Abstract
The adenovirus mutant ONYX-015 is in phase III clinical trials as a novel antitumor therapy. Its apparent efficacy is thought to be due to its ability to replicate selectively in tumor cells defective in the signaling pathway for p53. Recent data have shown that p14ARF, a positive regulator of p53, inhibits ONYX-015 replication in cells with a wild-type p53, a phenotype that characterizes normal cells. We, however, found that ONYX-015 activates p53 in tumor cells and in normal cells and that this can occur without p14ARF induction. We also show that ONYX-015 is not attenuated in cells with functional p53, whether or not p14ARF is expressed, and that where attenuation does occur, it is cell type specific.
Although human adenoviruses (Ads) were used nearly 50 years ago to treat cervical cancers, with some modest success (36), widespread clinical use did not occur due to the lack of tumor selectivity. One strategy to improve tumor cell selectivity centers on the well-established observation that tumor cells have a deregulated cell cycle due to loss of tumor suppressor gene function.
Ads express E1a proteins that bind to the tumor suppressor gene product pRb105 (42), releasing bound transcription factor E2F, which in turn induces cells into S phase (25, 27). Similarly, Ads also express proteins that bind and inactivate the p53 tumor suppressor gene product (5, 43, 45). These interactions are thought to maximize virus replication by creating an environment conducive to virus growth. Since tumor cells are frequently defective in p53 and/or genes that regulate pRb105 function, it is thought that Ads defective in genes that disable pRb105 and/or p53 may selectively replicate in tumors but would be attenuated in normal cells.
One such Ad is ONYX-015 (originally dl1520) (2). This virus has received considerable attention as a new cancer therapy (24), as it was originally shown to replicate only in tumor cells defective in the p53 tumor suppressor gene (4). This virus does not express the E1b 55-kDa protein due to a large deletion in the E1b gene (2). Following infection with wild-type (wt) Ad, the E1b 55-kDa protein, along with another viral protein (E4ORF6 gene product), binds p53, causing it to be degraded (13, 31, 37). After infection with viruses deficient in either of these viral proteins, such as ONYX-015, there is an increase in p53 levels due to the stabilizing influence of E1a gene products (13, 21). Although there are other mechanisms of p53 stabilization (10), one way that this can occur is through binding of E1a to pRb105, which leads to transactivation of the p14ARF gene by the released E2F (3). p14ARF in turn binds the MDM2 protein (29), which normally promotes the degradation of p53 through the ubiquitin pathway (23). Binding of p14ARF neutralizes or degrades MDM2, resulting in a rise in p53 (29, 46). Such a stabilization of p53 would result in either a cell cycle arrest due to p53 transactivating the cyclin-dependent kinase inhibitor p21CIP1/WAF1 or an induction of apoptosis. It has been argued (4) that infection of normal cells with ONYX-015 would induce only cell cycle arrest, as apoptosis would be prevented by the viral E1b 19-kDa antiapoptotic protein. The resultant cell cycle arrest would then restrict virus DNA synthesis, as ONYX-015 is unable to degrade p53 and overcome the arrest. Thus, it is predicted that ONYX-015 would be attenuated in normal cells. By contrast, in tumor cells deficient in p53 (or in the p53 pathway) cells would continue to replicate, thereby promoting ONYX-015 replication leading to tumor cell lysis (4). As p53 defects are characteristic of many tumors (19), if the hypothesis is correct, ONYX-015 would have a capacity to distinguish normal cells from tumor cells, a necessary criterion for tumor therapy.
Initial reports suggested that ONYX-015 did in fact selectively kill tumor cells harboring a defective p53 gene (4, 17). However, subsequent reports have shown that ONYX-015 replicates in, and kills, tumor cells with wt p53 at least as efficiently as cells defective in p53 (9, 11, 12, 15, 34, 39; reviewed in reference 8). To explain these observations, it has been suggested that all tumor cells are deficient either in p53 itself or in the p14ARF gene (32). The prevailing argument is that without p14ARF, stabilization of p53 by E1a would not occur. However, in normal cells, p14ARF is present, so E1a would induce p14ARF, leading to loss of MDM2 and allowing p53 to be activated. Activated p53 would then cause a cell cycle arrest, inhibiting ONYX-015 replication.
Although there is some evidence to support this model (32, 38), equally, there are aspects of it that presently lack experimental support. For example, it is not clear whether the E1b 19-kDa protein can completely prevent apoptosis in normal cells as is required for the virus to be attenuated. In addition, as there are other pathways of p53 stabilization and activation by viral E1a proteins (7, 10, 30) besides that involving p14ARF, it is not clear that ONYX-015 replication will always be controlled by p14ARF.
In this paper we have addressed the latter issue. We have asked whether p14ARF is essential for activation of p53 by ONYX-015 in tumor and normal cells and whether this activation affects ONYX-015 replication. Our data suggest that in some, but not all, cases p53 is transcriptionally activated independently of p14ARF in both normal and tumor cells and that neither p53 by itself nor p53 in combination with p14ARF selectively inhibits ONYX-015 replication.
MATERIALS AND METHODS
Cell lines.
293 cells (ATCC CRL-1573) were used for propagating wt Ad5 and ONYX-015, as they contain the E1a and E1b genes. Cell lines containing wt p53 were A549 lung cancer cells (ATCC CCL-185), HepG2 hepatoma cells (ATCC HB-8065), RKO colon carcinoma cells (35), U2OS osteosarcoma cells (22), normal mammary epithelial cells (Bre80) (20), and early-passage human diploid fibroblast (HDF) cells. Cell lines containing mutant p53 were T98G glioma cells (ATCC CRL-1690), C33A cervical carcinoma cells (4), J82 bladder carcinoma cells (ATCC HTB-1), and HT29 colon cancer cells (ATCC HTB-38). Cells that are null for p53 were Saos2 osteosarcoma cells (22), H1299 lung cancer cells (38), and IIICF/c Li Fraumeni skin fibroblasts (33). Other cells used were RKO p53.13 cells, which are RKO cells transfected with a dominant negative mouse p53 mutant (35); HeLa cells, which contain an integrated human papillomavirus genome (ATCC CCL-2), and GM639DM and GM847DM cells, which contain large T antigen from simian virus 40 (41). H24-H1299 cells are a variant of H1299 cells containing a tetracycline (TET-off)-inducible wt p53 (6). Most cells were grown in Dulbecco's modified Eagle's medium (Life Technologies) supplemented with 10% fetal bovine serum at 37°C with 10% CO2. Additionally, H24-H1299 cells were maintained with 2 μg of puromycin per ml, 300 μg of G418 per ml, and 4.5 μg of tetracycline per ml (6). Epithelial cells were grown in serum-free medium (MCDB.170; Invitrogen Life Technologies) supplemented with 5 μg of gentamicin per ml according to a published protocol (20). Cells were passaged to no more than 80% confluence and split at a ratio of 1:16. The medium was changed every 2 to 3 days.
Ads.
wt Ad5 and ONYX-015 (dl1520) (2) were used for the experiments described in this paper. All viruses were grown on 293 cells and titrated on 293 cells by using a cytopathic effect (CPE) assay (26). Virus titers were standardized in CPE units, defined as the virus dose which causes 50% CPE of 293 cells after 3 days of infection.
Virus replication.
Virus replication was carried out using a CPE assay as described previously(26) and in some cases also a plaque-forming assay. Briefly, various cell lines were seeded into six-well plates and then infected with the indicated doses of Ads. After 3 to 5 days of infection, virus was recovered from each cell type, serial dilutions were prepared, and CPE and plaque assays were set up in quadruplicate using 293 cells to estimate the amount of virus replication.
Transcriptional reporter assays.
Cells were seeded at 2 × 105 cells/well (six-well plates) and incubated overnight. Cells were transfected with 2 μg of plasmid according to the instructions of the manufacturer (FuGENE 6 transfection reagent; Roche). Constructs used were the p21CIP1/WAF1 promoter and its control, p21op53 (with the p53 response element deleted), both of which were linked to the luciferase reporter. Cells were infected 6 h later with the appropriate virus (wt Ad5 or ONYX-015) at 10 to 20 CPE units/cell (A549 and Bre80 cells), 50 CPE units/cell (RKO cells), or 300 CPE units/cell (HDF cells). Cells were harvested at the indicated times, cell numbers were determined, and cell pellets were frozen until required. Cells were lysed and assayed for luciferase activity according to the instructions of the manufacturer (Luciferase assay system; Promega). Results were standardized according to cell number, and all experiments were done in duplicate and repeated one or two times.
Immunoblotting.
In general, about 106 cells were seeded and infected with viruses as described above. At the indicated times, protein lysates were prepared in lysis buffer (10 mM Tris-HCl [pH 8.0], 140 mM NaCl, 5 mM dithiothreitol, 1 mM EDTA, 1% sodium dodecyl sulfate [SDS], 1% NP-40). For each sample, a volume corresponding to 8 × 104 cells was mixed with an equal volume of DSL loading buffer (50 mM Tris-HCl [pH 6.8], 200 mM dithiothreitol, 4% SDS, 40% glycerol, 0.08% bromophenol blue), boiled for 5 min, and then loaded on an SDS-12% polyacrylamide gel. Following electrophoresis, protein extracts were transferred to a polyvinylidene difluoride membrane (Pall). Detection was carried out by standard procedures, and bands were visualized by using the Immun-Star alkaline phosphatase chemiluminescent system (Bio-Rad).
Antibodies.
Immunoblotting experiments were carried out with antibodies to p53 (DO1; mouse monoclonal [Santa Cruz]), MDM2 (SMP-14; mouse monoclonal [Santa Cruz]), p14ARF (C-18; goat polyclonal [Santa Cruz]), and actin (C-11; goat polyclonal [Santa Cruz]).
RESULTS
p14ARF protein is often undetectable in tumor cells expressing wt p53.
To confirm the idea of there being a negative feedback loop in which overexpression of p53 reduces p14ARF (38), we used immunoblotting to determine p14ARF protein levels in a panel of tumor cell lines that vary in their p53 status. The results of these experiments are summarized in Table 1. We found that p14ARF protein was undetectable in 6 of 11 cell lines containing a wt p53 gene. Four of the cell lines in which p14ARF was detectable contain integrated viral genomes, from human papillomavirus (HeLa cells), Ad (293 cells) and simian virus 40 (GM639DM and GM847DM cells), all of which express proteins which neutralize p53 activity. Bre80, however, expressed both p53 and p14ARF, although the level of p14ARF was lower than that in several cell lines expressing a mutant p53 (Table 1; see Fig. 2).
TABLE 1.
p14ARF protein is absent from tumor cells with a wt p53 genea
| Cell line | p53 gene statusb | p14ARF gene status | p14ARF protein status |
|---|---|---|---|
| HDF | wt | + | NDc |
| Bre80 | wt | + | + |
| A549 | wt | + | ND |
| U20S | wt | + | ND |
| HepG2 | wt | + | ND |
| RKO | wt | + | ND |
| RKO p53.13 | wt/DN | + | ND |
| HeLa | wt/E6 | + | ++ |
| 293 | wt/E1b55k | + | + |
| GM 639 DM | wt/LT | + | + |
| GM 847 DM | wt/LT | + | + |
| T98G | Mutant | − | ND |
| C33A | Mutant | + | + |
| J82 | Mutant | + | ++ |
| HT29 | Mutant | + | ++ |
| Saos 2 | Null | + | + |
| IIICF/c | Null | − | ND |
| H1299 | Null | + | ++ |
p14ARF protein levels were determined by immunoblotting as described in Methods and Materials. Where p14ARF protein was not detected, the gene status was determined, either from the literature or by PCR. p53 status was determined from the literature (18, 20, 32, 33, 38, 41).
DN, dominant negative; E6, E6 gene product from human papillomavirus; E1b55k, E1b 55-kDa gene product from human adenovirus; LT, large T antigen from simian virus 40.
ND, not detected.
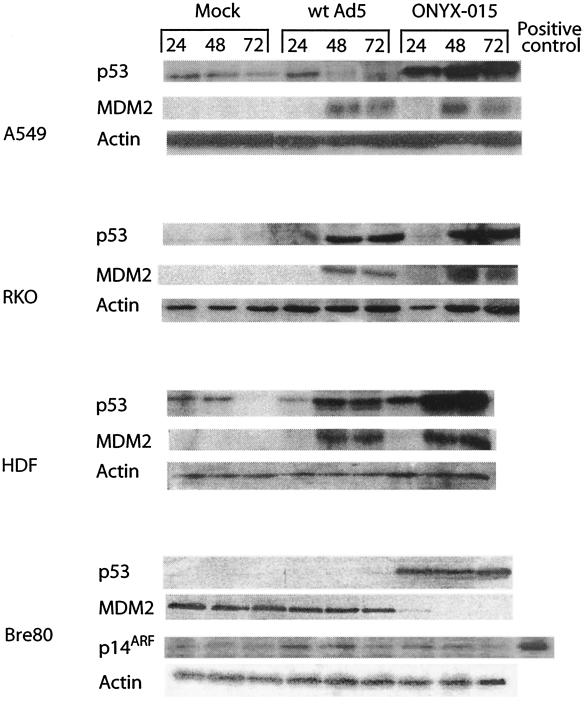
FIG. 2.
ONYX-015 can induce p53 in the absence of p14ARF after infection of both tumor and normal cells. A549, RKO, HDF, and Bre80 cells were infected as described in Materials and Methods, and at the indicated times (hours) they were harvested and immunoblotting was carried out with antibodies to p53, p14ARF, MDM2, and actin. Protein lysate from HT29 cells was used as a positive control for p14ARF.
In cells with mutant p53 genes or cells with p53 deleted, moderate to high levels of p14ARF protein were detected, but there were exceptions. The p53 null cell line IIICF/c and the p53 mutant cell line T98G expressed no p14ARF. In both of these cases there are reported defects in the INK4A gene, encoding p14ARF (18, 33), and so these cell lines are unable to express p14ARF. Cell lines RKO and RKO p53.13 are interesting, as RKO has a wt p53 and does not express p14ARF, consistent with the observed reciprocal relationship between p53 and p14ARF. However, its isogenic variant, RKO p53.13, expressing a dominant negative mutant p53 (35), shows no up-regulation of p14ARF, as might be expected if there is a negative feedback by p53 on p14ARF expression. Thus, in these cells, it is likely there are other alterations in the p14ARF/p53 pathway. In general, however, our data are consistent with the hypothesis that during tumor development there is selection against p53 itself or against p14ARF.
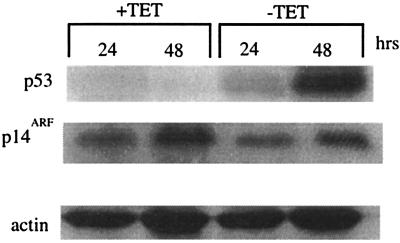
To explore the issue of feedback further, cell line H24-H1299, which contains an inducible p53 (6), was used in immunoblotting experiments to determine the relative levels of p14ARF after induction of p53. H24-H1299 cells contain an inducible wt p53 that becomes active upon removal of tetracycline. p14ARF was readily detected in uninduced cells, whereas p53 was not (Fig. 1). Following induction of p53, p14ARF levels were a little lower, but not markedly so. These data show that p53 does not have a profound effect on p14ARF expression. We similarly found no down-regulation of p14ARF after transient transfection of Saos2 cells with a wt p53 expression construct (data not shown).
FIG. 1.
Overexpression of p53 does not reduce the level of p14ARF. H24-H1299 cells were seeded and incubated for the indicated times in the absence (p53 induced) or presence of 4.5 μg of tetracycline per ml. Cells were harvested, and immunoblotting was carried out with antibodies to p53, p14ARF, and actin. Actin was used as a loading control.
ONYX-015 stabilizes p53 in tumor and normal cells independently of p14ARF.
In order to determine the relationship between p53 and p14ARF expression following infection with wt Ad5 and ONYX-015, cells were infected with these viruses, and the relative levels of p53 were determined by immunoblotting. p53 was readily detected in mock-infected A549 and HDF cells (Fig. 2), although this declined by 72 h, most likely due to contact inhibition since the cells were crowded by this time. However, p53 was barely detectable in RKO and Bre80 cells. p53 was readily detected 24 h after infection of A549 cells with wt Ad5 but disappeared thereafter. By contrast, a steady and marked increase in p53 was observed after infection with ONYX-015. In Bre80 cells, p53 was not detected after infection with wt Ad5, but a marked stabilization was observed after infection with ONYX-015. However, in RKO and HDF cells, p53 levels increased after infection with both wt Ad5 and ONYX-015.
MDM2 and p14ARF levels were measured in parallel with p53 (Fig. 2). MDM2 was not detected in uninfected A549, RKO, and HDF cells but was readily detected in Bre80 cells. After infection with wt Ad5 and ONYX-015, MDM2 levels rose dramatically with essentially the same kinetics in all cells except Bre80. In Bre80 cells, MDM2 levels did not change after wt Ad5 infection but declined markedly after infection with ONYX-015. In contrast, p14ARF was undetectable in A549, RKO, and HDF cells (data not shown), whether or not they were infected, but was detectable in Bre80 cells (Fig. 2). We conclude that p53 is stabilized by ONYX-015 in all cells and by wt Ad5 in some cases. Moreover, the postinfection stabilization of p53 can occur in the absence of p14ARF.
ONYX-015 induces transcriptionally active p53 in normal and tumor cells.
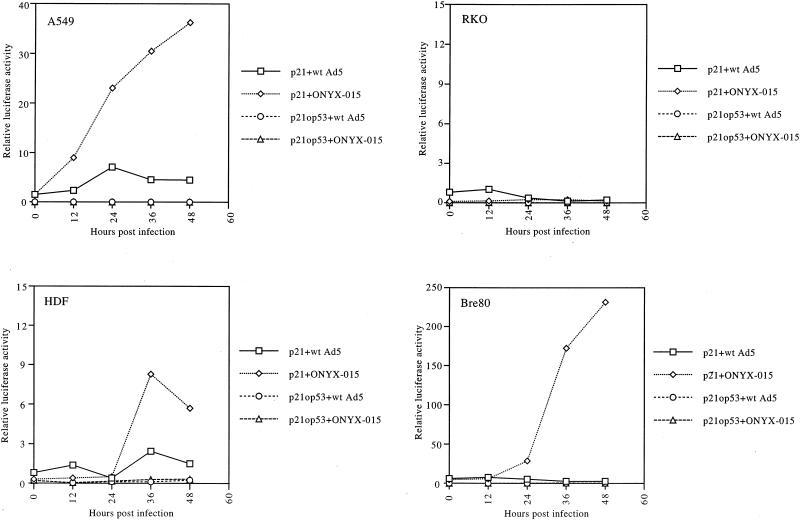
To determine whether the p53 induced by ONYX-015 infection was functional, a reporter assay was carried out with a plasmid containing the p21CIP1/WAF1 promoter linked to the luciferase reporter. The results for A549 cells (Fig. 3) show that the p21CIP1/WAF1 reporter activity steadily increased over time after ONYX-015 infection, to reach a maximum of about 15-fold above the starting level. In contrast, wt Ad5 had little effect on promoter activity. In the same experiment, activity from a p21CIP1/WAF1 reporter with the p53 response element deleted (p21op53), was not increased by either ONYX-015 or wt Ad5. These results show that p53 is transcriptionally active after infection with ONYX-015, but not with wt Ad5, as reported previously (14) and are consistent with the increased levels of p53 present after ONYX-015 infection of these cells (Fig. 2). In addition, after ONYX-015 infection of A549 cells, we detected binding of p53 to its cognate binding site in electrophoretic mobility shift assays but not under the other conditions (data not shown).
FIG. 3.
ONYX-015 can activate the p21CIP1/WAF1 promoter in tumor and normal cells. Cells were transfected with the p21CIP1/WAF1 promoter-luciferase reporter constructs with and without (op53) the p53 response element and subsequently infected with wt Ad5 or ONYX-015 as described in Materials and Methods. At different times postinfection, cells were harvested and luciferase activity was determined. All samples were standardized to the same cell number.
RKO cells were also transfected with the p21CIP1/WAF1 reporter constructs, and their activities were determined after infection (Fig. 3). In contrast to the A549 data, both wt Ad5 and ONYX-015 had little effect on promoter activity.
When HDF cells were infected with ONYX-015, p53 promoter activity increased over time (Fig. 3), in parallel with increased p53 protein (Fig. 2). However, little change in promoter activity was observed after infection with wt Ad5. Again, as for the A549 cells, the increased promoter activity was p53 dependent, as the promoter without the p53 binding site was not activated by either virus.
For Bre80 cells, promoter activity again increased after infection with ONYX-015 but not after wt Ad5 infection (Fig. 3).
We conclude from these experiments that p53 can be transcriptionally activated upon ONYX-015 infection independently of p14ARF. The data also show that p53 stabilization and transcriptional activation are not strictly linked.
ONYX-015 replication is independent of p53 and p14ARF status.
Next we measured the relative abilities of wt Ad5 and ONYX-015 to replicate in all of the above-described cells, as well as in the variant RKO cell line RKO p53.13, which expresses a dominant negative p53 (35). Cells were infected with wt Ad5 and ONYX-015, and at the indicated times after infection virus was harvested. The virus yield from each cell type was then estimated by using CPE and plaque assays on 293 cells. We found that titer estimates were different with the two assays (Table 2), although not markedly so, but the trends were nonetheless similar. ONYX-015 was found to be attenuated about 3- to 4-fold compared with wt Ad5 in A549 cells and about 10-fold in RKO cells, by both CPE and plaque assays (Table 2). No significant attenuation was observed in the RKO p53.13 or Bre80 cells. By contrast, ONYX-015 was markedly attenuated in HDF cells, with no CPE evident over the time course of the experiment.
TABLE 2.
ONYX-015 replication is independent of p53 and p14ARF statusa
| Cell type | wt Ad5
|
ONYX-015
|
||
|---|---|---|---|---|
| CPE (U/ml) | PFU/ml | CPE (U/ml) | PFU/ml | |
| A549 | 7.5 × 109 | 2.0 × 1010 | 2.0 × 109 | 6.0 × 109 |
| RKO | 7.5 × 109 | 1.8 × 1010 | 7.5 × 108 | 2.0 × 109 |
| RKO p53.13 | 7.5 × 109 | 1.5 × 1010 | 6.0 × 109 | 6.0 × 109 |
| HDF | 3.8 × 107 | NDb | None | ND |
| Bre80 | 2.2 × 108 | ND | 1.7 × 108 | ND |
CPE and plaque-forming assays were carried out as described in Methods and Materials.
ND, not determined.
Thus, ONYX-015 replicates variably depending on the cell type and, importantly, is no more attenuated in cells expressing p14ARFand a transcriptionally active p53 (Bre80) than in cells without either (e.g., RKO p53.13) and is markedly attenuated only in HDF cells, which do not express p14ARF.
High levels of p14ARF in combination with p53 do not selectively inhibit ONYX-015 replication.
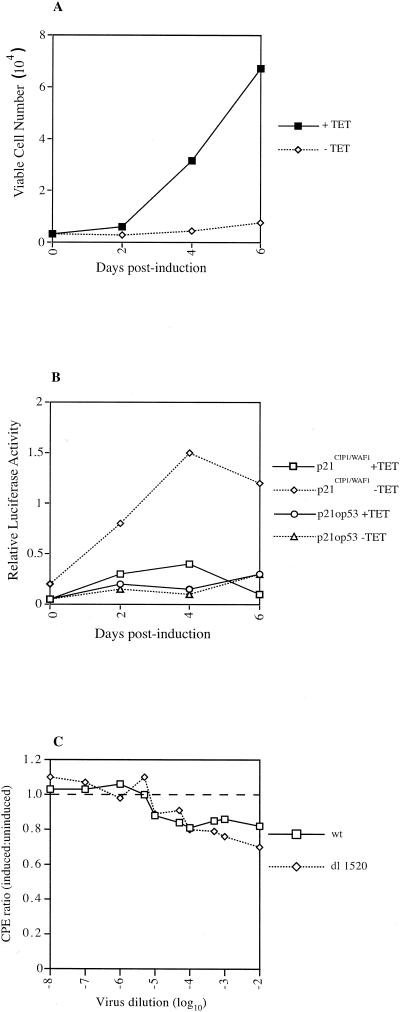
To determine if p14ARF can attenuate ONYX-015 in combination with p53 when p14ARF is highly expressed, we compared the abilities of ONYX-015 and wt Ad5 to replicate in H24-H1299 cells. As described above, H24-H1299 cells contain an inducible wt p53 that becomes active upon removal of tetracycline and also express comparatively large amounts of p14ARF that are only slightly reduced upon p53 induction (Fig. 1). To demonstrate that removal of tetracycline induces functional p53, two experiments were done. First, a cell growth experiment was carried out. The results (Fig. 4A) show that up to 6 days after removal of tetracycline, there was little change in the overall cell number, although this increased about threefold by day 8. These data indicate that the majority of the cell population is growth arrested. By contrast, cells maintained in tetracycline increased 20-fold over the time course of the experiment. Second, the cells were assayed for their ability to activate the p21CIP1/WAF1 promoter in a luciferase reporter assay. The results (Fig. 4B) show a time-dependent activation of p21CIP1/WAF1 promoter activity after removal of tetracycline. This activation is p53 dependent, as a control promoter which lacks the p53 response element (p21op53) was not activated.
FIG. 4.
High levels of p53 and p14ARF do not selectively inhibit ONYX-015 replication. H24-H1299 cells were seeded and incubated either in the presence of 4.5 μg of tetracycline per ml or in its absence (p53 induced; see Fig. 1). (A) Cell growth was determined over time by direct cell counting. Viable and nonviable cells were distinguished by using trypan blue staining. (B) Cells were seeded, grown in the presence and absence of tetracycline, and transfected with the p21CIP1/WAF1 luciferase reporter constructs. At the indicated times posttransfection, cells were harvested and luciferase activity was determined. All samples were standardized to the same cell number. (C) Cells were infected with serial dilutions of wt Ad5 and ONYX-015, and a CPE analysis was carried out at about 3 to 4 days postinfection on 293 cells. The ratio of CPE induced by each virus under induced conditions to that induced under uninduced conditions is plotted against virus dilution. A ratio of 1.0 (dashed line) indicates no preference for the different conditions.
Next, H24-H1299 cells were infected with wt Ad5 and ONYX-015 in the presence and absence of tetracycline. After 5 days of infection, virus was harvested and a CPE assay was carried out on 293 cells. The results (Fig. 4C) are expressed as a ratio of CPE under induced and uninduced conditions for each virus. If there is no difference in the replication ability of the viruses under each condition, then the CPE ratio would always be equal to 1.0. Any divergence from 1.0 indicates replication differences. The results show that after p53 induction there is a small decrease in the ability of both viruses to induce CPE, but there is little difference between wt Ad5 and ONYX-015. These data, as well as those above, do not support the hypothesis that p53 in combination with p14ARF selectively impairs ONYX-015 replication.
DISCUSSION
Recent reports (32, 38) have suggested that during tumor development there is selection against a functional p53 pathway and that this can occur either by inactivation of p53 itself or by inactivation of p14ARF. A negative feedback loop in which overexpression of p53 reduces p14ARF has also been postulated (38). It has been further suggested (32) that the antitumor adenovirus ONYX-015 is attenuated by p14ARF in normal cells expressing wt p53. Infection of normal cells with ONYX-015 would cause an up-regulation of p53, as MDM2 would be neutralized by an induction of p14ARF. The activation of p53 would then restrict replication of the virus. These reports go on to suggest that in tumor cells with wt p53, in which p14ARF is not present, the signaling pathway to p53 would be defective. Under these conditions, one might expect no p53 activation in response to infection, thus allowing ONYX-015 to replicate, causing tumor cell lysis. In this way, ONYX-015 would selectively replicate in and destroy tumor cells (32, 38).
Consistent with previous reports (32, 38), the data presented here (Table 1) show that in almost every case there is a reciprocal relationship between p53 and p14ARF expression. However, some of our data are not consistent with a direct feedback loop (38) in which a rise in p53 reduces p14ARF. Down-regulation of p53 by a dominant negative mechanism in RKO p53.13 cells does not increase p14ARF expression (Table 1), and we find that the induction of p53 in the cell line H24-H1299 has little influence on p14ARF expression (Fig. 1). Why there is such a marked difference between our data and the previous report is not clear, but it is unlikely to be due to the induction system or timing, as these are similar in both studies. Cell type differences may well be influencing the outcome of the result. For example, there may be some other defect in the p14ARF/p53 pathway in H24-H1299 cells that interferes with the feedback by p53 on p14ARF expression. However, we also observed no p53-directed down-regulation of p14ARF in ONYX-015-infected Bre80 cells (Fig. 2), and we have been unable to lower p14ARF expression after transient transfection of cells with a wt p53 expression construct (data not shown). These data raise some questions about the generality of the feedback loop.
Several reports have shown that p53 is degraded as a result of binding to the E1b 55-kDa and E4ORF6 proteins (13, 31, 37). Conversely, in the absence of either the E1b 55-kDa or E4ORF6 protein, p53 levels rise as a result of a stabilization event. Recent work has shown that this occurs in both tumor and normal cells. A report by Ries et al. (32) showed that infection of HCT116 tumor cells with ONYX-015 resulted in a rise in p53 but no marked change in MDM2 or p21CIP1/WAF1. That report also showed that there was no transcriptional activation of a p53 reporter construct after ONYX-015 infection and no induction of p14ARF. However, in normal endothelial cells, there was an induction of p53 and p14ARF.
To extend these observations, we carried out similar experiments using normal HDF cells, normal human mammary epithelial (Bre80) cells, and two tumor cell lines containing a wt p53 gene, A549 and RKO. We found an induction of p53 in all cell types examined after ONYX-015 infection (Fig. 2), as has been reported previously (13, 31, 37). This was paralleled by an induction of MDM2, except in Bre80 cells, in which MDM2 expression was lost. Where MDM2 induction was evident, it did not occur until 48 h after infection and was not observed at 24 h, the latest time examined by Ries et al. (32). p14ARF was not detected in either of the two tumor cell lines or in HDF cells and was not induced after infection. However, p14ARF was clearly detected in Bre80 cells.
Infection of A549 cells with wt Ad5 caused a decline in p53 expression, consistent with reports that the E1b 55-kDa protein facilitates p53 degradation (13, 31, 37). However, wt Ad5 induced p53 in both RKO and HDF cells. This was not expected, but it was reproducible. Like ONYX-015, wt Ad5 also induced MDM2 in A549, RKO, and HDF cells. This induction is most likely a direct effect of the virus, as it occurred only in infected cells (Fig. 2). In Bre80 cells, p53 was not detected following infection with wt Ad5. Taken together, these data suggest that in response to infection p14ARF promotes MDM2 degradation and that this is inhibited by the E1b 55-kDa protein. Thus, the E1b 55-kDa protein may interact directly with of p14ARF, MDM2, or both.
High levels of MDM2 concomitantly induced with p53, in the absence of p14ARF, would suggest that the induced p53 will be nonfunctional, as found previously in HCT116 cells (32). We, however, found that ONYX-015 substantially induced activity of the p21CIP1/WAF1 promoter in a p53-dependent manner in both HDF and A549 cells but not in RKO cells (Fig. 3). By contrast, wt Ad5 had little or no inductive effect in each case, consistent with the E1b 55-kDa protein inhibiting p53-dependent transactivation (43, 44). These data suggest that some of the p53 that is induced after ONYX-015 infection is transcriptionally active in some cells, irrespective of whether they are normal or tumor derived, and, moreover, that this does not always require p14ARF. This result is not entirely unexpected, as, in addition to stabilization of p53 by p14ARF, other mechanisms have been reported (10). These involve an interaction between N-terminal domains of E1a and transcription factor pRb105 or p300/CBP (7, 30) or between a similar region of E1a and the 26S proteasome (40). It is interesting that p53 in Bre80 cells is far more transcriptionally active after ONYX-015 infection, presumably reflecting the MDM2-neutralizing ability of the expressed p14ARF.
An alternative explanation for the high levels of MDM2 together with p53 is that the MDM2 is nonfunctional or that virus can subvert normal MDM2 function. Since we have seen the same pattern not only in tumor cells but also in HDF cells, which are considered to be normal, a defect in MDM2 probably cannot account for all of our observations.
Next we asked whether there were any differences in ONYX-015 replication in cells in which the virus induces functional p53 with (Bre80 cells) or without (A549 and HDF cells) p14ARF and in cells in which neither is induced (RKO and RKO p53.13 cells). Compared to that of wt Ad5, ONYX-015 replication was markedly reduced in the normal HDF cells but only modestly reduced in the other cells examined (Table 2). Importantly, there was no obvious restriction of ONYX-015 replication in Bre80 cells, in which both p14ARF and p53 are present and in which MDM2 expression has been lost. These data stand in stark contrast to the proposed mechanism for ONYX-015 selectivity. It would seem that where ONYX-015 attenuation occurs, it is cell type dependent and can be independent of the p53/p14ARF pathway. This conclusion is further supported by the data in Fig. 4, which show that high levels of p14ARF in conjunction with wt p53 (inducible H24-H1299 cells) also failed to have a significant restrictive effect on ONYX-015 replication.
The simplest explanation of the replication differences between wt Ad5 and ONYX-015 in the different cell types is that they are due to the absence of the E1b 55-kDa protein (1, 28), which has more or less impact on replication, depending on the cellular environment.
In summary, our data do not support the present model of attenuation of ONYX-015 replication in normal cells being due to p53 and/or p14ARF. Attenuation of ONYX-015 in normal cells, as indicated by some of the clinical trial data (24), may be due to cell type differences or some other unknown factor. Although it appears that ONYX-015 may be useful in treatment of cancers, particularly in combination with standard chemotherapeutic agents (16), we still do not understand how it is selective for tumor cells.
Acknowledgments
We thank Arnie Berk (UCSD) for dl1520 (ONYX-015), Moshe Oren (Weizmann) and Karen Vousden (NIH) for reporter constructs, Carol Prives (Columbia) for H24-H1299 cells, Mike Kastan (St. Jude's) for the RKO and RKO p53.13 cells, and Roger Reddel (Sydney) for IIICF/c and Bre80 cells. We also thank Craig Homer for help in figure preparation and the other members of the Cell Transformation Group for comments on the manuscript.
This work was supported by grants from the Health Research Council, the Cancer Society, the Royal Society, and the Lottery Board.
REFERENCES
- 1.Babiss, L. E., H. S. Ginsberg, and J. E. Darnell, Jr. 1985. Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol. Cell. Biol. 5:2552-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker, D. D., and A. J. Berk. 1987. Adenovirus proteins from both E1b reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology 156:107-121. [DOI] [PubMed] [Google Scholar]
- 3.Bates, S., A. C. Phillips, P. A. Clark, F. Stott, G. Peters, R. L. Ludwig, and K. H. Vousden. 1998. p14ARF links the tumour suppressors Rb and p53. Nature 395:124-125. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumour cells. Science 274:373-376. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite, A. W., G. E. Blair, C. C. Nelson, J. McGovern, and A. J. Bellett. 1991. Adenovirus E1b58kD antigen binds to p53 during infection of rodent cells: evidence for an N-terminal binding site on p53. Oncogene 6:781-787. [PubMed] [Google Scholar]
- 6.Chen, X., L. J. Ko, L. Jayaraman, and C. Prives. 1996. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10:2438-2451. [DOI] [PubMed] [Google Scholar]
- 7.Chiou, S. K., and E. White. 1997. p300 binding by E1A cosegregates with p53 induction but is dispensable for apoptosis. J. Virol. 71:3515-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dix, B. R., S. J. Edwards, and A. W. Braithwaite. 2001. Does the antitumor virus ONYX-015/dl1520 selectively target cells defective in the p53 pathway? J. Virol. 75:5443-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dix, B. R., S. J. O'Carroll, C. J. Myers, S. J. Edwards, and A. W. Braithwaite. 2000. Efficient induction of cell death by adenoviruses requires binding of E1B55k and p53. Cancer Res. 60:2666-2672. [PubMed] [Google Scholar]
- 10.Gallimore, P. H., and A. S. Turnell. 2001. Adenovirus E1A: remodelling the host cell, a life or death experience. Oncogene 20:7824-7835. [DOI] [PubMed] [Google Scholar]
- 11.Geoerger, B., J. Grill, P. Opolon, J. Morizet, G. Aubert, M. J. Terrier-Lacombe, B. Bressac De-Paillerets, M. Barrois, J. Feunteun, D. H. Kirn, and G. Vassal. 2002. Oncolytic activity of the E1B-55kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 62:764-772. [PubMed] [Google Scholar]
- 12.Goodrum, F. D., and D. A. Ornelles. 1998. p53 status does not determine outcome of E1b 55-kilodalton mutant adenovirus lytic infection. J. Virol. 72:9479-9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grand, R. J., M. L. Grant, and P. H. Gallimore. 1994. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology 203:229-240. [DOI] [PubMed] [Google Scholar]
- 14.Grand, R. J., D. Owen, S. M. Rookes, and P. H. Gallimore. 1996. Control of p53 expression by adenovirus 12 early region 1A and early region 1B 54K proteins. Virology 218:23-34. [DOI] [PubMed] [Google Scholar]
- 15.Hall, A. R., B. R. Dix, S. J. O'Carroll, and A. W. Braithwaite. 1998. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nat. Med. 4:1068-1072. [DOI] [PubMed] [Google Scholar]
- 16.Heise, C., M. Lemmon, and D. Kirn. 2000. Efficacy with a replication-selective adenovirus plus cisplatin-based chemotherapy: dependence on sequencing but not p53 functional status or route of administration. Clin. Cancer Res. 6:4908-4914. [PubMed] [Google Scholar]
- 17.Heise, C., A. Sampson-Johannes, A. Williams, F. McCormick, D. D. Von Hoff, and D. H. Kirn. 1997. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 3:639-645. [DOI] [PubMed] [Google Scholar]
- 18.Higashi, H., I. Suzuki-Takahashi, E. Yoshida, S. Nishimura, and M. Kitagawa. 1997. Expression of p16INK4a suppresses the unbounded and anchorage-independent growth of a glioblastoma cell line that lacks p16INK4a. Biochem. Biophys. Res. Commun. 231:743-750. [DOI] [PubMed] [Google Scholar]
- 19.Hollstein, M., D. Sidransky, B. Vogelstein, and C. Harris. 1991. p53 mutations in human cancers. Science 253:49-53. [DOI] [PubMed] [Google Scholar]
- 20.Huschtscha, L. I., J. R. Noble, A. A. Neumann, E. L. Moy, P. Barry, J. R. Melki, S. J. Clark, and R. R. Reddel. 1998. Loss of p16INK4 expression by methylation is associated with lifespan extension of human mammary epithelial cells. Cancer Res. 58:3508-3512. [PubMed] [Google Scholar]
- 21.Lowe, S., and H. Ruley. 1993. Stabilization of the p53 tumour suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 7:535-545. [DOI] [PubMed] [Google Scholar]
- 22.Masuda, H., C. Miller, H. P. Koeffler, H. Battifora, and M. J. Cline. 1987. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc. Natl. Acad. Sci. USA 84:7716-7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momand, J., G. Zambetti, D. Olson, D. George, and A. Levine. 1992. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69:1237-1245. [DOI] [PubMed] [Google Scholar]
- 24.Nemunaitis, J., I. Ganly, F. Khuri, J. Arseneau, J. Kuhn, T. McCarty, S. Landers, P. Maples, L. Romel, B. Randlev, T. Reid, S. Kaye, and D. Kirn. 2000. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 60:6359-6366. [PubMed] [Google Scholar]
- 25.Nevins, J. R. 1994. Cell cycle targets of the DNA tumor viruses. Curr. Opin. Genet. Dev. 4:130-134. [DOI] [PubMed] [Google Scholar]
- 26.O'Carroll, S. J., A. R. Hall, C. J. Myers, A. W. Braithwaite, and B. R. Dix. 2000. Quantifying adenoviral titers by spectrophotometry. BioTechniques 28:408-410. [DOI] [PubMed] [Google Scholar]
- 27.Phillips, A. C., S. Bates, K. M. Ryan, K. Helin, and K. H. Vousden. 1997. Induction of DNA synthesis and apoptosis are separable functions of E2F-1. Genes Dev. 11:1853-1863. [DOI] [PubMed] [Google Scholar]
- 28.Pilder, S., M. Moore, J. Logan, and T. Shenk. 1986. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol. Cell. Biochem. 6:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomerantz, J., N. Schreiber-Agus, N. J. Liegeois, A. Silverman, L. Alland, L. Chin, J. Potes, K. Chen, I. Orlow, H. W. Lee, C. Cordon-Cardo, and R. A. DePinho. 1998. The INK4a tumor suppressor gene product, p19ARF, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92:713-723. [DOI] [PubMed] [Google Scholar]
- 30.Querido, E., J. G. Teodoro, and P. E. Branton. 1997. Accumulation of p53 induced by the adenovirus E1A protein requires regions involved in the stimulation of DNA synthesis. J. Virol. 71:3526-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridgway, P. J., A. R. Hall, C. J. Myers, and A. W. Braithwaite. 1997. p53/E1b58kDa complex regulates adenovirus replication. Virology 237:404-413. [DOI] [PubMed] [Google Scholar]
- 32.Ries, S. J., C. H. Brandts, A. S. Chung, C. H. Biederer, B. C. Hann, E. M. Lipner, F. McCormick, and W. M. Korn. 2000. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl1520 (ONYX-015). Nat. Med. 6:1128-1133. [DOI] [PubMed] [Google Scholar]
- 33.Rogan, E. M., T. M. Bryan, B. Hukku, K. Maclean, A. C. Chang, E. L. Moy, A. Englezou, S. G. Warneford, L. Dalla-Pozza, and R. R. Reddel. 1995. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol. Cell. Biol. 15:4745-4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothmann, T., A. Hengstermann, N. J. Whitaker, M. Scheffner, and H. zur Hausen. 1998. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J. Virol. 72:9470-9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slichenmyer, W. J., W. G. Nelson, R. J. Slebos, and M. B. Kastan. 1993. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 53:4164-4168. [PubMed] [Google Scholar]
- 36.Smith, R., R. Heubner, and W. Rowe. 1956. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer Res. 9:1211-1218. [DOI] [PubMed] [Google Scholar]
- 37.Steegenga, W. T., N. Riteco, A. G. Jochemsen, F. J. Fallaux, and J. L. Bos. 1998. The large E1B protein together with the E4ORF6 protein target p53 for active degradation in adenovirus infected cells. Oncogene 16:349-357. [DOI] [PubMed] [Google Scholar]
- 38.Stott, F. J., S. Bates, M. C. James, B. B. McConnell, M. Starborg, S. Brookes, I. Palmero, K. Ryan, E. Hara, K. H. Vousden, and G. Peters. 1998. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 17:5001-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnell, A. S., R. J. Grand, and P. H. Gallimore. 1999. The replicative capacities of large E1B-null group A and group C adenoviruses are independent of host cell p53 status. J. Virol. 73:2074-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turnell, A. S., R. J. Grand, C. Gorbea, X. Zhang, W. Wang, J. S. Mymryk, and P. H. Gallimore. 2000. Regulation of the 26S proteasome by adenovirus E1A. EMBO J. 19:4759-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitaker, N. J., T. M. Bryan, P. Bonnefin, A. C. Chang, E. A. Musgrove, A. W. Braithwaite, and R. R. Reddel. 1995. Involvement of RB-1, p53, p16INK4 and telomerase in immortalisation of human cells. Oncogene 11:971-976. [PubMed] [Google Scholar]
- 42.Whyte, P., K. J. Buchkovich, J. M. Horowitz, S. H. Friend, M. Raybuck, R. A. Weinberg, and E. Harlow. 1988. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature 334:124-129. [DOI] [PubMed] [Google Scholar]
- 43.Yew, P. R., and A. J. Berk. 1992. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature 357:82-85. [DOI] [PubMed] [Google Scholar]
- 44.Yew, P. R., X. Liu, and A. J. Berk. 1994. Adenovirus E1B oncoprotein tethers a transcriptional repression domain to p53. Genes Dev. 8:190-202. [DOI] [PubMed] [Google Scholar]
- 45.Zantema, A., P. I. Schrier, A. Davis-Olivier, T. van Laar, R. T. Vaessen, and A. J. van der Eb. 1985. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol. Cell. Biol. 5:3084-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, Y., Y. Xiong, and W. G. Yarbrough. 1998. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92:725-734. [DOI] [PubMed] [Google Scholar]