19-5′ | L-8 | 610 | 510 | 1.5 | 860 | 580 | 1.4 | 1,020 | 480 | 0.9 | 0.40 | 0.04 | 0.25 |
| U22 | L-9 | 50 | 240 | 0.1 | 190 | 250 | 0.3 | 250 | 400 | 0.2 | 0.30 | 0.34 | 0.00 |
| U24 | L-10 | 270 | 320 | 0.7 | 850 | 300 | 1.4 | 1,320 | 620 | 1.2 | 0.98 | 0.00 | 0.44 |
| U25 | L-11 | 300 | 230 | 0.7 | 640 | 660 | 1.0 | 1,750 | 760 | 1.6 | 0.06 | 0.91 | 0.28 |
| U27/8 | L-12 | 490 | 440 | 1.2 | 1,310 | 550 | 2.1 | 2,670 | 1,640 | 2.5 | 0.24 | 0.30 | 0.11 |
| U27-5′ | L-13 | 230 | 210 | 0.6 | 660 | 550 | 1.1 | 1,290 | 840 | 1.2 | 0.20 | 0.48 | 0.94 |
| U31/34 | L-14 | 430 | 470 | 1.0 | 740 | 540 | 1.2 | 1,110 | 700 | 1.0 | 0.24 | 0.00 | 0.03 |
| U35 | L-15 | 410 | 240 | 1.0 | 480 | 370 | 0.8 | 860 | 610 | 0.8 | 0.20 | 0.17 | 0.06 |
| U38 | L-16 | 1,260 | 960 | 3.0 | 1,090 | 810 | 1.8 | 1,700 | 1,150 | 1.6 | 0.06 | 0.00 | 0.47 |
| U41 | L-17 | 30 | 190 | 0.1 | 30 | 310 | 0.0 | 60 | 340 | 0.1 | 0.79 | 0.50 | 0.08 |
| U44-5′ | L-18 | 500 | 710 | 1.2 | 720 | 440 | 1.2 | 1,140 | 620 | 1.1 | 0.06 | 0.00 | 0.87 |
| U44/45 | L-19 | 1,020 | 1,180 | 2.5 | 1,360 | 950 | 2.2 | 2,600 | 830 | 2.4 | 0.10 | 0.51 | 0.00 |
| U46/47 | L-20 | 4,430 | 1,180 | 10.7 | 3,720 | 2,400 | 6.0 | 5,280 | 3,560 | 4.9 | 0.00 | 0.00 | 0.00 |
| U48 | L-21 | 230 | 300 | 0.6 | 210 | 300 | 0.3 | 730 | 390 | 0.7 | 0.09 | 0.00 | 0.09 |
| U51 | L-22 | 1,390 | 1,460 | 3.4 | 1,870 | 1,270 | 3.0 | 4,310 | 2,580 | 4.0 | 0.12 | 0.00 | 0.00 |
| RLXY | L-23 | 110 | 210 | 0.3 | 20 | 560 | 0.0 | 20 | 410 | 0.0 | 0.21 | 0.74 | 0.19 |
| RLX | L-24 | 2,280 | 1,160 | 5.5 | 2,990 | 2,040 | 4.8 | 3,500 | 3,700 | 3.2 | 0.01 | 0.00 | 0.00 |
| RICP34.5 | L-25 | 590 | 410 | 1.4 | 740 | 450 | 1.2 | 890 | 870 | 0.8 | 0.15 | 0.17 | 0.00 |
| US5-5′ | L-26 | 1,210 | 490 | 2.9 | 1,810 | 1,170 | 2.9 | 2,240 | 2,020 | 2.1 | 0.93 | 0.01 | 0.00 |
| US8-5′ | L-27 | 920 | 630 | 2.2 | 1,580 | 1,000 | 2.6 | 2,460 | 1,950 | 2.3 | 0.37 | 0.03 | 0.03 |
| US8/9 | L-28 | 510 | 640 | 1.2 | 1,510 | 1,290 | 2.4 | 4,190 | 2,170 | 3.9 | 0.69 | 0.63 | 0.68 |
| RLAT-5′ | LT-1 | 430 | 180 | 1.0 | 660 | 280 | 1.1 | 940 | 430 | 0.9 | 0.14 | 0.00 | 0.00 |
| RHA6 | LT-2 | 420 | 300 | 1.0 | 450 | 320 | 0.7 | 600 | 360 | 0.6 | 0.12 | 0.00 | 0.65 |
| RLATX | LT-3 | 670 | 710 | 1.6 | 650 | 570 | 1.1 | 1,010 | 890 | 0.9 | 0.03 | 0.00 | 0.05 |
| RLAT-3′ | LT-4 | 1,420 | 1,110 | 3.4 | 1,810 | 1,050 | 2.9 | 3,150 | 1,650 | 2.9 | 0.12 | 0.00 | 0.00 |
| U1X | ? | 290 | 210 | 0.7 | 70 | 350 | 0.1 | 140 | 250 | 0.1 | 0.14 | 0.58 | 0.67 |
| U6/7 | L/? | 1,190 | 400 | 2.9 | 2,340 | 1,170 | 3.8 | 4,340 | 2,050 | 4.0 | 0.00 | 0.11 | 0.25 |
| U11/13 | L/E/L | 70 | 250 | 0.2 | 80 | 510 | 0.1 | 1,200 | 720 | 1.1 | 0.83 | 0.10 | 0.00 |
| U36 | E/L | 140 | 270 | 0.3 | 80 | 450 | 0.1 | 80 | 350 | 0.1 | 0.56 | 0.58 | 0.18 |
| U43.5-5′ | ? | 180 | 110 | 0.4 | 220 | 470 | 0.4 | 330 | 280 | 0.3 | 0.01 | 0.01 | 0.00 |
| U49 | E/L | 800 | 510 | 1.9 | 1,160 | 1,010 | 1.9 | 2,730 | 800 | 2.5 | 0.05 | 0.23 | 0.88 |
| U52/53 | E/L | 400 | 320 | 1.0 | 600 | 560 | 1.0 | 1,150 | 410 | 1.1 | 0.12 | 0.38 | 0.15 |
| RLAT-1 | LAT | 360 | 80 | 0.9 | 490 | 320 | 0.8 | 720 | 450 | 0.7 | 0.19 | 0.00 | 0.00 |
| ROP | ? | 780 | 220 | 1.9 | 940 | 550 | 1.5 | 1,150 | 780 | 1.1 | 0.00 | 0.00 | 0.00 |
| US3-5′ | L? | 1,240 | 640 | 3.0 | 2,060 | 1,080 | 3.3 | 2,740 | 1,520 | 2.5 | 0.23 | 0.06 | 0.00 |
| US3/4 | E/L? | 310 | 280 | 0.7 | 410 | 400 | 0.7 | 900 | 640 | 0.8 | 0.25 | 0.07 | 0.36 |
| US5/6/7 | EL/E/E | 550 | 550 | 1.3 | 1,040 | 950 | 1.7 | 2,280 | 950 | 2.1 | 0.92 | 0.50 | 0.08 |
| US10/11/12 | E/E/IE | 370 | 290 | 0.9 | 800 | 890 | 1.3 | 1,580 | 770 | 1.5 | 0.01 | 0.26 | 0.00 |
| Total | 41,350 | 61,820 | 108,540 | ||||||||||
Infection was initiated at an MOI of 0.05 PFU per cell. Bold entries represent either single transcripts or multiple transcripts of the same kinetic class.
See Table 1, footnote b.
Median values are based upon eight separate experiments. All signals were determined at a laser power of 90 with a photomultiplier at 85.
Median value is based upon 17 separate hybridizations.
Median value is based upon 16 separate hybridizations.
Relative values of transcript levels for infections by RP5R (wt) and RP5 at the various time points were compared using Student's t test as described in Materials and Methods. The null hypothesis is that the true values for the wt and mutant viruses are identical. A value of 0.00 is used to indicate P values of <0.01.
Given that the overall levels of gene expression were reduced in RP5 infection, we then asked whether the cascade of gene expression—that is, the relative abundance of each transcript in a given kinetic class—was affected by deletion of the VP16 activation domain. To make this comparison, we first calculated the relative abundance of each signal (relative to the total viral signal) for each replicate experiment at every time point. The sets of relative abundances for a given transcript in the two infections were then compared by using Student's t test, where the null hypothesis is that the true values from the two infections are the same. The results of this comparison are shown in Table 2. The null hypothesis is rejected (P < 0.05) for one-third or more of the genes in each kinetic class, indicating that the relative abundances of those transcripts are significantly different in RP5 infection than in a wt (RP5R) infection. We take this as strong evidence that the pattern of viral gene expression during RP5 infection is aberrant and does not simply reflect a reduced level of a normal gene expression pattern.
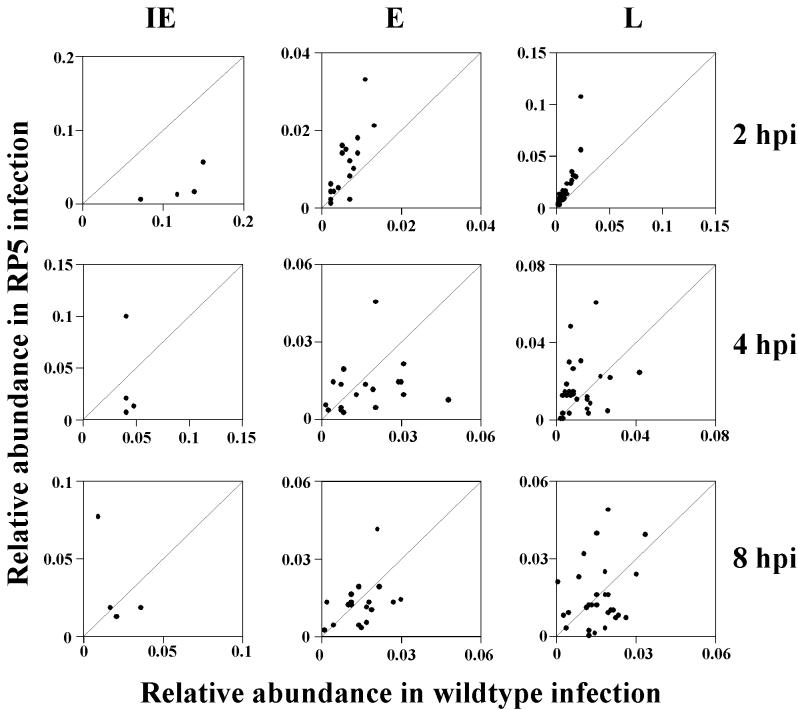
The difference in transcription patterns during infection by RP5 and wt viruses can also be demonstrated by scatter analysis by plotting the relative abundance of each transcript (based on the median values reported in Tables 1 and 2) in RP5 infection against corresponding values from RP5R infections (Fig. 2). Correlation coefficients calculated for each kinetic class at each time point are shown in Table 3. The weak correlations for all kinetic classes (IE, E, and L) at 4 and 8 hpi strongly imply that the patterns of viral gene expression are markedly different in cells infected by the RP5 and RP5R viruses. Thus, the expression seen in RP5 infection does not represent a low level of a normal gene expression pattern but an aberrant pattern altogether.
FIG. 2.
Correlation of transcript abundances between expression levels of RP5R (wt) and RP5 at 2, 4, and 8 hpi. The relative abundances of transcripts for RP5R infection are displayed on the abscissa, and the relative abundances of transcripts from an RP5 infection are on the ordinate. Each point represents a signal for IE, E, or L transcripts at 2, 4, or 8 hpi. For each plot, the ordinate and abscissa have the same units, and the expected locations of equivalent signals under both conditions (i.e., a correlation of 1.0) are indicated by the 45° diagonals.
TABLE 3.
Correlation between relative abundance of the different kinetic classes of HSV-1 transcripts following infection with wt versus mutant viruses under various conditionsa
| Virus and treatment | hpi | Correlation coefficient for kinetic class of transcripts
|
||||
|---|---|---|---|---|---|---|
| IE | E | L | LAT | Overall | ||
| RP5, 0.05 PFU/cell | 2 | 0.72 | 0.77 | 0.86 | 0.93 | 0.26 |
| 4 | −0.46 | 0.21 | 0.24 | 0.98 | 0.24 | |
| 8 | −0.67 | 0.38 | 0.19 | 0.57 | 0.25 | |
| RP5, 1.0 PFU/cell | 4 | −0.43 | 0.87 | 0.86 | 0.98 | 0.85 |
| 8 | 0.92 | 0.74 | 0.76 | 0.68 | 0.85 | |
| RP5 in HMBA | 4 | 0.09 | 0.82 | 0.86 | 0.93 | 0.83 |
| RP5/16-8 | 2 | 0.79 | 0.57 | 0.43 | 0.49 | 0.93 |
| 4 | 0.90 | 0.94 | 0.90 | −0.08 | 0.92 | |
| 8 | 0.84 | 0.85 | 0.76 | 0.89 | 0.86 | |
| RP3 | 2 | 0.79 | 0.70 | 0.87 | 0.88 | 0.92 |
| 4 | 0.25 | 0.92 | 0.90 | 0.93 | 0.87 | |
| 8 | 0.95 | 0.77 | 0.70 | 0.66 | 0.81 | |
| RP4 | 2 | 0.59 | 0.78 | 0.91 | 0.89 | 0.55 |
| 4 | 0.35 | 0.89 | 0.84 | 0.98 | 0.86 | |
| 8 | 0.81 | 0.94 | 0.76 | 0.68 | 0.88 | |
HMBA and MOI have differential effects on the levels of specific IE transcripts while activating a normal regulatory cascade.
The cell-differentiating agent HMBA can increase the titer of viruses with mutations in VP16. For example, the poorly replicating viral mutant strain in1814 contains a four-amino-acid insertion in VP16 that prevents interaction with Oct-1 and is unable to activate IE transcription in standard infection conditions (1). By mechanisms that are not yet well understood, HMBA compensates for the loss of activation by VP16 and permits viral replication and production (17).
To explore the nature of compensation by HMBA, we asked whether this agent could alter or revert the kinetic pattern of viral gene expression during RP5 infection to resemble that of a wt infection. We infected HeLa cells with RP5 at an MOI of 0.05 PFU/cell in the presence of 5 mM HMBA and harvested RNA at 4 hpi. Gene expression levels derived from four hybridization experiments are shown in Table 4. Overall viral expression was considerably greater than the level observed during RP5 infection in the absence of HMBA (Table 2) and approached the levels observed during infection by the wt RP5R virus (Table 1). Of the IE genes, only that encoding ICP0 showed a significantly lower median expression level (approximately 40%) in RP5-HMBA infection than in RP5R infection. In contrast, UL54 (ICP27) expression was relatively high in HMBA-treated cells. Very few E and L genes showed statistically significant differences in expression based on relative abundance (Student's t test) (Table 4). Thus, despite differences in expression of two of the IE transcripts, the overall levels and patterns of E and L gene expression during RP5 infection in HMBA and during wt RP5R infection were quite similar. This conclusion was supported by correlation coefficients calculated for the relative abundance values for RP5-HMBA versus RP5R (Table 3). Although the correlation coefficient for the IE genes was rather low, the correlation coefficients for the E and L genes were quite high. This result implies that, in the absence of activation by VP16, HMBA stimulates expression of the IE genes but not in normal ratios. Nonetheless, the IE gene expression induced by HMBA is sufficient to support later waves of viral gene expression in relatively normal patterns. Thus, considerable variation in relative levels of IE transcripts can be tolerated during HSV infection. This observation suggests an inherent plasticity in the viral regulatory network.
TABLE 4.
Abundance of HSV-1 transcripts at 4 h after infection with RP5 virus in HeLa cells treated with HMBAa
| Transcript set | Kinetic class and IDb | Abundance
|
Wt %d | t test valuee | ||
|---|---|---|---|---|---|---|
| Medianc | SD | % | ||||
| RICP0 | IE-1 | 13,130 | 5,830 | 3.2 | 4.1 | 0.04 |
| U54 | IE-2 | 42,970 | 11,840 | 10.5 | 4.1 | 0.03 |
| RICP4 | IE-3 | 21,430 | 11,580 | 5.2 | 4.0 | 0.32 |
| R/S22 | IE-4 | 27,730 | 6,770 | 6.8 | 4.8 | 0.16 |
| U4-5′ | E-1 | 810 | 930 | 0.2 | 0.1 | 0.20 |
| U4/5 | E-2 | 4,680 | 2,140 | 1.1 | 2.0 | 0.27 |
| U8/9 | E-3 | 880 | 1,090 | 0.2 | 1.3 | 0.08 |
| U8-5′ | E-4 | 2,410 | 1,360 | 0.6 | 0.4 | 0.44 |
| U21 | E-5 | 880 | 530 | 0.2 | 0.7 | 0.17 |
| U23 | E-6 | 13,670 | 1,810 | 3.3 | 3.1 | 0.47 |
| U29 | E-7 | 10,630 | 6,730 | 2.6 | 2.0 | 0.12 |
| U30 | E-8 | 10,770 | 6,930 | 2.6 | 2.9 | 0.99 |
| U37 | E-9 | 520 | 630 | 0.1 | 0.8 | 0.18 |
| U39-5′ | E-10 | 13,480 | 4,240 | 3.3 | 1.9 | 0.15 |
| U39/40 | E-11 | 13,220 | 4,030 | 3.2 | 4.8 | 0.11 |
| U42 | E-12 | 6,920 | 2,750 | 1.7 | 3.1 | 0.21 |
| U43 | E-13 | 2,300 | 690 | 0.6 | 0.8 | 0.13 |
| U50 | E-14 | 10,020 | 6,220 | 2.4 | 3.0 | 0.99 |
| U52-5′ | E-15 | 2,230 | 1,120 | 0.5 | 0.2 | 0.11 |
| U55 | E-16 | 1,520 | 740 | 0.4 | 0.7 | 0.18 |
| U56 | E-17 | 3,350 | 630 | 0.8 | 1.6 | 0.02 |
| US2 | E-18 | 1,360 | 330 | 0.3 | 0.7 | 0.25 |
| U1 | L-1 | 10,200 | 2,210 | 2.5 | 2.6 | 0.83 |
| U3 | L-2 | 850 | 520 | 0.2 | 0.6 | 0.21 |
| U10 | L-3 | 3,060 | 1,130 | 0.7 | 0.8 | 0.63 |
| U16/17 | L-4 | 1,980 | 400 | 0.5 | 0.8 | 0.30 |
| U15 | L-5 | 670 | 1,340 | 0.2 | 0.3 | 0.83 |
| U18/20 | L-6 | 4,510 | 2,300 | 1.1 | 1.5 | 0.31 |
| U19/20 | L-7 | 2,470 | 890 | 0.6 | 1.5 | 0.15 |
| U19-5′ | L-8 | 2,600 | 1,190 | 0.6 | 0.4 | 0.24 |
| U22 | L-9 | 1,500 | 510 | 0.4 | 0.6 | 0.29 |
| U24 | L-10 | 2,600 | 1,280 | 0.6 | 0.6 | 0.77 |
| U25 | L-11 | 3,350 | 1,360 | 0.8 | 1.0 | 0.30 |
| U27/8 | L-12 | 8,610 | 3,280 | 2.1 | 2.7 | 0.43 |
| U27-5′ | L-13 | 9,460 | 2,840 | 2.3 | 1.5 | 0.18 |
| U31/34 | L-14 | 2,850 | 740 | 0.7 | 0.5 | 0.12 |
| U35 | L-15 | 3,840 | 840 | 0.9 | 1.7 | 0.27 |
| U38 | L-16 | 1,190 | 750 | 0.3 | 0.5 | 0.30 |
| U41 | L-17 | 510 | 170 | 0.1 | 0.3 | 0.15 |
| U44-5′ | L-18 | 1,610 | 640 | 0.4 | 0.3 | 0.19 |
| U44/45 | L-19 | 5,710 | 1,330 | 1.4 | 2.2 | 0.18 |
| U46/47 | L-20 | 11,800 | 3,770 | 2.9 | 2.0 | 0.04 |
| U48 | L-21 | 730 | 750 | 0.2 | 1.6 | 0.00 |
| U51 | L-22 | 5,760 | 1,760 | 1.4 | 1.2 | 0.65 |
| RLXY | L-23 | 480 | 350 | 0.1 | 0.2 | 0.22 |
| RLX | L-24 | 6,540 | 2,130 | 1.6 | 0.7 | 0.01 |
| RICP34.5 | L-25 | 2,850 | 1,520 | 0.7 | 0.7 | 0.92 |
| US5-5′ | L-26 | 2,010 | 1,290 | 0.5 | 0.6 | 0.79 |
| US8-5′ | L-27 | 3,590 | 1,120 | 0.9 | 0.8 | 0.72 |
| US8/9 | L-28 | 26,120 | 10,630 | 6.4 | 4.2 | 0.07 |
| RLAT-5′ | LT-1 | 580 | 670 | 0.1 | 0.2 | 0.66 |
| RHA6 | LT-2 | 250 | 320 | 0.1 | 0.2 | 0.26 |
| RLATX | LT-3 | 1,590 | 1,510 | 0.4 | 0.2 | 0.12 |
| RLAT-3′ | LT-4 | 3,750 | 2,800 | 0.9 | 0.4 | 0.04 |
| U1X | ? | 130 | 120 | 0.0 | 0.1 | 0.20 |
| U6/7 | L/? | 2,450 | 770 | 0.6 | 1.5 | 0.10 |
| U11/13 | L/E/L | 4,150 | 2,820 | 1.0 | 2.1 | 0.22 |
| U36 | E/L | 350 | 220 | 0.1 | 0.4 | 0.20 |
| U43.5-5′ | ? | 260 | 150 | 0.1 | 0.0 | 0.14 |
| U49 | E/L | 14,880 | 7,440 | 3.6 | 2.7 | 0.04 |
| U52/53 | E/L | 3,170 | 2,240 | 0.8 | 1.3 | 0.01 |
| RLAT-1 | LAT | 890 | 560 | 0.2 | 0.1 | 0.14 |
| ROP | ? | 1,740 | 830 | 0.4 | 0.1 | 0.01 |
| US3-5′ | L? | 8,360 | 3,340 | 2.0 | 1.0 | 0.22 |
| US3/4 | E/L? | 3,130 | 2,180 | 0.8 | 2.4 | 0.10 |
| US5/6/7 | EL/E/E | 16,570 | 7,360 | 4.0 | 3.6 | 0.27 |
| US10/11/12 | E/E/IE | 15,730 | 6,790 | 3.8 | 4.4 | 0.96 |
| Total | 410,340 | |||||
Infection was initiated at an MOI of 0.05 PFU (50 virions) per cell in the presence of 5 mM HMBA. Bold entries represent either single transcripts or multiple transcripts of the same kinetic class.
See Table 1, footnote b.
Median values are based upon eight separate experiments. All values were determined at a laser power of 80 with a photomultiplier at 75%.
Taken from Table 1.
Student's two-tailed t test (assuming unequal variance) was used to compare the relative abundance values of transcripts in all experiments of RP5-HMBA infection with corresponding set of values for wt infection at 4 hpi (Table 1). The null hypothesis is that the values are identical.
We have previously shown that infection by wt virus at MOIs ranging from 0.05 to 5 PFU/cell results in essentially the same temporal profile of transcript abundance (33). The infections described above used 50 virions per cell, which corresponds to an MOI of 5 PFU for wt virus or 0.05 PFU for the RP5 mutant grown on noncomplementing cells (29). These conditions were chosen to allow a comparison of equivalent numbers of particles (and thus of viral DNA templates) on gene expression. However, in some cases, high-multiplicity infections can overcome defects in viral gene expression. Therefore, we tested whether infecting cells with greater numbers of RP5 virion particles would affect viral transcription. HeLa cells were infected with RP5 at an MOI of 1 PFU per cell (a 20-fold increase). RNA was extracted at 4 and 8 hpi, and viral mRNA levels were assessed by hybridization to DNA microarrays. Data derived from eight separate hybridizations are shown in Table 5. The higher-multiplicity infections led to significantly higher levels of viral transcripts, so that by 8 h most viral transcript levels were essentially equivalent to those in a wt infection. Moreover, most probes revealed time-based differences in transcript abundance consistent with a rather normal kinetic cascade of expression. Despite this, some statistically significant differences were observed. For example, at 4 h, the median signals for ICP0 and ICP22 transcripts (and a number of E and L transcripts) were lower in high-multiplicity RP5 infection than in wt (RP5R) infection. In contrast, by 8 h the levels of these transcripts were typically indistinguishable in the two infections, but other genes (including that for ICP4) showed differences at the later times. Correlation coefficients comparing RP5 at a high MOI with wt virus were calculated for the various kinetic classes (Table 3). At 4 h, the pattern of IE gene expression in the high-multiplicity RP5 infection did not correlate well with the pattern seen with the RP5R infection. At both 4 and 8 h, however, the relative expression levels for the E and L genes correlated well with the levels seen in wt RP5R infection. We conclude that both HMBA and high-multiplicity infections result in IE gene expression patterns noticeably different from those in wt infections, but nonetheless the patterns of viral E and L gene expression at later times are not significantly affected.
TABLE 5.
Abundance of HSV-1 transcripts following high-multiplicity infection of HeLa cells with RP5a
| Transcript set | Kinetic class and IDb | Abundance at:
|
Variance (t test)e
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 hpi
|
8 hpi
|
||||||||
| Medianc | SD | % | Mediand | SD | % | 4 hpi | 8 hpi | ||
| RICP0 | IE-1 | 6,270 | 2,360 | 2.7 | 19,880 | 5,650 | 1.4 | 0.00 | 0.59 |
| U54 | IE-2 | 14,770 | 3,830 | 6.3 | 19,940 | 12,820 | 1.4 | 0.21 | 0.37 |
| RICP4 | IE-3 | 17,770 | 3,260 | 7.5 | 18,320 | 4,190 | 1.3 | 0.19 | 0.01 |
| R/S22 | IE-4 | 9,390 | 2,970 | 4.0 | 41,210 | 7,430 | 2.9 | 0.05 | 0.89 |
| U4-5′ | E-1 | 1,280 | 750 | 0.5 | 7,810 | 4,220 | 0.5 | 0.93 | 0.22 |
| U4/5 | E-2 | 5,480 | 890 | 2.3 | 24,030 | 11,880 | 1.7 | 0.06 | 0.58 |
| U8/9 | E-3 | 790 | 720 | 0.3 | 9,010 | 3,480 | 0.6 | 0.03 | 0.01 |
| U8-5′ | E-4 | 1,230 | 1,310 | 0.5 | 5,950 | 2,250 | 0.4 | 0.40 | 0.14 |
| U21 | E-5 | 670 | 390 | 0.3 | 10,540 | 4,620 | 0.7 | 0.09 | 0.04 |
| U23 | E-6 | 5,940 | 1,380 | 2.5 | 18,710 | 10,650 | 1.3 | 0.01 | 0.12 |
| U29 | E-7 | 4,300 | 2,240 | 1.8 | 17,760 | 10,330 | 1.2 | 0.12 | 0.88 |
| U30 | E-8 | 5,600 | 1,940 | 2.4 | 15,720 | 3,230 | 1.1 | 0.01 | 0.13 |
| U37 | E-9 | 640 | 300 | 0.3 | 12,540 | 15,500 | 0.9 | 0.12 | 0.76 |
| U39-5′ | E-10 | 7,550 | 1,530 | 3.2 | 24,220 | 8,650 | 1.7 | 0.27 | 0.25 |
| U39/40 | E-11 | 7,740 | 1,540 | 3.3 | 40,610 | 12,890 | 2.8 | 0.01 | 0.43 |
| U42 | E-12 | 4,220 | 740 | 1.8 | 40,630 | 11,740 | 2.8 | 0.03 | 0.70 |
| U43 | E-13 | 1,190 | 670 | 0.5 | 4,420 | 11,920 | 0.3 | 0.00 | 0.48 |
| U50 | E-14 | 5,560 | 1,490 | 2.4 | 39,870 | 9,320 | 2.8 | 0.02 | 0.08 |
| U52-5′ | E-15 | 340 | 190 | 0.1 | 740 | 610 | 0.1 | 0.25 | 0.99 |
| U55 | E-16 | 880 | 680 | 0.4 | 10,000 | 15,330 | 0.7 | 0.03 | 0.52 |
| U56 | E-17 | 2,300 | 340 | 1.0 | 14,410 | 6,590 | 1.0 | 0.01 | 0.48 |
| U52 | E-18 | 1,340 | 710 | 0.6 | 34,670 | 5,630 | 2.4 | 0.16 | 0.00 |
| U1 | L-1 | 8,330 | 1,710 | 3.5 | 24,410 | 10,690 | 1.7 | 0.01 | 0.97 |
| U3 | L-2 | 840 | 370 | 0.4 | 11,930 | 8,810 | 0.8 | 0.11 | 0.43 |
| U10 | L-3 | 1,810 | 1,150 | 0.8 | 29,170 | 6,710 | 2.0 | 0.00 | 0.20 |
| U16/17 | L-4 | 880 | 430 | 0.4 | 18,300 | 3,790 | 1.3 | 0.08 | 0.25 |
| U15 | L-5 | 290 | 200 | 0.1 | 5,060 | 2,630 | 0.4 | 0.01 | 0.81 |
| U18/20 | L-6 | 2,300 | 770 | 1.0 | 40,780 | 17,780 | 2.8 | 0.05 | 0.15 |
| U19/20 | L-7 | 3,370 | 1,020 | 1.4 | 40,710 | 14,840 | 2.8 | 0.07 | 0.02 |
| U19-5′ | L-8 | 1,240 | 1,000 | 0.5 | 8,830 | 5,280 | 0.6 | 0.42 | 0.37 |
| U22 | L-9 | 460 | 100 | 0.2 | 18,890 | 8,530 | 1.3 | 0.08 | 0.10 |
| U24 | L-10 | 1,510 | 1,120 | 0.6 | 18,690 | 3,850 | 1.3 | 0.00 | 0.68 |
| U25 | L-11 | 1,790 | 910 | 0.8 | 54,310 | 25,870 | 3.8 | 0.04 | 0.03 |
| U27/8 | L-12 | 6,990 | 2,540 | 3.0 | 45,830 | 7,590 | 3.2 | 0.05 | 0.00 |
| U27-5′ | L-13 | 5,750 | 1,290 | 2.4 | 34,730 | 6,140 | 2.4 | 0.33 | 0.06 |
| U31/34 | L-14 | 1,840 | 670 | 0.8 | 21,320 | 6,020 | 1.5 | 0.03 | 0.67 |
| U35 | L-15 | 1,030 | 760 | 0.4 | 38,080 | 16,260 | 2.6 | 0.06 | 0.32 |
| U38 | L-16 | 1,190 | 1,810 | 0.5 | 19,590 | 10,180 | 1.4 | 0.14 | 0.74 |
| U41 | L-17 | 80 | 40 | 0.0 | 10,110 | 4,190 | 0.7 | 0.03 | 0.39 |
| U44-5′ | L-18 | 1,080 | 310 | 0.5 | 38,340 | 16,420 | 2.7 | 0.05 | 0.01 |
| U44/45 | L-19 | 2,610 | 1,890 | 1.1 | 45,960 | 21,900 | 3.2 | 0.04 | 0.17 |
| U46/47 | L-20 | 8,620 | 6,060 | 3.7 | 48,260 | 22,220 | 3.3 | 0.23 | 0.04 |
| U48 | L-21 | 960 | 690 | 0.4 | 25,500 | 8,130 | 1.8 | 0.00 | 0.97 |
| U51 | L-22 | 2,620 | 1,350 | 1.1 | 22,930 | 9,720 | 1.6 | 0.00 | 0.35 |
| RLXY | L-23 | 40 | 150 | 0.0 | 9,320 | 14,730 | 0.6 | 0.07 | 0.82 |
| RLX | L-24 | 5,280 | 2,230 | 2.2 | 17,920 | 9,300 | 1.2 | 0.92 | 0.17 |
| RICP34.5 | L-25 | 1,150 | 780 | 0.5 | 2,600 | 2,580 | 0.2 | 0.03 | 0.63 |
| US5-5′ | L-26 | 930 | 820 | 0.4 | 1,230 | 1,290 | 0.1 | 0.27 | 0.06 |
| US8-5′ | L-27 | 2,160 | 1,790 | 0.9 | 10,660 | 4,680 | 0.7 | 0.36 | 0.81 |
| US8/9 | L-28 | 19,550 | 8,010 | 8.3 | 50,660 | 21,210 | 3.5 | 0.47 | 0.16 |
| RLAT-5′ | LT-1 | 730 | 5,820 | 0.3 | 1,550 | 8,450 | 0.1 | 0.50 | 0.28 |
| RHA6 | LT-2 | 430 | 5,750 | 0.2 | 970 | 1,540 | 0.1 | 0.56 | 0.20 |
| RLATX | LT-3 | 430 | 510 | 0.2 | 12,960 | 4,440 | 0.9 | 0.35 | 0.02 |
| RLAT-3′ | LT-4 | 1,690 | 2,310 | 0.7 | 21,870 | 5,480 | 1.5 | 0.55 | 0.00 |
| U1X | ? | 110 | 110 | 0.0 | 350 | 700 | 0.0 | 0.15 | 0.31 |
| U6/7 | L/? | 2,400 | 860 | 1.0 | 18,090 | 7,910 | 1.3 | 0.04 | 0.24 |
| U11/13 | L/E/L | 4,100 | 560 | 1.7 | 35,560 | 18,760 | 2.5 | 0.06 | 0.24 |
| U36 | E/L | 200 | 120 | 0.1 | 14,450 | 6,040 | 1.0 | 0.14 | 0.81 |
| U43.5-5′ | ? | 110 | 120 | 0.0 | 950 | 5,770 | 0.1 | 0.63 | 0.19 |
| U49 | E/L | 7,590 | 2,000 | 3.2 | 48,390 | 24,410 | 3.4 | 0.03 | 0.03 |
| U52/53 | E/L | 1,770 | 450 | 0.8 | 21,300 | 5,440 | 1.5 | 0.00 | 0.71 |
| RLAT-1 | LAT | 380 | 1,050 | 0.2 | 1,590 | 4,660 | 0.1 | 0.98 | 0.34 |
| ROP | ? | 980 | 1,620 | 0.4 | 5,390 | 2,200 | 0.4 | 0.39 | 0.01 |
| US3-5′ | L? | 3,280 | 310 | 1.4 | 1,520 | 980 | 0.1 | 0.36 | 0.24 |
| US3/4 | E/L? | 2,680 | 1,080 | 1.1 | 14,230 | 6,310 | 1.0 | 0.03 | 0.12 |
| US5/6/7 | EL/E/E | 8,570 | 2,690 | 3.6 | 42,870 | 15,220 | 3.0 | 0.15 | 0.02 |
| US10/11/12 | E/E/1E | 10,170 | 3,890 | 4.3 | 55,710 | 28,620 | 3.9 | 0.17 | 0.02 |
| Total | 235,570 | 1,442,860 | |||||||
Infection was initiated at an MOI of 1 PFU (approximately 1,000 virions) of RP5 per cell. Bold entries represent either single transcripts or multiple transcripts of the same kinetic class.
See Table 1, footnote b.
Median values are based upon eight separate experiments. All values were determined at a laser power of 80 with a photomultiplier at 75%.
Median values are based upon seven separate experiments.
The relative abundance values for high-multiplicity infection by RP5 (this table) were compared with values determined at 4 and 8 hpi for wt infection (Table 1). The null hypothesis is that the true values for a given timepoint in the two infections are identical. The value 0.00 is used to indicate P values of <0.01.
VP16 produced during productive infection has no apparent effect on E or L gene expression.
Although virion-borne VP16 is best known as an activator of IE gene expression, the possibility also exists that the VP16 produced in infected cells as a leaky-late protein might contribute to IE gene expression at later times in infection or might directly influence transcription of E or L genes. To distinguish between the effects of virion-borne and newly synthesized VP16 in infected cells, we used RP5 virions grown in a complementing cell line, 16-8 (36). These virions, designated RP5/16-8, contain normal amounts of wt VP16 protein in the tegument (expressed from the host cell) but express the truncated VP16 (lacking the activation domain) from the infecting viral genome (29). Oligonucleotide microarrays were used to assess the changes in abundance of viral transcripts in HeLa cells infected for 2, 4, and 8 h with RP5/16-8. The hybridization data reported in Table 6 were compared with the results for a wt (RP5R) infection (Table 1) for each gene at each time point, and correlation coefficients were calculated for the major kinetic classes (Table 3). The results reveal that during infection by RP5/16-8, all classes of viral genes are expressed in essentially normal patterns. No significant difference in IE gene expression was noted at early or late times, nor were any E or L genes significantly altered during this infection. From this, we conclude that the VP16 protein translated at late times in infected cells has little direct impact on the gene expression in that cell.
TABLE 6.
Abundance of HSV-1 transcripts following infection by RP5 virus grown in 16-8 cellsa
| Transcript set | Kinetic class and IDb | Abundance atc:
|
t-test value, RP5/16-8 vs wtd
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 hpi
|
4 hpi
|
8 hpi
|
|||||||||||
| Median | SD | % | Median | SD | % | Median | SD | % | 2 hpi | 4 hpi | 8 hpi | ||
| RICP0 | IE-1 | 11,220 | 12,980 | 22.0 | 19,520 | 7,630 | 4.5 | 76,190 | 1,590 | 3.0 | 0.74 | 0.70 | 0.44 |
| U54 | IE-2 | 1,960 | 5,240 | 3.9 | 11,440 | 3,410 | 2.6 | 38,230 | 15,900 | 1.5 | 0.22 | 0.36 | 0.82 |
| RICP4 | IE-3 | 6,520 | 10,400 | 12.8 | 12,930 | 3,430 | 3.0 | 39,130 | 17,350 | 1.5 | 0.27 | 0.40 | 0.37 |
| R/S22 | IE-4 | 6,050 | 11,040 | 11.9 | 28,540 | 10,280 | 6.5 | 81,750 | 3,380 | 3.2 | 0.85 | 0.32 | 0.53 |
| U4-5′ | E-1 | 60 | 20 | 0.1 | 930 | 500 | 0.2 | 7,870 | 420 | 0.3 | 0.60 | 0.97 | 0.42 |
| U4/5 | E-2 | 620 | 510 | 1.2 | 8,210 | 1,910 | 1.9 | 60,560 | 580 | 2.4 | 0.17 | 0.87 | 0.52 |
| U8/9 | E-3 | 300 | 170 | 0.6 | 3,410 | 730 | 0.8 | 43,120 | 7,580 | 1.7 | 0.75 | 0.27 | 0.56 |
| U8-5′ | E-4 | 30 | 100 | 0.1 | 80 | 120 | 0.0 | 1,420 | 990 | 0.1 | 0.18 | 0.20 | 0.25 |
| U21 | E-5 | 20 | 60 | 0.0 | 2,360 | 180 | 0.5 | 41,230 | 7,590 | 1.6 | 0.00 | 0.49 | 0.15 |
| U23 | E-6 | 170 | 270 | 0.3 | 11,450 | 2,940 | 2.6 | 22,340 | 4,050 | 0.9 | 0.17 | 0.44 | 0.18 |
| U29 | E-7 | 40 | 80 | 0.1 | 5,250 | 1,530 | 1.2 | 17,330 | 7,470 | 0.7 | 0.09 | 0.40 | 0.23 |
| U30 | E-8 | 390 | 610 | 0.8 | 15,740 | 6,220 | 3.6 | 30,010 | 13,430 | 1.2 | 0.30 | 0.58 | 0.70 |
| U37 | E-9 | 660 | 650 | 1.3 | 4,920 | 2,060 | 1.1 | 48,750 | 6,160 | 1.9 | 0.26 | 0.72 | 0.47 |
| U39-5′ | E-10 | 120 | 160 | 0.2 | 5,440 | 2,800 | 1.2 | 25,070 | 14,630 | 1.0 | 0.45 | 0.44 | 0.73 |
| U39/40 | E-11 | 650 | 360 | 1.3 | 18,440 | 6,970 | 4.2 | 62,640 | 9,270 | 2.5 | 0.15 | 0.95 | 0.43 |
| U42 | E-12 | 730 | 620 | 1.4 | 18,010 | 4,640 | 4.1 | 92,800 | 12,530 | 3.6 | 0.34 | 0.60 | 0.32 |
| U43 | E-13 | 500 | 250 | 1.0 | 2,220 | 680 | 0.5 | 21,460 | 2,260 | 0.8 | 0.85 | 0.20 | 0.26 |
| U50 | E-14 | 230 | 70 | 0.5 | 11,860 | 1,120 | 2.7 | 45,440 | 17,540 | 1.8 | 0.92 | 0.75 | 0.47 |
| U52-5′ | E-15 | 0 | 40 | 0.0 | 0 | 30 | 0.0 | 0 | 0 | 0.0 | 0.16 | 0.19 | 0.19 |
| U55 | E-16 | 60 | 80 | 0.1 | 1,670 | 180 | 0.4 | 26,620 | 10,480 | 1.0 | 0.08 | 0.15 | 0.40 |
| U56 | E-17 | 170 | 210 | 0.3 | 4,340 | 710 | 1.0 | 38,200 | 23,050 | 1.5 | 0.68 | 0.10 | 0.59 |
| US2 | E-18 | 670 | 900 | 1.3 | 3,050 | 1,580 | 0.7 | 67,210 | 9,780 | 2.6 | 0.65 | 0.69 | 0.29 |
| U1 | L-1 | 720 | 1,460 | 1.4 | 15,050 | 2,440 | 3.4 | 64,900 | 7,470 | 2.5 | 0.35 | 0.12 | 0.30 |
| U3 | L-2 | 250 | 70 | 0.5 | 3,560 | 1,560 | 0.8 | 60,180 | 6,970 | 2.4 | 0.18 | 0.75 | 0.70 |
| U10 | L-3 | 840 | 1,240 | 1.7 | 3,000 | 860 | 0.7 | 68,500 | 2,280 | 2.7 | 0.43 | 0.26 | 0.14 |
| U16/17 | L-4 | 60 | 120 | 0.1 | 2,190 | 410 | 0.5 | 43,110 | 12,450 | 1.7 | 0.07 | 0.33 | 0.41 |
| U15 | L-5 | 70 | 110 | 0.1 | 1,000 | 800 | 0.2 | 20,010 | 7,240 | 0.8 | 0.04 | 0.97 | 0.27 |
| U18/20 | L-6 | 70 | 90 | 0.1 | 13,140 | 660 | 3.0 | 57,670 | 15,980 | 2.3 | 0.26 | 0.11 | 0.45 |
| U19/20 | L-7 | 110 | 1,120 | 0.2 | 11,340 | 930 | 2.6 | 54,870 | 14,320 | 2.1 | 0.43 | 0.20 | 0.88 |
| U19-5′ | L-8 | 10 | 80 | 0.0 | 70 | 90 | 0.0 | 990 | 1,020 | 0.0 | 0.16 | 0.20 | 0.19 |
| U22 | L-9 | 120 | 70 | 0.2 | 1,700 | 520 | 0.4 | 40,390 | 2,450 | 1.6 | 0.45 | 0.48 | 0.03 |
| U24 | L-10 | 100 | 170 | 0.2 | 2,300 | 170 | 0.5 | 30,540 | 4,790 | 1.2 | 0.07 | 0.09 | 0.40 |
| U25 | L-11 | 120 | 150 | 0.2 | 7,590 | 3,310 | 1.7 | 50,950 | 5,840 | 2.0 | 0.33 | 0.27 | 0.96 |
| U27/8 | L-12 | 290 | 350 | 0.6 | 11,800 | 2,970 | 2.7 | 39,840 | 9,960 | 1.6 | 0.24 | 0.43 | 0.69 |
| U27-5′ | L-13 | 70 | 90 | 0.1 | 5,250 | 1,610 | 1.2 | 22,050 | 17,730 | 0.9 | 0.24 | 0.61 | 0.55 |
| U31/34 | L-14 | 300 | 430 | 0.6 | 3,260 | 1,450 | 0.7 | 39,320 | 2,710 | 1.5 | 0.66 | 0.72 | 0.19 |
| U35 | L-15 | 360 | 440 | 0.7 | 8,610 | 3,380 | 2.0 | 68,000 | 2,920 | 2.7 | 0.35 | 0.99 | 0.97 |
| U38 | L-16 | 1,100 | 600 | 2.2 | 2,350 | 1,660 | 0.5 | 73,970 | 2,860 | 2.9 | 0.36 | 0.69 | 0.53 |
| U41 | L-17 | 470 | 1,100 | 0.9 | 1,180 | 2,650 | 0.3 | 45,320 | 660 | 1.8 | 0.33 | 0.57 | 0.60 |
| U44-5′ | L-18 | 60 | 130 | 0.1 | 810 | 470 | 0.2 | 24,850 | 12,210 | 1.0 | 0.24 | 0.39 | 0.70 |
| U44/45 | L-19 | 660 | 290 | 1.3 | 13,590 | 3,650 | 3.1 | 77,290 | 1,240 | 3.0 | 0.72 | 0.93 | 0.91 |
| U46/47 | L-20 | 410 | 680 | 0.8 | 11,220 | 1,280 | 2.6 | 54,760 | 16,730 | 2.1 | 0.16 | 0.24 | 0.84 |
| U48 | L-21 | 110 | 120 | 0.2 | 1,200 | 480 | 0.3 | 12,520 | 3,820 | 0.5 | 0.25 | 0.00 | 0.08 |
| U51 | L-22 | 200 | 430 | 0.4 | 2,730 | 600 | 0.6 | 48,010 | 14,060 | 1.9 | 0.13 | 0.02 | 0.39 |
| RLXY | L-23 | 260 | 1,490 | 0.5 | 2,020 | 1,010 | 0.5 | 45,680 | 3,130 | 1.8 | 0.39 | 0.78 | 0.54 |
| RLX | L-24 | 1,070 | 1,610 | 2.1 | 2,050 | 490 | 0.5 | 39,220 | 5,800 | 1.5 | 0.43 | 0.10 | 0.03 |
| RICP34.5 | L-25 | 160 | 90 | 0.3 | 1,280 | 310 | 0.3 | 10,900 | 880 | 0.4 | 0.26 | 0.11 | 0.42 |
| US5-5′ | L-26 | 10 | 240 | 0.0 | 0 | 90 | 0.0 | 0 | 0 | 0.0 | 0.16 | 0.19 | 0.18 |
| US8-5′ | L-27 | 50 | 260 | 0.1 | 330 | 210 | 0.1 | 3,980 | 2,260 | 0.2 | 0.16 | 0.20 | 0.23 |
| US8/9 | L-28 | 1,260 | 1,550 | 2.5 | 21,560 | 6,110 | 4.9 | 67,640 | 1,820 | 2.6 | 0.66 | 0.32 | 0.12 |
| RLAT-5′ | LT-1 | 20 | 100 | 0.0 | 0 | 10 | 0.0 | 5,000 | 3,630 | 0.2 | 0.19 | 0.19 | 0.40 |
| RHA6 | LT-2 | 380 | 1,000 | 0.7 | 1,580 | 1,270 | 0.4 | 16,050 | 3,750 | 0.6 | 0.44 | 0.40 | 0.83 |
| RLATX | LT-3 | 360 | 2,870 | 0.7 | 90 | 90 | 0.0 | 22,420 | 15,630 | 0.9 | 0.46 | 0.21 | 0.56 |
| RLAT-3′ | LT-4 | 290 | 460 | 0.6 | 620 | 80 | 0.1 | 34,490 | 17,560 | 1.3 | 0.34 | 0.09 | 0.50 |
| UIX | ? | 330 | 1,410 | 0.6 | 1,600 | 1,230 | 0.4 | 9,690 | 3,280 | 0.4 | 0.40 | 0.41 | 0.23 |
| U6/7 | L/? | 590 | 610 | 1.1 | 5,520 | 1,320 | 1.3 | 69,070 | 4,790 | 2.7 | 0.09 | 0.52 | 0.54 |
| U11/13 | L/E/L | 430 | 830 | 0.8 | 15,430 | 2,450 | 3.5 | 64,810 | 4,330 | 2.5 | 0.34 | 0.24 | 0.45 |
| U36 | E/L | 500 | 700 | 1.0 | 3,140 | 1,710 | 0.7 | 53,710 | 6,950 | 2.1 | 0.33 | 0.83 | 0.29 |
| U43.5-5′ | ? | 20 | 90 | 0.0 | 0 | 40 | 0.0 | 90 | 130 | 0.0 | 0.19 | 0.31 | 0.37 |
| U49 | E/L | 320 | 270 | 0.6 | 11,550 | 3,110 | 2.6 | 37,000 | 5,730 | 1.4 | 0.09 | 0.82 | 0.19 |
| U52/53 | E/L | 150 | 190 | 0.3 | 4,100 | 860 | 0.9 | 37,030 | 12,820 | 1.4 | 0.08 | 0.02 | 0.51 |
| RLAT-1 | LAT | 40 | 100 | 0.1 | 0 | 20 | 0.0 | 3,520 | 740 | 0.1 | 0.18 | 0.19 | 0.59 |
| ROP | ? | 50 | 120 | 0.1 | 0 | 40 | 0.0 | 290 | 190 | 0.0 | 0.11 | 0.20 | 0.07 |
| US3-5′ | L? | 60 | 180 | 0.1 | 450 | 210 | 0.1 | 0 | 0 | 0.0 | 0.15 | 0.21 | 0.18 |
| US3/4 | E/L? | 590 | 730 | 1.2 | 12,540 | 2,260 | 2.9 | 6,480 | 330 | 0.3 | 0.61 | 0.80 | 0.16 |
| US5/6/7 | EL/E/E | 1,260 | 1,750 | 2.5 | 18,790 | 6,750 | 4.3 | 66,540 | 15,940 | 2.6 | 0.66 | 0.11 | 0.96 |
| US10/11/12 | E/E/IE | 5,110 | 8,820 | 10.0 | 21,630 | 8,230 | 4.9 | 75,830 | 11,280 | 3.0 | 0.81 | 0.33 | 0.85 |
| Total | 50,950 | 437,060 | 2,554,860 | ||||||||||
HeLa cells were infected at an MOI of 5 PFU per cell using RP5 virus grown in the complementing cell line 16-8. Bold entries represent either single transcripts or multiple transcripts of the same kinetic class.
See Table 1, footnote b.
Median values are based on three, three, and two separate experiments at 2, 4, and 8 hpi, respectively.
Relative values for each transcript at each time point during RP5/16-8 infection were compared with values for wt infection (Table 1) by using Student's two-tailed t test assuming unequal variance. The value 0.00 is used to indicate a P value of <0.01.
Cycloheximide stimulates IE gene expression independently of the VP16 activation domain.
Cycloheximide has frequently been used as a protein synthesis inhibitor to block translation of viral IE proteins and thus to arrest HSV infection at the IE stage. Other laboratories have previously reported that the presence of cycloheximide itself (or other translation inhibitors) can stimulate expression of individual IE genes as measured by steady-state levels of IE or reporter gene mRNAs (22). We tested the effects of cycloheximide on the global pattern of viral gene expression in the absence or presence of stimulation by the VP16 activation domain. Cycloheximide-treated cells were infected with either RP5 or RP5/16-8. RNA was isolated at 2 hpi and hybridized to “first-generation” oligonucleotide array chips, with the results shown in Table 7. The overall signal strength for RP5/16-8 was much higher than that from the mutant infection, so relative values rather than absolute median values were used for comparisons with infections in the absence of cycloheximide. The analysis indicates that during RP5 infection, cycloheximide caused a pronounced increase (20-fold) in ICP4 and ICP0 expression and modest increases (approximately 6-fold) in ICP22 and ICP27 expression. The relatively elevated expression of transcripts hybridizing to probes L-20, L22, L-24, and LT-4 seen at 2 h in the RP5 infection (Table 1) was also seen here. In cells infected with viruses carrying intact VP16 protein (RP5/16-8), total IE gene expression was greater in the presence of cycloheximide than in its absence, but the impact on specific IE genes was less apparent. These results suggest that cycloheximide can stimulate IE gene expression above that induced by VP16. Moreover, the VP16-independent effect of cycloheximide is more pronounced on some IE genes than others, implying that some transcript-specific mechanism involving either mRNA stabilization or transcription effects (or both) might be involved. To date, the contributions of these factors to this cycloheximide effect on ICP4 and ICP0 abundance have not been identified.
TABLE 7.
Abundance of HSV-1 transcripts at 2 h after infection of HeLa cells treated with cycloheximidea
| Transcript set | Kinetic class and IDb | Abundance
|
|||||
|---|---|---|---|---|---|---|---|
| RP5
|
RP5 (16-8)
|
||||||
| Medianc | SD | % | Mediand | SD | % | ||
| RICP0 | IE-1 | 11,930 | 3,080 | 5.1 | 104,140 | 23,580 | 24.2 |
| U54 | IE-2 | 1,250 | 740 | 0.5 | 26,880 | 7,680 | 6.2 |
| RICP4 | IE-3 | 39,970 | 13,250 | 17.2 | 82,180 | 45,810 | 19.1 |
| R/S22 | IE-4 | 3,650 | 2,230 | 1.6 | 69,390 | 36,500 | 16.1 |
| US10/11/12 | E/E/IEe | 3,950 | 1,390 | 1.7 | 55,850 | 16,870 | 13.0 |
| U4/5 | E-2 | 9,110 | 1,850 | 3.9 | 2,780 | 640 | 0.6 |
| U8/9 | E-3 | 2,610 | 650 | 1.1 | 1,230 | 280 | 0.3 |
| U21 | E-5 | 820 | 90 | 0.4 | 900 | 390 | 0.2 |
| U23 | E-6 | 5,070 | 1,110 | 2.2 | 1,280 | 280 | 0.3 |
| U29 | E-7 | 410 | 630 | 0.2 | 1,590 | 890 | 0.4 |
| U30 | E-8 | 2,010 | 500 | 0.9 | 2,370 | 1,390 | 0.6 |
| U37 | E-9 | 630 | 450 | 0.3 | 2,630 | 1,070 | 0.6 |
| U39/40 | E-11 | 870 | 130 | 0.4 | 3,490 | 930 | 0.8 |
| U42 | E-12 | 2,030 | 840 | 0.9 | 2,530 | 700 | 0.6 |
| U43 | E-13 | 5,750 | 820 | 2.5 | 3,370 | 1,560 | 0.8 |
| U50 | E-14 | 2,910 | 660 | 1.3 | 1,290 | 410 | 0.3 |
| U55 | E-16 | 410 | 250 | 0.2 | 1,310 | 370 | 0.3 |
| U56 | E-17 | 3,170 | 720 | 1.4 | 1,860 | 900 | 0.4 |
| US2 | E-18 | 1,340 | 300 | 0.6 | 2,810 | 1,990 | 0.7 |
| U1 | L-1 | 560 | 480 | 0.2 | 1,550 | 710 | 0.4 |
| U3 | L-2 | 700 | 180 | 0.3 | 1,690 | 460 | 0.4 |
| U10 | L-3 | 4,580 | 760 | 2.0 | 1,280 | 990 | 0.3 |
| U16/17 | L-4 | 2,550 | 760 | 1.1 | 1,610 | 600 | 0.4 |
| U15 | L-5 | 1,680 | 840 | 0.7 | 1,350 | 450 | 0.3 |
| U18/20 | L-6 | 530 | 320 | 0.2 | 1,270 | 480 | 0.3 |
| U19/20 | L-7 | 1,320 | 820 | 0.6 | 1,400 | 580 | 0.3 |
| U22 | L-9 | 360 | 190 | 0.2 | 1,520 | 660 | 0.4 |
| U24 | L-10 | 2,760 | 920 | 1.2 | 1,040 | 350 | 0.2 |
| U25 | L-11 | 1,670 | 520 | 0.7 | 1,100 | 420 | 0.3 |
| U27/8 | L-12 | 3,000 | 920 | 1.3 | 1,350 | 570 | 0.3 |
| U31/34 | L-14 | 2,100 | 640 | 0.9 | 2,180 | 550 | 0.5 |
| U35 | L-15 | 1,830 | 500 | 0.8 | 2,190 | 770 | 0.5 |
| U38 | L-16 | 4,160 | 1,240 | 1.8 | 2,250 | 480 | 0.5 |
| U41 | L-17 | 50 | 100 | 0.0 | 2,060 | 630 | 0.5 |
| U44/45 | L-19 | 5,090 | 1,550 | 2.2 | 2,050 | 810 | 0.5 |
| U46/47 | L-20 | 24,490 | 3,540 | 10.5 | 3,610 | 1,590 | 0.8 |
| U48 | L-21 | 1,050 | 450 | 0.5 | 1,750 | 810 | 0.4 |
| U51 | L-22 | 18,580 | 4,900 | 8.0 | 2,060 | 620 | 0.5 |
| RLXY | L-23 | 250 | 130 | 0.1 | 1,220 | 1,160 | 0.3 |
| RLX | L-24 | 16,640 | 3,260 | 7.2 | 1,850 | 4,570 | 0.4 |
| RICP34.5 | L-25 | 3,370 | 300 | 1.5 | 2,330 | 1,050 | 0.5 |
| US8/9 | L-28 | 3,590 | 1,520 | 1.5 | 2,410 | 860 | 0.6 |
| RHA6 | LT-2 | 2,240 | 740 | 1.0 | 2,350 | 2,090 | 0.5 |
| RLAT-3′ | LT-4 | 14,220 | 6,440 | 6.1 | 2,690 | 550 | 0.6 |
| UIX | 390 | 430 | 0.2 | 1,290 | 320 | 0.3 | |
| U6/7 | 6,110 | 1,670 | 2.6 | 3,310 | 180 | 0.8 | |
| U11/13 | 640 | 320 | 0.3 | 1,420 | 450 | 0.3 | |
| U36 | 310 | 330 | 0.1 | 2,600 | 840 | 0.6 | |
| U49 | 2,850 | 2,380 | 1.2 | 1,280 | 450 | 0.3 | |
| U52/53 | 2,680 | 1,080 | 1.2 | 2,170 | 720 | 0.5 | |
| US3/4 | 1,420 | 360 | 0.6 | 2,060 | 780 | 0.5 | |
| US5/6/7 | 2,600 | 1,250 | 1.1 | 2,230 | 430 | 0.5 | |
| Total | 232,260 | 430,400 | |||||
HeLa cells were infected with equivalent virion numbers of either RP5 virus grown in Vero cells (at 0.05 PFU/cell) or RP5 virus grown in VP16-expressing 16-8 cells (at 5 PFU/cell). Cycloheximide (60 μg/ml) was present in culture media starting 2 h prior to infection. RNA was harvested at 2 hpi. Bold entries represent probes for IE transcripts.
See Table 1, footnote b.
Median values are based upon four separate experiments. All signals were determined at a laser power of 90 with a photomultiplier at 85%.
Median values are based upon four separate experiments. All signals were determined at a laser power of 80 with a photomultiplier at 75. These values were then multiplied by 8 to allow direct comparison with the values for the RP5 infection alone (see the text) (33).
Although this probe nominally detects transcripts from several genes of different kinetic classes, the signal at IE times arises predominantly from the US12 (ICP47) gene.
Deletion of either of two subregions of the VP16 activation domain results in quantitative and qualitative changes in viral gene expression.
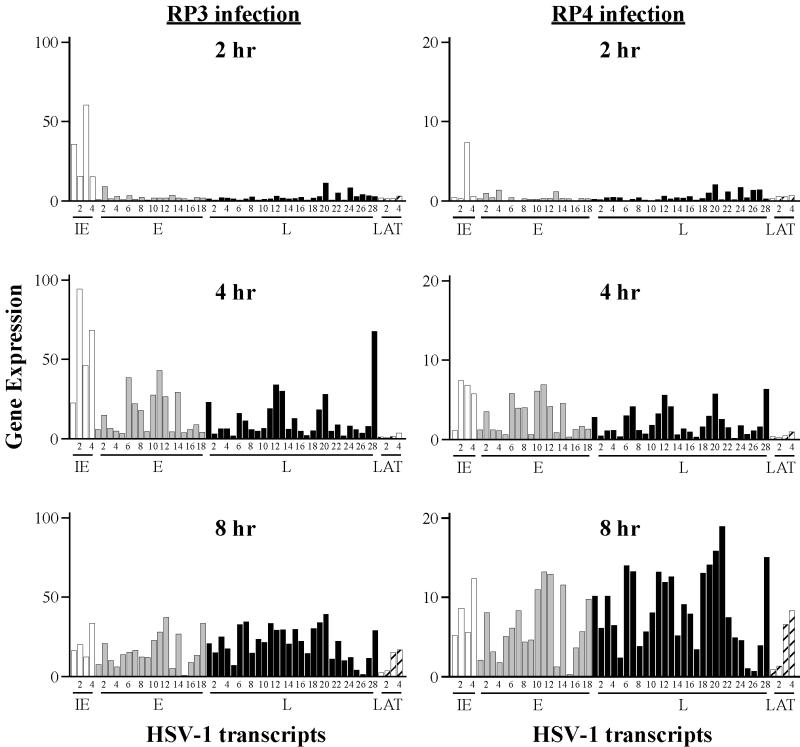
The activation domain of VP16 extends from amino acids 413 to 490, within which two subregions have been defined, each of which can function independently to activate transcription (23, 27, 35). To ask whether these two subregions support identical patterns of viral gene expression, we used two recombinant viruses in which only one subregion or the other is present on VP16. Strain RP3 retains VP16 amino acids 1 to 456 (and lacks residues 457 to 490), whereas in strain RP4 the amino acids 413 to 452 were deleted (29). We infected HeLa cells with each virus and harvested RNA at 2, 4, and 8 h. We then used DNA chip hybridization analysis to determine the median and relative values for abundance of each viral transcript (Table 8). The abundance of transcripts at the three time intervals, summarized in Fig. 3, permits three observations. First, the absolute expression levels of transcripts in cells infected with RP3 were essentially indistinguishable from those in wt infections, whereas transcript levels during RP4 infection were significantly reduced. Secondly, the IE gene expression patterns differ significantly for the two viruses. At 2 h, ICP4 was the only IE gene abundantly expressed during RP4 infection, whereas RP3 displayed a more balanced pattern of IE gene expression. The differences in expression of the ICP27, ICP0, and ICP22 transcripts at 2 hpi by these two viruses are statistically significant (t test, P < 0.05) (Table 8). Especially for RP4, the ICP0 expression levels at 4 and 8 h were notably low compared with those in wt infections. Third, despite these differences in IE gene expression, both viruses exhibited a regulated pattern of changes in relative transcript abundance that was generally similar to that seen in a wt infection. By 4 hpi, while the correlation between relative levels of IE transcripts as a group was very low between either RP3 or RP4 and the RP5R (wt) infection, expression of the E and L genes from each mutant correlated well with the wt pattern (Table 3). After 8 h of infection, the relative levels of all classes of transcripts were well correlated with the wt level.
TABLE 8.
Abundance of HSV-1 transcripts at various times following infection of HeLa cells with RP3 or RP4 virusa
| Transcript set | Kinetic class and IDb | Abundance
|
% RP3 vs % RP4c
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection by RP3
|
Infection by RP4
|
|||||||||||||||||||||
| 2 hpi
|
4 hpi
|
8 hpi
|
2 hpi
|
4 hpi
|
8 hpi
|
|||||||||||||||||
| Median | SD | % | Median | SD | % | Median | SD | % | Median | SD | % | Median | SD | % | Median | SD | % | 2 hpi | 4 hpi | 8 hpi | ||
| RICP0 | IE-1 | 34,380 | 27,220 | 13.4 | 21,780 | 12,930 | 2.2 | 15,560 | 4,720 | 1.4 | 320 | 540 | 1.0 | 1,010 | 480 | 0.7 | 4,970 | 1,140 | 1.1 | 0.00 | 0.00 | 0.32 |
| U54 | IE-2 | 14,610 | 29,530 | 5.7 | 91,290 | 65,700 | 9.1 | 19,290 | 3,070 | 1.7 | 220 | 90 | 0.7 | 7,110 | 3,800 | 4.7 | 8,310 | 2,120 | 1.8 | 0.04 | 0.10 | 0.50 |
| RICP4 | IE-3 | 58,490 | 30,190 | 22.8 | 44,550 | 25,330 | 4.4 | 11,730 | 6,170 | 1.0 | 7,070 | 3,080 | 21.1 | 6,580 | 2,480 | 4.4 | 5,370 | 1,730 | 1.2 | 0.36 | 0.73 | 0.96 |
| R/S22 | IE-4 | 14,420 | 31,680 | 5.6 | 66,120 | 37,570 | 6.6 | 32,250 | 5,070 | 2.8 | 510 | 280 | 1.5 | 5,520 | 1,160 | 3.7 | 11,920 | 2,740 | 2.6 | 0.03 | 0.00 | 0.20 |
| U4-5′ | E-1 | 450 | 190 | 0.2 | 5,470 | 2,360 | 0.5 | 7,180 | 3,000 | 0.6 | 210 | 620 | 0.6 | 1,110 | 180 | 0.7 | 1,990 | 1,100 | 0.4 | 0.20 | 0.11 | 0.68 |
| U4/5 | E-2 | 8,290 | 4,340 | 3.2 | 14,180 | 7,170 | 1.4 | 19,850 | 6,180 | 1.7 | 890 | 450 | 2.7 | 3,350 | 1,110 | 2.2 | 7,800 | 3,260 | 1.7 | 0.69 | 0.14 | 0.89 |
| U8/9 | E-3 | 840 | 370 | 0.3 | 6,060 | 2,470 | 0.6 | 9,440 | 5,480 | 0.8 | 370 | 390 | 1.1 | 1,100 | 220 | 0.7 | 3,000 | 1,440 | 0.6 | 0.08 | 0.25 | 0.57 |
| U8-5′ | E-4 | 2,380 | 970 | 0.9 | 4,510 | 2,160 | 0.4 | 5,590 | 1,840 | 0.5 | 1,300 | 650 | 3.9 | 980 | 280 | 0.6 | 1,690 | 930 | 0.4 | 0.02 | 0.39 | 0.86 |
| U21 | E-5 | 330 | 160 | 0.1 | 2,890 | 1,790 | 0.3 | 13,080 | 2,820 | 1.1 | 30 | 70 | 0.1 | 510 | 210 | 0.3 | 4,850 | 1,180 | 1.0 | 0.67 | 0.46 | 0.96 |
| U23 | E-6 | 2,770 | 1,820 | 1.1 | 37,180 | 25,550 | 3.7 | 14,610 | 5,940 | 1.3 | 340 | 180 | 1.0 | 5,550 | 1,200 | 3.7 | 5,900 | 4,590 | 1.3 | 0.13 | 0.22 | 0.37 |
| U29 | E-7 | 660 | 300 | 0.3 | 21,030 | 11,940 | 2.1 | 15,840 | 5,590 | 1.4 | 0 | 70 | 0.0 | 3,760 | 1,570 | 2.5 | 8,050 | 3,160 | 1.7 | 0.11 | 0.83 | 0.74 |
| U30 | E-8 | 1,760 | 840 | 0.7 | 16,980 | 4,830 | 1.7 | 11,650 | 4,590 | 1.0 | 220 | 330 | 0.7 | 3,820 | 860 | 2.5 | 4,160 | 1,440 | 0.9 | 0.34 | 0.00 | 0.53 |
| U37 | E-9 | 280 | 140 | 0.1 | 4,200 | 2,230 | 0.4 | 11,360 | 6,180 | 1.0 | 140 | 230 | 0.4 | 530 | 180 | 0.4 | 4,460 | 1,360 | 1.0 | 0.14 | 0.69 | 0.47 |
| U39-5′ | E-10 | 1,370 | 630 | 0.5 | 26,410 | 9,300 | 2.6 | 21,850 | 1,830 | 1.9 | 160 | 90 | 0.5 | 5,850 | 1,800 | 3.9 | 10,580 | 3,550 | 2.3 | 0.66 | 0.06 | 0.41 |
| U39/40 | E-11 | 1,360 | 750 | 0.5 | 41,560 | 9,980 | 4.1 | 26,890 | 4,010 | 2.3 | 260 | 150 | 0.8 | 6,630 | 2,440 | 4.4 | 12,780 | 3,310 | 2.7 | 0.13 | 0.17 | 0.43 |
| U42 | E-12 | 1,280 | 220 | 0.5 | 25,500 | 3,190 | 2.5 | 35,930 | 3,550 | 3.1 | 250 | 210 | 0.7 | 3,940 | 660 | 2.6 | 12,470 | 2,350 | 2.7 | 0.63 | 0.77 | 0.12 |
| U43 | E-13 | 3,090 | 740 | 1.2 | 3,900 | 1,570 | 0.4 | 4,550 | 6,050 | 0.4 | 1,070 | 570 | 3.2 | 780 | 270 | 0.5 | 1,120 | 980 | 0.2 | 0.03 | 0.16 | 0.41 |
| U50 | E-14 | 1,480 | 450 | 0.6 | 28,180 | 8,820 | 2.8 | 25,800 | 6,390 | 2.2 | 280 | 610 | 0.8 | 4,340 | 1,120 | 2.9 | 11,160 | 2,990 | 2.4 | 0.29 | 0.50 | 0.74 |
| U52-5′ | E-15 | 870 | 1,800 | 0.3 | 3,420 | 2,710 | 0.3 | 560 | 660 | 0.0 | 200 | 150 | 0.6 | 220 | 310 | 0.1 | 230 | 170 | 0.0 | 0.75 | 0.13 | 0.98 |
| U55 | E-16 | 290 | 160 | 0.1 | 5,550 | 3,890 | 0.6 | 8,300 | 5,170 | 0.7 | 0 | 20 | 0.0 | 1,180 | 500 | 0.8 | 3,460 | 2,180 | 0.7 | 0.04 | 0.90 | 0.98 |
| U56 | E-17 | 1,530 | 1,130 | 0.6 | 8,370 | 6,810 | 0.8 | 12,680 | 5,690 | 1.1 | 250 | 130 | 0.7 | 1,550 | 490 | 1.0 | 5,440 | 3,330 | 1.2 | 0.74 | 0.65 | 0.46 |
| US2 | E-18 | 1,410 | 450 | 0.6 | 3,750 | 2,970 | 0.4 | 32,200 | 7,130 | 2.8 | 220 | 240 | 0.7 | 1,210 | 380 | 0.8 | 9,410 | 1,960 | 2.0 | 0.44 | 0.03 | 0.04 |
| U1 | L-1 | 940 | 1,060 | 0.4 | 22,140 | 12,220 | 2.2 | 19,910 | 5,200 | 1.7 | 170 | 100 | 0.5 | 2,640 | 1,230 | 1.7 | 9,820 | 5,050 | 2.1 | 0.68 | 0.63 | 0.32 |
| U3 | L-2 | 280 | 150 | 0.1 | 2,680 | 1,920 | 0.3 | 14,400 | 6,020 | 1.3 | 110 | 350 | 0.3 | 360 | 200 | 0.2 | 5,880 | 3,110 | 1.3 | 0.18 | 0.64 | 0.70 |
| U10 | L-3 | 1,670 | 670 | 0.7 | 5,830 | 3,910 | 0.6 | 24,100 | 4,960 | 2.1 | 370 | 230 | 1.1 | 1,000 | 720 | 0.7 | 9,830 | 3,410 | 2.1 | 0.13 | 0.46 | 0.99 |
| U16/17 | L-4 | 1,480 | 500 | 0.6 | 5,810 | 3,130 | 0.6 | 16,670 | 2,800 | 1.5 | 400 | 230 | 1.2 | 1,070 | 310 | 0.7 | 6,250 | 1,480 | 1.3 | 0.08 | 0.76 | 0.36 |
| U15 | L-5 | 780 | 280 | 0.3 | 1,440 | 1,870 | 0.1 | 6,690 | 2,760 | 0.6 | 360 | 300 | 1.1 | 250 | 210 | 0.2 | 2,240 | 1,820 | 0.5 | 0.08 | 0.90 | 0.89 |
| U18/20 | L-6 | 270 | 130 | 0.1 | 15,410 | 6,920 | 1.5 | 31,520 | 23,200 | 2.7 | 0 | 70 | 0.0 | 2,850 | 980 | 1.9 | 13,560 | 4,150 | 2.9 | 0.85 | 0.59 | 0.65 |
| U19/20 | L-7 | 930 | 210 | 0.4 | 10,690 | 6,970 | 1.1 | 33,360 | 22,220 | 2.9 | 140 | 370 | 0.4 | 3,970 | 1,100 | 2.6 | 12,820 | 4,220 | 2.8 | 0.32 | 0.04 | 0.46 |
| U19-5′ | L-8 | 2,040 | 690 | 0.8 | 5,410 | 4,600 | 0.5 | 14,100 | 4,090 | 1.2 | 350 | 480 | 1.0 | 1,060 | 580 | 0.7 | 3,650 | 1,250 | 0.8 | 0.36 | 0.58 | 0.48 |
| U22 | L-9 | 210 | 140 | 0.1 | 4,320 | 3,090 | 0.4 | 22,670 | 3,470 | 2.0 | 60 | 40 | 0.2 | 610 | 240 | 0.4 | 5,460 | 2,020 | 1.2 | 0.12 | 0.92 | 0.04 |
| U24 | L-10 | 720 | 320 | 0.3 | 6,190 | 2,890 | 0.6 | 20,710 | 3,770 | 1.8 | 20 | 100 | 0.1 | 1,690 | 600 | 1.1 | 7,760 | 1,220 | 1.7 | 0.27 | 0.01 | 0.96 |
| U25 | L-11 | 740 | 190 | 0.3 | 18,180 | 6,010 | 1.8 | 32,310 | 20,880 | 2.8 | 80 | 200 | 0.2 | 3,100 | 890 | 2.0 | 12,770 | 3,500 | 2.7 | 0.56 | 0.74 | 0.55 |
| U27/28 | L-12 | 2,500 | 570 | 1.0 | 32,810 | 10,760 | 3.3 | 28,170 | 2,280 | 2.5 | 530 | 370 | 1.6 | 5,380 | 700 | 3.6 | 11,490 | 860 | 2.5 | 0.14 | 0.91 | 0.60 |
| U27-5′ | L-13 | 1,280 | 490 | 0.5 | 28,950 | 14,040 | 2.9 | 28,330 | 2,950 | 2.5 | 190 | 90 | 0.6 | 3,960 | 1,070 | 2.6 | 12,200 | 1,510 | 2.6 | 0.63 | 0.17 | 0.27 |
| U31/34 | L-14 | 980 | 780 | 0.4 | 5,620 | 1,930 | 0.6 | 19,620 | 6,520 | 1.7 | 340 | 220 | 1.0 | 540 | 390 | 0.4 | 4,950 | 1,880 | 1.1 | 0.06 | 0.81 | 0.26 |
| U35 | L-15 | 1,130 | 190 | 0.4 | 12,270 | 3,280 | 1.2 | 28,660 | 3,810 | 2.5 | 290 | 290 | 0.9 | 1,260 | 340 | 0.8 | 8,780 | 2,980 | 1.9 | 0.24 | 0.13 | 0.61 |
| U38 | L-16 | 1,720 | 710 | 0.7 | 4,460 | 2,690 | 0.4 | 21,530 | 6,230 | 1.9 | 480 | 410 | 1.4 | 840 | 470 | 0.6 | 7,620 | 2,680 | 1.6 | 0.11 | 0.45 | 0.37 |
| U41 | L-17 | 90 | 90 | 0.0 | 1,710 | 1,600 | 0.2 | 13,950 | 6,080 | 1.2 | 50 | 70 | 0.1 | 200 | 90 | 0.1 | 3,270 | 2,770 | 0.7 | 0.10 | 0.27 | 0.54 |
| U44-5′ | L-18 | 1,330 | 650 | 0.5 | 4,670 | 2,970 | 0.5 | 29,200 | 26,940 | 2.5 | 260 | 120 | 0.8 | 1,460 | 440 | 1.0 | 12,610 | 4,420 | 2.7 | 0.76 | 0.03 | 0.86 |
| U44/45 | L-19 | 2,350 | 480 | 0.9 | 17,420 | 4,000 | 1.7 | 32,830 | 10,510 | 2.9 | 930 | 430 | 2.8 | 2,810 | 290 | 1.9 | 13,620 | 2,920 | 2.9 | 0.02 | 0.39 | 0.56 |
| U46/47 | L-20 | 10,500 | 2,700 | 4.1 | 26,870 | 15,430 | 2.7 | 37,800 | 17,800 | 3.3 | 1,970 | 1,350 | 5.9 | 5,480 | 980 | 3.6 | 15,340 | 3,790 | 3.3 | 0.39 | 0.13 | 0.87 |
| U48 | L-21 | 300 | 140 | 0.1 | 4,330 | 1,740 | 0.4 | 10,550 | 4,940 | 0.9 | 100 | 140 | 0.3 | 2,400 | 1,070 | 1.6 | 18,340 | 8,100 | 3.9 | 0.26 | 0.00 | 0.01 |
| U51 | L-22 | 4,510 | 1,520 | 1.8 | 8,280 | 3,040 | 0.8 | 21,320 | 3,110 | 1.9 | 1,080 | 1,130 | 3.2 | 1,410 | 530 | 0.9 | 7,180 | 2,940 | 1.5 | 0.12 | 0.19 | 0.64 |
| RLXY | L-23 | 140 | 100 | 0.1 | 1,600 | 1,470 | 0.2 | 9,540 | 2,630 | 0.8 | 120 | 210 | 0.4 | 80 | 100 | 0.1 | 4,730 | 1,990 | 1.0 | 0.18 | 0.01 | 0.90 |
| RLX | L-24 | 7,670 | 1,650 | 3.0 | 7,560 | 5,410 | 0.8 | 11,410 | 5,970 | 1.0 | 1,620 | 1,500 | 4.8 | 1,620 | 700 | 1.1 | 4,360 | 1,790 | 0.9 | 0.09 | 0.33 | 0.90 |
| RICP34.5 | L-25 | 2,460 | 610 | 1.0 | 5,310 | 3,300 | 0.5 | 3,630 | 1,200 | 0.3 | 350 | 290 | 1.0 | 560 | 190 | 0.4 | 940 | 710 | 0.2 | 0.91 | 0.22 | 0.99 |
| US5-5′ | L-26 | 3,610 | 970 | 1.4 | 3,130 | 1,700 | 0.3 | 980 | 530 | 0.1 | 1,260 | 460 | 3.7 | 1,010 | 380 | 0.7 | 580 | 320 | 0.1 | 0.01 | 0.03 | 0.16 |
| US8-5′ | L-27 | 2,870 | 620 | 1.1 | 7,370 | 4,510 | 0.7 | 10,860 | 4,030 | 0.9 | 1,330 | 1,090 | 4.0 | 1,480 | 430 | 1.0 | 3,780 | 1,810 | 0.8 | 0.07 | 0.76 | 0.78 |
| US8/9 | L-28 | 2,300 | 1,640 | 0.9 | 65,390 | 59,470 | 6.5 | 27,980 | 13,230 | 2.4 | 230 | 300 | 0.7 | 6,060 | 5,120 | 4.0 | 14,540 | 3,990 | 3.1 | 0.48 | 0.58 | 0.35 |
| RLAT-5′ | LT-1 | 1,320 | 280 | 0.5 | 650 | 800 | 0.1 | 2,080 | 650 | 0.2 | 200 | 100 | 0.6 | 270 | 180 | 0.2 | 800 | 430 | 0.2 | 0.38 | 0.04 | 0.63 |
| RHA6 | LT-2 | 970 | 630 | 0.4 | 260 | 720 | 0.0 | 3,320 | 2,380 | 0.3 | 520 | 520 | 1.6 | 160 | 140 | 0.1 | 1,240 | 640 | 0.3 | 0.10 | 0.09 | 0.90 |
| RLATX | LT-3 | 1,130 | 380 | 0.4 | 1,060 | 730 | 0.1 | 14,620 | 3,580 | 1.3 | 450 | 290 | 1.3 | 360 | 150 | 0.2 | 6,310 | 3,120 | 1.4 | 0.16 | 0.00 | 0.15 |
| RLAT-3′ | LT-4 | 2,510 | 1,000 | 1.0 | 3,150 | 1,590 | 0.3 | 16,010 | 5,720 | 1.4 | 630 | 330 | 1.9 | 870 | 600 | 0.6 | 8,020 | 1,170 | 1.7 | 0.04 | 0.02 | 0.29 |
| U1X | ? | 360 | 250 | 0.1 | 170 | 250 | 0.0 | 350 | 260 | 0.0 | 50 | 290 | 0.1 | 30 | 120 | 0.0 | 140 | 340 | 0.0 | 0.37 | 0.34 | 0.38 |
| U6/7 | L/? | 4,000 | 1,170 | 1.6 | 7,620 | 3,010 | 0.8 | 16,880 | 7,430 | 1.5 | 890 | 230 | 2.7 | 1,950 | 450 | 1.3 | 6,370 | 2,680 | 1.4 | 0.15 | 0.10 | 0.89 |
| U11/13 | L/E/L | 310 | 290 | 0.1 | 22,000 | 9,380 | 2.2 | 24,870 | 7,260 | 2.2 | 180 | 140 | 0.5 | 3,500 | 720 | 2.3 | 9,310 | 1,600 | 2.0 | 0.05 | 0.80 | 0.91 |
| U36 | E/L | 170 | 40 | 0.1 | 2,010 | 1,260 | 0.2 | 14,810 | 1,820 | 1.3 | 80 | 220 | 0.2 | 210 | 110 | 0.1 | 5,070 | 1,710 | 1.1 | 0.20 | 0.41 | 0.21 |
| U43.5-5′ | ? | 440 | 190 | 0.2 | 300 | 480 | 0.0 | 830 | 800 | 0.1 | 30 | 70 | 0.1 | 70 | 80 | 0.0 | 400 | 160 | 0.1 | 0.95 | 0.46 | 0.77 |
| U49 | E/L | 1,870 | 900 | 0.7 | 34,250 | 9,110 | 3.4 | 29,940 | 25,440 | 2.6 | 370 | 200 | 1.1 | 5,140 | 630 | 3.4 | 14,270 | 4,350 | 3.1 | 0.22 | 0.61 | 0.79 |
| U52/53 | E/L | 1,070 | 220 | 0.4 | 12,790 | 6,410 | 1.3 | 19,520 | 3,890 | 1.7 | 180 | 170 | 0.5 | 1,820 | 670 | 1.2 | 6,780 | 1,680 | 1.5 | 0.77 | 0.98 | 0.90 |
| RLAT-1 | LAT | 910 | 130 | 0.4 | 770 | 660 | 0.1 | 3,440 | 1,040 | 0.3 | 200 | 120 | 0.6 | 220 | 130 | 0.1 | 1,470 | 390 | 0.3 | 0.54 | 0.09 | 0.63 |
| ROP | ? | 2,190 | 870 | 0.9 | 1,350 | 1,870 | 0.1 | 1,460 | 870 | 0.1 | 800 | 420 | 2.4 | 250 | 170 | 0.2 | 820 | 200 | 0.2 | 0.02 | 0.50 | 0.48 |
| US3-5′ | L? | 5,910 | 1,870 | 2.3 | 11,220 | 5,490 | 1.1 | 1,500 | 1,030 | 0.1 | 700 | 300 | 2.1 | 2,420 | 940 | 1.6 | 790 | 490 | 0.2 | 0.81 | 0.07 | 0.46 |
| US3/4 | E/L? | 1,080 | 200 | 0.4 | 13,900 | 6,660 | 1.4 | 6,810 | 4,890 | 0.6 | 280 | 210 | 0.8 | 2,140 | 1,360 | 1.4 | 2,770 | 1,480 | 0.6 | 0.05 | 0.58 | 0.92 |
| US5/6/7 | EL/E/E | 1,340 | 800 | 0.5 | 36,670 | 16,300 | 3.6 | 27,750 | 4,410 | 2.4 | 170 | 260 | 0.5 | 4,470 | 3,520 | 3.0 | 10,520 | 2,810 | 2.3 | 0.79 | 0.93 | 0.60 |
| US10/11/12 | E/E/IE | 22,870 | 15,800 | 8.9 | 38,180 | 16,250 | 3.8 | 34,200 | 23,200 | 3.0 | 340 | 230 | 1.0 | 5,550 | 870 | 3.7 | 16,760 | 4,060 | 3.6 | 0.00 | 0.16 | 0.83 |
| Total | 256,590 | 1,005,080 | 1,147,380 | 33,570 | 151,210 | 465,330 | ||||||||||||||||
HeLa cells were infected with equivalent virion numbers of RP3 or RP4, corresponding to 5 or 0.5 PFU per cell. Median values are based on six, ten, or five experiments for RP3 (at 2, 4, and 8 hpi, respectively) and on six, nine, or five experiments for RP4 (at 2, 4, and 8 hpi, respectively). Bold entries represent either single transcripts or multiple transcripts of the same kinetic class.
See Table 1, footnote b.
Relative abundance values of each transcript at each time point in infections by RP3 and RP4 were compared by using Student's t test with two-tail distribution and assuming unequal variance. The null hypothesis is that the values for the RP3 and RP4 infections will be identical. The value 0.00 is used to indicate P values of <0.01.
FIG. 3.
Gene expression levels during infection with RP3 or RP4. Microarray chips were hybridized with probes prepared from RNA harvested from HeLa cells infected with RP3 or RP4 at 2, 4, or 8 hpi. The expression levels of IE (open), E (shaded), L (solid), and latency-associated (LAT) (hatched) transcripts are in arbitrary units.
DISCUSSION
In this study, we applied microarray technology to quantitatively measure global HSV-1 transcript abundance to provide an overall picture of the viral gene expression program as a function of the conditions of infection. Specifically, we examined the effect of VP16 activation domain mutations on the HSV-1 transcription program in toto. The virus designated RP5 lacks the entire VP16 activation domain and is defective in stimulating IE promoters on either transfected reporter genes or endogenous viral genomes (29). As shown here, infection with this mutant results in a severe disruption in both the level and timing of all viral gene expression. The requirement for the VP16 activation domain can be largely bypassed by infection at a high MOI or in the presence of HMBA. Two other viral strains that retain either of two subregions of the activation domain display quantitative and qualitative differences in IE gene expression patterns, suggesting that the two subregions have preferential activities at the various IE promoters. Despite these differences, and notwithstanding the overall reduction in expression levels during RP4 infection, the temporal patterns of E and L gene expression were relatively unaffected. While the effects of these mutations on IE gene expression have been previously documented, the downstream effects on E and L gene expression had not been thoroughly examined prior to this study.
Although a number of viral genes were expressed with measurable, if low, abundance following infection by RP5, the patterns of viral transcript abundance as a function of time were inconsistent with a normal transcriptional program. This inconsistency is apparent qualitatively, as shown in Fig. 1 and 2. More importantly, we show for the first time the extensive quantitative variance in the relative abundance of viral transcripts compared on a transcript-by-transcript basis between RP5R (wt) and RP5 (mutant) infections at each of three time points after infection (Tables 1 and 2).
The overall pattern of transcript accumulation during RP5 infection indicates an abortive process. Nonetheless, the presence of low levels of E transcripts encoding DNA replication machinery implies the potential inception of viral DNA replication. Also, expression of L transcripts suggests that some low level of infectious virus production can occur under these conditions. This conclusion is consistent with the known slow-growth phenotype of RP5 in noncomplementing cells (29).
The aberrant program of viral gene expression during RP5 infection supports the hypothesis that efficient virus replication typically depends on VP16-induced expression of IE genes. Despite this requirement, the expression of viral E and L genes is relatively normal in RP5-infected cells treated with HMBA or infected at high multiplicities (Tables 3 to 5). These observations indicate that other mechanisms exist that can induce adequate IE gene expression in the absence of the VP16 activation domain.
Our results also imply that high levels of E and L gene expression do not depend on the expression of IE genes in precise ratios. The IE expression patterns seen in HMBA or high-multiplicity infections differ significantly from those seen in wt infections, and yet the E and L gene expression patterns eventually converge on a normal pattern. Similarly, the distinct differences in IE patterns during infection by RP3 and RP4 do not result in marked differences in the subsequent pattern of E and L gene expression.
These two patterns of gene expression may correspond to gene expression during spontaneous and induced viral reactivation in several model systems. In this view, the rare spontaneous reactivation would be the result of a stochastic process, as observed in RP5 infection at a low multiplicity. In contrast, stress-induced changes in the transcriptional capacity of latently infected neurons would result in inception of a “normal” replication cascade, as observed during RP5 infection at a high multiplicity or during HMBA treatment. In either case, once the initial burst of newly enveloped virus is released, reinfection during recrudescence would follow the normal VP16-activated route. Clearly, analysis of neuronal tissue harboring spontaneous or induced reactivating viral genomes will be an important future goal of our global analyses.
While the exact mechanism by which stress induces the HSV-1 transcriptional cascade in the absence of VP16 activation is unknown, it is likely to be manifold. Thus, protein synthesis inhibitors such as cycloheximide can increase the expression of viral IE genes (6, 22) by a means likely to involve cellular stress responses that influence either RNA synthesis or RNA stability. During RP5 infection at low multiplicity, cycloheximide enhanced the transcription of only two IE genes, those encoding ICP0 and ICP4. Thus, one can argue that productive viral infection under cell stress conditions is the result of activation by these two “primary” IE transcriptional activators. From this, the other VP16-induced IE gene products may be evolutionary consequences of viral replication in the face of robust host defensive responses during reactivation and therefore have no obligate roles in the initial stages of reactivation from latency. There is accumulating genetic data to support this view (2, 8, 24).
The RP3 and RP4 viruses encode VP16 proteins lacking either the C-terminal half (amino acids 452 to 490) or the N-terminal half (amino acids 412 to 456) of the VP16 activation domain, respectively. RP3 effectively stimulated transcription of all IE genes and launched a cascade of viral gene expression quite similar to that of a wt virus. In contrast, the ICP4 promoter was the only IE promoter significantly stimulated at 2 h following RP4 infection. Expression of ICP0 transcripts was notably reduced in RP4 infection, even at 4 hpi, consistent with previous assays by reverse transcription and PCR (29). The delay or reduction of ICP0 expression during RP4 infection may be responsible for the delay but not elimination of the normal viral regulatory cascade. At least two models can be proposed to explain the failure of ICP0 expression in RP4 infection. The activation of ICP0 expression might require a critical threshold of ICP4, which itself is activated by VP16. Alternatively, the mutant VP16 protein encoded by RP4 might be defective in directly stimulating the transcription from the ICP0 promoter. Regardless of the exact nature of the delay in ICP0 expression, at later times many of the E and L genes show patterns of relative abundance that are not significantly different in RP4 infection than in RP3 infection. This operationally confirms the requirement for coactivation of both ICP0 and ICP4 in order for the virus to induce a rapid productive cascade, despite the auxiliary role of ICP0 in viral replication.
The experiments reported here were performed with mutations affecting only one of the viral regulatory proteins and in a cultured cell line under actively replicating conditions. We expect that further analysis of the perturbations of the viral transcriptional cascade with other regulatory mutants and in cells more representative of differentiated tissue will provide further insights into aspects of HSV pathogenesis and latency. Quantitative analysis of these transcription patterns will provide important corroboration of existing models as well as providing insight for the formulation of new ones.
Acknowledgments
The first two authors contributed equally to this work.
This work was supported by NIH grants AI-27323 to S.J.T. and CA-11861 and CA-90287 to E.W. and by a grant from the British Biotechnology Science Research Council and Scottish Higher Education Funding Council to P.G.
Marcia Rice and J. S. Aguilar (UCI) and Douglas Roy (GTI, Edinburgh, United Kingdom) provided technical assistance. Søren Ottosen and Francisco Herrera (MSU) provided useful criticism of the manuscript.
REFERENCES
- 1.Ace, C. I., T. A. McKee, J. M. Ryan, J. M. Cameron, and C. M. Preston. 1989. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J. Virol. 63:2260-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai, W., T. L. Astor, L. M. Liptak, C. Cho, D. M. Coen, and P. A. Schaffer. 1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 67:7501-7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers, J., A. Angulo, D. Amaratunga, H. Guo, Y. Jiang, J. S. Wan, A. Bittner, K. Frueh, M. R. Jackson, P. A. Peterson, M. G. Erlander, and P. Ghazal. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 73:5757-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousens, D. J., R. Greaves, C. R. Goding, and P. O'Hare. 1989. The C-terminal 79 amino acids of the herpes simplex virus regulatory protein, Vmw65, efficiently activate transcription in yeast and mammalian cells in chimeric DNA-binding proteins. EMBO J. 8:2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cress, W. D., and S. J. Triezenberg. 1991. Critical structural elements of the VP16 transcriptional activation domain. Science 251:87-90. [DOI] [PubMed] [Google Scholar]
- 6.Elshiekh, N. A., E. Harris-Hamilton, and S. L. Bachenheimer. 1991. Differential dependence of herpes simplex virus immediate-early gene expression on de novo-infected cell protein synthesis. J. Virol. 65:6430-6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flint, J., and T. Shenk. 1997. Viral transactivating proteins. Annu. Rev. Genet. 31:177-212. [DOI] [PubMed] [Google Scholar]
- 8.Harris, R. A., and C. M. Preston. 1991. Establishment of latency in vitro by the herpes simplex virus type 1 mutant in 1814. J. Gen. Virol. 72:907-913. [DOI] [PubMed] [Google Scholar]
- 9.Herr, W. 1998. The herpes simplex virus VP16-induced complex: mechanisms of combinatorial transcriptional regulation. Cold Spring Harbor Symp. Quant. Biol. 63:599-607. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs, W. E., and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenner, R. G., M. M. Alba, C. Boshoff, and P. Kellam. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khodarev, N. N., S. J. Advani, N. Gupta, B. Roizman, and R. R. Weichselbaum. 1999. Accumulation of specific RNAs encoding transcriptional factors and stress response proteins against a background of severe depletion of cellular RNAs in cells infected with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 96:12062-12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaMarco, K. L., and S. L. McKnight. 1989. Purification of a set of cellular polypeptides that bind to the purine-rich cis-regulatory element of herpes simplex virus immediate early genes. Genes Dev. 3:1372-1383. [DOI] [PubMed] [Google Scholar]
- 16.Manger, I. D., and D. A. Relman. 2000. How the host ′sees' pathogens: global gene expression responses to infection. Curr. Opin. Immunol. 12:215-218. [DOI] [PubMed] [Google Scholar]
- 17.McFarlane, M., J. I. Daksis, and C. M. Preston. 1992. Hexamethylene bisacetamide stimulates herpes simplex virus immediate early gene expression in the absence of trans-induction by Vmw65. J. Gen. Virol. 73:285-292. [DOI] [PubMed] [Google Scholar]
- 18.Morgan, R. W., L. Sofer, A. S. Anderson, E. L. Bernberg, J. Cui, and J. Burnside. 2001. Induction of host gene expression following infection of chicken embryo fibroblasts with oncogenic Marek's disease virus. J. Virol. 75:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Hare, P. 1993. The virion transactivator of herpes simplex virus. Semin. Virol. 4:145-155. [Google Scholar]
- 20.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulose-Murphy, M., N. K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preston, C. M., A. Rinaldi, and M. J. Nicholl. 1998. Herpes simplex virus type 1 immediate early gene expression is stimulated by inhibition of protein synthesis. J. Gen. Virol. 79:117-124. [DOI] [PubMed] [Google Scholar]
- 23.Regier, J. L., F. Shen, and S. J. Triezenberg. 1993. Pattern of aromatic and hydrophobic amino acids critical for one of two subdomains of the VP16 transcriptional activator. Proc. Natl. Acad. Sci. USA 90:883-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samaniego, L. A., L. Neiderhiser, and N. A. DeLuca. 1998. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J. Virol. 72:3307-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spector, D., F. Purves, and B. Roizman. 1990. Mutational analysis of the promoter region of the alpha-27 gene of herpes simplex virus 1 within the context of the viral genome. Proc. Natl. Acad. Sci. USA 87:5268-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stingley, S. W., J. J. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan, S. M., P. J. Horn, V. A. Olson, A. H. Koop, W. Nu, R. H. Ebright, and S. J. Triezenberg. 1998. Mutational analysis of a transcriptional activation region of the VP16 protein of herpes simplex virus. Nucleic Acids Res. 26:4487-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanstrom, R. I., and E. K. Wagner. 1974. Regulation of synthesis of herpes simplex type 1 virus mRNA during productive infection. Virology 60:522-533. [DOI] [PubMed] [Google Scholar]
- 29.Tal-Singer, R., R. Pichyangkura, E. Chung, T. M. Lasner, B. P. Randazzo, J. Q. Trojanowski, N. W. Fraser, and S. J. Triezenberg. 1999. The transcriptional activation domain of VP16 is required for efficient infection and establishment of latency by HSV-1 in the murine peripheral and central nervous systems. Virology 259:20-33. [DOI] [PubMed] [Google Scholar]
- 30.Triezenberg, S. J. 1995. Structure and function of transcriptional activation domains. Curr. Opin. Genet. Dev. 5:190-196. [DOI] [PubMed] [Google Scholar]
- 31.Triezenberg, S. J., R. C. Kingsbury, and S. L. McKnight. 1988. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 2:718-729. [DOI] [PubMed] [Google Scholar]
- 32.Triezenberg, S. J., K. L. LaMarco, and S. L. McKnight. 1988. Evidence of DNA:protein interactions that mediate HSV-1 immediate early gene activation by VP16. Genes Dev. 2:730-743. [DOI] [PubMed] [Google Scholar]
- 33.Wagner, E. K., J. J. Garcia Ramirez, S. W. Stingley, J. S. Aguilar, L. Buehler, G. B. Devi-Rao, and P. Ghazal. 2002.. Practical approaches to long oligonucleotide-based DNA microarrays: lessons from herpes viruses. Prog. Nucleic Acid Res. 71:449-491. [DOI] [PubMed]
- 34.Wagner, E. K., J. F. Guzowski, and J. Singh. 1995. Transcription of the herpes simplex virus genome during productive and latent infection. Prog. Nucleic Acid Res. Mol. Biol. 51:123-165. [DOI] [PubMed] [Google Scholar]
- 35.Walker, S., R. Greaves, and P. O'Hare. 1993. Transcriptional activation by the acidic domain of Vmw65 requires the integrity of the domain and involves additional determinants distinct from those necessary for TFIIB binding. Mol. Cell. Biol. 13:5233-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinheimer, S. P., B. A. Boyd, S. K. Durham, J. L. Resnick, and D. R. O'Boyle. 1992. Deletion of the VP16 open reading frame of herpes simplex virus type 1. J. Virol. 66:258-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weir, J. P. 2001. Regulation of herpes simplex virus gene expression. Gene 271:117-130. [DOI] [PubMed] [Google Scholar]
- 38.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]