Abstract
The hepatitis C virus (HCV) RNA-dependent RNA polymerase (RdRp) belongs to a class of membrane proteins termed tail-anchored proteins. Here, we show that the HCV RdRp C-terminal membrane insertion sequence traverses the phospholipid bilayer as a transmembrane segment. Moreover, the HCV RdRp was found to be retained in the endoplasmic reticulum (ER) or an ER-derived modified compartment both following transient transfection and in the context of a subgenomic replicon. An absolutely conserved GVG motif was not essential for membrane insertion but possibly provides a docking site for transmembrane protein-protein interactions. These findings have important implications for the functional architecture of the HCV replication complex.
The hepatitis C virus (HCV) RNA-dependent RNA polymerase (RdRp), represented by nonstructural protein 5B (NS5B), is believed to form a membrane-associated replication complex together with the other viral nonstructural proteins and as-yet-unidentified host cell components (8, 22). It was shown previously that the HCV RdRp belongs to a class of membrane proteins termed tail-anchored proteins (33). Characteristic features of these proteins include (i) posttranslational membrane targeting via a hydrophobic C-terminal insertion sequence (which in the case of NS5B was mapped to the C-terminal 21-amino-acid [aa] residues), (ii) integral membrane association, and (iii) cytosolic orientation of the functional protein domain (reviewed in references 21 and 36). The prototype of this class of proteins is cytochrome b5 (Cb5). Other examples include members of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins such as the vesicle-associated membrane proteins (15, 16, 20), microsomal aldehyde dehydrogenase (25), and Bcl-2 (14). The C-terminal location of insertion sequences implies that these proteins are targeted to membranes posttranslationally via a signal recognition particle-independent mechanism. However, the actual mechanism of membrane targeting and integration of tail-anchored proteins is unknown.
The topology of the membrane insertion sequence of tail-anchored proteins has been a matter of debate. Both transmembrane and hairpin loop conformations have been proposed in the case of Cb5, which was most intensely studied in this regard. In the transmembrane model, the insertion sequence spans the phospholipid bilayer with the C terminus in the lumen of the endoplasmic reticulum (ER) (13, 19, 35). In the hairpin loop model, the hydrophobic segment penetrates only the external leaflet of the ER membrane and the C terminus loops back to the cytosol (5, 30). In the case of the HCV RdRp, both topologies would be conceivable in view of an absolutely conserved GVG motif (aa 582 to 584) which could serve as a flexible hinge in the center of the insertion sequence (Fig. 1). However, structure predictions of an α-helix (33) and thermodynamic considerations strongly favor a transmembrane topology. Thus, the aim of this study was to define the membrane topology of the NS5B insertion sequence.
FIG. 1.
C-terminally tagged NS5B constructs. NS5B amino acid positions are indicated at the top (17) (GenBank accession number AF009606). Identical, strongly similar, and weakly similar residues found among 269 HCV isolates are symbolized by an asterisk, a colon, and a dot, respectively (reference 33 and references therein). #, minimal transmembrane segment as deduced from various prediction methods (33). Engineered tag sequences are shown by dotted underlining. MAb epitopes and potential glycosylation acceptor sites are in bold. Potentially glycosylated Asn residues are marked by a bold asterisk, and the Gly residues mutated to Leu at positions 582 and 584 of the NS5B-LVL-gt construct are underlined.
In a first set of experiments, epitope tags for well-defined monoclonal antibodies (MAbs) were fused to the C terminus of NS5B. In brief, NS5B fragments with engineered c-myc or hemagglutinin (HA) epitope tags were derived by PCR from pBRTM/HCV1-3011con (17) (kindly provided by Charles M. Rice, Rockefeller University, New York, N.Y.) by using forward primer NS5B139fwd (26) and reverse primer NS5Bmycrev or NS5BHArev, respectively (Table 1). Amplification products were digested with EcoRI (recognition site at nucleotide position 604 of NS5B) and XbaI and inserted together with the BamHI-EcoRI fragment of pCMVNS5Bcon (33) (representing the 5′ 604 nucleotides of NS5B) into the BamHI-XbaI sites of pcDNA3.1 (Invitrogen, San Diego, Calif.) to yield constructs pCMVNS5Bcon-myc and pCMVNS5Bcon-HA, respectively (Fig. 1). These constructs were transiently transfected into U-2 OS human osteosarcoma cells. The location of the engineered epitope tag was subsequently investigated by selective permeabilization techniques.
TABLE 1.
Primer sequences
| Primer | Restriction enzyme | Sequencea |
|---|---|---|
| NS5Bmycrev | Xbal | 5′ GCTCTAGACCAGATCTTCCTCCGAGATTAGCTTCTGTTCCGATCCTCGGTTGGGGAGGAGGTAGATGCCTACCCCTGCAGCGAG 3′ |
| NS5BHArev | Xbal | 5′ GCTCTAGACAGCATAGTCAGGTACGTCATATGGATACGATCCTCGGTTGGGGAGGAGGTAGATGCCTACCCCTGCAGCGAG 3′ |
| 5B-gt-1 | 5′ GTAGAAGTTTGGTCCCTCTGTTCCGTTCATTCGGTTGGGGAGGAGGTAGATG 3′ | |
| 5B-gt-2 | Xbal | 5′ GCTCTAGATTAGTCCACTGTCTTGTTTGAGAATGGTACGTAGAAGTTTGGTCCCTCTGTTCCGTTCATTCGGTTG 3′ |
| 5B-myc-gt | Xbal | 5′ GCTCTAGATTAGTCCACTGTCTTGTTTGAGAATGGTACGTAGAACAGATCTTCCTCCGAGATTAGCTTCTGTTCCGATCCTCG 3′ |
| 5B-myc-sw | Xbal | 5′ GCTCTAGATTACTGGAATGTCGAGTTGATCTGCGAGATCTGCGACAGATCTTCCTCCGAGATTAGCTTCTGTTCCGATCCTCG 3′ |
| S8467 | 5′ GTAATACCCTCACATGTTACTTGA 3′ | |
| A9413 | 5′ CAGGATGGCCTATTGGCCTGGAG 3′ |
Restriction enzyme recognition sites are underlined, and start codons are set in bold.
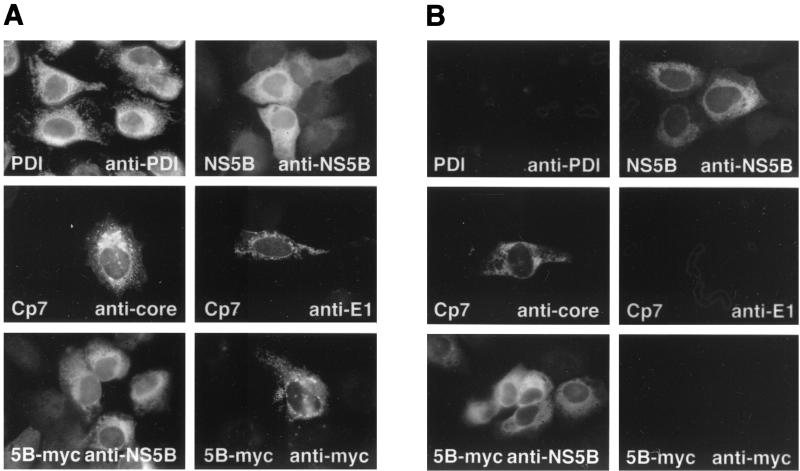
Cellular membranes have to be permeabilized to make antigenic sites accessible to the antibodies used for immunostaining. Detergents such as saponin or Triton X-100 are commonly used for this purpose at concentrations yielding total permeabilization of cells. By contrast, by using low concentrations of the mild nonionic detergent digitonin it is possible to selectively permeabilize the plasma membrane while leaving the ER membrane intact (4, 29). Thus, selective permeabilization can be used to distinguish cytosolic from ER luminal antigenic sites. This strategy was carefully validated by using proteins of known topology on the ER membrane, namely protein disulfide isomerase (PDI), a chaperone found in the ER lumen, the HCV core (cytosolic orientation), and E1 (ER luminal orientation) proteins, as well as NS5B (cytosolic orientation). Optimal discrimination of ER luminal and cytosolic epitopes was observed at a digitonin concentration of 0.000125% (1.25 μg per ml). Cells were fixed with 2% paraformaldehyde for 40 min at 20°C. Total permeabilization was achieved with 0.05% saponin for 10 min at 20°C as described previously (27). Selective permeabilization was performed for 15 min at 4°C with 0.000125% digitonin (Calbiochem, La Jolla, Calif.) in a buffer containing 10 mM HEPES (pH 6.8), 1 mM EDTA, 0.3 M sucrose, 0.1 M KCl, and 2.5 mM MgCl2. Indirect immunofluorescence microscopy was subsequently performed as described previously (27) by using phosphate-buffered saline containing 3% bovine serum albumin as a blocking buffer.
As shown in Fig. 2A, all antigens were stained following permeabilization with 0.05% saponin. By contrast, under selective permeabilization conditions with digitonin (Fig. 2B), only the cytosolically oriented HCV core and NS5B epitopes recognized by MAbs C7-50 (27) and 5B-12B7 (26), respectively, were accessible. A polyclonal antiserum directed against PDI (StressGen, Victoria, British Columbia, Canada) and the MAb A4 directed against an epitope located on the E1 ectodomain (6) (kindly provided by Jean Dubuisson, Institut Pasteur de Lille, Lille, France, and Harry Greenberg, Stanford University, Stanford, Calif.) did not react with their target antigens under these conditions. Importantly, the MAb 9E10 (9) against the c-myc epitope reacted with NS5B-myc only under total but not under selective permeabilization conditions. This suggests that the epitope tag fused to the C terminus of NS5B was translocated to the ER lumen. Identical results were obtained for construct NS5B-HA stained with a MAb specific for the HA tag (data not illustrated). Thus, these results indicate that the NS5B insertion sequence spans the membrane bilayer as a transmembrane segment.
FIG. 2.
Selective permeabilization experiments. U-2 OS cells were transiently transfected with pCMVNS5Bcon (NS5B) or pCMVNS5Bcon-myc (5B-myc). For expression of the HCV structural proteins, cells were cotransfected with pUHD15-1, which codes for a tetracycline-controlled transactivator (tTA), and pUHDCp7con (Cp7), which allows expression of the entire HCV structural region under the control of a tTA-dependent promoter (Moradpour et al., unpublished). Thirty-six hours posttransfection, cells were fixed and subjected to total permeabilization with 0.05% saponin (A) or selective permeabilization with 0.000125% digitonin (B), followed by indirect immunofluorescence microscopy with MAbs 5B-12B7 (anti-NS5B), C7-50 (anticore), A4 (anti-E1), or 9E10 (anti-myc), as indicated in the panels. In addition, nontransfected U-2 OS cells were stained with a polyclonal antiserum against PDI.
To confirm and further extend these findings, we investigated the membrane topology by a second experimental strategy. This approach is based on the fact that the oligosaccharyltransferase present in the ER lumen transfers carbohydrate moieties to Asn-X-Thr/Ser (NXT/S) consensus acceptor sites (12). The 14-saccharide glycan moiety transferred by N-linked glycosylation increases the mass of a protein by about 3 kDa. Acceptor sites for N-linked glycosylation should be located at a minimum distance of 12 to 14 aa residues from the transmembrane domain (28), as dictated by the location of the oligosaccharyltransferase active site relative to the luminal membrane surface. Thus, the spacer sequences and glycosylation acceptor sites illustrated in Fig. 1 were fused to the C terminus of NS5B. Constructs NS5B-gt and NS5B-LVL-gt contain a spacer sequence derived from the N-terminal domain of bovine opsin (25). These constructs possess two potential glycosylation sites (Fig. 1), but only the C-terminal site is expected to be glycosylated because of the required distance from the luminal membrane surface. The N-terminal 8 aa of the bovine opsin spacer sequence were replaced by the c-myc epitope in NS5B-myc-gt. NS5B-myc-sw contains a spacer sequence composed of the c-myc epitope and of 6 aa residues that have been shown previously to be inert in translocation assays (28).
In brief, a two-step PCR protocol using pCMVNS5Bcon (33) as a template was employed. The first portion of the glycosylation tag was added by using primer pair NS5B46fwd (26) and 5B-gt1 (Table 1). This was completed by a second PCR using forward primer NS5B139fwd and reverse primer 5B-gt2. The amplification product was digested with EcoRI and XbaI and inserted into the EcoRI-XbaI sites of pCMVNS5Bcon to yield plasmid pCMVNS5Bcon-gt. Plasmid pCMVNS5Bcon-LVL-gt was constructed similarly by using pCMVNS5Bcon-LVL as a PCR template. In this construct the GVG motif (aa 582 to 584) was mutated to LVL (D. Moradpour et al., unpublished data). Plasmids pCMVNS5Bcon-myc-gt and pCMVNS5Bcon-myc-sw were derived from pCMVNS5Bcon-myc by PCR with forward primer NS5B46fwd and reverse primers 5B-myc-gt or 5B-myc-sw, respectively, followed by digestion of the amplification products with EcoRI and XbaI and ligation into the EcoRI-XbaI sites of pCMVNS5Bcon-myc.
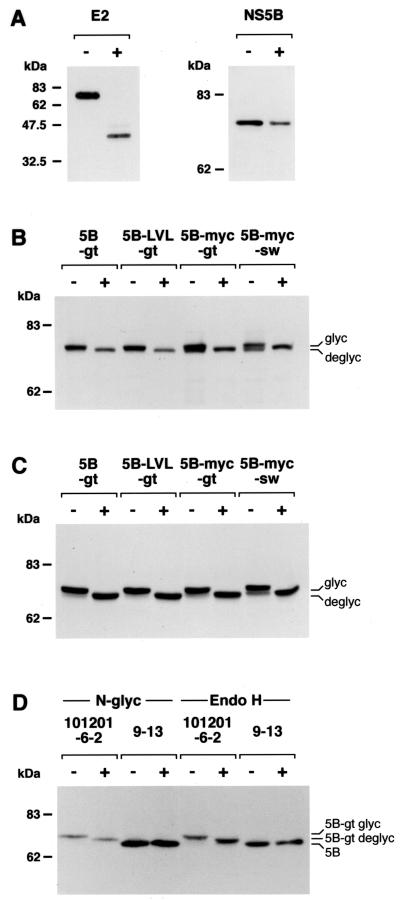
As glycoproteins pass through the Golgi apparatus, N-linked carbohydrate moieties are trimmed and modified to complex oligosaccharides. These can be distinguished by their different susceptibility to glycosidases, which allows to define the localization of a glycoprotein. N-Glycosidase F cleaves all types of N-linked glycans, whereas endoglycosidase H targets only unmodified high mannose oligosaccharides. Plasmids pCMVNS5Bcon, pCMVNS5Bcon-gt, pCMVNS5Bcon-LVL-gt, pCMVNS5Bcon-myc-gt, and pCMVNS5Bcon-myc-sw were transiently transfected into U-2 OS human osteosarcoma cells. Transfected cells were lysed in a buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, and protease inhibitors. Subsequently, cell lysates were treated with N-glycosidase F or endoglycosidase H, followed by immunoblotting using the NS5B-specific MAb 5B-3B1 (26). Digestions with N-glycosidase F (Roche Molecular Biochemicals, Mannheim, Germany) and endoglycosidase H (New England Biolabs, Beverly, Mass.) were performed for 18 or 3 h, respectively, at 37°C as recommended by the manufacturers. HCV E2, which carries 11 N-linked carbohydrate moieties and is retained in the ER (6, 7), served as a control for the deglycosylation procedure.
In line with previous reports, deglycosylation of E2 with N-glycosidase F (Fig. 3A) and endoglycosidase H (data not illustrated) led to a reduction of the apparent molecular mass from 70 to about 35 kDa. As expected, treatment of NS5B with N-glycosidase F did not alter its migration pattern (Fig. 3A). By contrast, a clear difference in apparent molecular mass was found in all NS5B constructs with engineered glycosylation acceptor sites, indicating that these were efficiently translocated and recognized by oligosaccharyltransferase present in the ER lumen. Indeed, both treatment with N-glycosidase F (Fig. 3B) and endoglycosidase H (Fig. 3C) reduced the mass of these proteins by about 3 kDa. The endoglycosidase H-sensitive nature of these carbohydrate moieties indicates that the constructs are retained in the ER. Interestingly, mutation of the GVG motif in the insertion sequence to the more rigid LVL sequence did not impair translocation, suggesting that the GVG motif is not essential for membrane integration. Of note, constructs NS5B-myc-gt and NS5B-myc-sw seemed to be translocated somewhat less efficiently because a second band, presumably corresponding to the unglycosylated construct, was observed by immunoblotting (Fig. 3B and C).
FIG. 3.
Glycosylation mapping. U-2 OS cells were transiently transfected with pCMVNS5Bcon (NS5B), pCMVNS5Bcon-gt (5B-gt), pCMVNS5B-LVL-gt (5B-LVL-gt), pCMVNS5Bcon-myc-gt (5B-myc-gt), pCMVNS5Bcon-myc-sw (5B-myc-sw), or with pUHD15-1 and pUHDCp7con (E2). Thirty-six hours posttransfection, cell lysates were digested with N-glycosidase F (A and B) or endoglycosidase H (C). In addition, lysates from cell clone 101201-6-2 harboring a replicon with NS5B-gt and 9-13 replicon cells expressing NS5B without an engineered glycosylation acceptor site were treated with N-glycosidase F (D, left panel) and endoglycosidase H (D, right panel). Undigested (−) and glycosidase-digested (+) samples were separated by 12% (panel A, E2), 8% (panel A [NS5B] and panel B), or 6% (panels C and D) sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblotting with MAbs A11 against E2 or 5B-3B1 against NS5B. Positions of glycosylated (glyc) and deglycosylated (deglyc) proteins are shown on the right. Molecular mass standards in kilodaltons are shown on the left.
To investigate whether the C terminus of NS5B is translocated to the ER lumen also in the context of a functional HCV replication complex, the NS5B-gt construct was introduced into a subgenomic replicon. In brief, an XbaI site was generated directly after the stop codon of the HCV open reading frame in pFKnt341-sp-PI-neoEI3420-9605 (10) by site-directed mutagenesis. This replicon construct contained three cell culture-adaptive mutations (E1202G, T1280I, and a deletion of S2202 [reference 18; V. Lohmann et al., submitted for publication]) to increase replication efficiency. The C terminus of NS5B with the artificial glycosylation site and the spacer sequence derived from bovine opsin were introduced into the replicon by insertion of the 436-bp NcoI-XbaI fragment from pCMVNS5Bcon-gt. After restriction with AseI and ScaI, runoff transcripts were generated by using T7 RNA polymerase (Promega, Mannheim, Germany) and electroporated into HuH-7 cells as described previously (18). Transfected cells were subjected to selection with 250 μg of G418/ml. After several weeks, eight colonies were obtained following transfection of 100 ng of replicon RNA. Thus, the G418 transduction efficiency was about 4 orders of magnitude lower than that of the parental replicon, suggesting that the C-terminal glycosylation sequence was poorly tolerated. The C-terminal region of NS5B from two clones was amplified by reverse transcription (RT)-PCR using primer A9413 for RT and primer pair S8467/A9413 for PCR, followed by subcloning and sequencing of the region from nucleotide 9084 (corresponding to amino acid residue 2914) to the poly(U/UC) tract. Interestingly, in one HuH-7 cell clone, a single thymidine insertion was found after four codons of the glycosylation sequence, leading to a translational frameshift that removed the distal glycosylation site. In the other HuH-7 cell clone, designated 101201-6-2, an isoleucine substitution for the methionine at the beginning of the glycosylation tag was found whereas the remainder of this sequence was completely retained. The levels of RNA replication in this cell clone were comparable to those obtained with other bicistronic replicons that lack a C-terminal extension of NS5B, indicating that the glycosylation tag per se did not interfere with RNA replication or that a second site mutation was required for efficient RNA replication. In order to analyze the glycosylation state of NS5B present in the replicon of cell clone 101201-6-2, lysates from this as well as cell clone 9-13 harboring a subgenomic HCV replicon with an authentic NS5B (23) were treated with N-glycosidase F and endoglycosidase H and analyzed by Western blotting using MAb 5B-3B1. As shown in Fig. 3D, this treatment led to a decrease in the apparent molecular mass of NS5B-gt present in cell line 101201-6-2 whereas the apparent molecular mass of NS5B from cell line 9-13 carrying unmodified NS5B was not affected. Thus, the glycosylation acceptor site at the C terminus of NS5B was functional even in a cell clone harboring a persistent replicon, indicating that within a native replication complex the C terminus of NS5B is located in the lumen of the ER.
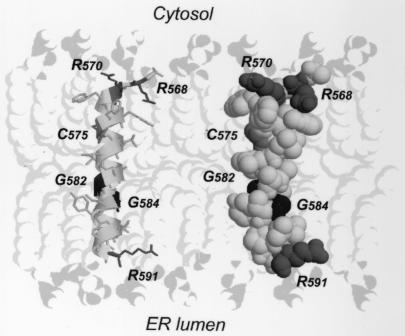
Taken together, the results obtained by two independent experimental strategies unequivocally demonstrate that the HCV RdRp insertion sequence traverses the phospholipid bilayer of the ER or an ER-derived modified compartment as a bona fide transmembrane segment. These data, together with previous structure predictions indicating that this segment adopts an α-helical fold in the hydrophobic membrane context (33), encouraged us to propose the three-dimensional molecular model shown in Fig. 4. Modeling of the segment of NS5B containing aa 567 to 591 (NS5B[567-591]) was performed by using the Swiss Model server facilities (http://www.expasy.ch/swissmod/) (11). The segment of aa 5 to 30 (segment 5-30) of bacteriorhodopsin (Protein Data Bank [PDB] entry 1BHB [31]) was used as a structure template because it displays 41% sequence identity with NS5B[567-591] (detailed in reference 33) and forms a transmembrane α-helix.
FIG. 4.
Theoretical three-dimensional model of the HCV NS5B membrane insertion sequence. The molecular model of NS5B[567-591] was constructed by using segment 5-30 of bacteriorhodopsin as a template (PDB entry 1BHB) and Swiss Model server facilities (http://www.expasy.ch/swissmod/). A ribbon representation including residue side chains and a space-filling representation are shown on the left and the right, respectively. Gly, Arg, and Cys residues are represented in black, dark grey, and grey, respectively. The model was manually positioned in the phospholipid bilayer that was built by using coordinates of phospholipids reported in PDB entry 1BCC (37). The polar heads and the aliphatic tails of phospholipids are in light grey and very light grey, respectively. Figures were generated with Rasmol 2.7 (32).
Figure 4 illustrates the putative position of the NS5B[567-591] segment in a theoretical phospholipid bilayer. This positioning was based on the assumption that the basic Arg residues at both helix ends interact with the negatively charged polar head of phospholipids. As expected (33), this model shows that the α-helix has the right size to form a classical transmembrane segment that likely represents the thermodynamically stable final state of the NS5B insertion sequence. However, the presence of flexible Gly residues in the GVG motif within the central part of the helix and the presence of Pro at amino acid position 589 clearly indicate that the C-terminal segment of the helix could exhibit some flexibility. One can speculate that these structural features could play a role in the mechanism of insertion of the NS5B membrane. However, efficient translocation of the LVL mutant indicates that the GVG motif is not essential for membrane insertion, suggesting that it may play a role in protein-protein interactions within the lipid bilayer. Indeed, it has been well documented that Gly residues are frequently involved in transmembrane helix-helix interactions (34). As can be seen in the space-filling model of Fig. 4, the absence of side chains for the Gly residues yields “holes” along the helix. In helix-helix interactions, such holes are often filled by large hydrophobic residues (particularly Phe or Leu) present in the transmembrane segments of interaction partners, yielding a “knobs into holes” hydrophobic-interaction pattern (24). Although such interaction has been essentially reported for the GxxxG dimerization motif (34), examination of membrane protein three-dimensional structures available in the PDB revealed various examples where Gly residues of GxG sequences belonging to transmembrane α-helices are involved in helix-helix interactions. As a typical example, in the S chain of the photosynthetic reaction center from Rhodobacter sphaeroides (PDB entry 1DV6 [1]), Gly 281 and Gly 283 of the GIG sequence in transmembrane helix 13 interact with Leu 155 of helix 5 and Phe 201 of helix 8, respectively. These observations suggest that the GVG motif in the HCV RdRp insertion sequence may be involved in intermolecular transmembrane helix-helix interactions.
No evidence for ER-distal carbohydrate modifications of the engineered glycosylation acceptor sites was found following transient transfection or in the context of a functional HCV replication complex, indicating that NS5B is retained in the ER or an ER-derived modified compartment. Static retention in the ER mediated by the C-terminal insertion sequence of a tail-anchored protein was also demonstrated for microsomal aldehyde dehydrogenase (25). ER retention signals in the transmembrane domains of the HCV envelope glycoproteins E1 and E2 have been described (2, 3, 7). Thus, both structural and nonstructural HCV proteins harbor determinants for retention in the ER or an ER-derived modified compartment. This is in agreement with recent ultrastructural analyses showing that all HCV proteins colocalize to a seemingly ER-derived membranous web representing a candidate HCV replication complex (8).
In conclusion, the HCV RdRp C-terminal insertion sequence forms a putative transmembrane α-helix that not only may serve as a membrane anchor but also probably has additional functions in the context of the HCV replication complex.
Acknowledgments
N.I. and B.W. contributed equally to this study.
We gratefully acknowledge Elke Bieck, Sandra Hoffmann, and Ulrike Herian for expert technical advice and assistance, Juliane Schmidt-Mende for helpful discussions, Jean Dubuisson for sharing selective permeabilization protocols, Volker Brass and Rainer Gosert for critical reading of the manuscript, Charles M. Rice for plasmid pBRTM/HCV1-3011con, and Jean Dubuisson and Harry Greenberg for MAbs A4 and A11.
N.I. acknowledges Vladimir T. Ivashkin for supporting her doctoral fellowship at the University of Freiburg. This work was supported by grants Mo 799/1-2 and Mo 799/1-3 (D.M. and H.E.B.) as well as SFB 490/Teilprojekt A2 (R.B.) from the Deutsche Forschungsgemeinschaft, QLK2-CT1999-00356 from the European Commission (D.M., H.E.B., R.B., and F.P.), 01 KI 9951 from the Bundesministerium für Bildung und Forschung (D.M. and H.E.B.), the Centre National de la Recherche Scientifique (F.P.), the Lucie Bolte Foundation, and the Wissenschaftliche Gesellschaft in Freiburg im Breisgau.
REFERENCES
- 1.Axelrod, H. L., E. C. Abresch, M. L. Paddock, M. Y. Okamura, and G. Feher. 2000. Determination of the binding sites of the proton transfer inhibitors Cd2+ and Zn2+ in bacterial reaction centers. Proc. Natl. Acad. Sci. USA 97:1542-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocquerel, L., S. Duvet, J. C. Meunier, A. Pillez, R. Cacan, C. Wychowski, and J. Dubuisson. 1999. The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J. Virol. 73:2641-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cocquerel, L., J.-C. Meunier, A. Pillez, C. Wychowski, and J. Dubuisson. 1998. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glykoprotein E2. J. Virol. 72:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocquerel, L., A. Op de Beeck, M. Lambot, J. Roussel, D. Delgrange, A. Pillez, C. Wychowski, F. Penin, and J. Dubuisson. 2002. Topologic changes in the transmembrane domains of hepatitis C virus envelope glycoproteins. EMBO J. 21:2893-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dailey, H. A., and P. Strittmatter. 1981. Orientation of the carboxyl and NH2 termini of the membrane-binding segment of cytochrome b5 on the same side of phospholipid bilayers. J. Biol. Chem. 256:3951-3955. [PubMed] [Google Scholar]
- 6.Dubuisson, J., H. H. Hsu, R. C. Cheung, H. B. Greenberg, D. G. Russell, and C. M. Rice. 1994. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 68:6147-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvet, S., A. Pillez, L. Cocquerel, R. Cacan, A. Verbert, D. Moradpour, C. Wychowski, and J. Dubuisson. 1998. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J. Biol. Chem. 273:32088-32095. [DOI] [PubMed] [Google Scholar]
- 8.Egger, D., B. Wölk, R. Gosert, L. Bianchi, H. E. Blum, D. Moradpour, and K. Bienz. 2002. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 76:5974-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evan, G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friebe, P., V. Lohmann, N. Krieger, and R. Bartenschlager. 2001. Sequences in the 5′ nontranslated region of hepatitis C virus required for RNA replication. J. Virol. 75:12047-12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guex, N., A. Diemand, and M. C. Peitsch. 1999. Protein modelling for all. Trends Biochem. Sci. 24:364-367. [DOI] [PubMed] [Google Scholar]
- 12.Helenius, A., and M. Aebi. 2001. Intracellular functions of N-linked glycans. Science 291:2364-2369. [DOI] [PubMed] [Google Scholar]
- 13.Honsho, M., J. Y. Mitoma, and A. Ito. 1998. Retention of cytochrome b5 in the endoplasmic reticulum is transmembrane and luminal domain-dependent. J. Biol. Chem. 273:20860-20866. [DOI] [PubMed] [Google Scholar]
- 14.Janiak, F., B. Leber, and D. W. Andrews. 1994. Assembly of Bcl-2 into microsomal and outer mitochondrial membranes. J. Biol. Chem. 269:9842-9849. [PubMed] [Google Scholar]
- 15.Kim, P. K., C. Hollerbach, S. W. Trimble, B. Leber, and D. W. Andrews. 1999. Identification of the endoplasmic reticulum targeting signal in vesicle-associated membrane proteins. J. Biol. Chem. 274:36876-36882. [DOI] [PubMed] [Google Scholar]
- 16.Kim, P. K., F. Janiak-Spens, W. S. Trimble, B. Leber, and D. W. Andrews. 1997. Evidence for multiple mechanisms for membrane binding and integration via carboxy-terminal insertion sequences. Biochemistry 36:8873-8882. [DOI] [PubMed] [Google Scholar]
- 17.Kolykhalov, A. A., E. V. Agapov, K. J. Blight, K. Mihalik, S. M. Feinstone, and C. M. Rice. 1997. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277:570-574. [DOI] [PubMed] [Google Scholar]
- 18.Krieger, N., V. Lohmann, and R. Bartenschlager. 2001. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 75:4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuroda, R., J.-Y. Kinoshita, M. Honsho, J.-Y. Mitoma, and A. Ito. 1996. In situ topology of cytochrome b5 in the endoplasmic reticulum membrane. J. Biol. Chem. 120:828-833. [DOI] [PubMed] [Google Scholar]
- 20.Kutay, U., G. Ahnert-Hilger, E. Hartmann, B. Wiedenmann, and T. A. Rapoport. 1995. Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J. 14:217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutay, U., E. Hartmann, and T. A. Rapoport. 1993. A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 3:72-75. [DOI] [PubMed] [Google Scholar]
- 22.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 23.Lohmann, V., F. Körner, J.-O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 24.MacKenzie, K. R., J. H. Prestegard, and D. M. Engelman. 1997. A transmembrane helix dimer: structure and implications. Science 276:131-133. [DOI] [PubMed] [Google Scholar]
- 25.Masaki, R., A. Yamamoto, and Y. Tashiro. 1996. Membrane topology and retention of microsomal aldehyde dehydrogenase in the endoplasmic reticulum. J. Biol. Chem. 271:16939-16944. [DOI] [PubMed] [Google Scholar]
- 26.Moradpour, D., E. Bieck, T. Hügle, W. Wels, J. Z. Wu, Z. Hong, H. E. Blum, and R. Bartenschlager. 2002. Functional properties of a monoclonal antibody inhibiting the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:593-601. [DOI] [PubMed] [Google Scholar]
- 27.Moradpour, D., C. Englert, T. Wakita, and J. R. Wands. 1996. Characterization of cell lines allowing tightly regulated expression of hepatitis C virus core protein. Virology 222:51-63. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson, I., and G. von Heijne. 1993. Determination of the distance between the oligosaccharyltransferase active site and the endoplasmic reticulum membrane. J. Biol. Chem. 268:5798-5801. [PubMed] [Google Scholar]
- 29.Otto, J. C., and W. L. Smith. 1994. The orientation of prostaglandin endoperoxide synthases-1 and -2 in the endoplasmic reticulum. J. Biol. Chem. 269:19868-19875. [PubMed] [Google Scholar]
- 30.Ozols, J. 1989. Structure of cytochrome b5 and its topology in the microsomal membrane. Biochim. Biophys. Acta 997:121-130. [DOI] [PubMed] [Google Scholar]
- 31.Pervushin, K. V., V. Y. Orekhov, A. I. Popov, L. Y. Musina, and A. S. Arseniev. 1994. Three-dimensional structure of (1-71)bacterioopsin solubilized in methanol/chloroform and SDS micelles determined by15N-1H heteronuclear NMR spectroscopy. Eur. J. Biochem. 219:571-583. [DOI] [PubMed] [Google Scholar]
- 32.Sayle, R. A., and E. J. Milner-White. 1995. RASMOL: biomolecular graphics for all. Trends Biochem. Sci. 20:374.. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt-Mende, J., E. Bieck, T. Hügle, F. Penin, C. M. Rice, H. E. Blum, and D. Moradpour. 2001. Determinants for membrane association of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 276:44052-44063. [DOI] [PubMed] [Google Scholar]
- 34.Senes, A., M. Gerstein, and D. M. Engelman. 2000. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J. Mol. Biol. 296:921-936. [DOI] [PubMed] [Google Scholar]
- 35.Vergères, G., J. Ramsden, and L. Waskell. 1995. The carboxyl terminus of the membrane-binding domain of cytochrome b5 spans the bilayer of the endoplasmic reticulum. J. Biol. Chem. 270:3414-3422. [DOI] [PubMed] [Google Scholar]
- 36.Wattenberg, B., and T. Lithgow. 2001. Targeting of C-terminal (tail)-anchored proteins: understanding how cytoplasmic activities are anchored to intracellular membranes. Traffic 2:66-71. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Z., L. Huang, V. M. Shulmeister, Y. I. Chi, K. K. Kim, L. W. Hung, A. R. Crofts, E. A. Berry, and S. H. Kim. 1998. Electron transfer by domain movement in cytochrome bc1. Nature 392:677-684. [DOI] [PubMed] [Google Scholar]