Abstract
Arsenic trioxide (As2O3) increased human immunodeficiency virus type 1 (HIV-1) infectivity when particular Homo sapiens and Cercopithecus aethiops cell lines were used as targets. Knockdown of human TRIM5α by RNA interference eliminated the As2O3 effect, demonstrating that the drug acts by modulating the activity of this retroviral restriction factor. In contrast, HIV-1 infectivity in target cell lines from other primate species (Cercopithecus tantalus, Macaca mulatta, and Aotus trivirgatus) was not increased by As2O3, despite the potent TRIM5-dependent HIV-1 restriction activity that these cells exhibit. To determine if As2O3 responsiveness is characteristic of particular TRIM5 orthologues and not others, TRIM5 cDNAs from these five primate species were transduced into cat fibroblasts, which lack endogenous HIV-1 restriction activity and, therefore, responsiveness to As2O3. In this context, the HIV-1 restriction activity conferred by all TRIM5 orthologues was largely eliminated by As2O3. The effect of As2O3 on HIV-1 restriction is thus shared by different TRIM5 orthologues but dependent on factors specific to the cell line in which TRIM5 is expressed.
Human immunodeficiency virus type 1 (HIV-1) infectivity is inhibited in cells from Old World monkeys, such as rhesus macaques and African green monkeys, and in New World owl monkeys (7). The block to replication occurs early, at a point after virus entry but before integration of the viral DNA (5, 9). The cellular factor responsible for retroviral restriction in Old World monkeys is TRIM5α (18), a member of the tripartite motif family of proteins (14). In owl monkeys, characterization of the HIV-1 CA-binding protein cyclophilin A (CypA) led to the discovery of TRIMCyp (16), an HIV-1-specific restriction factor that was generated by cyclophilin A cDNA retrotransposition into the TRIM5 locus (11, 16). Though HIV-1 replicates well enough in humans to cause AIDS, human TRIM5α possesses a modest anti-HIV-1 restriction activity (6, 8, 13, 18, 21) that perhaps contributes to the long clinical latency observed in most HIV-1-infected people (12).
When added to target cells at the time of infection, arsenic trioxide (As2O3) stimulates retroviral infectivity (3, 19). The As2O3 effect has only been observed in the context of target cells bearing TRIM5α-mediated retrovirus restriction activity (2, 3). Consistent with the hypothesis that As2O3 counteracts TRIM5α-mediated restriction, the transfer of human TRIM5α cDNA to nonrestrictive mouse fibroblasts rendered viral titers on these cells responsive to As2O3 (8). Conversely, selection of a human cell line for loss of retrovirus restriction activity resulted in cells that were As2O3 unresponsive (15).
As2O3 counteracts HIV-1 restriction in some cell lines.
We previously reported that HIV-1 titers on certain restrictive target cell lines were As2O3 responsive but that the drug had no effect on HIV-1 titers in other, equally restrictive cell lines (2). To examine the effect of As2O3 in more detail, a panel of five primate cell lines that restrict HIV-1 to various extents was tested for the potential of As2O3 to relieve HIV-1 restriction. As previously described (2), cells were exposed to vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped HIV-1NL4-3 in which the envelope was deleted and green fluorescent protein (GFP) reporter gene replaced nef (HIV-1GFP). Each cell line was challenged with a constant amount of virus such that a few percent of cells were transduced and rendered GFP positive. As2O3 was prepared as described previously (3) and added to media for the first 12 h of infection at the concentrations indicated in Fig. 1.
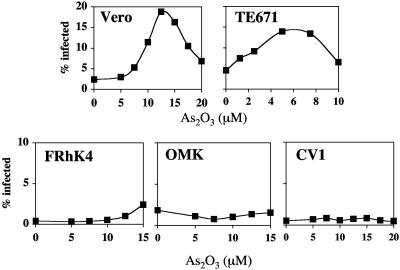
FIG. 1.
As2O3 counteracts HIV-1 restriction in Vero and TE671 cells but not in CV1, OMK, or FRhK4 cells. Cells were infected with VSV-G-pseudotyped HIV-1NL4-3 bearing an env deletion and GFP in place of nef (HIV-1GFP), such that <5% of target cells were infected. The indicated concentrations of As2O3 were maintained for the first 12 h that cells were exposed to the virus, at which time the medium was replaced without the drug. The percentage of GFP-positive (infected) cells was determined at 48 h postinfection by flow cytometry.
As2O3 increased HIV-1 titer 10-fold in Vero cells (Cercopithecus aethiops), with a maximal effect observed at a concentration of 12.5 μM (Fig. 1). HIV-1 titer was increased threefold by As2O3 when human TE671 cells were used as targets, with a maximal drug effect at 5 μM (Fig. 1). The decline in HIV-1 titer with higher As2O3 concentrations can be attributed to target cell toxicity (3). In contrast, As2O3 did not stimulate HIV-1 titer in Macaca mulatta FRhK4 cells, Aotus trivirgatus OMK cells, or Cercopithecus tantalus CV1 cells (Fig. 1), even at drug concentrations high enough to cause grossly evident target cell death in these particular cell lines (Fig. 1 and data not shown). Using the XTT assay (3), no significant differences in the sensitivities of the cell lines to As2O3 were detected that would explain the differential drug responsiveness.
TRIM5α is required for the HIV-1 stimulatory effect of As2O3.
To determine if the positive effect of As2O3 on the HIV-1 titer is related to effects of the drug on TRIM5α, we created a TE671 cell line in which TRIM5 was knocked down by stable transduction of a short hairpin RNA (shRNA), as previously described (18). Successful TRIM5 knockdown in these cells was documented previously by demonstrating complete removal of the potent restriction activity that targets N-tropic murine leukemia virus (MLV) (17). Cells transduced with shRNA targeting luciferase were generated concurrently to serve as a control.
TRIM5 knockdown resulted in a threefold increase in HIV-1 titer (Fig. 2). The same magnitude of increase in HIV-1 infectivity was observed with the control, luciferase knockdown cells when infection was conducted in the presence of 2.5 μM As2O3 (Fig. 2). However, As2O3 had no effect on HIV-1 titer in TRIM5 knockdown cells (Fig. 2), demonstrating that As2O3 counteracts HIV-1 restriction mediated by TRIM5α. Although we knocked down TRIM5 expression in FRhK4 cells (1) and others did the same in a CV1-derived cell line (8), multiple attempts to knock down TRIM5 in Vero cells were unsuccessful, precluding confirmation of these results in another As2O3-responsive cell line.
FIG. 2.
TRIM5 knockdown in human cells eliminates the stimulatory effect of As2O3 on HIV-1 infectivity. TE671 cells were transduced with pSUPER.retro.puro (Oligoengine), encoding an shRNA against TRIM5 (target sequence, 5′-GCCUUACGAAGUCUGAAAC-3′) or against luciferase as a control (target sequence, 5′-CGUACGCGGAAUACUUCGA-3′). After selection in puromycin, cells were transduced with HIV-1GFP in the presence or absence of 2.5 μM As2O3, and the percentage of GFP-positive (infected) cells was determined at 48 h postinfection. RT units, reverse transcriptase units.
HIV-1 restriction in cat cells transduced with TRIM5 cDNA is suppressed by As2O3.
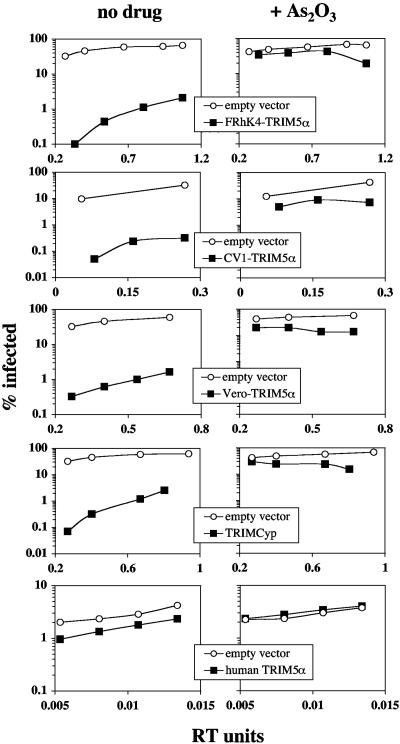
One possible explanation for our findings with the five cell lines is that As2O3 suppresses the restriction activity of certain TRIM5 orthologues and not others. To test this hypothesis, TRIM5 cDNAs from the two As2O3-responsive cell lines (TE671 and Vero) and the three As2O3-unresponsive cell lines (CV-1, FRhK4, and OMK) were transduced into CRFK, a cat fibroblast cell line without apparent retroviral restriction activity (6, 8). This was done by using murine stem cell virus-derived vectors as previously described (16). Each cDNA was expressed as a fusion to a C-terminal FLAG epitope tag. Despite the range in expression of the different orthologues (Fig. 3), all exhibited HIV-1 restriction activity that corresponded in relative magnitude to what is observed in the cell lines that served as the sources for the TRIM5 cDNAs (Fig. 4, left column).
FIG. 3.
Western blot showing synthesis of TRIM5 orthologues in cat fibroblasts. CRFK cells were transduced with retroviral vectors bearing TRIM5α cDNAs from the primate cell lines TE671, FRhK4, CV1, and Vero or TRIMCyp cDNA from OMK cells. Each cDNA encoded a C-terminal FLAG epitope tag. The vector used for transduction was pMIP, which is pMSCV-IRES-GFP (20) with a puromycin resistance cassette in place of the GFP coding sequence. Lysate from each cell population was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-FLAG M2 antibody (Sigma).
FIG. 4.
As2O3 counteracts the restriction of HIV-1 that is conferred by TRIM5 orthologues from various species. CRFK cells expressing TRIM5α from Homo sapiens (TE671), Macaca mulatta (FRhK4), Cercopithecus aethiops (Vero), or Cercopithecus tantalus (CV-1), or Aotus trivirgatus TRIMCyp (OMK), were infected with HIV-1GFP in the absence or presence of 15 μM As2O3. The drug was present for the first 12 h of infection. The relative quantity of virus used to infect each sample is shown in RT units on the x axis. The percentage of GFP-positive (infected) cells was determined at 48 h postinfection.
The HIV-1 titer on cells transduced with empty vector was not increased by any concentration of As2O3 that was applied to target cells (Fig. 4 and data not shown). This is consistent with our other data showing a requirement for TRIM5-mediated restriction in order to see the effect of As2O3 (Fig. 2) and with the apparent lack of HIV-1 restriction activity in cat cells (6, 8). As2O3 treatment of cat cells transduced with any of the TRIM5 orthologues, however, resulted in a potent increase in the HIV-1 titer (Fig. 4). A 15 μM As2O3 concentration was found empirically to be the drug concentration with the largest effect on HIV-1 titers in TRIM5-transduced CRFK cells (data not shown). The magnitude of the increase in titer due to As2O3 correlated with the magnitude of HIV-1 restriction that a given cell line possessed. For example, the biggest effect of As2O3 was found with cells transduced with Macaca mulatta TRIM5α or Aotus trivirgatus TRIMCyp, the two cDNAs that conferred the greatest HIV-1 restriction activities on cat cells (Fig. 4). Of the TRIM5 cDNAs, human TRIM5α cDNA conferred the least HIV-1 restriction activity and, correspondingly, the smallest response to As2O3 (Fig. 4). Thus, HIV-1 restriction activity attributable to transduction of any of the TRIM5 cDNAs into cat cells was inhibited by As2O3.
As2O3 overcomes TRIM5α-mediated restriction of SIVMAC239.
To determine if the findings with HIV-1 might be extended to other retroviruses, the effect of As2O3 on SIVMAC239 infectivity was tested.Of the five primate cell lines examined here, only CV1 cells exhibit significant restriction activity against SIVMAC239 (4-6, 10). The SIVMAC239 titer in CV1 cells did not respond to As2O3 treatment (Fig. 5A), though, confirming our previous findings for this cell line with HIV-1 (Fig. 1). As2O3 had no effect on SIVMAC239 titer in any of the other four cell lines (data not shown), as expected since these cells do not possess significant restriction activity against SIVMAC239. The cat cell line transduced with the TRIM5α cDNA derived from CV1 cells exhibited a very potent restriction activity against SIVMAC239 which was effectively suppressed by As2O3 (Fig. 5B). Cat cell lines transduced with the other TRIM5 orthologue cDNAs exhibited more modest SIVMAC239 restriction activity and, correspondingly, smaller responses to As2O3 (data not shown). Similarly, As2O3 stimulated N-tropic MLV infectivity, but not B-MLV infectivity, in those TRIM5-transduced cat cell lines that possessed N-MLV-specific restriction activity (data not shown). Thus, the cell-specific suppression of TRIM5α restriction activity by As2O3 is not unique to HIV-1.
FIG. 5.
As2O3 does not increase the titer of SIVMAC239 on CV1 cells but counteracts the SIVMAC239 restriction that is conferred on cat cells by CV1 TRIM5α cDNA. (A) CV1 cells were infected with VSV-G-pseudotyped, GFP-encoding SIVMAC239 (SIVGFP) while being treated with the indicated amounts of As2O3. Infectivity was measured as described in the legend to Fig. 1. (B) Experiments were performed as described in the legend to Fig. 4, using SIVGFP instead of HIV-1GFP.
Conclusions.
Here we have directly shown that the stimulatory effect of As2O3 on HIV-1 titers involves suppression of TRIM5-mediated restriction activity. It has been suggested that the variable As2O3 responsiveness observed with different cell lines is a property of the particular TRIM5α orthologue expressed by those cells, but this conclusion was reached with single data points for two orthologues that differed in restriction activity by almost 2 orders of magnitude (8). The results presented here clearly demonstrate that all TRIM5 orthologues that exhibit retroviral restriction activity are likely to respond to As2O3 when they are expressed in the proper cell type. The particular property of a cell which determines whether TRIM5-mediated restriction will be As2O3 responsive remains to be determined. Given that human cells, monkey cells, and even cat cells can be As2O3 responsive, it seems unlikely that this is a species-specific property. Another possibility to consider is that some cells may be As2O3 unresponsive because they possess a retroviral restriction factor other than TRIM5 which is not sensitive to inhibition by As2O3. The molecular basis for the As2O3 effect on TRIM5-mediated restriction is unknown. As2O3 had no detectable effect on the half-life or subcellular localization of TRIM5 proteins in cat cells (data not shown). Ultimately, studies with As2O3 may aid attempts to elucidate the mechanism of TRIM5-mediated restriction.
Acknowledgments
This work was supported by National Institutes of Health grant RO1AI36199 to J.L. and used core facilities of the Columbia Center for AIDS Research. S.S. was supported by NIH training grant T32AI007161.
REFERENCES
- 1.Berthoux, L., S. Sebastian, E. Sokolskaja, and J. Luban. 2005. Cyclophilin A is required for TRIM5α-mediated resistance to HIV-1 in Old World monkey cells. Proc. Natl. Acad. Sci. USA 102:14849-14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoux, L., S. Sebastian, E. Sokolskaja, and J. Luban. 2004. Lv1 inhibition of human immunodeficiency virus type 1 is counteracted by factors that stimulate synthesis or nuclear translocation of viral cDNA. J. Virol. 78:11739-11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthoux, L., G. J. Towers, C. Gurer, P. Salomoni, P. P. Pandolfi, and J. Luban. 2003. As(2)O(3) enhances retroviral reverse transcription and counteracts Ref1 antiviral activity. J. Virol. 77:3167-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatziioannou, T., D. Perez-Caballero, A. Yang, S. Cowan, and P. D. Bieniasz. 2004. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5α. Proc. Natl. Acad. Sci. USA 101:10774-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann, W., D. Schubert, J. LaBonte, L. Munson, S. Gibson, J. Scammell, P. Ferrigno, and J. Sodroski. 1999. Species-specific, postentry barriers to primate immunodeficiency virus infection. J. Virol. 73:10020-10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keckesova, Z., L. M. Ylinen, and G. J. Towers. 2004. The human and African green monkey TRIM5α genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc. Natl. Acad. Sci. USA 101:10780-10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munk, C., S. M. Brandt, G. Lucero, and N. R. Landau. 2002. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 99:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama, E. E., H. Miyoshi, Y. Nagai, and T. Shioda. 2005. A specific region of 37 amino acid residues in the SPRY (B30.2) domain of African green monkey TRIM5α determines species-specific restriction of simian immunodeficiency virus SIVmac infection. J. Virol. 79:8870-8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nisole, S., C. Lynch, J. P. Stoye, and M. W. Yap. 2004. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc. Natl. Acad. Sci. USA 101:13324-13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantaleo, G., and A. Fauci. 1996. Immunopathogenesis of HIV infection. Annu. Rev. Microbiol. 50:825-854. [DOI] [PubMed] [Google Scholar]
- 13.Perron, M. J., M. Stremlau, B. Song, W. Ulm, R. C. Mulligan, and J. Sodroski. 2004. TRIM5α mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc. Natl. Acad. Sci. USA 101:11827-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reymond, A., G. Meroni, A. Fantozzi, G. Merla, S. Cairo, L. Luzi, D. Riganelli, E. Zanaria, S. Messali, S. Cainarca, A. Guffanti, S. Minucci, P. G. Pelicci, and A. Ballabio. 2001. The tripartite motif family identifies cell compartments. EMBO J. 20:2140-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayah, D. M., and J. Luban. 2004. Selection for loss of Ref1 activity in human cells releases human immunodeficiency virus type 1 from cyclophilin A dependence during infection. J. Virol. 78:12066-12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayah, D. M., E. Sokolskaja, L. Berthoux, and J. Luban. 2004. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 430:569-573.15243629 [Google Scholar]
- 17.Sokolskaja, E. Promotion of HIV-1 infectivity in human cells by cyclophilin A is independent of TRIM5α. Submitted for publication.
- 18.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 19.Turelli, P., V. Doucas, E. Craig, B. Mangeat, N. Klages, R. Evans, G. Kalpana, and D. Trono. 2001. Cytoplasmic recruitment of INI1 and PML on incoming HIV preintegration complexes: interference with early steps of viral replication. Mol. Cell 7:1245-1254. [DOI] [PubMed] [Google Scholar]
- 20.Van Parijs, L., Y. Refaeli, J. D. Lord, B. H. Nelson, A. K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11:281-288. [DOI] [PubMed] [Google Scholar]
- 21.Yap, M. W., S. Nisole, C. Lynch, and J. P. Stoye. 2004. Trim5α protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 101:10786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]