Abstract
We studied the motion of pigment organelles driven by myosin-V in Xenopus melanophores using a tracking technique with precision of 2 nm. The organelle trajectories showed occasional steps with a distribution centered at 35 nm and a standard deviation of 13 nm, in agreement with the step size of myosin-V determined in vitro. In contrast, trajectories of melanosomes in cells expressing a dominant negative form of myosin-V did not show steps. The step duration was in the range 20–80 ms, slower than what it would be expected from in vitro results. We speculate that the cytoplasm high viscosity may affect significantly the melanosomes' motion.
The organization of the eukaryotic cells cytoplasm is regulated by molecular motors that distribute organelles and other cargoes along cytoskeleton tracks to their correct destination in the cytoplasm.
In contrast to the detailed information regarding the biophysical properties of motors in vitro, little is know about their function in living cells. Moreover, different authors reported that some properties determined for motors in the cell cytoplasm cannot be deduced from in vitro measurements (1,2).
Melanophore cells are an exceptionally convenient model system to study intracellular transport (3). Xenopus melanophores have pigment organelles called melanosomes, which are filled with the black pigment melanin. Therefore, they can be easily imaged using bright-field transmission light microscopy.
Pigment organelles can be distributed in the cells in two configurations: either aggregated in the perinuclear region or homogeneously dispersed in the cytoplasm. The transport of pigment organelles during aggregation and dispersion is regulated by signaling mechanisms initiated by the binding of specific hormones to cell surface receptors, which results in the modulation of cAMP concentrations (4,5). Hence, one can stimulate melanosome movement toward or away from the cell center by using appropriate hormones to decrease or increase the concentration of cAMP in the cytoplasm, respectively.
Pigment dispersion requires the plus-end directed microtubule motor kinesin-2 (6) and the actin motor myosin-V (7), whereas aggregation is powered by the minus-end directed motor cytoplasmic dynein (8). The net movement of melanosomes results from the combined action of these motors.
In a recent work, we studied organelles transport along microtubules by using a new particle tracking technique with 2 nm accuracy (2). In this work, we used a similar approach to investigate pigment organelle transport along actin filaments.
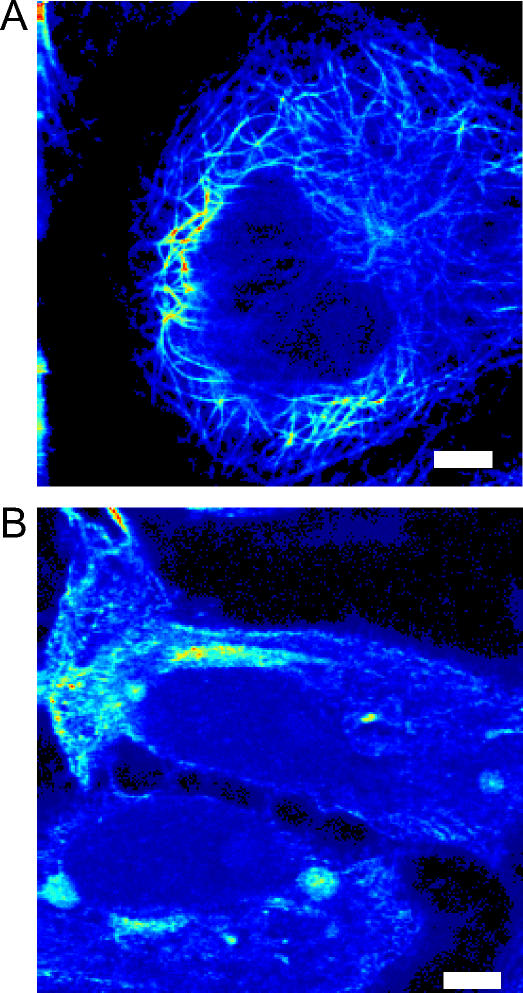
To eliminate the transport along microtubules, we treated the cells with nocodazole. We verified that the microtubules were completely depolymerized after this treatment by staining the cells with the tubulin antibody DM1 α and a fluorescein-5-isothiocyanate-labeled secondary antibody (Fig. 1).
FIGURE 1.
Depolymerization of microtubules in Xenopus melanophores. Cells grown in the presence of phenylthiourea were incubated at 0°C for 30 min with 10 μM nocodazole. Control (A) and nocodazole-treated (B) samples were fixed and stained as described in the text. The fluorescence images were registered in the two-photon microscope previously described (9). Bars, 2 μm.
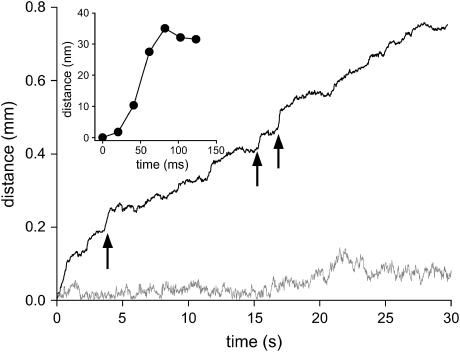
Nocodazole-treated melanophores were stimulated for dispersion by incubation with 100 nM melanocyte-stimulating hormone. Movies of the cells were recorded at 50 frames per second and analyzed as described previously (2). Fig. 2 shows a representative time course of motion of a melanosome. Abrupt jumps in the position or steps can be observed, exceeding the noise of the trajectory.
FIGURE 2.
Movement of melanosomes in control cells (solid line) and cells expressing a dominant-negative myosin-V (shaded line). The arrows point to some steps in the trajectory. (Inset) Details of one step.
To determine if the steps are due to the activity of myosin-V, we studied the motion of melanosomes in cells transfected with a plasmid encoding a green fluorescent protein-tagged myosin-V tail. Expression of this plasmid results in a dominant-negative inhibition of myosin-V driven melanosome transport (7). Before the tracking experiment, the cells were treated with nocodazole and stimulated for dispersion as described above. The trajectories did not show steps (Fig. 2), confirming that they are due to myosin-V. The comparison of these trajectories with those obtained in control cells shows that myosin-V prevents high-frequency oscillations on melanosome position, likely because myosin-V tethers organelles to actin.
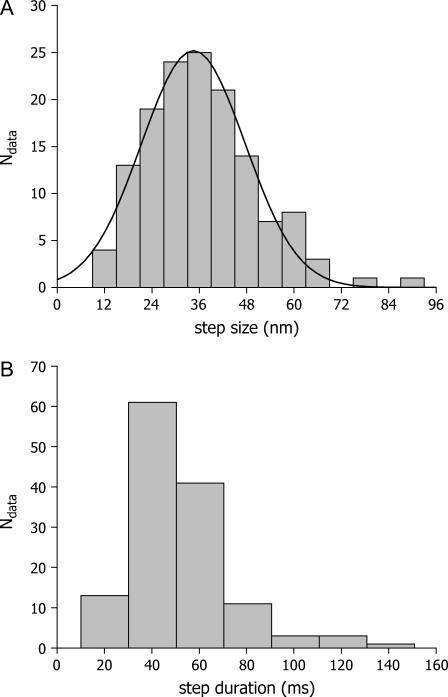
We constructed histograms of the size and duration of the steps (N = 143). The mean distance was 35 nm (Fig. 3 A), very close to the step size determined for myosin-V in vitro (10). Surprisingly, the steps occurred in 20–80 ms (Fig. 3 B).
FIGURE 3.
Step characterization. (A) Step size distribution of melanosome driven by myosin-V. The solid curve is the fitting of a Gaussian function centered at 34.5 ± 0.6 nm with a standard deviation of 13.1 ± 0.5 nm. (B) Step duration distribution. The duration was estimated from the number of data points during the steps.
To our knowledge, there are no reports on the step duration of myosin-V. A recent in vitro study describes a substep within the 36 nm step with lifetime of ∼8 ms (11), setting a limit to the step duration. In the case of kinesin, the step occurs in <0.1 ms (12). These results suggest that the steps determined in Xenopus melanophores are slower than what would have been expected from the reported in vitro measurements.
Carter et al. (12) reported that attaching a large bead to kinesin significantly decreases the step velocity, indicating that this velocity depends on the motor load. Since the cell cytoplasm is highly crowded and the viscosity can be three orders higher than that of the buffer used in in vitro assays (1), we would expect slower steps for organelles transported by myosin-V in the cytoplasm than those measured for the motor attached to beads in vitro.
In conclusion, we showed that it is possible to measure individual steps of myosin-V in vivo and that the step size of this motor in Xenopus melanophores agrees with the value determined in vitro. Also, the steps of melanosomes transported by myosin-V are slower than what it is expected from in vitro assays, suggesting that the high viscosity of the cytoplasm slows down the steps of melanosomes moving along actin filaments.
METHODS
Immortalized Xenopus laevis melanophores were cultured as described (13). The number of melanosomes in the cell was reduced by treatment with phenylthiourea (14) to track the movement of individual organelles. For microscopy measurements, the cells were grown as previously described (2). The samples were observed at 21°C, between 5 and 15 min after stimulation.
Acknowledgments
This research was supported by the Division of Research Resources of the National Institutes of Health (PHS 5 P41-RR03155), by a grant from the National Institute of Health to V.I.G. (GM-52111), and by the University of Illinois at Urbana-Champaign.
References
- 1.Hill, D. B., M. J. Plaza, K. Bonin, and G. Holzwarth. 2004. Fast vesicle transport in PC12 neurites: velocities and forces. Eur. Biophys. J. 33:623–632. [DOI] [PubMed] [Google Scholar]
- 2.Levi, V., A. S. Serpinskaya, E. Gratton, and V. Gelfand. 2005. Organelle transport along microtubules in Xenopus melanophores: evidence for cooperation between multiple motors. Biophys. J. 318–327. [DOI] [PMC free article] [PubMed]
- 3.Nascimento, A. A., J. T. Roland, and V. I. Gelfand. 2003. Pigment cells: a model for the study of organelle transport. Annu. Rev. Cell Dev. Biol. 19:469–491. [DOI] [PubMed] [Google Scholar]
- 4.Rozdzial, M. M., and L. T. Haimo. 1986. Bidirectional pigment granule movements of melanophores are regulated by protein phosphorylation and dephosphorylation. Cell. 47:1061–1070. [DOI] [PubMed] [Google Scholar]
- 5.Sammak, P. J., S. R. Adams, A. T. Harootunian, M. Schliwa, and R. Y. Tsien. 1992. Intracellular cyclic AMP not calcium, determines the direction of vesicle movement in melanophores: direct measurement by fluorescence ratio imaging. J. Cell Biol. 117:57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuma, M. C., A. Zill, N. Le Bot, I. Vernos, and V. Gelfand. 1998. Heterotrimeric kinesin II is the microtubule motor protein responsible for pigment dispersion in Xenopus melanophores. J. Cell Biol. 143:1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers, S. L., R. L. Karcher, J. T. Roland, A. A. Minin, W. Steffen, and V. I. Gelfand. 1999. Regulation of melanosome movement in the cell cycle by reversible association with myosin V. J. Cell Biol. 146:1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson, H., and M. Wallin. 1997. Evidence for several roles of dynein in pigment transport in melanophores. Cell Motil. Cytoskeleton. 38:397–409. [DOI] [PubMed] [Google Scholar]
- 9.Levi, V., Q. Ruan, and E. Gratton. 2005. 3-D particle tracking in a two photon microscope. Application to the study of molecular dynamics in cells. Biophys. J. 88:2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta, A. D., R. S. Rock, M. Rief, J. A. Spudich, M. S. Mooseker, and R. E. Cheney. 1999. Myosin-V is a processive actin-based motor. Nature. 400:590–593. [DOI] [PubMed] [Google Scholar]
- 11.Uemura, S., H. Higuchi, A. O. Olivares, E. M. De La Cruz, and S. Ishiwata. 2004. Mechanochemical coupling of two substeps in a single myosin V motor. Nat. Struct. Mol. Biol. 11:877–883. [DOI] [PubMed] [Google Scholar]
- 12.Carter, N. J., and R. A. Cross. 2005. Mechanics of the kinesin step. Nature. 435:308–312. [DOI] [PubMed] [Google Scholar]
- 13.Rogers, S. L., I. S. Tint, P. C. Fanapour, and V. I. Gelfand. 1997. Regulated bidirectional motility of melanophore pigment granules along microtubules in vitro. Proc. Natl. Acad. Sci. USA. 94:3720–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross, S. P., M. C. Tuma, S. W. Deacon, A. S. Serpinskaya, A. R. Reilein, and V. I. Gelfand. 2002. Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 156:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]