Abstract
Instability in the intracellular Ca2+ handling system leading to Ca2+ alternans is hypothesized to be an underlying cause of electrical alternans. The highly coupled nature of membrane voltage and Ca2+ regulation suggests that there should be reciprocal effects of membrane voltage on the stability of the Ca2+ handling system. We investigated such effects using a mathematical model of the cardiac intracellular Ca2+ handling system. We found that the morphology of the action potential has a significant effect on the stability of the Ca2+ handling system at any given pacing rate, with small changes in action potential morphology resulting in a transition from stable nonalternating Ca2+ transients to stable alternating Ca2+ transients. This bifurcation occurs as the alternans eigenvalue of the system changes from absolute value <1 to absolute value >1. These results suggest that the stability of the intracellular Ca2+ handling system and the occurrence of Ca2+ alternans are not dictated solely by the Ca2+ handling system itself, but are also modulated to a significant degree by membrane voltage (through its influence on sarcolemmal Ca2+ currents) and, therefore, by all ionic currents that affect membrane voltage.
INTRODUCTION
Cardiac electrical alternans appears to be a diagnostic indicator for vulnerability to arrhythmias (1–4). Recent evidence also demonstrates that electrical alternans can provide a suitable substrate for the initiation of reentry, suggesting a potential mechanistic link to the onset of arrhythmias (5–8). Recent research has focused on understanding the physiological mechanisms underlying alternans (9–19), and on developing new ways of eliminating alternans (15,20–25).
Particular interest has been paid to the role of the intracellular Ca2+ handling system in electrical alternans (26). This focus is due in part to experiments conducted in isolated rabbit ventricular myocytes, where it was demonstrated that the intracellular Ca2+ transient could alternate on a beat-to-beat basis during voltage clamping even though the applied voltage waveform was a train of identical action potentials (27). This demonstration, that Ca2+ alternans may occur independently of electrical alternans, appears to support the hypothesis that electrical alternans is caused by Ca2+ alternans (26). Based on this hypothesis, mathematical modeling has suggested that the intracellular Ca2+ handling system instability that leads to Ca2+ alternans during voltage clamping (i.e., in the absence of electrical alternans) can be explained by a steep and nonlinear dependence of Ca2+ release from the sarcoplasmic reticulum (SR) on SR Ca2+ load and diastolic Ca2+ concentration (19,28,29). Recent experimental results also point to the importance of the steep dependence of Ca2+ release on SR Ca2+ load and diastolic Ca2+ concentration (13,30).
Although the properties of the SR are important in understanding the regulation of intracellular Ca2+ concentration, other processes such as the passage of Ca2+ through voltage- and Ca2+-sensitive L-type Ca2+ channels (ICa) and Na+-Ca2+ exchangers (INaCa) also play a role in Ca2+ regulation. The voltage-sensitive gating of these sarcolemmal currents suggests that the morphology of the action potential should influence their activity and, therefore, the occurrence of Ca2+ alternans. Action potential clamp experiments conducted using rat and rabbit ventricular myocytes have shown that the morphology of the voltage waveform does have a marked influence on the shape of the intracellular Ca2+ transient (31). However, these experiments were conducted at excitation rates that were too slow for Ca2+ alternans to develop. In a mathematical model of intracellular Ca2+ handling that reproduces the rabbit ventricular myocyte action potential clamp experiments discussed above (29), and in recent experiments investigating Ca2+ alternans in rat myocytes during voltage clamping (13), it was demonstrated that the Ca2+-selective sarcolemmal currents alternate during Ca2+ alternans. These currents alternated despite being clamped by voltage waveforms that were identical on a beat-to-beat basis. However, the influence of different voltage waveforms on the behavior of the intracellular Ca2+ handling system and the incidence of Ca2+ alternans was not investigated in these studies. Recent experiments with epicardial and endocardial myocytes isolated from guinea pig hearts investigated the onset of Ca2+ alternans in response to two different action potential morphologies during action potential clamping (30). This study found that the excitation rate at which Ca2+ alternans was initiated during clamping did not depend upon which of the two action potentials were used to perform clamping (Figs. 2 and 4 in Wan et al. (30)). However, the influence of action potential morphology on Ca2+ handling system stability was not systematically investigated in these experiments.
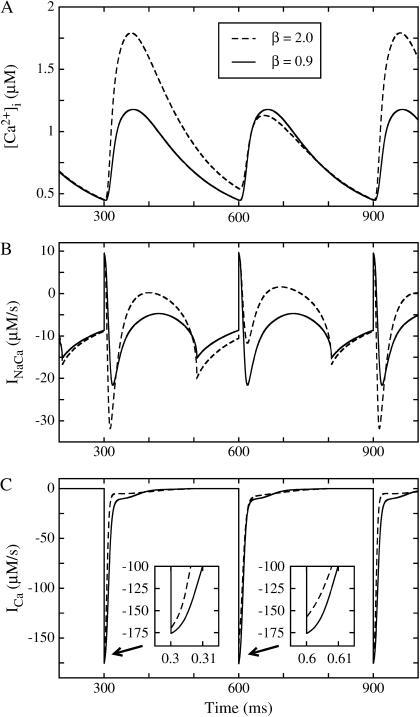
FIGURE 2.
(A) Intracellular Ca2+ concentration, (B) Na+-Ca2+ exchange current, and (C) L-type Ca2+ channel current for two different values of β at a pacing period of T = 300 ms. When β = 2.0, all three components exhibit a stable period-2 alternans rhythm. For β = 0.9 alternans does not occur in any component, indicating that the period-1, nonalternating rhythm is stable.
FIGURE 4.
(A) Alternans eigenvalue (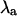 ) as a function of APD50 at pacing periods T = 250 ms (black crosses), T = 320 ms (red circles), and T = 400 ms (blue triangles). For T = 250 ms and T = 320 ms, the transition from no alternans to alternans is characterized by a rapid change in
) as a function of APD50 at pacing periods T = 250 ms (black crosses), T = 320 ms (red circles), and T = 400 ms (blue triangles). For T = 250 ms and T = 320 ms, the transition from no alternans to alternans is characterized by a rapid change in 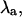 with the switch from a very stable Ca2+ handling system to an unstable system occurring in response to a very small change in action potential morphology (as measured by the action potential duration at 50% repolarization [APD50]). For T = 400 ms, the period-1 solution becomes much less stable in response to changes in action potential morphology, but does not become unstable. (B) The relationships between β and APD50 for T = 250 ms (black line), T = 320 ms (red line), and T = 400 ms (blue line).
with the switch from a very stable Ca2+ handling system to an unstable system occurring in response to a very small change in action potential morphology (as measured by the action potential duration at 50% repolarization [APD50]). For T = 400 ms, the period-1 solution becomes much less stable in response to changes in action potential morphology, but does not become unstable. (B) The relationships between β and APD50 for T = 250 ms (black line), T = 320 ms (red line), and T = 400 ms (blue line).
The influence of action potential morphology at any given excitation rate on the stability of the Ca2+ handling system and the development of Ca2+ alternans therefore remains poorly understood. Using the mathematical model that reproduces the action potential clamp experiments discussed above (29), we explored the stability of the Ca2+-handling system in response to different action potential morphologies. We demonstrate that the morphology of the action potential during voltage clamping is a significant factor in determining the stability of the Ca2+-handling system, and therefore plays an important role in determining whether Ca2+ alternans occurs. Given that the morphology of the action potential waveform during voltage clamping reflects the contribution of every membrane current, this suggests that every membrane current, and not just those of the Ca2+ handling system, influences the conditions under which alternans occurs.
MATERIALS AND METHODS
Mathematical model
Simulations were performed using a model of the intracellular Ca2+ handling system of a ventricular myocyte subjected to action potential voltage clamping (29). The model incorporates cytosolic, submembrane, junctional SR, and network SR Ca2+ concentrations, L-type Ca2+ (ICa), Na+-Ca2+ exchange (INaCa), and SR uptake (Iup) currents, Ca2+-induced Ca2+ release from the SR, and Ca2+ buffering. The membrane voltage was clamped according to
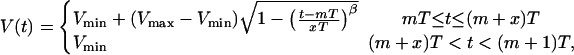 |
(1) |
where 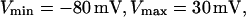 T is the pacing period (in seconds), m denotes beat number, and β and x are parameters that characterize the morphology of the action potential. The parameter β allows the shape of the action potential in the plateau and repolarization phases to be varied while changing the action potential duration at 90% repolarization only slightly (if at all). β was varied between 0.8 and 2.0 in our simulations (β= 2.0 was the value used in the original modeling study (29)). The parameter x, which is the ratio of action potential duration to pacing period, varies with T according to
T is the pacing period (in seconds), m denotes beat number, and β and x are parameters that characterize the morphology of the action potential. The parameter β allows the shape of the action potential in the plateau and repolarization phases to be varied while changing the action potential duration at 90% repolarization only slightly (if at all). β was varied between 0.8 and 2.0 in our simulations (β= 2.0 was the value used in the original modeling study (29)). The parameter x, which is the ratio of action potential duration to pacing period, varies with T according to
 |
(2) |
Stability analysis
The stability of the intracellular Ca2+ handling system was characterized using the “eigenmode” method (10), which was adapted to analyze the intracellular Ca2+-handling model used in this study. Briefly, the method involves: i), locating the period-1 (i.e., no alternans) solution for a given pacing period T (which may be inherently stable, as in situations where no Ca2+ alternans naturally occurs at that value of T, or inherently unstable, as in situations where Ca2+ alternans naturally occurs at that value of T); ii), systematically applying small perturbations to each state variable (i.e., Ca2+ concentrations, ion channel gating variables, etc.); and iii), calculating the eigenvalues of the system from the response of the state variables to the perturbations. As there are nine state variables in the model (V, [Ca2+]s, [Ca2+]i, [Ca2+]j, [Ca2+]j′, Irel, f, q, [CaT]i, and [CaT]s; see Shiferaw et al. (29) for details), nine eigenvalues are calculated for each set of conditions. The alternans eigenvalue (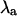 ), defined as the largest negative eigenvalue (10), characterizes the stability of the corresponding alternans eigenmode, and hence characterizes the stability of the period-1, nonalternating rhythm. If
), defined as the largest negative eigenvalue (10), characterizes the stability of the corresponding alternans eigenmode, and hence characterizes the stability of the period-1, nonalternating rhythm. If  a stable, period-1, nonalternating rhythm will be present, whereas if
a stable, period-1, nonalternating rhythm will be present, whereas if  the period-1 rhythm is unstable. Systems characterized by larger values of
the period-1 rhythm is unstable. Systems characterized by larger values of 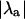 are less stable than systems characterized by smaller values of
are less stable than systems characterized by smaller values of 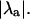
RESULTS
Fig. 1 illustrates the effect of changing the value of β in Eq. 1 on the morphology of the action potential waveform applied during voltage clamping at a pacing period T = 300 ms. As Fig. 1 A illustrates, varying β markedly changes the slope of the plateau phase of the action potential (as indicated by the different action potential duration (APD) restitution curves calculated at 50% repolarization). Over this range of β-values the APD at 90% repolarization is practically identical (as indicated by the overlapping APD restitution curves at 90% repolarization).
FIGURE 1.
(A) Action potential morphology as a function of the parameter β (see Eq. 1) at a pacing period of T = 300 ms. (B) Action potential duration (APD) restitution curves for the three values of β in panel A, measured at both 50% (APD50) and 90% (APD90) repolarization. As panel B indicates, APD and diastolic interval (DI) measured at 90% repolarization are practically identical for the values of β shown.
Action potential morphology and Ca2+ alternans
Fig. 2 shows how changing the morphology of the action potential waveform at a given excitation rate influences the occurrence of Ca2+ alternans. As Fig. 2 A indicates, β = 2.0 at a pacing period T = 300 ms results in stable, period-2 Ca2+ alternans, whereas β = 0.9 at the same pacing period results in stable, period-1, nonalternating behavior. Fig. 2 also illustrates the activity of the Ca2+ currents INaCa (Fig. 2 B) and ICa (Fig. 2 C), with both currents alternating for β = 2.0 but neither current alternating for β = 0.9. Given that the intrinsic properties of the system (e.g., ionic current conductances, pump, and exchanger strengths) and the pacing period are identical for both values of β, Fig. 2 suggests that, at any given pacing period, the voltage waveform sensed by the sarcolemmal Ca2+ channels and exchangers (and not just the APD, diastolic interval (DI), or pacing period) is intimately involved in determining whether the intracellular Ca2+ handling system generates nonalternating or alternating Ca2+ transients.
Ca2+ handling system stability
The results in Fig. 2 demonstrate the effect of changing the morphology of the action potential waveform during voltage clamping at a single pacing period. Fig. 3 explores the effect of changing the morphology of the action potential on the stability of the Ca2+ handling system over a range of values of T. Simulations using three values of β (0.9, 1.5, and 2.0) are shown, with the bifurcation diagrams in Fig. 3 A depicting the peak value of [Ca2+]i from two consecutive beats at steady state plotted as a function of T. The variety of β values reflects different combinations of ionic conductances that generate a range of action potential morphologies.
FIGURE 3.
(A) Bifurcation diagrams illustrating peak [Ca2+]i from two consecutive beats as a function of pacing period for the three values of β shown in Fig. 1. Ca2+ alternans occurs over a range of pacing periods for β = 2.0 and β = 1.5, but alternans is absent at all pacing periods for β = 0.9. (B) Alternans eigenvalue (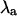 ) as a function of pacing period for the same values of β and T as in panel A. As panel B shows,
) as a function of pacing period for the same values of β and T as in panel A. As panel B shows, 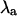 for each value of β and T in panel A indicates the presence or absence of alternans, with the presence of alternans characterized by
for each value of β and T in panel A indicates the presence or absence of alternans, with the presence of alternans characterized by  and the absence of alternans characterized by
and the absence of alternans characterized by 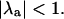
Fig. 3 A shows that, for β = 1.5 and β = 2.0, peak [Ca2+]i alternates over a range of pacing periods. For β = 2.0, alternans occurs between T = 320 ms and T = 200 ms, whereas for β = 1.5 alternans occurs between T = 340 ms and T = 180 ms. In contrast, there is no alternans for β = 0.9 at any value of T. This indicates that how the action potential morphology changes as a function of the pacing period is important in determining whether Ca2+ alternans will occur at any given value of T.
Fig. 3 B illustrates the alternans eigenvalue ( ) calculated for the same values of β and T as in Fig. 3 A. Fig. 3 B shows that
) calculated for the same values of β and T as in Fig. 3 A. Fig. 3 B shows that 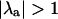 for every set of conditions (i.e., each combination of β and T) where Ca2+ alternans occurs in Fig. 3 A, whereas
for every set of conditions (i.e., each combination of β and T) where Ca2+ alternans occurs in Fig. 3 A, whereas  corresponds to the stable period-1 behavior seen in Fig. 3 A. Although
corresponds to the stable period-1 behavior seen in Fig. 3 A. Although  indicates the absence or presence of alternans (i.e., the degree of stability of the system), Fig. 3 also shows that there is no correlation between
indicates the absence or presence of alternans (i.e., the degree of stability of the system), Fig. 3 also shows that there is no correlation between 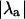 and the magnitude of alternans (measured as the difference between the peak [Ca2+]i from two consecutive beats in Fig. 3 A). For example, the period-1 solution for β = 1.5 becomes more unstable than the period-1 solution for β = 2.0 (indicated by the larger
and the magnitude of alternans (measured as the difference between the peak [Ca2+]i from two consecutive beats in Fig. 3 A). For example, the period-1 solution for β = 1.5 becomes more unstable than the period-1 solution for β = 2.0 (indicated by the larger 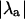 in Fig. 3 B), but the magnitude of alternans is always smaller for β = 1.5 than for β = 2.0. Therefore, alternans magnitude cannot be used as a measure of the degree of instability of the system.
in Fig. 3 B), but the magnitude of alternans is always smaller for β = 1.5 than for β = 2.0. Therefore, alternans magnitude cannot be used as a measure of the degree of instability of the system.
Transition to Ca2+ alternans
As Fig. 3 B indicates, the nonalternating period-1 behavior becomes very stable (i.e.,  ) for action potential waveforms corresponding to β = 0.9 at pacing periods shorter than T = 300 ms. This is in stark contrast to the situation for β = 1.5, where the period-1 solution is unstable between T = 340 ms and T = 180 ms. At each pacing period over this range, the nonalternating period-1 solution becomes unstable at some value of β between 0.9 and 1.5. To explore the transition to instability, the value of β was varied at three different values of T, and
) for action potential waveforms corresponding to β = 0.9 at pacing periods shorter than T = 300 ms. This is in stark contrast to the situation for β = 1.5, where the period-1 solution is unstable between T = 340 ms and T = 180 ms. At each pacing period over this range, the nonalternating period-1 solution becomes unstable at some value of β between 0.9 and 1.5. To explore the transition to instability, the value of β was varied at three different values of T, and 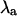 for each value of β and T was calculated. The resulting values of
for each value of β and T was calculated. The resulting values of  shown in Fig. 4 A, are plotted as a function of the action potential duration at 50% repolarization (APD50). For reference, the relationships between APD50 and β for each value of T are illustrated in Fig. 4 B.
shown in Fig. 4 A, are plotted as a function of the action potential duration at 50% repolarization (APD50). For reference, the relationships between APD50 and β for each value of T are illustrated in Fig. 4 B.
As Fig. 4 A illustrates, the stability of the nonalternating period-1 solution is highly sensitive to the morphology of the action potential (as measured by APD50) at each pacing period, with the transition to instability resulting from small changes in APD50 when T = 250 ms and T = 320 ms. For both of these values of T, the nonalternating period-1 rhythm reaches a maximal level of instability as APD50 is lengthened, and in both cases the period-1 rhythm remains unstable until the last data point shown. At T = 400 ms, changing the morphology of the action potential is not sufficient to force the system to cross the stability boundary ( ). However, the system does become markedly less stable as the action potential morphology changes, with this reduction in stability similarly occurring in response to a very small change in morphology.
). However, the system does become markedly less stable as the action potential morphology changes, with this reduction in stability similarly occurring in response to a very small change in morphology.
Stability sensitivity
Fig. 5 illustrates, for two action potential morphologies (corresponding to β = 1.0 and β = 2.0) and pacing periods (T = 250 ms and T = 333 ms, respectively), the sensitivity of  to changes in the contribution of particular components of the Ca2+ handling system (changes that mimic, for example, the effects of pharmacological agents). Two of the components (u, vup) are related to the sarcoplasmic reticulum, and two (gCa, gNaCa) are related to the sarcolemmal Ca2+ currents. At baseline (corresponding to the 0% line) in both cases,
to changes in the contribution of particular components of the Ca2+ handling system (changes that mimic, for example, the effects of pharmacological agents). Two of the components (u, vup) are related to the sarcoplasmic reticulum, and two (gCa, gNaCa) are related to the sarcolemmal Ca2+ currents. At baseline (corresponding to the 0% line) in both cases,  indicating that the system is sitting on the stability boundary.
indicating that the system is sitting on the stability boundary.
FIGURE 5.
Sensitivity of the transition to Ca2+ alternans to changes in the contribution of various components of the Ca2+ handling system. (A) β = 1.0, T = 250 ms; (B) β = 2.0, T = 333 ms. At baseline (i.e., 0% change) in both cases, the system is sitting on the stability boundary (i.e., 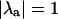 ). Increasing or decreasing the contribution of any part of the Ca2+ handling system (i.e., moving to the left or the right along the axis) changes the stability of the entire system. u = slope of the sarcoplasmic reticulum (SR) Ca2+ release function; vup = SR uptake pump strength; gCa = maximum conductance of the L-type Ca2+ channels; gNaCa = Na+-Ca2+ exchanger strength.
). Increasing or decreasing the contribution of any part of the Ca2+ handling system (i.e., moving to the left or the right along the axis) changes the stability of the entire system. u = slope of the sarcoplasmic reticulum (SR) Ca2+ release function; vup = SR uptake pump strength; gCa = maximum conductance of the L-type Ca2+ channels; gNaCa = Na+-Ca2+ exchanger strength.
Fig. 5 suggests that: i), changing any parameter in the model affects the stability of the Ca2+ handling system (i.e., changes the value of  ) in the neighborhood of the transition from no alternans to alternans (i.e., in the neighborhood of the transition to the unstable period-1 solution); ii), the degree of sensitivity of the stability of the Ca2+ handling system is parameter dependent; and iii), particular parameter changes can have qualitatively different stability effects for different baseline states (e.g., increasing gCa or gNaCa from baseline causes an increase in stability when β = 1.0, but a decrease in stability when β = 2.0).
) in the neighborhood of the transition from no alternans to alternans (i.e., in the neighborhood of the transition to the unstable period-1 solution); ii), the degree of sensitivity of the stability of the Ca2+ handling system is parameter dependent; and iii), particular parameter changes can have qualitatively different stability effects for different baseline states (e.g., increasing gCa or gNaCa from baseline causes an increase in stability when β = 1.0, but a decrease in stability when β = 2.0).
Fig. 6 further explores the sensitivity of the system by systematically changing the four components studied in Fig. 5 while also varying the pacing period and action potential morphology. Fig. 6 illustrates the influence of increasing and decreasing by 20% the maximum conductance of the L-type Ca2+ channels (Fig. 6 A), the Na+-Ca2+ exchanger strength (Fig. 6 B), the SR uptake pump strength (Fig. 6 C), and the slope of the SR Ca2+ release function (Fig. 6 D) on the alternans eigenvalue over a range of pacing periods when β = 0.9 and β = 2.0. In both cases, the eigenvalues at baseline (i.e., 0% change) are identical to those shown in Fig. 3 B.
FIGURE 6.
Sensitivity of 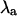 to +20% (▵) and −20% (∇) changes in the contribution of (A) maximum conductance of the L-type Ca2+ channels (gCa), (B) the Na+-Ca2+ exchanger strength (gNaCa), (C) the SR uptake pump strength (vup), and (D) the slope of the sarcoplasmic reticulum Ca2+ release function (u) for β = 0.9 (top panels) and β = 2.0 (bottom panels). Crosses indicate
to +20% (▵) and −20% (∇) changes in the contribution of (A) maximum conductance of the L-type Ca2+ channels (gCa), (B) the Na+-Ca2+ exchanger strength (gNaCa), (C) the SR uptake pump strength (vup), and (D) the slope of the sarcoplasmic reticulum Ca2+ release function (u) for β = 0.9 (top panels) and β = 2.0 (bottom panels). Crosses indicate 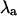 at baseline (identical to Fig. 3 B).
at baseline (identical to Fig. 3 B).
Fig. 6 reveals that when the action potential morphology is changed, the same percentage change in the contribution of a component of the Ca2+ handling system can have markedly different effects on the stability of the system as a function of pacing period. When β = 2.0, 20% changes in each of the four parameters in either direction result in fairly small changes to the stability of the system. However, when β = 0.9, the same Ca2+ handling system subjected to the same 20% changes but a different action potential morphology results in markedly different stability properties. For example, a reduction of the Na+-Ca2+ exchanger strength (Fig. 6 B) by 20% causes a very slight stabilization of the Ca2+ handling system compared to baseline for all pacing periods when β = 2.0. However, the same 20% reduction in INaCa for action potentials whose morphology is characterized by β = 0.9 destabilizes the system compared to baseline, with the amount of destabilization (as measured by the distance between the 0 and −20% alternans eigenvalues) varying widely as a function of pacing period.
Restitution curve slope
Fig. 3 indicates that how action potential morphology changes as a function of the pacing period is important in determining whether alternans will occur at a given value of T. To determine whether a relationship exists between the occurrence of Ca2+ alternans and the slope of the APD restitution curve, the slopes of the APD restitution curves characterizing the action potentials imposed during voltage clamping were calculated, and these slopes were plotted as a function of DI as shown in Fig. 7 A. The slopes of the restitution curves at 50% repolarization were used, because (as Fig. 1 B shows) the restitution curves at 90% repolarization for the three values of β used in Fig. 1 are identical. Fig. 7 B illustrates the alternans eigenvalues from Fig. 3 B at the same values of β as in Fig. 7 A, also plotted as a function of DI.
FIGURE 7.
(A) Slopes of the restitution curves from Fig. 1 B (50% repolarization). (B)  for the same values of β as shown in panel A, plotted as a function of diastolic interval.
for the same values of β as shown in panel A, plotted as a function of diastolic interval.
Fig. 7 shows that, for β = 2.0 and β = 1.5, Ca2+ alternans occurs under conditions corresponding to the APD restitution curve slope being at times <1 and at times >1. For β = 0.9, however, Ca2+ alternans is absent for all values of DI (as Fig. 7 B indicates), even for values of DI corresponding to a slope of the APD restitution curve >1. These results indicate that there is no correlation between the slope of the APD50 restitution curve and the occurrence of Ca2+ alternans. Although not shown, no correlation was found between the slopes of the APD90 restitution curves and the presence of Ca2+ alternans.
DISCUSSION
The results of this study indicate that, at any given excitation rate, the stability of the intracellular Ca2+ handling system is intimately linked to the temporal dynamics of membrane voltage. By changing the morphology of the action potential at a given excitation rate, qualitatively different behaviors of the Ca2+ handling system (either nonalternating or alternating Ca2+ transients) can be induced.
Whole-cell dependence of Ca2+ handling
The Ca2+ handling system in healthy cardiac myocytes possesses the ability to exquisitely control [Ca2+]i (28). Normally, the amount of Ca2+ that enters a myocyte during each beat is exactly equal to the amount of Ca2+ that is pumped out, and the amount of Ca2+ that is released by the SR during each beat is equal to the amount that is returned to the SR (28). If a single beat provides insufficient time to allow this Ca2+ cycling process to reach steady state (as may occur at rapid excitation rates), the Ca2+ cycling process may instead reach steady state after every second beat. This will be manifested as an alternation in the shape of the Ca2+ transient on a beat-to-beat basis.
Certain elements of the Ca2+ handling system appear to be important in determining the conditions under which Ca2+ alternans occurs. Particular emphasis has recently been placed on the characteristics of the SR (32), with this emphasis stemming from insights both from experimental work (13) and from mathematical modeling (19,28,29). In this study we have instead focused on the influence of membrane voltage on the behavior of the Ca2+ handling system. We found that membrane voltage, which affects the system through the sarcolemmal currents ICa and INaCa, plays an important role in determining when Ca2+ alternans occurs.
Our results suggest that the conditions under which Ca2+ alternans occurs are governed not by any one part of the Ca2+ handling system, but by the entire interconnected system. From a dynamical systems perspective, this result is not surprising. The highly interconnected nature of the Ca2+ handling system means that every component must be considered when attempting to understand the stability properties of the system in the neighborhood of the transition from a stable to unstable period-1 solution. It is not sufficient to draw conclusions about the stability properties of the entire system by studying each component of the system in isolation, as this does not allow for the compensatory effects of other components. For this reason, techniques such as the “eigenmode” method are crucial for understanding complex physiological processes in their entirety (10). By taking into account every component of the model Ca2+ handling system with this method, we are able to analyze the stability properties of the system in the neighborhood of the transition to alternans, and thereby predict the occurrence of Ca2+ alternans.
Slope of the restitution curve
Several studies have suggested a relationship between the slope of the APD restitution curve and the occurrence of electrical alternans (33–39). These studies are based on the restitution hypothesis, which states that electrical alternans will occur when the slope of the APD restitution curve is >1. We sought to determine whether a relationship exists between the slope of the APD restitution curve during action potential voltage clamping and the occurrence of Ca2+ alternans.
As Fig. 7 indicates, there is no simple relationship between the slope of the APD restitution curve and the occurrence of Ca2+ alternans in this model. Even though the slopes of the three restitution curves shown in Fig. 1 all became >1 at short DIs, Ca2+ alternans did not uniformly occur for all action potential morphologies when the slopes of the respective APD restitution curves were >1. This finding is consistent with a number of recent studies that have found that the slope of the APD restitution curve is not an accurate predictor of the conditions under which alternans occurs (10,12,16,40,41). As our results indicate, the only accurate predictor of whether Ca2+ alternans will occur is the alternans eigenvalue.
Membrane currents and Ca2+ handling
During action potential voltage clamping, the predominant membrane currents influencing the dynamics of the Ca2+ handling system are Ca2+ currents (such as ICa and INaCa in the model used here). During unclamped action potentials, however, every membrane current in the myocyte contributes to shaping the membrane voltage as a function of time, and thus every membrane current contributes to the voltage that is sensed by the Ca2+-selective currents. Given that our results suggest that the morphology of the action potential plays a key role in determining the stability of the Ca2+ handling system, it follows that every membrane current in the myocyte (through its influence on membrane voltage) plays a role in modulating the stability of the Ca2+ handling system.
This has important implications for electrical alternans. If the hypothesis that intracellular Ca2+ alternans leads to electrical alternans is correct (26), the unclamped action potential would be expected to alternate when the Ca2+-selective currents such as ICa and INaCa begin to alternate. Therefore, a change in the contribution of any membrane current when the action potential is not clamped should alter the stability of the Ca2+ handling system and, in turn, should change the conditions under which Ca2+ alternans and, therefore, electrical alternans, occurs. This prediction is borne out in Fig. 8.
FIGURE 8.
Stability of the complete ionic model of the ventricular action potential, incorporating the Ca2+ handling system used in Figs. 1–7. Action potentials in the model were elicited by brief suprathreshold current injections at a pacing period of 365 ms. A change in the contribution of any of the membrane currents shown causes the morphology of the action potential to change (see insets a–d; APD50 for a = 194 ms, b = 185 ms, c = 172 ms, d = 151 ms), which causes the stability of the system to change as well (as reflected in  ). In some cases, the system becomes more stable, and in other cases the system moves into the unstable regime. It should be noted that action potentials a–d demonstrate that stability is not simply a function of APD, as the longer (a) and shorter (d) action potentials are more stable than the intermediate (b and c) action potentials. It is also worth noting that the stabilization seen as gKr increases is consistent with previous experimental findings demonstrating that increased IKr expression can suppress alternans (15). In the complete ionic model, u = 11.3 (same as for the voltage clamp model), γ = 0.7, and τf = 30 ms. gKr = maximum conductance of the fast component of the delayed rectifier potassium current; gKs = maximum conductance of the slow component of the delayed rectifier potassium current; gto = maximum conductance of the transient outward potassium current; gNa = maximum conductance of the fast sodium current; gK1 = maximum conductance of the inward rectifier potassium current.
). In some cases, the system becomes more stable, and in other cases the system moves into the unstable regime. It should be noted that action potentials a–d demonstrate that stability is not simply a function of APD, as the longer (a) and shorter (d) action potentials are more stable than the intermediate (b and c) action potentials. It is also worth noting that the stabilization seen as gKr increases is consistent with previous experimental findings demonstrating that increased IKr expression can suppress alternans (15). In the complete ionic model, u = 11.3 (same as for the voltage clamp model), γ = 0.7, and τf = 30 ms. gKr = maximum conductance of the fast component of the delayed rectifier potassium current; gKs = maximum conductance of the slow component of the delayed rectifier potassium current; gto = maximum conductance of the transient outward potassium current; gNa = maximum conductance of the fast sodium current; gK1 = maximum conductance of the inward rectifier potassium current.
Fig. 8 was generated using a complete ionic model of the myocyte that incorporates the Ca2+ handling system used in this study (19). Instead of being clamped by a predetermined waveform, the membrane voltage in the complete ionic model varies dynamically in response to the contribution of a number of membrane currents including INa, IKr, IKs, Ito, and IK1. During action potential voltage clamping, the Ca2+ handling system of the complete ionic model exhibits alternans (just as the action potential clamp model used elsewhere in this study does). When membrane voltage is not clamped, however, all components of the model alternate simultaneously, and 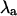 becomes a measure of the stability of the complete interconnected system. In Fig. 8, the stability of the entire system, including the contribution of membrane currents, is shown.
becomes a measure of the stability of the complete interconnected system. In Fig. 8, the stability of the entire system, including the contribution of membrane currents, is shown.
The simulations in Fig. 8 are very similar to those in Fig. 3, where the morphology of the action potential during voltage clamping was changed directly and the effect on the stability of the system was determined. In Fig. 8, however, the morphology of the action potential is changed indirectly by varying the conductances of five sodium and potassium currents (to mimic, for example, the effects of pharmacological agents, or the effects of altered channel protein expression (15)). At baseline (i.e., at 0%),  indicating that the system is sitting on the stability boundary. As predicted, changing the contribution of any membrane current at a given pacing period changes the stability characteristics of the system, and thereby changes the conditions under which Ca2+ alternans (and, therefore, electrical alternans) occurs. This suggests that every membrane current contributes to the stability of the Ca2+ handling system in the neighborhood of the transition from a stable to unstable period-1 solution and, hence, every membrane current contributes to establishing the conditions under which electrical alternans occurs.
indicating that the system is sitting on the stability boundary. As predicted, changing the contribution of any membrane current at a given pacing period changes the stability characteristics of the system, and thereby changes the conditions under which Ca2+ alternans (and, therefore, electrical alternans) occurs. This suggests that every membrane current contributes to the stability of the Ca2+ handling system in the neighborhood of the transition from a stable to unstable period-1 solution and, hence, every membrane current contributes to establishing the conditions under which electrical alternans occurs.
Limitations
The results in this study were obtained using a mathematical model of the rabbit ventricular myocyte intracellular Ca2+ handling system. Although it is likely that there are quantitative differences between the model system and the human ventricular myocyte Ca2+ handling system, we expect the basic finding of this work—that the stability of the Ca2+ handling system is modulated by the morphology of the action potential and, therefore, by all membrane currents—to be valid. Although our results were obtained from simulated cardiac myocytes, this finding can, in principle, be tested experimentally.
Acknowledgments
This research was supported by a Whitaker Foundation Biomedical Engineering Research Grant (No. RG-02-0369). Peter Jordan was also supported by a Predoctoral Fellowship from the Howard Hughes Medical Institute.
References
- 1.Adam, D. R., J. M. Smith, S. Akselrod, S. Nyberg, A. O. Powell, and R. J. Cohen. 1984. Fluctuations in T-wave morphology and susceptibility to ventricular fibrillation. J. Electrocardiol. 17:209–218. [DOI] [PubMed] [Google Scholar]
- 2.Smith, J. M., E. A. Clancy, C. R. Valeri, J. N. Ruskin, and R. J. Cohen. 1988. Electrical alternans and cardiac electrical instability. Circulation. 77:110–121. [DOI] [PubMed] [Google Scholar]
- 3.Nearing, B. D., A. H. Huang, and R. L. Verrier. 1991. Dynamic tracking of cardiac vulnerability by complex demodulation of the T wave. Science. 252:437–440. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum, D. S., L. E. Jackson, J. M. Smith, H. Garan, J. N. Ruskin, and R. J. Cohen. 1994. Electrical alternans and vulnerability to ventricular arrhythmias. N. Engl. J. Med. 330:235–241. [DOI] [PubMed] [Google Scholar]
- 5.Tachibana, H., I. Kubota, M. Yamaki, T. Watanabe, and H. Tomoike. 1998. Discordant S-T alternans contributes to formation of reentry: a possible mechanism of reperfusion arrhythmia. Am. J. Physiol. 275:H116–H121. [DOI] [PubMed] [Google Scholar]
- 6.Pastore, J. M., S. D. Girouard, K. R. Laurita, F. G. Akar, and D. S. Rosenbaum. 1999. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 99:1385–1394. [DOI] [PubMed] [Google Scholar]
- 7.Qu, Z., A. Garfinkel, P. S. Chen, and J. N. Weiss. 2000. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 102:1664–1670. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe, M. A., F. H. Fenton, S. J. Evans, H. M. Hastings, and A. Karma. 2001. Mechanisms for discordant alternans. J. Cardiovasc. Electrophysiol. 12:196–206. [DOI] [PubMed] [Google Scholar]
- 9.Walker, M. L., X. Wan, G. E. Kirsch, and D. S. Rosenbaum. 2003. Hysteresis effect implicates calcium cycling as a mechanism of repolarization alternans. Circulation. 108:2704–2709. [DOI] [PubMed] [Google Scholar]
- 10.Li, M., and N. F. Otani. 2003. Ion channel basis for alternans and memory in cardiac myocytes. Ann. Biomed. Eng. 31:1213–1230. [DOI] [PubMed] [Google Scholar]
- 11.Laurita, K. R., R. Katra, B. Wible, X. Wan, and M. H. Koo. 2003. Transmural heterogeneity of calcium handling in canine. Circ. Res. 92:668–675. [DOI] [PubMed] [Google Scholar]
- 12.Cherry, E. M., and F. H. Fenton. 2004. Suppression of alternans and conduction blocks despite steep APD restitution: electrotonic, memory, and conduction velocity restitution effects. Am. J. Physiol. 286:H2332–H2341. [DOI] [PubMed] [Google Scholar]
- 13.Diaz, M. E., S. C. O'Neill, and D. A. Eisner. 2004. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ. Res. 94:650–656. [DOI] [PubMed] [Google Scholar]
- 14.Sipido, K. R. 2004. Understanding cardiac alternans: the answer lies in the Ca2+ store. Circ. Res. 94:570–572. [DOI] [PubMed] [Google Scholar]
- 15.Hua, F., D. C. Johns, and R. F. Gilmour, Jr. 2004. Suppression of electrical alternans by overexpression of HERG in canine ventricular myocytes. Am. J. Physiol. 286:H2342–H2351. [DOI] [PubMed] [Google Scholar]
- 16.Pruvot, E. J., R. P. Katra, D. S. Rosenbaum, and K. R. Laurita. 2004. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ. Res. 94:1083–1090. [DOI] [PubMed] [Google Scholar]
- 17.Goldhaber, J. I., L. H. Xie, T. Duong, C. Motter, K. Khuu, and J. N. Weiss. 2005. Action potential duration restitution and alternans in rabbit ventricular myocytes: the key role of intracellular calcium cycling. Circ. Res. 96:459–466. [DOI] [PubMed] [Google Scholar]
- 18.Lakireddy, V., P. Baweja, A. Syed, G. Bub, M. Boutjdir, and N. El-Sherif. 2005. Contrasting effects of ischemia on the kinetics of membrane voltage and intracellular calcium transient underlie electrical alternans. Am. J. Physiol. 288:H400–H407. [DOI] [PubMed] [Google Scholar]
- 19.Shiferaw, Y., D. Sato, and A. Karma. 2005. Coupled dynamics of voltage and calcium in paced cardiac cells. Phys Rev E. 71:021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rappel, W. J., F. Fenton, and A. Karma. 1999. Spatiotemporal control of wave instabilities in cardiac tissue. Phys. Rev. Lett. 83:456–459. [Google Scholar]
- 21.Hall, G. M., and D. J. Gauthier. 2002. Experimental control of cardiac muscle alternans. Phys. Rev. Lett. 88:198102. [DOI] [PubMed] [Google Scholar]
- 22.Echebarria, B., and A. Karma. 2002. Spatiotemporal control of cardiac alternans. Chaos. 12:923–930. [DOI] [PubMed] [Google Scholar]
- 23.Allexandre, D., and N. F. Otani. 2004. Preventing alternans-induced spiral wave breakup in cardiac tissue: an ion-channel-based approach. Phys Rev E. 70:061903. [DOI] [PubMed] [Google Scholar]
- 24.Li, M., and N. F. Otani. 2004. Controlling alternans in cardiac cells. Ann. Biomed. Eng. 32:784–792. [DOI] [PubMed] [Google Scholar]
- 25.Jordan, P. N., and D. J. Christini. 2004. Adaptive diastolic interval control of cardiac action potential duration alternans. J. Cardiovasc. Electrophysiol. 15:1177–1185. [DOI] [PubMed] [Google Scholar]
- 26.Walker, M. L., and D. S. Rosenbaum. 2003. Repolarization alternans: implications for the mechanism and prevention of sudden cardiac death. Cardiovasc. Res. 57:599–614. [DOI] [PubMed] [Google Scholar]
- 27.Chudin, E., J. Goldhaber, A. Garfinkel, J. Weiss, and B. Kogan. 1999. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys. J. 77:2930–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisner, D. A., H. S. Choi, M. E. Diaz, S. C. O'Neill, and A. W. Trafford. 2000. Integrative analysis of calcium cycling in cardiac muscle. Circ. Res. 87:1087–1094. [DOI] [PubMed] [Google Scholar]
- 29.Shiferaw, Y., M. A. Watanabe, A. Garfinkel, J. N. Weiss, and A. Karma. 2003. Model of intracellular calcium cycling in ventricular myocytes. Biophys. J. 85:3666–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan, X., K. R. Laurita, E. J. Pruvot, and D. S. Rosenbaum. 2005. Molecular correlates of repolarization alternans in cardiac myocytes. J. Mol. Cell. Cardiol. 39:419–428. [DOI] [PubMed] [Google Scholar]
- 31.Sah, R., R. J. Ramirez, and P. H. Backx. 2002. Modulation of Ca2+ release in cardiac myocytes by changes in repolarization rate: role of phase-1 action potential repolarization in excitation-contraction coupling. Circ. Res. 90:165–173. [DOI] [PubMed] [Google Scholar]
- 32.Eisner, D. A., M. E. Diaz, Y. Li, C. O'Neill, and A. W. Trafford. 2005. Stability and instability of regulation of intracellular calcium. Exp. Physiol. 90:3–12. [DOI] [PubMed] [Google Scholar]
- 33.Nolasco, J. B., and R. W. Dahlen. 1968. A graphic method for the study of alternation in cardiac action potentials. J. Appl. Physiol. 25:191–196. [DOI] [PubMed] [Google Scholar]
- 34.Guevara, M. R., G. Ward, A. Shrier, and L. Glass. 1984. Electrical alternans and period-doubling bifurcations. Comput. Cardiol. 11:167–170. [Google Scholar]
- 35.Karma, A. 1994. Electrical alternans and spiral wave breakup in cardiac tissue. Chaos. 4:461–472. [DOI] [PubMed] [Google Scholar]
- 36.Koller, M. L., M. L. Riccio, and R. F. Gilmour, Jr. 1998. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am. J. Physiol. 275:H1635–H1642. [DOI] [PubMed] [Google Scholar]
- 37.Tolkacheva, E. G., D. G. Schaeffer, D. J. Gauthier, and W. Krassowska. 2003. Condition for alternans and stability of the 1:1 response pattern in a “memory” model of paced cardiac dynamics. Phys Rev E. 67:031904. [DOI] [PubMed] [Google Scholar]
- 38.Tolkacheva, E. G., M. M. Romeo, M. Guerraty, and D. J. Gauthier. 2004. Condition for alternans and its control in a two-dimensional mapping model of paced cardiac dynamics. Phys Rev E. 69:031904. [DOI] [PubMed] [Google Scholar]
- 39.Kalb, S. S., H. M. Dobrovolny, E. G. Tolkacheva, S. F. Idriss, W. Krassowska, and D. J. Gauthier. 2004. The restitution portrait: a new method for investigating rate-dependent restitution. J. Cardiovasc. Electrophysiol. 15:698–709. [DOI] [PubMed] [Google Scholar]
- 40.Banville, I., and R. A. Gray. 2002. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. J. Cardiovasc. Electrophysiol. 13:1141–1149. [DOI] [PubMed] [Google Scholar]
- 41.Fox, J. J., E. Bodenschatz, and R. F. Gilmour, Jr. 2002. Period-doubling instability and memory in cardiac tissue. Phys. Rev. Lett. 89:138101. [DOI] [PubMed] [Google Scholar]