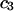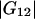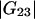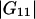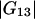In a recent article (Schurr, J. M., D. P. Rangel, and S. R. Aragon. 2005. A contribution to the theory of preferential interaction coefficients. Biophys. J. 89:2258–2276), a detailed derivation of an expression for the preferential binding coefficient via the Kirkwood-Buff theory of solutions was presented. The authors of this Comment (Shulgin, I. L., and E. Ruckenstein. 2005. A protein molecule in an aqueous mixed solvent: fluctuation theory outlook. J. Chem. Phys. 123:054909) also recently established on the basis of the Kirkwood-Buff theory of solutions an equation for the preferential binding of a cosolvent to a protein. There are other publications that relate the preferential binding parameter to the Kirkwood-Buff theory of solutions for protein + binary mixed solvents. The expressions derived in the two articles mentioned above are different because the definitions of the preferential binding parameter are different. However, there are articles in which the definitions of the preferential binding parameter are the same, but the derived equations that relate the preferential binding parameter to the Kirkwood-Buff integrals are different. The goal of this Comment is to examine the various expressions that relate the preferential binding parameter to the Kirkwood-Buff theory.
INTRODUCTION
An important characteristic of a solution of a protein (component 2) in a mixture water (1) + cosolvent (3) is the preferential binding parameter 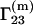 (1–6)
(1–6)
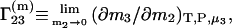 |
(1) |
where 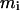 is the molality of component i, P is the pressure, T is the absolute temperature, and
is the molality of component i, P is the pressure, T is the absolute temperature, and 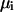 is the chemical potential of component i. The preferential binding parameter can be also defined at a molarity scale by
is the chemical potential of component i. The preferential binding parameter can be also defined at a molarity scale by
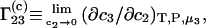 |
(2) |
where 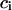 is the molar concentration of component i. It should be emphasized that
is the molar concentration of component i. It should be emphasized that 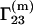 and
and 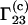 are defined at infinite protein dilution.
are defined at infinite protein dilution.
The preferential binding parameter 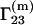 was determined experimentally (5–7) and provides information regarding the interactions between a protein and the components of the mixed solvent. As a rule (1–5),
was determined experimentally (5–7) and provides information regarding the interactions between a protein and the components of the mixed solvent. As a rule (1–5), 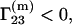 the protein is preferentially hydrated, for cosolvents such as glycerol, sucrose, etc., which can stabilize at high concentrations the protein structure and preserve its enzymatic activity (3–5), and
the protein is preferentially hydrated, for cosolvents such as glycerol, sucrose, etc., which can stabilize at high concentrations the protein structure and preserve its enzymatic activity (3–5), and 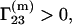 the protein is preferentially solvated by cosolvents (such as urea), which can cause protein denaturation.
the protein is preferentially solvated by cosolvents (such as urea), which can cause protein denaturation.
In literature (8) a number of different definitions of the preferential binding parameter (coefficient) have been employed. They can be connected by thermodynamic relations for ternary mixtures (8). In this Comment the preferential binding parameter will be mostly defined by Eqs. 1 and 2.
Because the preferential binding parameter is a meaningful physical quantity, attempts have been made to relate it to a general theory of solutions, such as the Kirkwood-Buff theory of solutions (9). Several authors reported results in this direction (10–17). The authors of this Comment derived the following equation for 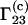 (16):
(16):
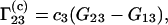 |
(3) |
where 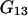 and
and 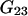 are the Kirkwood-Buff integrals defined as (9)
are the Kirkwood-Buff integrals defined as (9)
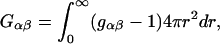 |
(4) |
where 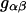 is the radial distribution function between species α and β, and r is the distance between the centers of molecules α and β.
is the radial distribution function between species α and β, and r is the distance between the centers of molecules α and β.
Equation 3 differs from the expression of 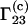 employed in Shimizu (10,11):
employed in Shimizu (10,11):
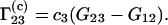 |
(5) |
In a recent article in this journal (17), the Kirkwood-Buff theory of solutions was used to express the preferential binding coefficient 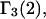 defined as
defined as
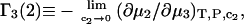 |
(6) |
in terms of the Kirkwood-Buff integrals. It was found (17) that
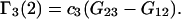 |
(7) |
As noted in Schurr et al. (17) the preferential binding coefficient 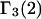 defined by Eq. 6 differs from the preferential binding parameter
defined by Eq. 6 differs from the preferential binding parameter 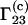 defined by Eq. 2.
defined by Eq. 2.
However, Eqs. 3 and 5 are different equations even though they are based on the same definition of the preferential binding parameter and have the same theoretical basis: the Kirkwood-Buff theory of solutions. To make a selection between Eqs. 3 and 5 a simple limiting case, the ideal ternary mixture, will be examined using the traditional thermodynamics, and the results will be compared to those provided by Eqs. 3 and 5.
IDEAL TERNARY MIXTURE
Let us consider an ideal ternary mixture. According to the definition of an ideal mixture (18), the activities of the components (ai) are equal to their mol fractions (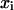 ) and their partial molar volumes are equal to those of the pure components (
) and their partial molar volumes are equal to those of the pure components ( ).
).
Because
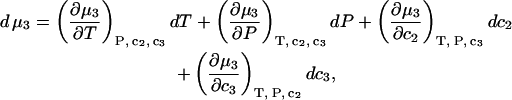 |
(8) |
one can write for an ideal mixture
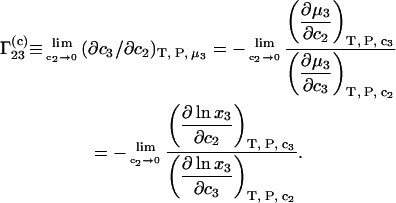 |
(9) |
For isothermal-isobaric conditions
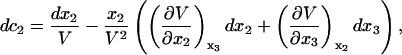 |
(10) |
and
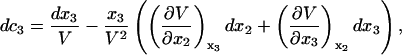 |
(11) |
where V is the molar volume of the ternary mixture.
When 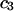 is a constant, Eqs. 10 and 11 lead to
is a constant, Eqs. 10 and 11 lead to
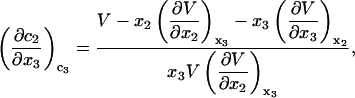 |
(12) |
and when 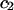 is a constant, Eqs. 10 and 11 lead to
is a constant, Eqs. 10 and 11 lead to
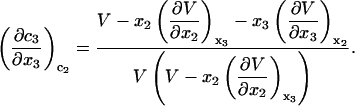 |
(13) |
By inserting Eqs. 12 and 13 into Eq. 9 at infinite dilution of component 2, one obtains the following expression for 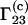 of an ideal ternary mixture:
of an ideal ternary mixture:
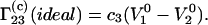 |
(14) |
On the other hand, expressions for 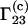 for an ideal ternary solution can be also derived by combining Eq. 3 or Eq. 5 with the following Kirkwood-Buff integrals for ideal ternary mixtures (16):
for an ideal ternary solution can be also derived by combining Eq. 3 or Eq. 5 with the following Kirkwood-Buff integrals for ideal ternary mixtures (16):
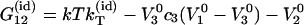 |
(15) |
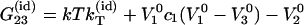 |
(16) |
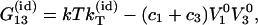 |
(17) |
where k is the Boltzmann constant and 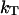 is the isothermal compressibility.
is the isothermal compressibility.
Equation 3 leads to
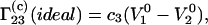 |
(18) |
whereas Eq. 5 to
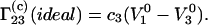 |
(19) |
DISCUSSION
One can see that the result obtained on the basis of Eq. 3 (Eq. 18) coincides with Eq. 14 derived from general thermodynamic considerations, whereas that based on Eq. 5 does not. The numerical difference between the two expressions is very large because the molar volume of a protein is, usually, much larger than the molar volume of the cosolvent.
Whereas the above discussion involves 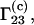 the quantity
the quantity 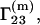 which is usually determined experimentally (2–7), is related to
which is usually determined experimentally (2–7), is related to 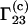 through the equation (1,16)
through the equation (1,16)
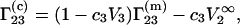 |
(20) |
where 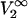 is the partial molar volume of the protein at infinite dilution.
is the partial molar volume of the protein at infinite dilution. 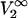 and
and 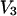 can be expressed at infinite dilution of component 2 in terms of the Kirkwood-Buff integrals as follows (19):
can be expressed at infinite dilution of component 2 in terms of the Kirkwood-Buff integrals as follows (19):
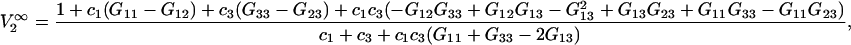 |
(21) |
and (9)
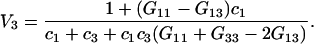 |
(22) |
By combining Eqs. 3, 20, 21, and 22, one obtains after some algebra the following simple expression:
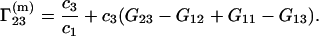 |
(23) |
Whereas 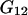 and
and 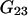 depend on the protein characteristics,
depend on the protein characteristics, 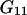 and
and 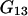 depend only on the characteristics of the protein-free mixed solvent.
depend only on the characteristics of the protein-free mixed solvent.
For usual cosolvents (organic solvents, salts, etc.), one can use the following approximation of Eq. 23 in the dilute cosolvent range:
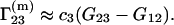 |
(24) |
Indeed, 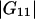 and
and 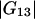 are much smaller than the Kirkwood-Buff integrals for the pairs involving the protein (
are much smaller than the Kirkwood-Buff integrals for the pairs involving the protein (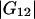 and
and 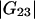 ). Table 1 provides their values for the system water (1) + lysozyme (2) + urea (3) (pH 7.0, 20°C).
). Table 1 provides their values for the system water (1) + lysozyme (2) + urea (3) (pH 7.0, 20°C).
TABLE 1.
Numerical values of the Kirkwood-Buff integrals for the water (1) + lysozyme (2) + urea (3) (pH 7.0, 20°C) system
However, when 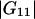 and
and 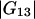 are large, and this occurs when the cosolvent is, for example, a polymer (
are large, and this occurs when the cosolvent is, for example, a polymer (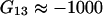 (cm3/mol) for the system water/polyethylene glycol 2000 at a weight fraction of polyethylene glycol of 0.02 (21)), the complete Eq. 23 should be used. This conclusion is valid for all large cosolvent molecules (polymers, biomolecules, etc.).
(cm3/mol) for the system water/polyethylene glycol 2000 at a weight fraction of polyethylene glycol of 0.02 (21)), the complete Eq. 23 should be used. This conclusion is valid for all large cosolvent molecules (polymers, biomolecules, etc.).
Let us consider the biochemical equilibrium between infinitely dilute native (N) and denaturated (D) states of a protein in a mixed solvent. The changes of the preferential binding parameters 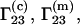 and
and 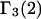 in this process are given by
in this process are given by
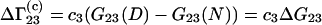 |
(25) |
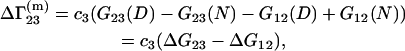 |
(26) |
and
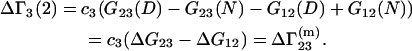 |
(27) |
Equations 25 and 26 follow from Eqs. 3 and 23 by taking into account that 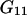 and
and 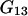 are characteristics of the protein-free mixed solvent at infinite protein dilution.
are characteristics of the protein-free mixed solvent at infinite protein dilution.
The equilibrium constant K of biochemical equilibrium between infinitely dilute native (N) and denaturated (D) states of a protein in a mixed solvent can be expressed in terms of 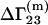 (22)
(22)
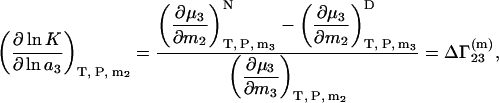 |
(28) |
where 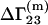 can be provided by experiment (23).
can be provided by experiment (23).
Using for 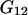 and
and 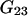 expressions from Shulgin and Ruckenstein (16), Eq. 28 can be also rewritten in the form
expressions from Shulgin and Ruckenstein (16), Eq. 28 can be also rewritten in the form
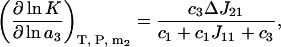 |
(29) |
where 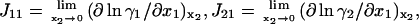 and
and 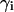 is the activity coefficient of component i at a mol fraction scale. Let us note that
is the activity coefficient of component i at a mol fraction scale. Let us note that 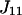 is characteristic of the protein-free mixed solvent at infinite protein dilution.
is characteristic of the protein-free mixed solvent at infinite protein dilution.
Acknowledgments
We are indebted to Prof. J. Michael Schurr (Dept. of Chemistry, University of Washington, Seattle, WA) for helpful comments regarding this manuscript and for drawing our attention to the fact that the coefficients 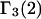 and
and 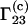 are different.
are different.
References
- 1.Casassa, E. F., and H. Eisenberg. 1964. Thermodynamic analysis of multi-component solutions. Adv. Protein Chem. 19:287–395. [DOI] [PubMed] [Google Scholar]
- 2.Noelken, M. E., and S. N. Timasheff. 1967. Preferential solvation of bovine serum albumin in aqueous guanidine hydrochloride. J. Biol. Chem. 1967:5080–5085. [PubMed] [Google Scholar]
- 3.Kuntz, I. D., and W. Kauzmann. 1974. Hydration of proteins and polypeptides. Adv. Protein Chem. 28:239–345. [DOI] [PubMed] [Google Scholar]
- 4.Timasheff, S. N. 1993. The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu. Rev. Biophys. Biomol. Struct. 22:67–97. [DOI] [PubMed] [Google Scholar]
- 5.Timasheff, S. N. 1998. Control of protein stability and reactions by weakly interacting cosolvents: the simplicity of the complicated. Adv. Protein Chem. 51:355–432. [DOI] [PubMed] [Google Scholar]
- 6.Record, M. T., W. T. Zhang, and C. F. Anderson. 1998. Analysis of effects of salts and uncharged solutes on protein and nucleic acid equilibria and processes: a practical guide to recognizing and interpreting polyelectrolyte effects, Hofmeister effects and osmotic effects of salts. Adv. Protein Chem. 51:281–353. [DOI] [PubMed] [Google Scholar]
- 7.Zhang, W. T., M. W. Capp, J. P. Bond, C. F. Anderson, and M. T. Record, Jr. 1996. Thermodynamic characterization of interactions of native bovine serum albumin with highly excluded (glycine betaine) and moderately accumulated (urea) solutes by a novel application of vapor pressure osmometry. Biochemistry. 35:10506–10516. [DOI] [PubMed] [Google Scholar]
- 8.Anderson, C. F., E. S. Courtenay, and M. T. Record, Jr. 2002. Thermodynamic expressions relating different types of preferential interaction coefficients in solutions containing two solute components. J. Phys. Chem. B. 106:418–433. [Google Scholar]
- 9.Kirkwood, J. G., and F. P. Buff. 1951. The statistical mechanical theory of solutions. J. Chem. Phys. 19:774–777. [Google Scholar]
- 10.Shimizu, S. 2004. Estimating hydration changes upon biomolecular reactions from osmotic stress, high pressure, and preferential interaction coefficients. Proc. Natl. Acad. Sci. USA. 101:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu, S. 2004. Estimation of excess solvation numbers of water and cosolvents from preferential interaction and volumetric experiments. J. Chem. Phys. 120:4989–4990. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu, S., and D. J. Smith. 2004. Preferential hydration and the exclusion of cosolvents from protein surfaces. J. Chem. Phys. 121:1148–1154. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu, S., and C. L. Boon. 2004. The Kirkwood-Buff theory and the effect of cosolvents on biochemical reactions. J. Chem. Phys. 121:9147–9155. [DOI] [PubMed] [Google Scholar]
- 14.Smith, P. E. 2004. Local chemical potential equalization model for cosolvent effects on biomolecular equilibria. J. Phys. Chem. B. 108:16271–16278. [Google Scholar]
- 15.Smith, P. E. 2004. Cosolvent interactions with biomolecules: relating computer simulation data to experimental thermodynamic data. J. Phys. Chem. B. 108:18716–18724. [Google Scholar]
- 16.Shulgin, I. L., and E. Ruckenstein. 2005. A protein molecule in an aqueous mixed solvent: fluctuation theory outlook. J. Chem. Phys. 123:054909. [DOI] [PubMed] [Google Scholar]
- 17.Schurr, J. M., D. P. Rangel, and S. R. Aragon. 2005. A contribution to the theory of preferential interaction coefficients. Biophys. J. 89:2258–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prausnitz, J. M., R. N. Lichtenthaler, and E. Gomes de Azevedo. 1986. Molecular Thermodynamics of Fluid-Phase Equilibria, 2nd Ed. Prentice-Hall, Englewood Cliffs, NJ.
- 19.Ruckenstein, E., and I. Shulgin. 2001. Entrainer effect in supercritical mixtures. Fluid Phase Equilib. 180:345–359. [Google Scholar]
- 20.Chitra, R., and P. E. Smith. 2002. Molecular association in solution: a Kirkwood-Buff analysis of sodium chloride, ammonium sulfate, guanidinium chloride, urea, and 2,2,2-trifluoroethanol in water. J. Phys. Chem. B. 106:1491–1500. [Google Scholar]
- 21.Vergara, A., L. Paduano, and R. Sartorio. 2002. Kirkwood-Buff integrals for polymer solvent mixtures. Preferential solvation and volumetric analysis in aqueous PEG solutions. Phys. Chem. Chem. Phys. 4:4716–4723. [Google Scholar]
- 22.Timasheff, S. N. 1992. Water as ligand: preferential binding and exclusion of denaturants in protein unfolding. Biochemistry. 31:9857–9864. [DOI] [PubMed] [Google Scholar]
- 23.Timasheff, S. N., and G. Xie. 2003. Preferential interactions of urea with lysozyme and their linkage to protein denaturation. Biophys. Chem. 105:421–448. [DOI] [PubMed] [Google Scholar]