Abstract
Infection of cells by the highly anemogenic feline leukemia virus subgroup C (FeLV-C) is mediated by the heme exporter FLVCR1, a cell surface protein containing 12 potential transmembrane segments with six presumptive extracellular loops (ECLs). To identify FLVCR1 residues critical for mediating FeLV-C infection, we first independently isolated a human cDNA encoding the FLVCR2 protein that shares 52% identity to human FLVCR1, and we show that FLVCR2 does not function as a receptor for FeLV-C. Then, by generating specific hybrids between FLVCR1 and FLVCR2 and testing susceptibility of mouse cells expressing these hybrids to β-galactosidase encoding FeLV-C, we identify FLVCR1 ECLs 1 and 6 as critical for mediating FeLV-C infection. Mouse cells expressing a hybrid protein containing FLVCR2 backbone with the ECL6 sequence from FLVCR1 were highly susceptible to FeLV-C infection. Using site-directed mutagenesis, we show that a single mutation of Asn463 in FLVCR2 ECL6 to an acidic Asp residue (a residue present in the corresponding position 487 in FLVCR1 ECL6) is sufficient to render FLVCR2 functional as an FeLV-C receptor. However, an Asp487Asn mutation in FLVCR1 ECL6 or substitution of the entire FLVCR1 ECL6 sequence for FLVCR2 ECL6 sequence does not disrupt receptor function. Subsequent substitutions show that residues within FLVCR1 ECL1 also contribute to mediating FeLV-C infection. Furthermore, our results suggest that FLVCR1 regions that mediate FeLV-C surface unit binding are distinct from ECL1 and ECL6. Our results are consistent with previous conclusions that infection of cells by gammaretroviruses involves interaction of virus with multiple receptor regions.
An interesting feature of cell surface receptors used for entry by gammaretroviruses (γ-retroviruses) is that they all contain multiple transmembrane (TM) segments (reviewed in reference 30). Furthermore, these receptors have been identified as transporters of important nutrients or have been shown to belong to transporter families (4, 12, 13, 20, 24-26, 33-37). Mapping receptor domains and residues critical for γ-retrovirus infection has often relied on generating specific hybrid constructs between a functional receptor, isolated from cells sensitive to virus infection, and a nonfunctional receptor homologue, normally isolated from cells resistant to retrovirus infection. This functional approach has been successfully used to map critical receptor-functioning domains and residues in many gammaretroviral receptors (reviewed in reference 30) and have allowed identification of authentic virus binding sites and of sites that are critical for membrane orientation of the receptor (10). These studies have shown that multiple receptor domains are required for mediating efficient γ-retrovirus binding and infection and suggest a common mechanism of interaction between γ-retroviruses and their respective receptors. The only exception is the interaction of ecotropic murine leukemia virus with the cationic amino acid transporter, CAT1 (3, 13, 36). To date, only presumptive extracellular loop 3 (ECL3) has been identified as critical for virus infection (2, 9, 23, 38).
The identification and characterization of a receptor for feline leukemia virus subgroup C (FeLV-C) has been of considerable interest because of its possible implication in feline and human pure red cell aplasia (PRCA) (25). This disease is characterized by a specific block in development of erythroid progenitor cells (1, 8), which are important precursor cells for erythrocytes. Feline PRCA is caused by FeLV-C (1, 8), which arises in infected cats from the weakly pathogenic progenitor FeLV subgroup A through mutations in the envelope gene responsible for receptor recognition (19, 28). These studies have led to the hypothesis that PRCA is caused by interaction of FeLV-C envelope with its cell surface receptor. The receptor for FeLV-C has been identified as FLVCR1 (also termed FLVCR) (24, 35), which is a member of the major facilitator superfamily of transporters (22). Hydropathy algorithms (11, 14) predict FLVCR1 to contain 12 potential membrane-spanning segments with six presumptive ECLs. The cellular function of FLVCR1 was recently identified as an exporter of heme (25). A block in surface expression of FLVCR1 causes a build-up of heme in erythroid progenitor cells and subsequent death of these cells by apoptosis.
In this study, we aimed to identify FLVCR1 domains that are critical for mediating efficient FeLV-C binding and infection and to ascertain whether interaction of FeLV-C with its receptor was consistent with interaction of other γ-retroviruses with their respective receptors. In our previous attempt to map human FLVCR1 residues critical for mediating FeLV-C infections, we had focused on isolating an FLVCR1 homologue from Mus dunni tail fibroblast (MDTF) cells, which are resistant to FeLV-C infection, with the hypothesis that murine FLVCR1 was a nonfunctional receptor for FeLV-C. However, we found that MDTF FLVCR1 functions as an efficient FeLV-C receptor when overexpressed in MDTF cells (32). In this study, we independently isolated a human cDNA encoding FLVCR2, a protein that is highly related in sequence and topology to FLVCR1, and we show that this protein does not function as a receptor for FeLV-C when overexpressed in MDTF cells. By generating specific hybrids and mutants between FLVCR1 and FLVCR2, our results suggest that multiple FLVCR1 regions are critical for mediating efficient FeLV-C infection and that these regions are distinct from the FeLV-C envelope binding site.
MATERIALS AND METHODS
Cells and viruses.
Human TE671, MDTF, and TELCeB6 cells (7) were all maintained in Dulbecco's minimal essential medium with low glucose and supplemented with 10% fetal bovine serum (FBS). Human embryonic kidney 293 cells and phoenix ampho packaging cells (provided by Garry Nolan, Stanford University) were maintained in Dulbecco's minimal essential medium with high glucose and 10% FBS. TELCeB6 cells are retroviral-packaging cells that do not contain retroviral envelope genes but produce noninfectious virus (7). Phoenix ampho cells produce replication-defective amphotropic murine leukemia virus.
lacZ(FeLV-C) pseudotype virus was generated by transfection (calcium phosphate precipitation [Stratagene]) of TELCeB6 cells with the FBCsalf retroviral expression vector (FeLV-C Sarma envelope gene [27] cloned into the FBsalf retroviral expression vector [7]). Transfectants were selected with phleomycin (50 μg/ml), and resistant colonies were pooled. Infection of target cells with lacZ(FeLV-C) was carried out as described below.
Isolation of human FLVCR2 cDNA.
Homologous sequences to human FLVCR1 cDNA were identified by BLAST (Basic Local Alignment Search Tool, available at http://www.ncbi.nlm.nih.gov/BLAST/) sequence comparison. A cDNA sequence isolated from HepG2 human hepatoma cells, showing high sequence identity to human FLVCR1 cDNA, was identified. Specific primers were subsequently designed complementary to the 5′ end (upstream primer) and 3′ end (downstream primer) of the potential coding region of HepG2 FLVCR1-related (FLVCR2) cDNA sequence (upstream primer, 5′-ACTGTGGCGATGGTGAATGAAGGTCCCAAC-3′; downstream primer, 5′-TTCCTCTCAGAGATGATCCTCTGACACAGC-3′). These primers were used in the amplification of the homologous FLVCR2 cDNA from human TE671 cells. Briefly, total RNA from TE671 cells was isolated (QIAGEN RNA midiprep isolation system; Mississauga, Ontario, Canada) and subsequently used to generate a cDNA library (Stratagene, Cedar Creek, TX). TE671 FLVCR2 cDNA was isolated by PCR using the primers described above and the TE671 cDNA library as a template. The PCR was run for 30 cycles at an annealing temperature of 57°C for 1.5 min and an extension temperature of 68°C for 1.5 min. The isolated FLVCR2 cDNA was cloned into the mammalian expression vector pcDNA3.1V5HisTOPO (pcDNA3.1VH) (Invitrogen, Burlington, Ontario, Canada), in frame with the V5 epitope sequence, and subsequently sequenced on a PE/ABD 377 sequencer by using dye terminator cycle sequencing chemistry (Applied Biosystems, Foster City, CA). V5-tagged FLVCR1 was generated by PCR amplification of human FLVCR1 cDNA and subsequent cloning in pcDNA3.1VH vector to obtain the plasmid pcDNA3.1FLVCR1V5.
Construction of hybrid FLVCR1/FLVCR2 cDNA expression vectors.
Hybrid and mutant human FLVCR1/FLVCR2 cDNAs were generated by PCR. Specific forward and complementary primers were designed to the specified region to generate hybrid and mutant FLVCR1/FLVCR2 cDNAs (see Fig. 2). For V5-tagged constructs the amplified DNA was initially cloned into the pcDNA3.1VH vector in frame with sequence encoding the V5 epitope. The V5-tagged constructs were cloned into the pFBneo retroviral vector (Stratagene) as follows. Two oligonucleotide primers containing EcoRI restriction enzyme sites flanking XcmI restriction sites were generated. These primers specifically prime upstream and downstream of the multiple cloning site in pCDNA3.1FLVCR1V5 vector. These primers were used to amplify human FLVCR1V5 fusion cDNA. The amplified cDNA was digested with EcoRI and subsequently cloned into an EcoRI-digested pFBneo vector to generate the pFBneoTFLVCR1V5 construct. A pFBneo clone containing the FLVCR1V5 in the forward orientation was subsequently used to clone other FLVCR1/FLVCR2 hybrid constructs. Hybrid FLVCR1/FLVCR2V5 fusion constructs were isolated from pcDNA3.1VH vector by digestion with BstXI restriction enzyme. The cDNAs were subsequently cloned into a BstXI-digested pFBneoTFLVCR1V5 vector. A BstXI digest removes the FLVCR1 sequence and subsequently allows cloning of mutant or hybrid FLVCR1/FLVCR2 constructs in the pFBneo retroviral vector while maintaining a fusion with the V5 epitope. Hemagglutinin (HA)-tagged constructs were amplified by PCR and subsequently cloned into an XcmI-cut pFBneoTFLVCR1V5 vector. An XcmI digest of the pFBneoTFLVCR1V5 vector removes the FLVCR1V5 sequence, resulting in the generation of a pFBneo vector with T overhangs. All PCR-amplified HA receptor cDNAs were cloned into this vector.
FIG. 2.
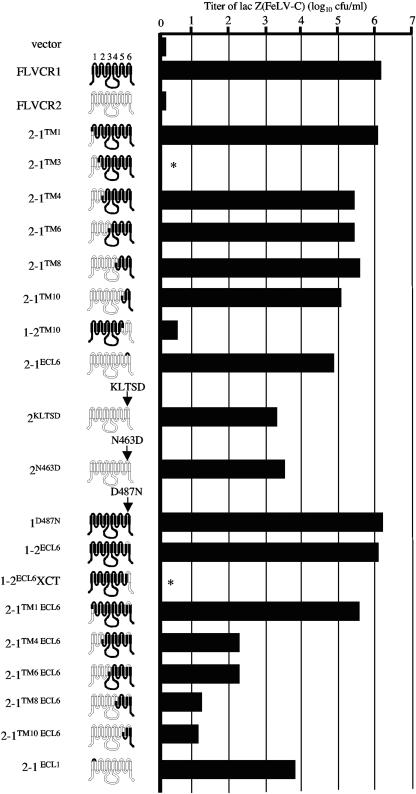
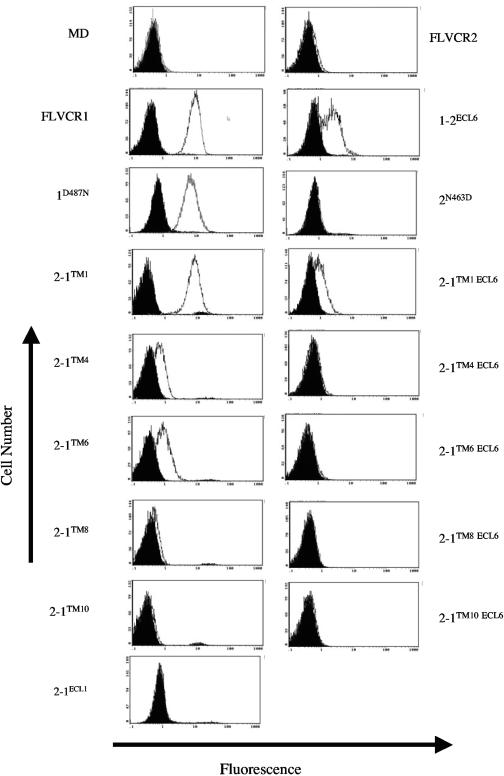
Susceptibility of MDTF cells expressing FLVCR1, FLVCR2, or FLVCR1/FLVCR2 hybrid or mutant constructs to lacZ(FeLV-C). These structures are hypothetical based on hydrophobicity algorithms. Titers are the averages of three independent experiments. The potential extracellular loops are numbered above FLVCR1. Proteins that were not expressed on the cell surface are represented by asterisks.
Expression of hybrid and mutant FLVCR1/FLVCR2 in MDTF cells.
FLVCR1/FLVCR2 hybrid and mutant constructs in pFBneo retroviral vector were introduced in phoenix ampho retroviral packaging cells by transfection using PolyFect reagent (QIAGEN). Two days posttransfection, supernatant was harvested and filtered using a 0.45-μm-pore-size filter. Filtered viral supernatant was used to infect MDTF cells to stably introduce FLVCR1/FLVCR2 constructs. Infected cells were selected using G418 (1.5 mg/ml). Resistant cells were pooled and used for infection and binding assays.
Virus infection.
Target cells were seeded in 24-well plates (1.0 × 104 cells/well) and incubated overnight at 37°C. The following day the cells were incubated with 1 ml of serially diluted lacZ(FeLV-C) supernatant for 4 h in the presence of polybrene (8 μg/ml). The virus supernatant was then replaced with fresh growth medium, and cells were allowed to incubate for a further 2 days before X-Gal (5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside) (Sigma-Aldrich, Canada) staining. lacZ(FeLV-C) pseudotype titers were determined by counting the number of blue CFU, and titers were expressed as the number of CFU obtained per milliliter of virus supernatant.
Protein analysis.
Approximately, 1 × 107 receptor-expressing cells grown in a 75-cm2 tissue culture flask were lysed using 200 μl of cell lysis buffer (20 mM Tris-HCl, pH 7.5, 1% Triton X-100, 0.05% sodium dodecyl sulfate [SDS], 5 mg/ml sodium deoxycholate, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride) at 4°C for 10 min. Cell genomic DNA was pelleted by centrifugation at 13,000 × g for 10 min at 4°C. Cell lysate supernatant was either stored at −80°C or used for protein analysis. Approximately 100 μg of total protein was run on a 10% SDS-polyacrylamide gel, and proteins were subsequently transferred to a nitrocellulose membrane (Pall, Pensacola, FL). V5-tagged FLVCR1/FLVCR2 proteins were detected by incubation of nitrocellulose membranes with anti-V5 monoclonal antibody (Invitrogen) diluted 1 in 500 in phosphate-buffered saline (PBS) containing 0.1% Tween 20. This was followed by incubation with rabbit anti-mouse antibody conjugated to horseradish peroxidase (HRP) (Sigma) diluted 1 in 1,000 in PBS-0.1% Tween 20. HA-tagged proteins were detected by incubation of nitrocellulose membranes with the anti-HA HRP antibody (Sigma-Aldrich). Signals were detected using chemiluminescence reagent (Perkin Elmer, Boston, MA), followed by exposure to Kodak Biomax MR film. For loading control of cell lysate samples, the nitrocellulose membrane was incubated with anti-actin monoclonal antibody (diluted 1 in 1,000; Sigma-Aldrich) followed by goat anti-mouse HRP (diluted 1 in 1,000; Sigma-Aldrich). Cell membrane samples were prepared from cells grown to confluence in 150-mm diameter tissue culture plates. Cells were initially washed with PBS and then resuspended in 3 ml of cold membrane lysis buffer (20 mM Tris [pH 7.4], 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 20 mM aprotinin). The cells were scraped from the tissue culture dish using a cell scraper and then homogenized using a Dounce homogenizer. The nuclear fraction was pelleted by centrifugation at 1,000 × g for 20 min at 4°C. Membrane fractions were pelleted by centrifugation of the nucleus-free supernatant at 30,000 rpm for 1 h at 4°C in a Beckman SW41 rotor. The membrane pellet was resuspended in 40 μl of PBS. Twenty microliters of membrane sample was run on a 10% SDS-polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane, and V5- and HA-tagged receptor proteins were detected as described above. For loading control, the remaining 20 μl of membrane sample was run on another 10% gel and transferred to a nitrocellulose membrane, which was subsequently incubated with a monoclonal antibody against the α subunit of the sodium potassium ATPase membrane protein (Sigma-Aldrich).
For analysis of glycosylated proteins, approximately 100 μg of total protein from cell lysates was incubated with or without N-glycosidase F enzyme PNGase F (Sigma) for 2 h at 37°C. Samples were run on a 10% SDS-polyacrylamide gel and the V5- and HA-tagged receptor proteins were detected as described above.
Determining relative surface expression of FLVCR1/FLVCR2 receptor proteins.
The relative surface expression of FLVCR1/FLVCR2 proteins was determined by estimating the band intensities of the proteins on scans of the immunoblots using the National Institutes of Health Image J (image processing) software (http://rsb.info.nih.gov/ij/) and then normalizing these band intensities to the band intensities of the control Na+K+ ATPase. The surface expression of hybrid FLVCR1/FLVCR2 proteins was then compared to expression of FLVCR1 and 2-1TM1 (a construct containing the amino intracellular region of FLVCR2 and the remaining TM-containing regions from FLVCR1 with the cross-junction at TM1), which were assigned a factor of 1.
FeLV-C SU envelope binding assay.
A pCS-FSCHA expression construct containing FeLV-C surface unit (SU) cDNA fused in frame with a double HA epitope was kindly provided by Julie Overbaugh. This construct was generated as described previously by Sugai and colleagues (29). Human 293 cells were seeded at 1 × 106 cells in a 100-mm culture dish 1 day prior to transfection. The cells were then transfected with 10 μg of pCS-FSCHA using PolyFect transfection reagent. Two days posttransfection, the supernatant was harvested and filtered using a 0.45-μm-pore-size filter. The HA-tagged FeLV-C SU envelope was subsequently stored at −80°C and used for envelope binding studies.
MDTF cells stably expressing high levels of FLVCR1/FLVCR2 hybrid or mutant proteins were treated with a cell dissociation buffer (Invitrogen) to dislodge cells. Approximately 1 × 106 cells were used for each binding assay. The cells were first incubated with 1 ml of FeLV-C SU HA-tagged envelope in the presence of polybrene (8 μg/ml) for 30 min at 37°C. Cells were then centrifuged at 4,000 rpm for 3 min. All subsequent spins were carried out at 4,000 rpm for 3 min. Cells were then washed two times with cold PBS containing 2% FBS (2% PFBS). Target cells were then incubated on ice for 35 min with 100 μl 2% PFBS containing monoclonal HA.11 antibody (diluted 1:200; Covance, Berkley, CA). Cells were washed again two times with 2% PFBS before incubation for 35 min on ice with 100 μl of PFBS containing donkey anti-mouse antibody, at a dilution of 1:25, conjugated to fluorescein isothiocyanate (1 mg/ml) (Sigma). After 30 min of incubation, 1 μl of propidium iodide (2 mg/ml) was added and cells, were incubated on ice for a further 5 min before being washed two times with cold PFBS. Target cells were then incubated for 10 min with 1% paraformaldehyde to fix the cells. Cells were then analyzed for envelope binding using a fluorescence-activated cell sorter (Beckman Coultier, Mississauga, Ontario, Canada).
Nucleotide sequence accession number.
The sequence of TE671 FLVCR2 cDNA was deposited in the GenBank database under accession number AF456126.
RESULTS
Isolation and characterization of human FLVCR2.
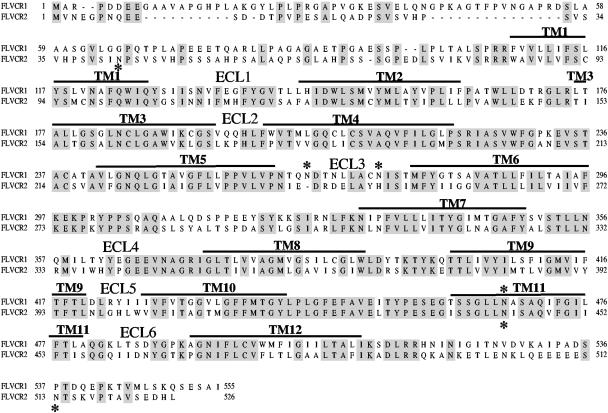
To identify FLVCR1 domains critical for mediating FeLV-C infection, we searched the NCBI database for an FLVCR1-related sequence with the aim of generating hybrids between FLVCR1 and the FLVCR1-related sequence and testing these hybrids for FeLV-C receptor function. We found a sequence isolated from human HepG2 hepatoma cells (accession no. NP_060261) that shared 52% amino acid identity with human FLVCR1. We termed this sequence FLVCR2 because of its strong sequence identity (Fig. 1) and similar predicted membrane topology to human FLVCR1 (not shown). We subsequently isolated a 1.578-kb human cDNA encoding FLVCR2 (Fig. 1) from human TE671 cells by PCR, using primers complementary to the coding region of HepG2 FLVCR2. Our FLVCR2 amino acid sequence is identical to the previously cloned sequences FLVCR14q (16) and CCT (6). FLVCR2 is a member of the major facilitator superfamily of transporters and contains the characteristic signature sequence between TM2 and TM3 (22). Kyte-Doolittle algorithms predict FLVCR2 to contain 12 TM segments with six presumptive ECLs (Fig. 1).
FIG. 1.
Sequence alignment of human FLVCR1 and human FLVCR2. Identical amino acids are shaded. Potential TM segments are shown as a line above amino acid sequence. The presumptive ECLs are also indicated above the sequence. Potential N-linked glycosylation sites are indicated by asterisks. Dashed lines are introduced for alignment. The assignment of TM segments is predicted by hydrophobicity algorithms (11, 14).
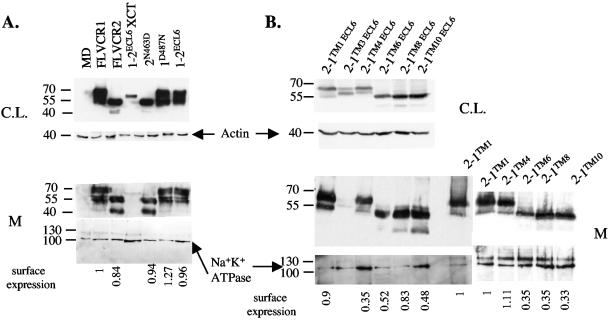
To test the ability of FLVCR2 to mediate infection of FeLV-C, we first cloned the FLVCR2 cDNA into the pFBneo retroviral expression vector. A V5 epitope sequence was tagged in frame with the coding region at the 3′ end of the FLVCR2 cDNA to allow detection of the encoded protein (see Materials and Methods). In addition, the human FLVCR1 cDNA was also tagged at the 3′ end with the V5 sequence and cloned into pFBneo. The FLVCR1 and FLVCR2 retroviral constructs were transfected into retroviral packaging cells, and the pseudotype viruses carrying the FLVCR1 or FLVCR2 sequences, produced from the packaging cells, were used to infect MDTF cells. MDTF cells expressing FLVCR1 or FLVCR2 constructs were subsequently tested for sensitivity to lacZ(FeLV-C). MDTF cells expressing FLVCR1 were highly sensitive to lacZ(FeLV-C), consistent with previous results showing that FLVCR1 is a receptor for FeLV-C. However, expression of FLVCR2 in MDTF cells did not cause an increase in lacZ(FeLV-C) infection (Fig. 2). Both FLVCR1 and FLVCR2 were efficiently expressed on the cell surface (Fig. 3A). Relative surface expression of receptor proteins was determined by first immunoblotting membrane samples prepared from MDTF cells expressing receptor proteins and then quantification using Image J software. These results clearly suggest that FLVCR2 does not function as a receptor for FeLV-C.
FIG. 3.
Western blot analysis of FLVCR1, FLVCR2, and hybrid FLVCR1/FLVCR2 proteins. Cell lysate (C.L.) and crude membrane (M) samples from MDTF cells infected with retrovirus carrying FLVCR1, FLVCR2, or hybrid FLVCR1/FLVCR2 constructs were analyzed for protein expression. The loading controls for actin and Na+ K+ ATPase are shown. (A) These proteins were tagged with V5 epitope and detected using monoclonal anti-V5 antibody and rabbit anti-mouse HRP antibody. Relative surface expression of proteins was determined by using Image J software (see Materials and Methods). Surface expression of proteins is relative to expression of FLVCR1. (B) These proteins were tagged with an HA epitope and detected using anti-HA HRP antibody. Surface expression is relative to the expression of 2-1TM1.
Asparagine 487 in FLVCR1 ECL6 is critical for FeLV-C receptor function.
To identify FLVCR1 receptor-functioning domains and residues, we generated and then expressed specific hybrid FLVCR1/FLVCR2 constructs in MDTF cells and subsequently tested the cells for sensitivity to lacZ(FeLV-C) infections (Fig. 2). We initially generated the hybrid construct 2-1TM1, which contains the amino intracellular region of FLVCR2 and the remaining TM-containing regions from FLVCR1 with the cross-junction at TM1 (Fig. 2). MDTF cells expressing 2-1TM1 were highly susceptible to lacZ(FeLV-C) (Fig. 2, 2-1TM1). This suggests that the receptor-functioning domains are located downstream of FLVCR1 TM1. We subsequently generated a series of FLVCR1/FLVCR2 hybrid constructs that were spliced at specific TM sequences. This allowed us to generate hybrid constructs that displayed specific FLVCR1 and FLVCR2 ECL sequences. For example, the 2-1TM3 construct spliced at TM3 displays presumptive ECL1 from FLVCR2 and ECLs 2, 3, 4, 5, and 6 from FLVCR1. We initially generated 2-1TM3, 2-1TM4, 2-1TM6, 2-1TM8, and 2-1TM10 hybrids (Fig. 2). All hybrids, with the exception of 2-1TM3, functioned as efficient receptors for FeLV-C when overexpressed in MDTF cells. Western blot analysis of membrane fractions from 2-1TM3-expressing cells showed that the 2-1TM3 protein was not expressed on the surface (data not shown; Fig. 3B shows a related Western blot for 2-1TM3 ECL6). Because the 2-1TM4, 2-1TM6, 2-1TM8, and 2-1TM10 hybrids all contain ECL6 from FLVCR1, we hypothesized that FLVCR1 residues critical for FeLV-C receptor function are located in the presumptive ECL6. To confirm this, we first generated and tested the 1-2TM10 hybrid, a converse hybrid of 2-1TM10 that contains FLVCR2 ECL6 instead of FLVCR1 ECL6. Cells expressing 1-2TM10 showed background levels of FeLV-C infection. Second, we replaced the FLVCR2 ECL6 residues (residues 459 to 468) with the corresponding residues from FLVCR1 ECL6 (Fig. 2, 2-1ECL6). This substitution was sufficient to allow FLVCR2 to function as an FeLV-C receptor. This clearly suggests that FLVCR1 ECL6 contains critical receptor-functioning residues.
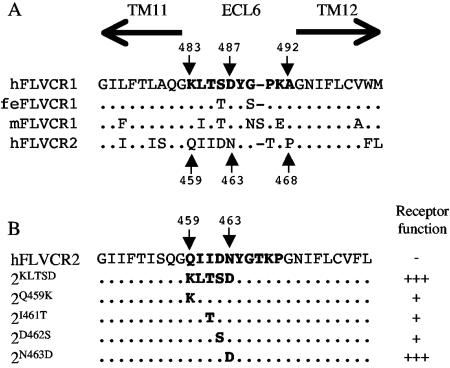
To identify the specific FLVCR1 ECL6 residue(s) responsible for receptor function, we first compared the ECL6 sequence of FLVCR1 isolated from human, cat, and mouse cells with FLVCR2 ECL6 sequence (Fig. 4A). The human, feline, and murine FLVCR1 proteins function as efficient receptors for FeLV-C (24, 32, 35). Human FLVCR1 ECL6 sequence differs from FLVCR2 ECL6 sequence in 7 out of 10 residues. Most of the variations occur in the first five residues of FLVCR1 ECL6 (residues 483 to 487). Furthermore, the first five residues in ECL6 of human, cat, and mouse FLVCR1 proteins (Fig. 4A, hFLVCR1, feFLVCR1, and mdFLVCR1, respectively) are highly conserved compared to FLVCR2 ECL6 sequence, suggesting that this region may contain residues critical for infection. To test our hypothesis, we mutated the QIIDN (residues 459 to 463) in FLVCR2 ECL6 to the corresponding KLTSD (residues 483 to 487) present in FLVCR1 ECL6 (Fig. 4B, mutant 2KLTSD). Cells expressing 2KLTSD were highly susceptible to FeLV-C infection (Fig. 2, 2KLTSD). To identify specific residues, we mutated individual residues within FLVCR2 QIIDN sequence to the respective FLVCR1 ECL6 residues (Fig. 4B). As shown in Fig. 2, a single mutation of N463D in FLVCR2 ECL6 was sufficient to render FLVCR2 strongly functional for FeLV-C (the titer was approximately 2,000-fold greater than the titer on FLVCR2-expressing cells), whereas single mutations of Q459K, I461T, or D462S in FLVCR2 (Fig. 3B) caused a 10-fold increase in infection (data not shown). Together, our results suggest that the negatively charged D487 in FLVCR1 ECL6 is critical for FeLV-C receptor function.
FIG. 4.
(A) Comparison of presumptive ECL 6 sequences from human (h), feline (fe), and murine (m) FLVCR1 and from human FLVCR2. TM11 and TM12 are indicated by arrows above the sequence. FLVCR1 and FLVCR2 ECL6 residue numbers are indicated. Dots represent identical amino acids. (B) Mutations of human FLVCR2 ECL6 residues to FLVCR1 ECL6 residues. Dots represent identical amino acids. Also shown is the receptor function of the mutant FLVCR2, indicated by a plus sign, with each plus sign representing a 10-fold increase in virus infection titer on cells expressing the respective mutant FLVCR2 proteins. The minus symbol represents background infection.
Residues located in FLVCR1 ECL1 also contribute to receptor function.
To confirm the role of FLVCR1 D487 in controlling FeLV-C infection, we generated a mutant FLVCR1 construct in which the D487 residue was mutated to a neutral asparagine. Surprisingly, a D487N mutation in FLVCR1 did not disrupt FeLV-C receptor function (Fig. 2, 1D487N). FeLV-C titer on 1D487N-expressing cells was equivalent to virus titer on FLVCR1-expressing cells (Fig. 2). Furthermore, a substitution of the entire FLVCR1 ECL6 sequence for the FLVCR2 ECL6 sequence also did not significantly disrupt FeLV-C infection (Fig. 2, 1-2ECL6). These results strongly suggest that additional FLVCR1 residues located outside the proposed ECL6 are important for FeLV-C infection. Because the hybrid FLVCR 1-2TM10, which contains FLVCR2 sequence downstream of TM10 including the FLVCR2 ECL6 and the C terminus, does not function as a receptor (Fig. 2, 1-2TM10), we hypothesized that the C terminus of FLVCR1 may contain additional residues necessary for mediating FeLV-C infection. Consequently, we replaced the FLVCR1 C terminal residues (XCT region) downstream of the proposed ECL6 in 1-2ECL6 with the corresponding FLVCR2 XCT region (Fig. 2, 1-2ECL6XCT). This substitution was sufficient to reduce FeLV-C infection to background levels. However, we found that the XCT substitution severely disrupted surface expression of 1-2ECL6XCT protein (Fig. 3A, membrane blot), even though this protein was expressed (Fig. 3A, cell lysate blot). We also analyzed surface expression of 1-2TM10 and found this protein to be weakly expressed on the surface (data not shown). Thus, the resistance of 1-2ECL6XCT-expressing cells and 1-2TM10-expressing cells to FeLV-C is likely caused by a severe disruption in the surface expression of these proteins.
To determine whether other FLVCR1 ECLs are involved in receptor function, we used the 2-1TM1, 2-1TM4, 2-1TM6, 2-1TM8, and 2-1TM10 constructs (Fig. 2) as templates to generate additional mutants in which the critical receptor-functioning FLVCR1 ECL6 sequence was replaced by the FLVCR2 ECL6 sequence (Fig. 2, 2-1TM1 ECL6, 2-1TM4 ECL6, 2-1TM6 ECL6, 2-1TM8 ECL6, and 2-1TM10 ECL6). The 2-1TM1, 2-1TM4, 2-1TM6, 2-1TM8, and 2-1TM10 constructs contain specific FLVCR1 ECL sequences, and all function as efficient receptors for FeLV-C (Fig. 2). Therefore, if other ECLs are important for receptor function, then a replacement of the critical FLVCR1 ECL6 should not disrupt receptor function. All of the above receptors and subsequent ECL6 mutants generated from these receptors were tagged with an HA epitope at the C terminus for protein detection. Replacing the FLVCR1 ECL6 sequence in 2-1TM4, 2-1TM6, 2-1TM8 and 2-1TM10 with the FLVCR2 ECL6 sequence caused a 1,000- to 10,000-fold reduction in FeLV-C infection (Fig. 2, compare FeLV-C titers of 2-1TM4 to 2-1TM4 ECL6, 2-1TM6 to 2-1TM6 ECL6, 2-1TM8 to 2-1TM8 ECL6, and 2-1TM10 to 2-1TM10 ECL6). The reduction in virus titer was not caused by a disruption in surface expression, as surface expression of the above ECL6 mutant was comparable to expression of the respective parental receptors (Fig. 3B). ECL6 substitution in 2-1TM1 did not significantly disrupt FeLV-C infection (Fig. 2, compare titer on 2-1TM1 to 2-1TM1 ECL6). These results, first, confirm our initial observation that FLVCR1 ECL6 is critical for mediating FeLV-C infection, and, second, suggest that FLVCR1 ECL1 is also critical for receptor function. To confirm the importance of ECL1 in receptor function, we generated a FLVCR2 mutant in which the entire ECL1 sequence was substituted for FLVCR1 ECL1 sequence (Fig. 2, 2ECL1). Cells expressing 2-1ECL1 were approximately 7,000-fold more susceptible to FeLV-C infection than cells expressing FLVCR2. In summary, our results suggest that residues within ECL1 and ECL6 of human FLVCR1 are critical for receptor function.
FeLV-C envelope binding.
To assess the importance of FLVCR1 ECL1 and ECL6 in binding FeLV-C envelope protein, we used an FeLV-C surface envelope protein fused to a double HA epitope (CSU-HA) for binding to MDTF cells expressing specific hybrid or mutant proteins. As shown in Fig. 5, CSU-HA efficiently bound to FLVCR1-expressing cells, whereas binding was not detected on FLVCR2-expressing cells or on control MDTF cells. This correlates with the ability of FLVCR1 and the inability of FLVCR2 and MDTF cells to mediate FeLV-C infection. To assess the importance of FLVCR1 ECL6 in envelope binding, we compared CSU-HA binding on cells expressing FLVCR1 and cells expressing 1-2ECL6. Both FLVCR1 and 1-2ECL6 function as efficient receptors for FeLV-C (Fig. 2) and show similar levels of receptor expression on the surface (Fig. 3A). CSU-HA binding was reduced on 1-2ECL6-expressing cells compared to binding on FLVCR1-expressing cells. We also analyzed envelope binding on cells expressing 2-1TM1, 2-1TM4, and 2-1TM6, and we compared it to envelope binding on their respective ECL6 mutants 2-1TM1 ECL6, 2-1TM4 ECL6, and 2-1TM6 ECL6 (Fig. 5). As mentioned above, surface expression of these ECL6 mutants was comparable to surface expression of their respective parental receptor. CSU-HA binding on cells expressing ECL6 mutants was significantly reduced compared to binding on cells expressing their respective parental receptor (Fig. 5, compare CSU-HA binding between 2-1TM1 and 2-1TM1ECL6, 2-1TM4 and 2-1TM4ECL6, and between 2-1TM6 and 2-1TM6ECL6). We did not detect CSU-HA binding on cells expressing 2-1TM8 or 2-1TM10 or on cells expressing the respective ECL6 mutants (Fig. 5). Taken together, these results suggest that ECL6 is important for FeLV-C envelope binding. To determine whether the D487 residue in FLVCR1 ECL6 was critical for envelope binding, we analyzed CSU-HA binding on 2N463D-expressing cells. We did not detect FeLV-C binding on 2N463D-expressing cells even though surface expression of 2N463D was comparable to FLVCR1 surface expression. We tested CSU-HA binding on 1D487N-expressing cells and observed a small but not a significant reduction in binding. These observations suggest that FLVCR1 D487 residue is not critical for strong envelope binding.
FIG. 5.
FeLV-C surface envelope binding on MDTF cells expressing FLVCR1, FLVCR2, or FLVCR1/FLVCR2 hybrids or mutants. Receptor-expressing cells were incubated with (white histogram) or without (black histogram) FeLV-C surface envelope protein tagged with a double HA epitope. Bound SU protein was detected using mouse anti-HA antibody (HA.11) and fluorescein-conjugated donkey anti-mouse. An increase in fluorescence (white histogram) denotes FeLV-C SU binding.
We assessed the importance of FLVCR1 ECL1 in its ability to bind FeLV-C envelope by testing CSU-HA binding on 2-1ECL1-expressing cells. As mentioned above, the 2-1ECL1 construct contains an FLVCR2 backbone with FLVCR1 ECL1 sequence. This protein functions as a receptor for FeLV-C (Fig. 2). However, we did not detect CSU-HA binding on 2-1ECL1-expressing cells (Fig. 5). Surface expression of 2-1ECL1 was comparable to surface expression of 2-1TM6 (data not shown). This result suggests that FLVCR1 ECL1 alone is not critical for strong envelope binding.
FLVCR1 is N-linked glycosylated.
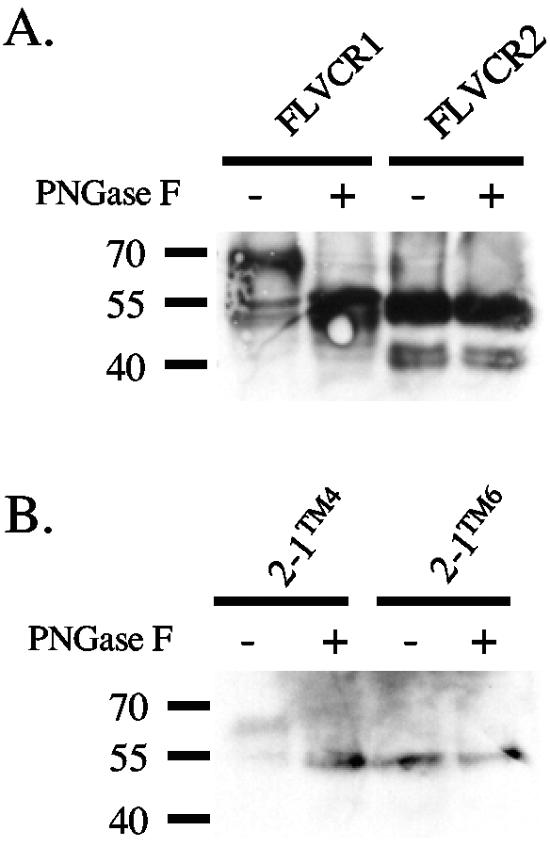
As shown in Fig. 3A, we observed two FLVCR1 products, one product of approximately 70 kDa in size and another at approximately 55 to 60 kDa. The calculated molecular size of FLVCR1 is approximately 60 kDa. FLVCR1 contains three potential N-linked glycosylation motifs, with two motifs located in presumptive ECL3 and one motif in TM11 (Fig. 1, see asterisks above potential glycosylation sites). We also observed a difference in the molecular weights of the hybrid proteins 2-1TM1 and 2-1TM4 compared to 2-1TM6, 2-1TM8, and 2-1TM10. A major difference in these two sets of proteins is that the former set of proteins display FLVCR1 ECL3 that contains the two potential N-linked glycosylation motifs, whereas the latter set of proteins contain FLVCR2 ECL3 that lacks N-linked glycosylation motifs. We hypothesized that the FLVCR1 ECL3 was N-linked glycosylated. To test this, we treated cell lysate samples isolated from MDTF cells expressing FLVCR1, 2-1TM4, or 2-1TM6 with or without the N-glycosidase F enzyme PNGase F to remove N-linked oligosaccharides. As shown in Fig. 6A, PNGase F treatment of FLVCR1 caused a reduction in the molecular mass of the protein from 70 kDa to 55 kDa, suggesting that FLVCR1 is N-linked glycosylated. Furthermore, PNGase F treatment of 2-1TM4 reduced the molecular mass of this protein, whereas PNGase F treatment of 2-1TM6 did not cause a shift in the molecular mass (Fig. 6B). This result clearly suggests that FLVCR1 ECL3 is N-linked glycosylated. We also observed two FLVCR2 products of 55 kDa and 40 kDa (Fig. 3A). FLVCR2 also contains three potential N-linked glycosylation motifs (Fig. 1), suggesting that FLVCR2 may also be N-linked glycosylated. However, PNGase F treatment of FLVCR2 did not cause a reduction in either the 55 kDa or 40 kDa proteins, suggesting that FLVCR2 is not N-linked glycosylated.
FIG. 6.
Glycosylation studies of FLVCR1, FLVCR2, and FLVCR1/FLVCR2 hybrid proteins. Cell lysates from MDTF cells expressing FLVCR1, FLVCR2, or hybrid proteins were either not treated (−) or treated (+) with N-glycosidase F (PNGase F), and proteins were analyzed by Western blotting (see Materials and Methods). Proteins in panel A were detected using mouse anti-V5 antibody. Proteins in panel B were detected using anti-HA HRP.
DISCUSSION
Characterization of human FLVCR1.
In this study, we have independently isolated a human cDNA encoding FLVCR2 protein that shares 52% amino acid identity to human FLVCR1, and we have shown that FLVCR2 does not function as a receptor for FeLV-C when it is overexpressed in murine cells. Furthermore, by generating specific FLVCR1/FLVCR2 hybrids and mutants, we have identified FLVCR1 ECL1 and Asp487 in ECL6 as critical for mediating FeLV-C infection (Fig. 2). Our results also suggest the possibility that other FLVCR1 regions are involved in receptor function. First, our results indicate that the presumptive FLVCR1 ECL4 provides some contribution to receptor function. FeLV-C titers on cells expressing 2-1TM4 ECL6 or 2-1TM6 ECL6, both of which contain FLVCR1 ECL4, were consistently 10-fold greater than titers on 2-1TM8 ECL6-expressing cells, which contain FLVCR2 ECL4. The difference in virus titer is not explained by differences in the surface expression of these proteins (Fig. 3B). Second, the weak receptor function of 2-1TM10 ECL6, which contains all presumptive ECLs from FLVCR2, suggests that other FLVCR1 residues outside ECL6 weakly contribute to receptor function. These residues could be located directly upstream or downstream of FLVCR1 ECL6 sequence, which would make ECL6 sequence longer than what we have proposed. Alternatively, additional receptor-functioning residues could still be located in the XCT region, which we were not able to ascertain using the 1-2ECL6XCT protein (Fig. 2) because it failed to be expressed on the cell surface (Fig. 3A). Finally, we cannot exclude the possibility that ECL2 or other FLVCR1 regions also contribute to receptor function.
Our FeLV-C SU binding studies show that neither FLVCR1 ECL1 nor ECL6 alone is sufficient for mediating strong SU binding even though these ECLs are critical for mediating virus infection. Our results suggest that ECL1 and ECL6 are important for mediating strong SU binding only in conjunction with other FLVCR1 regions. Substitution of FLVCR1 ECL6 sequence for FLVCR2 ECL6 in FLVCR1 or 2-1TM1 proteins caused a dramatic reduction in SU binding (Fig. 5, 1-2ECL6 and 2-1TM1 ECL6), and similar ECL6 substitution in 2-1TM4 or 2-1TM6 proteins reduced SU binding to background levels. This clearly shows that FLVCR1 ECL6 plays an important role in mediating SU binding. However, our observation that ECL6 substitution in FLVCR1 or 2-1TM1 does not completely abrogate SU binding suggests that additional FLVCR1 regions are involved in mediating binding. Our results suggest the involvement of FLVCR1 ECL4 because SU binding is detectable on cells expressing 2-1TM6 (displays FLVCR1 ECL4, 5, and 6) but not detectable on cells expressing 2-1TM8 (displays FLVCR1 ECL5 and 6). Other FLVCR1 residues located between ECL1 and ECL2 and possibly including ECL1 and ECL2 must also be involved because SU binding was more efficient on cells expressing 2-1TM1 than on cells expressing 2-1TM4 (Fig. 5). FLVCR1 ECL1 alone is not sufficient for mediating strong SU binding (Fig. 5, 2-1ECL1), suggesting that either ECL2 alone is critical or ECL1 in conjunction with ECL2 or with other FLVCR1 regions is critical. We were not able to ascertain the role of FLVCR1 ECL2 in SU binding because the 2-1TM3 protein failed to be expressed on the cell surface (Fig. 3B, 2-1TM3 ECL6). Taken together, our results clearly show that FLVCR1 ECL1 and ECL6 alone are sufficient for mediating efficient FeLV-C infection but are not sufficient for mediating strong SU binding. It is possible that SU binding can only be detected if ECL1 and ECL6 and/or additional ECLs are simultaneously present. Alternatively, ECL1 and ECL6 may be involved in triggering virus entry, and the authentic FeLV-C SU binding site is located elsewhere on FLVCR1. Such a mechanism of distinct receptor regions controlling SU binding and virus entry has been proposed for the human T-cell leukemia virus 1 receptor Glut-1 (17, 18) and for the retroviral receptor Pit1 (10). It is also conceivable that ECL1 and ECL6 influence the membrane topology of the authentic SU binding site in a similar manner that has been proposed for Pit1 (10). The topology we have proposed is based on Kyte-Doolittle (14) and TMPredict (11) algorithms, but this may not represent the correct configuration of FLVCR1 on the cell surface. Our glycosylation studies clearly indicate that ECL3 is extracellular, and we have determined by tagging the N and C termini of FLVCR1 with HA and V5 epitopes, respectively, and analyzing the expression of tagged FLVCR1 by confocal microscopy that the N and C termini are intracellular (data not shown). Additional studies are needed to identify the authentic FeLV-C SU binding site and to determine the correct membrane topology of FLVCR1.
Both the FLVCR1 cDNA and the FLVCR2 cDNA express two protein products. Whereas the higher-molecular-mass 70-kDa FLVCR1 protein is the N-linked glycosylated form of FLVCR1 that is reduced to the 55-kDa protein upon treatment with PNGase F, the higher-molecular-mass 55-kDa FLVCR2 protein appears to be the native FLVCR2. The 40-kDa FLVCR2 protein may, therefore, represent a cleaved form of FLVCR2, or it may represent a truncated FLVCR2 encoded by a spliced FLVCR2 transcript or by a FLVCR2 transcript that has an alternative translation initiation site(s). Such alternative translation initiation sites have been shown to be present in the ASCT2 mRNA, which encodes a receptor for the large group of retroviruses that include feline RD114 virus, baboon endogenous virus, several simian retroviruses, and the type W human endogenous retrovirus (5, 15, 26, 31, 34). The ASCT2 mRNA encodes the full-length ASCT2 protein as well as several truncated forms of ASCT2 that function as receptors for the above group of retroviruses (31).
Although FLVCR2 is not a heme exporter like FLVCR1 (25), this protein is sufficiently related to FLVCR1 to the extent that it can function as a receptor for FeLV-C by an Asn463Asp mutation in its ECL6 (Fig. 2, see 2N463D). This raises the possibility that FLVCR2 protein from other species could function as a receptor for FeLV-C. Alternatively, variants of the prototype FeLV-C Sarma strain, which have expanded tropisms (Brian Willett, personal communication), may have adapted to use FLVCR2 as a receptor. The use of closely related proteins as receptors is common among many retroviruses including γ-retroviruses (21, 30). This would ensure virus survival and would limit host escape mutations. In summary, our results suggest that distinct FLVCR1 regions control FeLV-C SU binding and virus infection. We propose that FeLV-C interacts with FLVCR1 using a mechanism that is highly related to other γ-retrovirus-receptor interactions.
Acknowledgments
The human FLVCR2 cDNA was isolated by C.S.T. in David Kabat's laboratory (Oregon Health Sciences University, Portland, OR). We are grateful to David Kabat, James Ellis, and Anne Opavsky for their helpful suggestions in preparing the manuscript. We are also grateful to Brian Willett (University of Glasgow, Scotland, United Kingdom) for providing the FeLV-C envelope construct, to Yasuhiro Takeuchi and Francois Cosset for providing the TELCeB6 packaging cells, and to Julie Overbaugh for providing the HA-tagged FeLV-C SU construct. We are also grateful to Anthony Au, Hafiza Pirani, Rati Rashmi, and Michelle Rey for their helpful suggestions and for preparing some constructs and to Sabah Asad for his assistance in using the fluorescence activated cell sorter.
This work was supported by the Canadian Institutes of Health Research. C.S.T. is a holder of a Canada Research Chair in Retrovirus and Gene Therapy.
REFERENCES
- 1.Abkowitz, J. L., R. D. Holly, and C. K. Grant. 1987. Retrovirus-induced feline pure red cell aplasia. Hematopoietic progenitors are infected with feline leukemia virus and erythroid burst-forming cells are uniquely sensitive to heterologous complement. J. Clin. Investig. 80:1056-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albritton, L. M., J. W. Kim, L. Tseng, and J. M. Cunningham. 1993. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J. Virol. 67:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albritton, L. M., L. Tseng, D. Scadden, and J. M. Cunningham. 1989. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell 57:659-666. [DOI] [PubMed] [Google Scholar]
- 4.Battini, J. L., J. E. Rasko, and A. D. Miller. 1999. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc. Natl. Acad. Sci. USA 96:1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blond, J. L., D. Lavillette, V. Cheynet, O. Bouton, G. Oriol, S. Chapel-Fernandes, B. Mandrand, F. Mallet, and F. L. Cosset. 2000. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 74:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasier, G., C. Tikellis, L. Xuereb, J. Craigie, D. Casley, C. S. Kovacs, N. J. Fudge, R. Kalnins, M. E. Cooper, and P. J. Wookey. 2004. Novel hexad repeats conserved in a putative transporter with restricted expression in cell types associated with growth, calcium exchange and homeostasis. Exp. Cell Res. 293:31-42. [DOI] [PubMed] [Google Scholar]
- 7.Cosset, F. L., Y. Takeuchi, J. L. Battini, R. A. Weiss, and M. K. L. Collins. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dornsife, R. E., P. W. Gasper, J. I. Mullins, and E. A. Hoover. 1989. Induction of aplastic anemia by intra-bone marrow inoculation of a molecularly cloned feline retrovirus. Leuk. Res. 13:745-755. [DOI] [PubMed] [Google Scholar]
- 9.Eiden, M. V., K. Farrell, J. Warsowe, L. C. Mahan, and C. A. Wilson. 1993. Characterization of a naturally occurring ecotropic receptor that does not facilitate entry of all ecotropic murine retroviruses. J. Virol. 67:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell, K. B., J. L. Russ, R. K. Murthy, and M. V. Eiden. 2002. Reassessing the role of region A in Pit1-mediated viral entry. J. Virol. 76:7683-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann, K., and W. Stoffel. 1993. Tmbase—a database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 374:166-170. [Google Scholar]
- 12.Kavanaugh, M. P., D. G. Miller, W. Zhang, W. Law, S. L. Kozak, D. Kabat, and A. D. Miller. 1994. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. USA 91:7071-7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, J. W., E. I. Closs, L. M. Albritton, and J. Cunningham. 1991. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 352:725-728. [DOI] [PubMed] [Google Scholar]
- 14.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 15.Lavillette, D., M. Marin, A. Ruggieri, F. Mallet, F. L. Cosset, and D. Kabat. 2002. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J. Virol. 76:6442-6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipovich, L., A. L. Hughes, M. C. King, J. L. Abkowitz, and J. G. Quigley. 2002. Genomic structure and evolutionary context of the human feline leukemia virus subgroup C receptor (hFLVCR) gene: evidence for block duplications and de novo gene formation within duplicons of the hFLVCR locus. Gene 286:203-213. [DOI] [PubMed] [Google Scholar]
- 17.Manel, N., J. L. Battini, and M. Sitbon. 2005. Human T cell leukemia virus envelope binding and virus entry are mediated by distinct domains of the glucose transporter GLUT1. J. Biol. Chem. 280:29025-29029. [DOI] [PubMed] [Google Scholar]
- 18.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449-459. [DOI] [PubMed] [Google Scholar]
- 19.Neil, J. C., R. Fulton, M. Rigby, and M. Stewart. 1991. Feline leukaemia virus: generation of pathogenic and oncogenic variants. Curr. Top. Microbiol. Immunol. 171:67-93. [DOI] [PubMed] [Google Scholar]
- 20.Olah, Z., C. Lehel, W. B. Anderson, M. V. Eiden, and C. A. Wilson. 1994. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J. Biol. Chem. 269:25426-25431. [PubMed] [Google Scholar]
- 21.Overbaugh, J., A. D. Miller, and M. V. Eiden. 2001. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol. Rev. 65:371-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian, Z., R. Donald, H. Wang, Q. Chen, and L. M. Albritton. 2003. Identification of a critical basic residue on the ecotropic murine leukemia virus receptor. J. Virol. 77:8596-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quigley, J. G., C. C. Burns, M. M. Anderson, E. D. Lynch, K. M. Sabo, J. Overbaugh, and J. L. Abkowitz. 2000. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood 95:1093-1099. [PubMed] [Google Scholar]
- 25.Quigley, J. G., Z. Yang, M. T. Worthington, J. D. Phillips, K. M. Sabo, D. E. Sabath, C. L. Berg, S. Sassa, B. L. Wood, and J. L. Abkowitz. 2004. Identification of a human heme exporter that is essential for erythropoiesis. Cell 118:757-766. [DOI] [PubMed] [Google Scholar]
- 26.Rasko, J. E., J. L. Battini, R. J. Gottschalk, I. Mazo, and A. D. Miller. 1999. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc. Natl. Acad. Sci. USA 96:2129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riedel, N., E. A. Hoover, P. W. Gasper, M. O. Nicholson, and J. I. Mullins. 1986. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, feline leukemia virus C-Sarma. J. Virol. 60:242-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigby, M. A., J. L. Rojko, M. A. Stewart, G. J. Kociba, C. M. Cheney, L. J. Rezanka, L. E. Mathes, J. R. Hartke, O. Jarrett, and J. C. Neil. 1992. Partial dissociation of subgroup C phenotype and in vivo behaviour in feline leukaemia viruses with chimeric envelope genes. J. Gen. Virol. 73:2839-2847. [DOI] [PubMed] [Google Scholar]
- 29.Sugai, J., M. Eiden, M. M. Anderson, N. Van Hoeven, C. D. Meiering, and J. Overbaugh. 2001. Identification of envelope determinants of feline leukemia virus subgroup B that permit infection and gene transfer to cells expressing human Pit1 or Pit2. J. Virol. 75:6841-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tailor, C. S., D. Lavillette, M. Marin, and D. Kabat. 2003. Cell surface receptors for gammaretroviruses. Curr. Top. Microbiol. Immunol. 281:29-106. [DOI] [PubMed] [Google Scholar]
- 31.Tailor, C. S., M. Marin, A. Nouri, M. Kavanaugh, and D. Kabat. 2001. Truncated forms of the dual function human ASCT2 neutral amino acid transporter/retroviral receptor are translationally initiated at multiple alternative CUG and GUG codons. J. Biol. Chem. 276:27221-27230. [DOI] [PubMed] [Google Scholar]
- 32.Tailor, C. S., A. Nouri, and D. Kabat. 2000. Cellular and species resistance to murine amphotropic, gibbon ape, and feline subgroup C leukemia viruses is strongly influenced by receptor expression levels and by receptor masking mechanisms. J. Virol. 74:9797-9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tailor, C. S., A. Nouri, C. G. Lee, C. Kozak, and K. Kabat. 1999. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc. Natl. Acad. Sci. USA 96:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tailor, C. S., A. Nouri, Y. Zhao, Y. Takeuchi, and D. Kabat. 1999. A sodium dependent neutral amino acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J. Virol. 73:4470-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tailor, C. S., B. J. Willet, and D. Kabat. 1999. A putative cell surface receptor for anemia-inducing subgroup C feline leukemia virus is a member of a transporter superfamily. J. Virol. 73:6500-6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, H., M. P. Kavanaugh, R. A. North, and D. Kabat. 1991. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature 352:729-731. [DOI] [PubMed] [Google Scholar]
- 37.Yang, Y. L., L. Guo, S. Xu, C. A. Holland, T. Kitamura, K. Hunter, and J. M. Cunningham. 1999. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nature Gen. 21:216-219. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto, T., E. Yoshimoto, and D. Meruelo. 1993. Identification of amino acid residues critical for infection with ecotropic murine leukemia retrovirus. J. Virol. 67:1310-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]