Abstract
The nef gene contributes to the replication of primate lentiviruses by altering the trafficking of cellular proteins involved in adaptive immunity (class I and II major histocompatibility complex [MHC]) and viral transmission (CD4 and DC-SIGN). A conserved acidic leucine-based sequence (E160xxxLL) within human immunodeficiency virus type 1 (HIV-1) Nef binds to the cellular adaptor protein (AP) complexes, which mediate protein sorting into endosomal vesicles. The leucine residues in this motif are required for the down-regulation of CD4 and for the up-regulation of DC-SIGN and the invariant chain of MHC class II, but the role of the acidic residue is unclear. Here, substitution of E160 with uncharged residues impaired the ability of Nef to up-regulate the expression of the invariant chain and DC-SIGN at the cell surface, whereas substitution with a basic residue was required for a similar effect on the down-regulation of CD4. All substitutions of E160 relieved the Nef-mediated block to transferrin uptake. E160 was required for the efficient interaction of Nef with AP-1 and AP-3 and for the stabilization of these complexes on endosomal membranes in living cells. Systematic mutation of the ExxxLL sequence together with correlation of binding and functional data leads to the hypotheses that AP-1 and AP-3 are major cofactors for the effect of Nef on the trafficking of transferrin, are less important but contribute to the modulation of the invariant chain and DC-SIGN, and are least critical for the modulation of CD4. The data suggest that the E160 residue plays a differential role in the modulation of leucine-dependent Nef-targets and support a model in which distinct AP complexes are used by Nef to modulate different cellular proteins.
The nef gene of primate lentiviruses is required for high-level viremia and the efficient pathogenesis of AIDS (12, 25, 44). These effects are at least partly due to the effect of Nef on the cellular protein trafficking environment. Nef alters the subcellular localization of a number of proteins, including CD4, DC-SIGN, transferrin receptor, tumor necrosis factor, LIGHT, CD28, class I major histocompatibility complex (MHC), and both mature and immature class II MHC (1, 27, 39, 40, 42, 43). These effects likely influence the efficiency of viral replication. For example, the down-regulation of the cell surface level of CD4 by Nef prevents the binding of the viral envelope glycoprotein (gp120) to CD4 on the surface of the virus-producing cell, preserving the infectivity of newly formed virions and potentially enhancing their release (26, 37). In contrast to CD4, Nef up-regulates the surface level of DC-SIGN, a C-type lectin expressed on dendritic cells that both allows the uptake of mannosylated antigens and serves as an adhesion molecule, facilitating the interaction of dendritic cells with T cells during antigen presentation (17, 40). Although DC-SIGN binds gp120, the human immunodeficiency virus virions internalized into dendritic cells remain infectious and are subsequently transmitted to T cells, a process that Nef may facilitate. Finally, Nef disrupts the presentation of viral antigens by down-regulating class I MHC and mature class II MHC from the cell surface, while up-regulating the surface expression of the invariant chain, which normally chaperones the immature class II complex to an endosomal compartment in which antigens derived from the extracellular space are processed (42).
The down-regulation of CD4, CD28, and transferrin receptor as well as the up-regulation of tumor necrosis factor, LIGHT, invariant chain, and DC-SIGN require two leucine residues within a C-terminal, solvent-exposed loop of the Nef protein (4, 9, 18, 27, 40, 42, 43). The leucine codons are conserved among human immunodeficiency virus type 1 (HIV-1) nef alleles, and their mutation leads to the complete loss of Nef's effect on these membrane proteins. The two leucines, together with the adjacent upstream sequence, conform to a class of intracellular sorting signals whose consensus sequence is E/DxxxLL. These sequences bind to the heterotetrameric adaptor protein (AP) complexes, which form part of the coat of a subset of vesicles that mediate transport within the endosomal system (21, 31). The family of AP complexes has four members: AP-1 mediates vesicular transport between the trans-Golgi network and endosomal compartments; AP-2 mediates transport between the plasma membrane and early/sorting endosomes; AP-3 mediates transport from the trans-Golgi network and early endosomes to late endosomes and lysosomes; and AP-4 may mediate transport from the trans-Golgi network to the basolateral plasma membrane of polarized cells.
The roles of these individual AP complexes in the effects of Nef on protein trafficking are unclear. The rate of internalization of CD4 from the cell surface is increased by Nef, suggesting involvement of AP-2 (1). In further support of a role for AP-2, Nef has been detected in clathrin-coated pits at the plasma membrane by electron microscopy (16), and a dominant-negative subunit of AP-2 reportedly blocks Nef-mediated down-regulation of CD4 (3). However, an obligate role for AP-2 in the down-regulation of CD4 by HIV-1 Nef has not been supported by knock-down experiments using RNA interference (36). Furthermore, microscopic colocalization with AP-2 is not a consistent feature of HIV-1 Nef proteins (10, 18). Indeed, HIV-1 Nef binds very weakly to intact AP-2 in vitro (5). In contrast, a relatively robust binding of HIV-1 Nef to intact AP-1 and AP-3 has been detected in vitro, and HIV-1 Nef colocalizes extensively in intact cells with AP-1 and AP-3 by immunofluorescence microscopy (10, 23). In further support of a role for AP-1 and AP-3, when HIV-1 Nef proteins from distinct isolates were compared to determine the generality of AP-binding preferences, all four interacted with hemicomplexes of AP-1, two of four interacted with AP-3, and none interacted with AP-2 (24).
This apparent preference of HIV-1 Nef for AP-1 (and to a lesser extent AP-3) suggests that a primary action of Nef is within the endosomal system. In support of this hypothesis, Nef blocks the recycling of internalized cell surface proteins such as CD4 and transferrin receptor from endosomes to the plasma membrane (29, 33). Recently, we reported that the block to recycling of transferrin receptor is dileucine dependent and is associated with the morphological distortion of a Rab11-positive, endosomal recycling compartment in which transferrin receptor accumulates (29). These effects may relate to the ability of Nef to induce the persistent attachment of AP-1 and AP-3 to endosomal membranes, a property revealed in cells treated with brefeldin A and which correlates with the binding of Nef to the AP complexes via its dileucine-based motif (7, 23).
Although the mechanisms by which similar dileucine-based sequences bind differentially to the distinct members of the AP complex family are not fully understood, residues upstream of the two leucines have been implicated in the binding to AP-3. For example, in Saccharomyces cerevisiae, an acidic residue at position −4 and/or −5 relative to the leucines is required for AP-3-mediated transport of the t-SNARE Vam3p to the vacuole, and a polar residue at position −2 is favored (11). Mammalian proteins show a similar preference: the leucine-dependent binding of LIMP II and the melanosomal protein tyrosinase to intact AP-3 in vitro requires an acidic residue at position −4 and/or −5 (22). Notably, progressive substitutions of the upstream residues in the DERAPLI sequence of LIMP II from acidic to neutral to basic amino acids causes progressive changes in subcellular localization, with basic residues causing mislocalization of LIMP II to the plasma membrane (38). This result suggests that the interaction with AP-2 may tolerate neutral but not basic residues at these positions.
HIV-1 Nef has a glutamic acid residue at position −4 relative to the leucines, E160. Despite the conservation of this residue among diverse viral isolates, alanine substitution at this position has been reported to have remarkably little effect on the Nef-mediated down-regulation of CD4 or the enhancement of viral infectivity (9, 24). These data leave open the possibility that this residue is more important for other Nef phenotypes.
Here, we investigated the roles of the sequence upstream of the two leucines both in Nef-functions and in the interaction of Nef with AP complexes. We hypothesized that this analysis might reveal contributions of these residues to the interactions with AP-1 and AP-3, and it might identify a more convincing phenotypic correlate for the genetic conservation of the E160 residue. The data indicate that residues upstream of the dileucine are required for efficient interaction of Nef with AP-1 and AP-3. They further show that the upstream acidic residue, while required for the optimal down-regulation of CD4, appears to play a more dramatic role in the modulation of transferrin, DC-SIGN, and the invariant chain.
MATERIALS AND METHODS
Plasmid constructs.
Overlap PCR was used to mutate the ENTSLL sequence of HIV-1NL4-3 nef, adding an EcoRI site to the 5′ end and a SalI site to the 3′ end of nef to insert the sequences into pCIneo (Promega) and pGEX 4T-1 (Pharmacia). The yeast three-hybrid plasmids pBridge, encoding Nef plus σ1 and Nef plus σ3, and pGADT7, encoding γ- or δ-adaptin, were a gift from Juan Bonifacino (24). The BspEI and BlpI sites were used to subclone the mutated nef sequences into the Nef-σ1 vector and the EcoRI/SalI sites were used for the Nef-σ3 vector. The BspEI and BlpI sites were used to subclone the mutated sequences into pRcCD8-Nef (15). PCR mutagenesis was used to create substitutions in the ENTSLL sequence of pCG-Nef-GFP (19). The expression vector for CD4 (pCMX-CD4) was the gift of Didier Trono (1). The expression vector for DC-SIGN-1 was the gift of Nathalie Sol-Foulon (40).
Antibodies.
The following antibodies were used: murine anti-γ-adaptin clone 100/3 (Sigma), murine anti-δ-adaptin (Transduction Laboratories), murine fluorescein isothiocyanate-conjugated anti-CD8 (Jackson Laboratories), rhodamine X-conjugated goat anti-mouse immunoglobulin G (Jackson Laboratories), phycoerythrin (PE)-conjugated anti-mouse immunoglobulin G (Jackson Immuno Research), Alexa-647-conjugated murine anti-CD4 (Molecular Probes, Invitrogen; and BD Pharmingen), sheep polyclonal anti-Nef (gift of Celsa Spina), murine anti-DC-SIGN (161-PE; R&D Systems), phycoerythrin-conjugated murine anti-CD74 (invariant chain) clone M-B741 (Ancell), murine anti-human HLA-A, -B, and -C clone W6/32 (Dako), murine anti-CD71 (transferrin receptor) clone DF1513 (Sigma), and murine antitubulin (Sigma).
Cells and transfections.
HEK 293T cells were the gift of Ned Landau and were maintained in Eagle's minimal essential medium with 10% fetal bovine serum and supplemental glutamine, penicillin, and streptomycin. HeLa clone P4.R5 cells were also the gift of Ned Landau and were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum and supplemental glutamine, penicillin, and streptomycin. HeLa-CIITA cells, which express class II MHC, were the gift of Philippe Benaroch and were maintained in DMEM with 10% fetal bovine serum and supplemental glutamine, penicillin, streptomycin, nonessential amino acids, and hygromycin B (300 μg/ml). HEK 293 T cells and HeLa-CIITA were transfected using cationic lipid Lipofectamine 2000 (Invitrogen), according to the manufacturer. Unmodified HeLa cells were transfected using Lipofectamine (Invitrogen) or by electroporation as described previously (23). HeLa P4.R5 cells were transfected using cationic lipid FuGene 6 (Roche) or Lipofectamine according to the manufacturer.
CD4 down-regulation.
HEK 293T cells (106) were transfected using Lipofectamine 2000 (Invitrogen). For each transfection, 0.5 μg pCMX-CD4; 0.03, 0.1, or 0.3 μg pCG-Nef-GFP or the pCG-Nef-GFP variants; pCG-GFP to a total of 0.3 μg of green fluorescent protein (GFP) expression construct; and pCIneo to a total of 4 μg of DNA were used. After 24 h, the cells were stained with Alexa-647-conjugated anti-CD4 at 4°C in phosphate-buffered saline (PBS) with 0.1% sodium azide and 2% fetal bovine serum, fixed with 1% paraformaldehyde, and analyzed by two-color flow cytometry.
MHC class I down-regulation.
HeLa cells (106) were transfected using Lipofectamine (Invitrogen) with 1 μg of pCG-GFP, pCG-Nef-GFP, or the pCG-Nef-GFP variants. After 24 h, the cells were stained at 4°C in PBS with a phycoerythrin-conjugated antibody to HLAs, fixed, and analyzed by two-color flow cytometry. The mean PE fluorescence intensity of the GFP-positive cells was used to calculate the activity of each mutant in reducing surface MHC class I relative to wild-type Nef.
Invariant chain up-regulation.
HeLa-CIITA cells (4 × 105) were cotransfected using Lipofectamine 2000 with plasmids expressing wild-type or mutated Nef (pCIneo-derived; 4 μg) and GFP (pCG-GFP; 0.1 μg). Alternatively, Nef-GFP was expressed from the pCG-derived vectors to allow assessment of the NefE160K mutant, which was poorly expressed as native Nef. After 48 h, an aliquot of the cells was lysed for analysis of Nef expression by Western blotting; the remainder was stained at 4°C in PBS with PE-conjugated anti-CD74, fixed, and analyzed by two-color flow cytometry. For the dose-response analysis, the total DNA in each transfection mixture was normalized to 4 μg using pCIneo.
DC-SIGN up-regulation.
HeLa cells (106) were cotransfected using Lipofectamine with plasmids expressing DC-SIGN-1 (0.5 μg) and Nef-GFP or the Nef-GFP variants (1 μg). After 24 h, an aliquot of the cells was lysed for analysis of Nef expression by Western blotting; the remainder was stained at 4°C in PBS with PE-conjugated anti-DC-SIGN, fixed, and analyzed by two-color flow cytometry as previously described (40).
Transferrin uptake.
HeLa cells (107) were transfected by electroporation with plasmid vectors expressing wild-type or mutated Nef-GFP (12 μg) and plated on glass slides. After 24 h, the cells were incubated in serum-free medium (containing 0.1% bovine serum albumin, 10 mM HEPES) for 2 h at 37°C, and then incubated at 37°C for 30 min in the same medium containing 10 μg /ml Alexa-594-conjugated transferrin. The cells were washed, fixed, and analyzed by confocal microscopy as previously described (29).
Transferrin receptor down-regulation.
HeLa cells (106) were transfected using Lipofectamine with 1 μg of pCG-GFP, pCG-Nef-GFP, or the pCG-Nef-GFP variants. After 24 h, the cells were stained at 4°C in PBS with an anti-CD71 antibody, followed by a secondary antibody conjugated to PE, fixed, and analyzed by two-color flow cytometry. The mean PE fluorescence intensity of the GFP-positive cells was used to calculate the activity of each mutant in reducing surface transferrin receptor relative to wild-type Nef.
GST pull-down assay.
Wild-type or mutated Nef proteins fused to glutathione-S-transferase (GST) were produced in Escherichia coli and purified as previously described (23). HeLa cells (107) were lysed in 50 mM Tris-HCl (pH 8), 5 mM EDTA, 150 mM NaCl, and 1% Triton X-100. The cytoplasmic lysates were incubated overnight at 4°C with 2 μg of GST or GST-Nef proteins, immobilized on glutathione-Sepharose beads (Amersham Biosciences). Beads were washed five times in lysis buffer, the bound cellular proteins were analyzed by Western blotting using anti-γ- or anti-δ-adaptin antibodies and chemiluminescent detection, and the signals were quantified using NIH Image software as previously described (23).
Yeast three-hybrid assays.
Yeast cells (strain HF7c) were cotransformed with pBridge (Clontech) containing Nef variants fused to the GAL4 DNA-binding domain plus σ1 or σ3 along with pGADT7 containing γ- or δ-adaptin fused to the GAL4 activation domain and streaked on medium that selected only for cotransformation (leucine- and tryptophan-minus). Five to 10 colonies from each plate were picked and patched in duplicate on leucine- and tryptophan-minus medium. After growth at 30°C, the patches were replica plated onto medium that selected for protein-protein interaction (leucine, tryptophan, and histidine minus) as well as medium that selected only for cotransformation (leucine and tryptophan minus), and incubated at 30°C for 3 to 5 days.
Nef-mediated membrane association of adaptor complexes in living cells.
HeLa cells (4 × 104; clone P4.R5) were plated on glass coverslips, then transfected with CD8-Nef expression constructs (1 μg) using FuGene 6 (Roche). After 24 h, the cells were treated with 10 μg /ml brefeldin A (Epicenter) for 5 min, then fixed with 3% paraformaldehyde and permeabilized with 0.1% NP-40. The cells were incubated with either murine anti-γ-adaptin or murine anti-δ-adaptin, then with rhodamine X-conjugated goat anti-mouse immunoglobulin G. After extensive washing to remove unbound secondary antibody, the cells were incubated with normal mouse serum to block exposed antigen binding sites on the goat anti-mouse immunoglobulin G secondary antibody, then stained with fluorescein isothiocyanate-conjugated anti-CD8. The cells were imaged using a spinning disk confocal fluorescence microscope (Olympus) and the data processed using Slidebook software.
RESULTS
Conservation of the ExxxLL motif among naturally occurring Nef sequences.
To document the conservation of residues within the ExxxLL motif of HIV-1 Nef, we analyzed over 600 Nef sequences from group M HIV-1 (the main genetic group responsible for the pandemic) obtained from the Los Alamos National Laboratory HIV Sequence Database. Table 1 indicates that a negatively charged residue is encoded by 99.1% of isolates at position −4 relative to the leucines. An uncharged, polar residue is encoded by 97.4% of isolates at position −2 and by 94.9% of isolates at position −1. These data support the importance of the −4, −2, and −1 positions, which correspond to E160, T162, and S163 in the NL4-3 Nef protein studied here. Position −3, here N161, is also conserved: an asparagine is encoded by 87.2% of isolates and an aspartic acid by 9.5%. Each of the leucine residues is conserved in 99.5% of isolates.
TABLE 1.
Characteristics of Nef amino acids 160 to 165 among HIV-1 group M isolates
| Parametera | Position
|
|||||
|---|---|---|---|---|---|---|
| 160 | 161 | 162 | 163 | 164 | 165 | |
| Residue in NL4-3 Nef | E | N | T | S | L | L |
| Residue in consensus Nef | E | N | N | C | L | L |
| % NP | 0.4 | 0.2 | 1.5 | 1.7 | 100.0 (99.5 L) | 99.8 (99.5 L) |
| % UP | 0.3 | 89.0 (87.2 N) | 97.4 (92.0 N, 2.7 S, 2.6 T) | 94.9 (51.7 C, 41.1 S) | 0.0 | 0.2 |
| % PC | 0.2 | 1.2 | 0.8 | 3.5 | 0.0 | 0.0 |
| % NC | 99.1 (97.7 E, 1.3 D) | 9.6 (9.5 D) | 0.3 | 0.0 | 0.0 | 0.0 |
NP, nonpolar (G, A, V, L, I, P, F, W, M); UP, uncharged, polar (S, T, C, Y, N, Q); PC, positively charged (K, R, H); NC, negatively charged (D, E).
Mutation of the ExxxLL sequence and expression of the Nef variants.
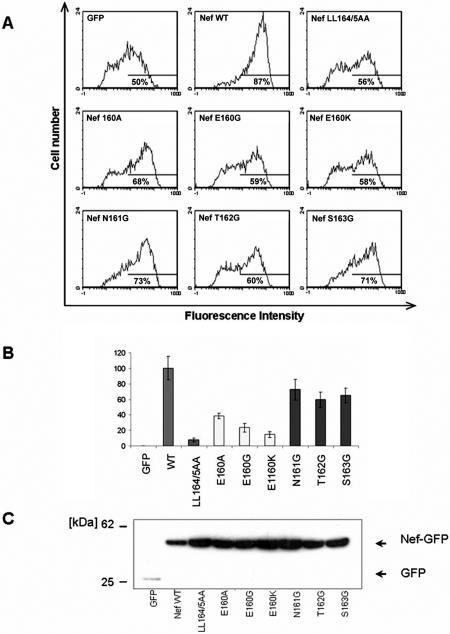
To investigate the roles of the residues upstream of the leucines, we mutated the HIV-1NL4-3 Nef sequence, E160NTSLL. Three different substitutions in the upstream acidic residue were constructed: E160A, E160G, and E160K. Glycine substitutions of residues N161, T162, and S163 were also constructed. The alanine substitution of L164 and L165 was constructed previously (9).
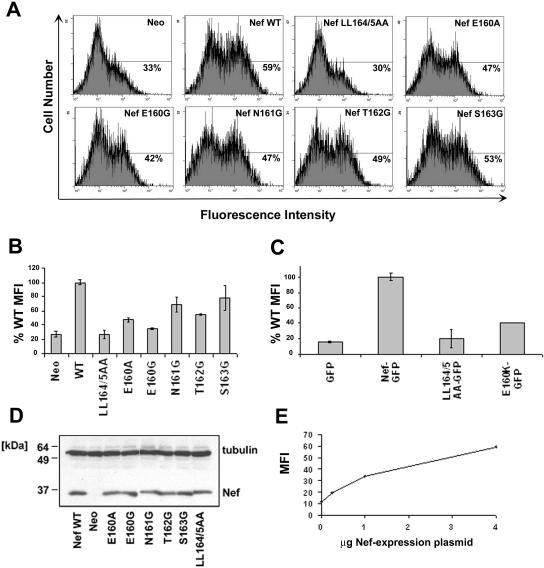
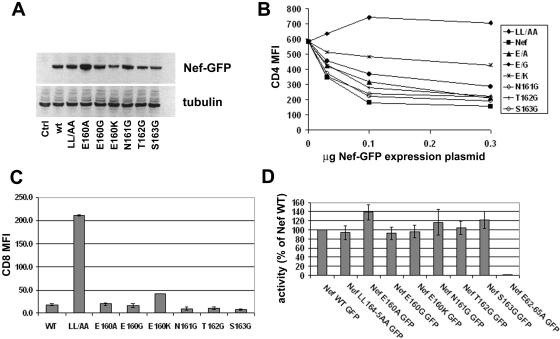
Initial analysis by immunoblotting after transient transfection of 293T cells with pCIneo-derived plasmids indicated that all the Nef proteins were reasonably well expressed at steady state with the exception of E160K, which was undetectable as native Nef (data not shown; see also Fig. 2). However, when fused to the N terminus of the green fluorescent protein, all the mutants, including E160K, were detectable (Fig. 1A). Although Nef-E160K, as well as Nef-T162G and Nef-S163G, appeared slightly less well expressed than wild-type Nef, these data on protein expression suggested that functional studies would be interpretable, with the caveat that GFP fusions would be required to incorporate the E160K substitution into the analyses.
FIG. 2.
Up-regulation of the invariant chain (Ii) of class II MHC to the cell surface. (A) HeLa-CIITA cells were cotransfected with pCG-GFP (0.1 μg) and pCIneo-derived plasmids expressing either native, wild-type Nef or the indicated Nef mutants (4 μg). After 2 days, the cells were stained for Ii with a PE-conjugated antibody and analyzed by two-color flow cytometry. Neo, cells cotransfected with the empty plasmid pCIneo. Representative histograms of relative cell number versus PE (Ii) fluorescence intensity for the GFP-positive cells are shown; the percentage of Ii-positive cells is indicated. (B) Relative levels of surface Ii in the transfected cells. The mean PE (Ii) fluorescence intensities (MFI) for the GFP-positive cells are expressed relative to cells expressing wild-type Nef. Wild-type (WT) Nef was set at 100%; the mean fluorescence intensity of the Nef-negative control is the actual value relative to that of the wild type (WT) and was not normalized to zero. Values are the means of two independent transfections; error bars are the standard deviation from the mean. (C) Phenotype of the E160K mutant as a fusion with GFP. HeLa-CIITA cells were transfected with pCG-based plasmids (4 μg) expressing wild-type Nef-GFP, the indicated missense mutants, or no Nef (“GFP”) and then stained for Ii 2 days later with PE-conjugated antibody and analyzed by two-color flow cytometry. The mean PE (Ii) fluorescence intensities for the GFP-positive cells are expressed relative to that of cells expressing wild-type Nef. Wild-type Nef (Nef-GFP) was set at 100%; the mean fluorescence intensity of the Nef-negative control (GFP) is the actual value relative to that of Nef-GFP and was not normalized to zero. Values are the mean of two independent transfections; error bars are the standard deviation from the mean. (D) Aliquots of the same populations of cells analyzed in panel A were analyzed for Nef expression by Western blotting; simultaneous detection of tubulin was used as a loading control. (E) Dose-response curve of Ii up-regulation using native Nef. The experiment was performed as in panels A and B, except that the amount of pCIneo-based plasmid expressing wild-type Nef was varied as shown; all transfections were normalized to 4 μg total of pCIneo-based plasmid using the empty pCIneo.
FIG. 1.
Initial characterization of the E, N, T, and S mutants: protein expression, modulation of CD4, surface expression of CD8 chimeras, and modulation of class I MHC. (A) Immunoblot analysis of Nef protein expression. Cells (HEK 293T) were transfected with pCG-based plasmids (0.5 μg) expressing wild-type Nef-GFP (wt) or the indicated missense mutants and then analyzed for Nef expression by Western blotting; simultaneous detection of tubulin was used as loading control. Ctrl, cells transfected with pCG-GFP. (B) Nef-mediated down-regulation of CD4. Cells (HEK 293T) were transfected with 0.5 μg pCMX-CD4; 0.03, 0.1, or 0.3 μg pCG-Nef-GFP or the pCG-Nef-GFP variants; and pCG-GFP as required to a total of 0.3 μg of GFP expression construct. One day later, the cells were stained for CD4 with an Alexa 647-conjugated antibody and analyzed by two-color flow cytometry. The mean Alexa 647 (CD4) fluorescence intensity of the GFP-positive cells is graphed. (C) Expression of CD8-Nef chimeras on the cell surface. Cells (HEK 293T) were transfected with plasmids expressing CD8-Nef chimeras in which the extracellular and transmembrane domains of the CD8 α chain are fused to Nef as the cytoplasmic domain. The cells were stained for CD8 using a fluorescein isothiocyanate-conjugated antibody at 4°C in the presence of azide, fixed, and then analyzed by flow cytometry. The mean fluorescence intensity of the cells is graphed; error bars are the standard deviation of the mean for duplicate transfections. (D) Nef-mediated modulation of class I MHC. Cells (HeLa) were transfected with the Nef-GFP expression plasmids described above (1 μg). One day later, the cells were stained with a phycoerythrin-conjugated antibody to HLA-A, -B, and -C and analyzed by two-color flow cytometry. The mean PE (class I MHC) fluorescence intensity of the GFP-positive cells was used to calculate activity relative to wild-type Nef; error bars are the standard deviation of the mean for duplicate transfections.
Role of the E160, N161, T162, and S163 residues in the Nef-mediated down-regulation of CD4 from the cell surface.
The effect of these mutations on the down-regulation of CD4 was tested by transfection of cells with plasmids expressing the Nef proteins as fusions with GFP in a dose-response format and measurement of CD4 at the cell surface by flow cytometry (Fig. 1B). As expected, the steady-state level of CD4 on cells expressing wild-type Nef-GFP was reduced compared to that of control cells that expressed only GFP, while the surface level of CD4 was not reduced in cells expressing Nef-GFP LL/AA. The impact of mutation of E160 on the down-regulation of CD4 was dose dependent and influenced by the specific substitution: Nef-GFP E160A was minimally impaired; Nef-GFP E160G was more impaired; and Nef-GFP E160K was the most impaired, retaining less than half the activity of wild-type Nef. These defects were most apparent at the lower doses of Nef expression. Notably, 0.1 μg of plasmid expressing wild-type Nef-GFP achieved the largest dynamic range while just reaching a saturating level of activity. Using this point for comparison, the E160A mutant retained 70% of wild-type activity; the E160G mutant retained 50% activity, and the E160K mutant retained only 30% of the activity. In contrast to E160, mutations of the N, T, and S residues had little or no effect on down-regulation of CD4. Notably, the similar expression of Nef-GFP S163G and Nef-GFP E160K argued against protein instability as the reason for the phenotypic defect of the E160K mutant.
Role of the E160, N161, T162, and S163 residues in the expression of CD8-Nef chimeras at the cell surface.
To directly assess protein trafficking, the effect of these mutations on the steady-state level of CD8-Nef chimeras at the surface of transiently transfected cells was measured using flow cytometry (Fig. 1C). In these chimeras, wild-type or mutated Nef was fused to the extracellular and transmembrane domains of CD8. Compared to wild-type Nef, CD8-Nef LL/AA was up-regulated at the cell surface, presumably reflecting a loss of its internalization signal. Although all the mutants had low surface levels compared to Nef-LL/AA, the surface level of Nef-E160K was increased significantly compared to wild-type Nef. Substitution of the N, T, and S residues had no detectable effect on the surface levels of the CD8-Nef chimera. These flow cytometric data agreed with the microscopic localizations of the chimeras shown in Fig. 7 and 8 below. These data on the surface expression of the chimeras were also consistent with the data on CD4 down-regulation above: both properties were LL dependent but markedly affected by mutation of E160 only in the case of the lysine substitution.
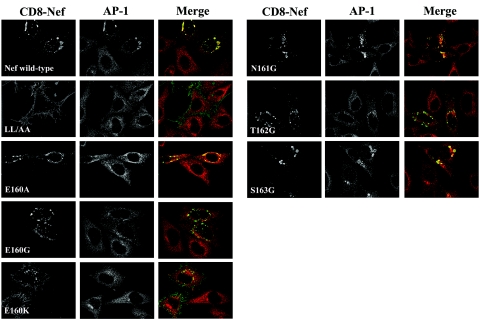
FIG. 7.
Nef-mediated stabilization of AP-1 on endosomal membranes. HeLa cells (clone P4.R5) were transfected with plasmids expressing CD8-Nef chimeras. One day later, the cells were treated with 10 μg /ml brefeldin A (BFA) for 5 min at 37°C and then fixed, permeabilized, and stained for γ-adaptin (AP-1) by indirect immunofluorescence with anti-γ-adaptin and rhodamine X-conjugated secondary antibody (red) and for CD8 by direct immunofluorescence with a fluorescein isothiocyanate-conjugated anti-CD8 antibody (green). For each field, a Z-series of images was acquired at 0.5-μm steps using an Olympus disk-scanning confocal microscope; the images were processed using no-neighbor deconvolution (Slidebook software); and the image shown is a projection of the Z-stack.
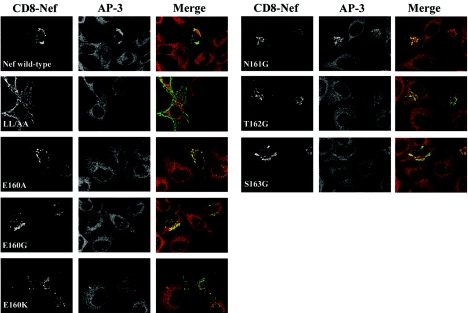
FIG. 8.
Nef-mediated stabilization of AP-3 on endosomal membranes. HeLa cells (clone P4.R5) were transfected with plasmids expressing the CD8-Nef chimeras, treated with brefeldin A, and imaged exactly as described in the legend to Fig. 7, except that the indirect immunofluorescence was done using a primary antibody to the δ subunit of AP-3.
Role of the E160, N161, T162, and S163 residues in the Nef-mediated down-regulation of class I MHC from the cell surface.
The leucine motif in Nef is dispensable for the down-regulation of class I MHC. We analyzed the contribution of the residues upstream of LL in Nef to the down-regulation of class I MHC by expressing transiently Nef-GFP and related mutants. The steady-state level of class I MHC A, B, and C at the cell surface was measured by flow cytometry (Fig. 1D). As expected, the down-regulation of class I MHC was markedly impaired by alanine substitution of residues E62-E65 but was unaffected by substitution of the LL sequence. Furthermore, the down-regulation of class I MHC was not impaired by substitution of the E, N, T, or S residue. These data argued against any marked instability of the mutants, and they support the specificity of the defects in the modulation of CD4 described above and of invariant chain, DC-SIGN, and transferrin described below.
Role of the E160, N161, T162, and S163 residues in the Nef-mediated up-regulation of the invariant chain of immature class II MHC to the cell surface.
In contrast to its down-regulating effects on CD4, Nef increases the surface-levels of the invariant chain (Ii) of class II MHC. We analyzed the contribution of the residues upstream of LL in Nef to the up-regulation of Ii by expressing native Nef transiently in HeLa-CIITA cells, which constitutively express the class II MHC (Fig. 2). The steady-state level of Ii at the cell surface was measured by flow cytometry. Wild-type Nef up-regulated the surface expression of Ii by four- to sixfold in this assay, whereas the LL/AA mutant had no effect. The E160 mutants were markedly impaired for Ii up-regulation: Nef-E160A retained only 30% of activity relative to wild-type Nef, and Nef-E160G retained only 10% of activity (Fig. 2A and B). Nef-T162G retained 40% activity, but Nef-S163G was 70% active. Nef-N161G was approximately 60% active. Nef-E160K was evaluated as a GFP fusion and retained 30% activity relative to wild-type Nef (Fig. 2C). These data indicated that the efficient up-regulation of Ii required not only the LL sequence but also E160 and to a lesser extent T162.
Role of the E160, N161, T162, and S163 residues in the Nef-mediated up-regulation of DC-SIGN to the cell surface.
Nef also increases the surface levels of DC-SIGN. We analyzed the contribution of the residues upstream of LL in Nef to the up-regulation of DC-SIGN by expressing transiently both Nef (as a GFP fusion) and DC-SIGN in HeLa cells (Fig. 3). The steady-state level of DC-SIGN at the cell surface was measured by flow cytometry. Each of the E160 mutants was impaired: Nef-E160A retained 40% activity relative to wild-type Nef, whereas Nef-E160G and Nef-E160K retained only 20 to 30% activity. Nef-N161G, Nef-T162G, and Nef-S163G were least impaired, retaining 60 to 80% activity. Overall, these data indicate a similarity between the genetic requirements for the up-regulation of DC-SIGN and for the up-regulation of Ii: both phenotypes were more dependent on the E160 residue than was the down-regulation of CD4.
FIG. 3.
Up-regulation of DC-SIGN to the cell surface. Cells (HeLa) were cotransfected with pCG-based plasmids expressing GFP, wild-type Nef-GFP, or the indicated Nef-GFP mutants, along with a plasmid expressing DC-SIGN (0.5 μg). One day later, the cells were stained for DC-SIGN with a phycoerythrin-conjugated antibody and analyzed by two-color flow cytometry. (A) Representative histograms of relative cell number versus PE fluorescence intensity for the GFP-positive cells; the percentage of positive cells is indicated. (B) The relative activity of each Nef mutant for the up-regulation of DC-SIGN. The mean fluorescence (PE) intensities for cells expressing the Nef-GFP mutants are expressed as percentages relative to that of cells expressing GFP (set to 0%) and cells expressing wild-type Nef-GFP (set to 100%). Values are the means of three independent transfections; error bars are the standard deviation from the mean. (C). Relative expression of Nef-GFP and the mutants; lysates of the cells analyzed in panel A were analyzed by Western blotting using an antibody to GFP.
Role of the E160, N161, T162, and S163 residues in the Nef-mediated modulation of transferrin trafficking.
The hypothesis that Nef has a general effect on endosomal trafficking has been supported by its ability to modulate both transferrin uptake and the expression of transferrin receptor at the cell surface (29). Transferrin receptor is normally internalized from the plasma membrane into early/sorting endosomes, from which it recycles to the plasma membrane (20). In the presence of Nef, the internalization rate of transferrin receptor is unaffected, but recycling to the plasma membrane is inhibited, resulting in a decrease in the surface level of transferrin receptor (29). We hypothesized that this decrease could account for the inability of Nef-expressing cells to efficiently internalize transferrin from the surrounding medium (29).
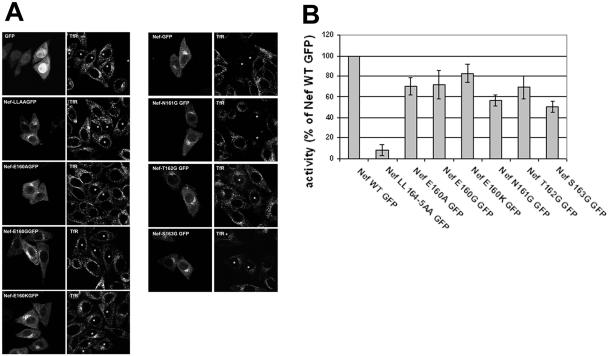
We tested the Nef mutants (expressed as GFP fusions) for their ability to affect transferrin uptake as detected by fluorescence microscopy (Fig. 4A). HeLa cells were transfected to transiently express the Nef-GFP proteins, depleted of endogenous transferrin, and allowed to internalize Alexa 594-transferrin for 30 min before fixation. As previously described, cells expressing wild-type Nef internalized little or no transferrin relative to untransfected cells or to cells transfected with the GFP control (29). In contrast, Nef-LL/AA had no effect on the uptake of transferrin. Strikingly, each of the E160 mutants was indistinguishable from the LL/AA mutant: Nef-E160A, Nef-E160G, and Nef-E160K each failed to block the uptake of transferrin. Nef-N161G was almost as effective as the wild type at blocking transferrin uptake. Nef-T162G failed to block the uptake of transferrin. Nef-S163G appeared to have an intermediate phenotype; it decreased transferrin uptake relative to Nef-LL/AA but not to the extent of wild-type Nef.
FIG. 4.
Nef-mediated modulation of transferrin trafficking. (A) Transferrin uptake. HeLa cells were transfected with plasmids expressing GFP, wild-type Nef-GFP, or the indicated Nef-GFP mutants. Twenty-four hours later, the cells were incubated for 2 h in serum-free medium at 37°C to deplete endogenous transferrin and then exposed to 10 μg /ml Alexa 594-conjugated transferrin in the same medium at 37°C for 30 min to allow uptake. The cells were then fixed and analyzed by confocal microscopy using a Bio-Rad MRC1000 microscope. The images shown are representative median optical sections. Asterisks indicate cells expressing any detectable Nef-GFP. (B) Down-regulation of transferrin receptor. HeLa cells were transfected with pCG-based expressing Nef-GFP or the indicated mutants (1 μg). One day later, the cells were stained for transferrin receptor with a phycoerythrin-conjugated antibody and analyzed by two-color flow cytometry. Using the mean PE (transferrin receptor) fluorescence intensity of the GFP-positive cells, the activity of each mutant in down-regulating transferrin receptor was calculated relative to that of wild-type Nef, which was set at 100%.
To test the hypothesis that the effect of Nef on transferrin uptake is due to a decrease in the surface levels of transferrin receptor at steady state, HeLa cells were transfected to transiently express the Nef-GFP proteins, and the surface expression of transferrin receptor was measured by flow cytometry (Fig. 4B). Whereas the Nef-LL/AA mutant was unable to down-regulate transferrin receptor, no selective defects were apparent in the mutants encoding the E, N, T, or S substitutions; these Nef proteins exhibited 50 to 80% activity in down-regulating transferrin receptor relative to wild-type Nef. Notably, the E160 mutants retained 70 to 80% activity in down-regulating transferrin receptor, despite their inactivity in blocking transferrin uptake. These data indicate that the down-regulation of transferrin receptor is insufficient for the Nef-mediated block to transferrin uptake.
Role of the E160, N161, T162, and S163 residues in the binding of Nef to AP-1 and AP-3 as detected in GST pull-down and yeast three-hybrid assays.
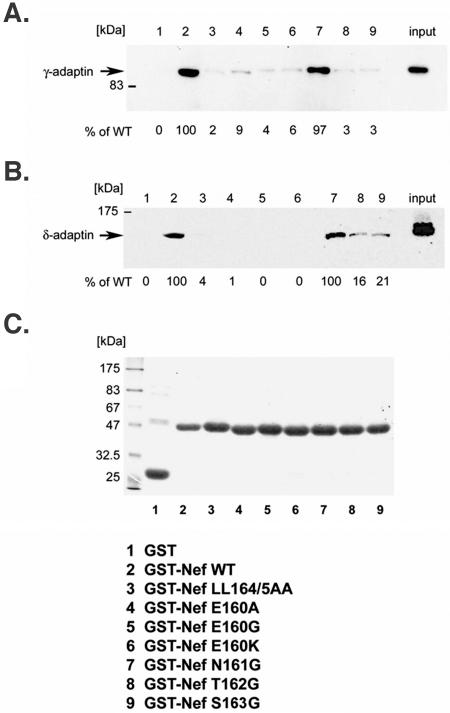
The binding of the Nef mutants to intact AP-1 and AP-3 was tested by pull-down of intact complexes from lysates of human cells using GST-Nef fusion proteins (Fig. 5). Mutation of the LL sequence abrogated binding to both complexes. The E160 mutations completely abrogated the pull-down of AP-3, and they markedly impaired the pull-down of AP-1, although trace residual binding of AP-1 to Nef-E160A was detected in some experiments (9% of wild-type level in Fig. 5). The N161G substitution had no effect on the interaction with either complex. The T162G and S163G substitutions impaired the binding of Nef to both intact AP-1 and AP-3, although residual binding (less than 25% of the wild-type level) was observed in the case of AP-3.
FIG. 5.
Pull-down of intact AP-1 or AP-3 from cytoplasmic lysates by GST-Nef. Lysates of HeLa cells were incubated with equal amounts of purified wild-type or mutated GST-Nef, previously immobilized on glutathione-Sepharose beads. Bound proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the association of AP complexes was analyzed by Western blotting with anti-γ-adaptin (panel A) or anti-δ-adaptin (panel B). Unfractionated cell lysates were run as a control for the detection of γ- and δ-adaptin (right lanes, input). The bands were quantified using NIH Image software and the values indicated are expressed as the percentage of binding relative to that of wild-type Nef. In panel (C), the Coomassie blue-stained gel indicates the relative loading of GST fusion proteins on the beads.
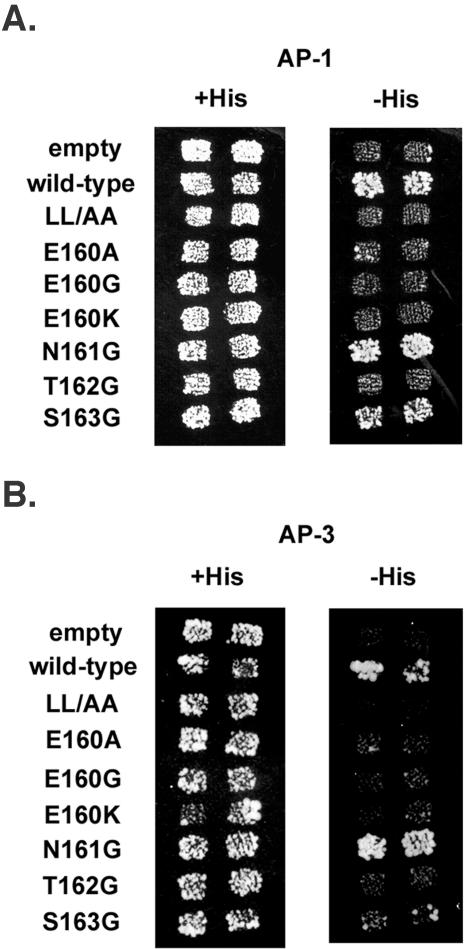
Although HIV-1 Nef was first reported to bind the medium (μ) subunits of AP-1 and AP-3, leucine-dependent binding to hemicomplexes consisting of the large, specific (γ or δ), and small (σ) subunits of AP-1 and AP-3 was recently shown using yeast three-hybrid analyses (10, 24, 28). In these experiments, Nef was fused to the GAL4 DNA-binding domain and coexpressed with σ from one plasmid, while the large, specific subunit was fused to the GAL4 activation domain and expressed from a second plasmid. The growth of cotransformed yeast on selective medium lacking histidine revealed an interaction between Nef and the adaptor hemicomplexes. Here, similar experiments were performed using our panel of Nef mutants (Fig. 6). As expected, wild-type Nef bound to the AP-1 and AP-3 hemicomplexes, whereas Nef-LL/AA did not. Substitution of E160 with A, G, or K abrogated binding to both the AP-1 and AP-3 hemicomplexes. In contrast, the N161G substitution did not affect binding to either complex. The T162G substitution abrogated binding to both hemicomplexes. Although the S163G substitution nearly eliminated binding to the AP-3 hemicomplex, its effect on the binding of Nef to the AP-1 hemicomplex was modest; Nef-S163 retained an ability to bind AP-1 in this assay.
FIG. 6.
Binding of Nef to AP-1 and AP-3 hemicomplexes detected by yeast three-hybrid assay. Yeast cells were cotransformed with two plasmids: one plasmid expressed Nef as a fusion with the GAL4 DNA binding domain together with the σ subunit of either AP-1 (A) or AP-3 (B), and the second plasmid expressed GAL4 activation domain fusions with either the γ subunit of AP-1 (A) or the δ subunit of AP-3 (B). Colonies of cotransformed yeast cells pooled, patch-plated on nonselective (+His) medium and then replica plated onto nonselective (+His) and selective (−His) medium. Growth over background on −His medium indicates a protein-protein interaction. Empty, yeast cotransformed with a vector expressing no Nef and no σ subunit, together with the vector expressing the γ-GAL4 activation domain fusion (A) or the δ-GAL4 activation domain fusion (B). Wild-type, native Nef, sequence ENTSLL. The mutants are indicated at the left.
Role of the E160, N161, T162, and S163 residues in the Nef-mediated stabilization of AP-1 and AP-3 on endosomal membranes in living human cells.
We previously reported that Nef stabilizes the association of AP-1 and AP-3 with endosomal membranes. This phenomenon was revealed in cells that are treated with brefeldin A, a fungal metabolite that inhibits guanine nucleotide exchange factors for ADP-ribosylation factor 1 (ARF1) (13). When cells are treated with brefeldin A, guanine nucleotide exchange on ARF1 is blocked, and AP-1 and AP-3 rapidly cycle off membranes and disperse throughout the cytosol (32, 41). However, when Nef-expressing cells are treated with brefeldin A, AP-1 and AP-3 remain colocalized with Nef in a juxtanuclear compartment, reflecting persistent endosomal association (23). This stabilization of AP-1 and AP-3 on endosomal membranes requires the dileucine sequence in Nef, suggesting that it is mediated by direct binding between Nef and the complexes.
Here, HeLa cells were transfected to express transiently either wild-type or mutated CD8-Nef and then treated with brefeldin A and stained for CD8 and either γ-adaptin (AP-1) (Fig. 7) or δ-adaptin (AP-3) (Fig. 8). As previously described, wild-type CD8-Nef was concentrated in a juxtanuclear region and often associated with abnormally large vesicles, whereas CD8-Nef LL/AA was expressed primarily at the cell surface (23). The E, N, T, and S mutants localized primarily to a juxtanuclear compartment, although CD8-Nef-E160K partially mislocalized to the plasma membrane, a finding consistent with the data of Fig. 1C. All the mutants except LL/AA localized to abnormally large endosomal vesicles. In untransfected cells, the distributions of AP-1 and AP-3 were diffuse and cytosolic, reflecting the block to membrane association mediated by brefeldin A. In cells expressing wild-type CD8-Nef, AP-1 and AP-3 retained a juxtanuclear concentration despite brefeldin A treatment; this stabilizing effect was not seen with CD8-Nef LL/AA.
All the substitutions of E160 blocked the ability of Nef to stabilize the membrane association of both AP-1 and AP-3 in brefeldin A-treated cells (Fig. 7 and 8), although trace activity was observed in the case of CD8-Nef-E160A and AP-1. In contrast, mutation of N161 had no effect on the stabilization of either complex. The T162G mutant was unable to stabilize the association of either complex with endosomal membranes. The S163G mutant was unable to stabilize AP-3 on endosomal membranes (Fig. 8), but it retained a substantial ability to stabilize AP-1 (Fig. 7).
Comparison of these data on endosomal stabilization with the AP-hemicomplex interactions (measured by yeast three-hybrid assay) and with the interactions with intact complexes (measured by GST pull-down) confirmed the critical role of the LL residues, but it further revealed a consistent and nearly obligatory role for E160 and T162 in the interactions between Nef and both AP-1 and AP-3. However, the activity of the S163G mutant in stabilizing the AP complexes on endosomes in human cells was most similar to the binding with the hemicomplexes in yeast: in both assays, the S163G mutant was unable to interact with AP-3, whereas it retained an ability to interact with AP-1.
DISCUSSION
Mutational analysis of the E160xxxLL sequence allowed us to correlate the modulation of distinct cellular proteins with the binding of Nef to specific members of the AP complex family (Table 2). GST pull-down, yeast three-hybrid, and endosomal stabilization assays indicated that, like residues L164 and L165, residues E160 and T162 are required for the efficient interaction of Nef with both AP-1 and AP-3. These assays also indicated that S163 is required for the interaction with AP-3 but may retain an ability to interact with AP-1. Phenotypically, E160 contributed to the down-regulation of CD4, but T162 and S163 did not. These data weigh against an obligatory role for AP-1 or AP-3 in the down-regulation of CD4 and suggest that the role of E160 in this phenotype is via another interaction (for example, with AP-2) that is particularly sensitive to substitution with a basic residue. In contrast, both E160 and T162 were essential for the inhibition of transferrin uptake by Nef, indicating a major role for AP-1 and/or AP-3 in this phenotype.
TABLE 2.
Summary of AP-binding and trafficking phenotypesdd
| Nef | Y3Ha with hemicomplexes
|
GST pull-down
|
Stabilizationb
|
CD4 down-regulation | Internalization as CD8-Nefc | Invariant chain up-reg. | DC-SIGN up-reg. | Tf uptake block | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AP-1 | AP-3 | AP-1 | AP-3 | AP-1 | AP-3 | ||||||
| Wild type | + | + | + | + | + | + | + | + | + | + | + |
| LL/AA | − | − | − | − | − | − | − | − | − | − | − |
| E160A | − | − | − | − | − | − | + | + | Int | Int | − |
| E160G | − | − | − | − | − | − | Int | + | − | − | − |
| E160K | − | − | − | − | − | − | − | Int | − | − | − |
| N161G | + | + | + | + | + | + | + | + | Int | Int | + |
| T162G | − | − | − | − | − | − | + | + | Int | Int | − |
| S163G | Int | − | − | − | + | − | + | + | Int | Int | Int |
Y3H, yeast three-hybrid.
Stabilization on endosomes in brefeldin-treated cells.
Inverse of surface expression.
+, positive; −, negative; Int, intermediate; reg, regulation.
In the cases of the invariant chain and DC-SIGN, the defects in up-regulation caused by uncharged substitutions of E160 and by substitutions of T162 and S163 were greater than the defects in the down-regulation of CD4, suggesting a more substantial contribution of AP-1 and/or AP-3 to these phenotypes. However, these complexes cannot be wholly responsible for these up-regulatory effects, because neither the T162G nor S163 mutant was fully defective. Consequently, as in the case of CD4, the disproportionate defects caused by the E160 mutations suggest a role for another interaction in the modulation of invariant chain and DC-SIGN.
Together, these data lead to two testable hypotheses. First, the E160 residue affects an interaction with an AP complex in addition to AP-1 and AP-3 that contributes to the modulation of CD4, invariant chain, and DC-SIGN; this may be AP-2. Second, Nef uses multiple AP family members to modulate different proteins in distinct but overlapping patterns: primarily AP-2 for CD4, AP-2 and AP-1 (and/or AP-3) for invariant chain and DC-SIGN, and AP-1 (and/or AP-3) for transferrin.
What are the implications of these data regarding the mechanisms of Nef action on different target proteins? As noted, the data suggest that interactions with AP-1 and AP-3 are largely dispensable for the down-regulation of CD4 from the cell surface (Table 2). This conclusion is supported by the persistent Nef-mediated down-regulation of CD4 observed in cells in which the μ subunit of either AP-1 or AP-3 has been depleted by RNA interference (35); it is also consistent with the finding that replacement of the ENTSLL sequence with DKQTLL yields a Nef protein unable to bind hemicomplexes of either AP-1 or AP-3 but still able to down-regulate CD4 (7). Similar to the case of down-regulation of CD4, the localization of CD8-Nef chimeras on internal membranes at steady state does not appear to require interactions between Nef and AP-1 or AP-3. Yet, both the down-regulation of CD4 and the internalization of the chimeras require the LL sequence and likely require AP complexes. Consequently, by eliminating major roles for AP-1 and AP-3, the data indirectly support the AP-2 complex as the most likely cofactor for the down-regulation of CD4 by Nef.
If AP-2 is the cofactor for the down-regulation of CD4, then the functional data predict that this interaction has less strict sequence requirements than the interaction with AP-1 and AP-3. The putative interaction must tolerate either a negatively charged or neutral amino acid at position −4 in the ExxxLL motif, because the E160A and E160G mutants retained 50% or greater activity in the down-regulation of CD4 and localized to internal membranes as effectively as the wild-type when expressed as CD8 chimeras. Similarly, this interaction must tolerate nonpolar residues at positions −1 and −2, because glycine substitutions at these sites had no apparent impact on the down-regulation of CD4 or the localization of the CD8 chimeras to internal membranes. However, if an interaction between Nef and AP-2 underlies the down-regulation of CD4, then it must be impaired by a positively charged residue at position −4: the E160K mutant inefficiently down-regulated CD4, and CD8-Nef E160K was mislocalized in part to the plasma membrane.
In support of this model, the effect of the Nef E160K substitution is analogous to that of substitution of the acidic residues in the LIMP II leucine-based motif with arginines: mislocalization from endosomal membranes to the cell surface, presumably due to a failure of internalization via AP-2 (38). Notwithstanding these considerations, the nearly undetectable direct interaction between HIV-1 Nef and AP-2, together with recent knock-down experiments indicating that AP-2 is not obligatory, leaves the role of AP-2 in the down-regulation of CD4 formally unproven (5, 36). Indeed, taken together, the published knock-down experiments suggest that no individual complex (neither AP-1, -2, or -3) is obligatory for the down-regulation of CD4 by HIV-1 Nef (35, 36); conceivably, HIV-1 Nef may be able to use more than one member of the AP family to reduce the expression of CD4 at the cell surface.
In contrast to the down-regulation of CD4, the block to transferrin uptake mediated by Nef correlated closely with the binding to AP-1 and/or AP-3: the E160, T162, and S163 residues each played an major role in this effect, just as they did in assays of interaction with AP-1 and AP-3 (Table 2). Notably, we recently hypothesized that the block to transferrin uptake is due to reduced expression of transferrin receptor at the cell surface, which in turn is due to an inhibition of the recycling of internalized transferrin receptor to the plasma membrane (29). However, data herein indicate that mutation of residue E160, T162, or S163 has relatively little effect on the down-regulation of transferrin receptor despite markedly impairing the block to transferrin uptake. These data leave the mechanism of the uptake block open to speculation.
Interestingly, the hierarchical contribution of individual residues in the ExxxLL motif to the up-regulation of the invariant chain was similar to that of DC-SIGN. Although the importance of the E160 residue supports a role for AP-1 and/or AP-3 in both of these properties, the modest impairment cause by the T162G and S163G substitutions (together with the detectable impairment caused by the N161G substitution) renders interpretation complex. Overall, this imprecise fit between the AP-binding data and the modulation of DC-SIGN and invariant chain suggests the involvement of leucine-dependent cofactors in addition to AP-1 and AP-3 in these effects. Notably, recent experiments using RNA interference to knock down the expression of AP complexes in HeLa-CIITA cells indicate that AP-2 is predominantly responsible for targeting the invariant chain to the endosomal class II antigen processing compartment (14). Consequently, part of the Nef-mediated up-regulation of invariant chain could be due to an inhibition of endocytosis mediated by AP-2. This hypothesis is consistent with the above conclusion that the disproportionate effects of the E160 substitutions on the up-regulation of the invariant chain indicate involvement of an AP complex in addition to AP-1 and AP-3. Similar considerations apply to the up-regulation of DC-SIGN. The data are best explained by a role for both AP-2 and AP-1/AP-3 in these phenotypes; such a model allows the modest effects of the T162 and S163 mutations to be attributed to impaired interactions with AP-1 and/or AP-3, while the quantitatively greater effects of the E160 mutations are attributed to an additional effect on the putative interaction with AP-2.
Notably, the mechanism by which Nef up-regulates the surface expression of host proteins is unclear. One hypothesis is that saturation of AP complexes by Nef causes the displacement of the target protein to the cell surface by default; we proposed this mechanism to explain the Nef-mediated increase in the surface expression of a Tac chimera containing the DKQTLL sequence (9, 30). Both the invariant chain and DC-SIGN have dileucine-based sorting signals in their cytoplasmic domains (34, 40). Interestingly, both motifs in the invariant chain have typically spaced upstream acidic residues, but the motif in DC-SIGN has no such residue. If only similar motifs compete with each other for specific AP complexes, then mutation of E160 would have affected only the up-regulation of invariant chain and not DC-SIGN. Consequently, the finding that E160 plays a major role in the up-regulation of both invariant chain and DC-SIGN weighs against a simple competition for AP complexes as the mechanism of up-regulation.
The data herein also provide potential insights into how individual residues in ExxxLL motifs contribute to interactions with AP-1 and AP-3. Previous studies have indicated that acidic residues at positions −4 and/or −5 relative to the leucines are important for binding to AP-3 (11, 22, 38). Here, three distinct binding assays (GST pull-down, yeast three-hybrid, and endosomal stabilization assays) confirmed the role of the glutamic acid at the −4 position in the Nef ExxxLL sequence for the binding to AP-3. However, this residue was almost equally important for the binding to AP-1, indicating that the role of acidic residues in ExxxLL motifs may extend to interactions with AP-1 as well as AP-3.
The original study using the three-hybrid assay to analyze dileucine-based interactions with AP complexes reported no effect of a T162A substitution on the interaction between Nef and hemicomplexes of AP-1 and AP-3 (24). In contrast, the T162G substitution herein markedly impaired the interactions of Nef with both complexes as observed not only using yeast-three hybrid but also using GST pull-down and endosomal stabilization assays. Notably, the results herein are consistent with a previous mutagenesis study in yeast that indicated a requirement for a polar residue at position −2 for the interaction with AP-3 (11). The data herein also indicated that position −1 relative to the leucines is constrained with respect to binding these complexes: the S163G mutation impaired the interaction with AP-3 in every assay, although at least partial activity in binding AP-1 was preserved in two of three assays. Together, the data indicate that the −1, −2, and −4 positions relative to the leucines in ExxxLL motifs are direct contributors to the interactions with the AP-1 and AP-3 complexes.
Taken together, the differential effects of the E, T, and S mutations reviewed above suggest that Nef uses different AP complexes, or different combinations of complexes, to modulate specific proteins. If this hypothesis is correct, then the complexes may be specified not only by Nef but also by the target protein itself. The cytoplasmic domains of CD4, transferrin receptor, DC-SIGN, and the invariant chain each contain sorting motifs (1, 8, 34, 40). The simplest mechanism by which these proteins can contribute to the utilization of specific AP complexes is by the involvement of their own sorting motifs in the selection process.
Notably, the concept that the sorting motifs in target proteins may interact directly with AP complexes during Nef-mediated modulation has been supported by two recent studies, each of which reported that the LL sequence in the cytoplasmic domain of CD4, while required for Nef-mediated down-regulation, does not contribute to the interaction between Nef and CD4 (2, 6). In this emerging model, the AP binding motifs in both Nef and the target protein are functional. Instead of serving as a simple connector between the cytoplasmic domains of target proteins and AP complexes (33), Nef would facilitate the interactions of its targets with the complexes via their own AP-binding signals. This facilitation may be based in the ability of Nef to stabilize the complexes on endosomal membranes, as shown herein. Although we can document this stabilization only in the case of AP-1 and AP-3, it may occur with AP-2 as well, explaining the ability of Nef to induce the formation of coated pits at the plasma membrane (16).
Interestingly, this “facilitator” model allows Nef to perform a catalytic-like function: it may stimulate the inclusion of a target protein in a transport vesicle without itself becoming part of that vesicle. This scenario is supported by a heretofore anomalous observation exemplified herein: although certain mutations in the ExxxLL motif clearly alter the subcellular distribution of CD8-Nef chimeras in which Nef is a covalent part of a transmembrane protein, specifically Nef-LL/AA and Nef-E160K (Fig. 7 and 8) (15, 23), the same mutations have no effect on the subcellular distribution of Nef-GFP (Fig. 4) (10), despite their ability to abrogate the effect of Nef-GFP on target proteins.
In summary, mutagenesis of the ExxxLL motif has revealed a differential role of the acidic residue in a subset of the leucine-dependent, Nef-induced perturbations of cellular protein trafficking. Binding studies implicated AP-1 and AP-3 in some of these effects but suggested by default a role for AP-2 in others. Together, the data are consistent with a model in which Nef uses different AP complexes, or different combinations of complexes, to modulate specific proteins. We speculate that the cytoplasmic domains of the proteins targeted by Nef participate in the selection of the complexes and that Nef facilitates sorting via a stabilizing effect on the vesicle coat.
Acknowledgments
Scott Coleman was in the Biomedical Sciences program of UCSD. Richard Mitchell was supported by an AIDS Training grant from the NIH (AI07384). This work was supported by grants from the National Institutes of Health (AI38201), the UCSD Center for AIDS Research (NIH AI36214), the Research Center for AIDS and HIV Infection of the San Diego Veterans Affairs Healthcare System, and the Agence Nationale de Recherche sur le SIDA and SIDACTION, France.
We thank Juan Bonifacino for the yeast three-hybrid vectors, Nathalie Sol-Foulon for the plasmid expressing DC-SIGN, Philippe Benaroch for the HeLa-CIITA cells, and Colleen Noviello and John Day for reviewing the manuscript.
REFERENCES
- 1.Aiken, C., J. Konner, N. R. Landau, M. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 2.Bentham, M., S. Mazaleyrat, and M. Harris. 2003. The di-leucine motif in the cytoplasmic tail of CD4 is not required for binding to human immunodeficiency virus type 1 Nef, but is critical for CD4 down-modulation. J. Gen. Virol. 84:2705-2713. [DOI] [PubMed] [Google Scholar]
- 3.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C.-H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853-866. [DOI] [PubMed] [Google Scholar]
- 4.Bresnahan, P. A., W. Yonemoto, S. S. Ferrell, D. G. R. Williams-Herman, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8:1235-1238. [DOI] [PubMed] [Google Scholar]
- 5.Bresnahan, P. A., W. Yonemoto, and W. C. Greene. 1999. Cutting edge: SIV Nef protein utilizes both leucine- and tyrosine-based protein sorting pathways for down-regulation of CD4. J. Immunol. 163:2977-2981. [PubMed] [Google Scholar]
- 6.Cluet, D., C. Bertsch, C. Beyer, L. Gloeckler, M. Erhardt, J. P. Gut, J. L. Galzi, and A. M. Aubertin. 2005. Detection of human immunodeficiency virus type 1 Nef and CD4 physical interaction in living human cells by using bioluminescence resonance energy transfer. J. Virol. 79:8629-8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman, S. H., N. Van Damme, J. R. Day, C. M. Noviello, D. Hitchin, R. Madrid, S. Benichou, and J. C. Guatelli. 2005. Leucine-specific, functional interactions between human immunodeficiency virus type 1 Nef and adaptor protein complexes. J. Virol. 79:2066-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collawn, J. F., M. Stangel, L. A. Kuhn, V. Esekogwu, S. Q. Jing, I. S. Trowbridge, and J. A. Tainer. 1990. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell 63:1061-1072. [DOI] [PubMed] [Google Scholar]
- 9.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig, H. M., T. R. Reddy, N. L. Riggs, P. P. Dao, and J. Guatelli. 2000. Interactions of HIV-1 Nef with the μ subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology 271:9-17. [DOI] [PubMed] [Google Scholar]
- 11.Darsow, T., C. G. Burd, and S. D. Emr. 1998. Acidic di-leucine motif essential for AP-3-dependent sorting and restriction of the functional specificity of the Vam3p vacuolar t-SNARE. J. Cell Biol. 142:913-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson, J. G., D. Finazzi, and R. D. Klausner. 1992. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360:350-352. [DOI] [PubMed] [Google Scholar]
- 14.Dugast, M., H. Toussaint, C. Dousset, and P. Benaroch. 2005. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J. Biol. Chem. 280:19656-19664. [DOI] [PubMed] [Google Scholar]
- 15.Erdtmann, L., K. Janvier, G. Raposo, H. M. Craig, P. Benaroch, C. Berlioz-Torrent, J. C. Guatelli, R. Bernarous, and S. Benichou. 2000. Two independent regions of HIV-1 Nef are required for connection with the endocytic pathway through binding to the μ1 chain of AP1 complex. Traffic 1:871-883. [DOI] [PubMed] [Google Scholar]
- 16.Foti, M., A. Mangasarian, V. Piguet, D. P. Lew, K.-H. Krause, D. Trono, and J.-L. Carpentier. 1997. Nef-mediated clathrin-coated pit formation. J. Cell Biol. 139:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8:1239-1242. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16:6964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao, M., and F. R. Maxfield. 2000. Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 275:15279-15286. [DOI] [PubMed] [Google Scholar]
- 21.Hirst, J., and M. S. Robinson. 1998. Clathrin and adaptors. Biochim. Biophys. Acta 1404:173-193. [DOI] [PubMed] [Google Scholar]
- 22.Honing, S., I. V. Sandoval, and K. von Figura. 1998. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 17:1304-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janvier, K., H. Craig, D. Hitchin, R. Madrid, N. Sol-Foulon, L. Renault, J. Cherfils, D. Cassel, S. Benichou, and J. Guatelli. 2003. HIV-1 Nef stabilizes the association of adaptor protein complexes with membranes. J. Biol. Chem. 275:8725-8732. [DOI] [PubMed] [Google Scholar]
- 24.Janvier, K., Y. Kato, M. Boehm, J. R. Rose, J. A. Martina, B. Y. Kim, S. Venkatesan, and J. S. Bonifacino. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 163:1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 26.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell surface expresion of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622-631. [DOI] [PubMed] [Google Scholar]
- 27.Lama, J., and C. F. Ware. 2000. Human immunodeficiency virus type 1 nef mediates sustained membrane expression of tumor necrosis factor and the related cytokine LIGHT on activated T cells. J. Virol. 74:9396-9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J.-M. Heard, and O. Schwartz. 1998. Nef interacts with the μ subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC 1 molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 29.Madrid, R., K. Janvier, D. Hitchin, J. Day, S. Coleman, C. Noviello, J. Bouchet, A. Benmerah, J. Guatelli, and S. Benichou. 2005. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 280:5032-5044. [DOI] [PubMed] [Google Scholar]
- 30.Marks, M. S., L. Woodruff, H. Ohno, and J. S. Bonifacino. 1996. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J. Cell Biol. 135:341-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 32.Ooi, C. E., E. C. Dell'Angelica, and J. S. Bonifacino. 1998. ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J. Cell Biol. 142:291-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piguet, V., Y.-L. Chen, A. Mangasarian, M. Foti, J.-L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the μ chain of adaptor complexes. EMBO J. 17:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pond, L., L. A. Kuhn, L. Teyton, M.-P. Schutze, J. A. Tainer, M. R. Jackson, and P. A. Peterson. 1995. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J. Biol. Chem. 270:19989-19997. [DOI] [PubMed] [Google Scholar]
- 35.Roeth, J. F., M. Williams, M. R. Kasper, T. M. Filzen, and K. L. Collins. 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J. Cell Biol. 167:903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose, J. J., K. Janvier, S. Chandrasekhar, R. P. Sekaly, J. S. Bonifacino, and S. Venkatesan. 2005. CD4 down-regulation by HIV-1 and simian immunodeficiency virus (SIV) Nef proteins involves both internalization and intracellular retention mechanisms. J. Biol. Chem. 280:7413-7426. [DOI] [PubMed] [Google Scholar]
- 37.Ross, T. M., A. E. Oran, and B. R. Cullen. 1999. Inhibition of HIV-1 progeny virion release by cell surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9:613-621. [DOI] [PubMed] [Google Scholar]
- 38.Sandoval, I. V., S. Martinez-Arca, J. Valdueza, S. Palacios, and G. D. Holman. 2000. Distinct reading of different structural determinants modulates the dileucine-mediated transport steps of the lysosomal membrane protein LIMPII and the insulin-sensitive glucose transporter GLUT4. J. Biol. Chem. 275:39874-39885. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J.-M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 40.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16:145-155. [DOI] [PubMed] [Google Scholar]
- 41.Stamnes, M. A., and J. E. Rothman. 1993. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell 73:999-1005. [DOI] [PubMed] [Google Scholar]
- 42.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobiume, M., M. Takahoko, T. Yamada, M. Tatsumi, A. Iwamoto, and M. Matsuda. 2002. Inefficient enhancement of viral infectivity and CD4 downregulation by human immunodeficiency virus type 1 Nef from Japanese long-term nonprogressors. J. Virol. 76:5959-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]