Abstract
Because the vaccine vectors currently being evaluated in human populations all have significant limitations in their immunogenicity, novel vaccine strategies are needed for the elicitation of cell-mediated immunity. The nonpathogenic, rapidly growing mycobacterium Mycobacterium smegmatis was engineered as a vector expressing full-length human immunodeficiency virus type 1 (HIV-1) HXBc2 envelope protein. Immunization of mice with recombinant M. smegmatis led to the expansion of major histocompatibility complex class I-restricted HIV-1 epitope-specific CD8+ T cells that were cytolytic and secreted gamma interferon. Effector and memory T lymphocytes were elicited, and repeated immunization generated a stable central memory pool of virus-specific cells. Importantly, preexisting immunity to Mycobacterium bovis BCG had only a marginal effect on the immunogenicity of recombinant M. smegmatis. This mycobacterium may therefore be a useful vaccine vector.
An effective human immunodeficiency virus (HIV)-AIDS vaccine will likely need to elicit virus-specific neutralizing antibodies and cytotoxic T-lymphocyte (CTL) responses. Although an immunogen that induces antibodies that neutralize a diversity of primary HIV type 1 (HIV-1) isolates has not yet been defined, a number of strategies are being developed for generating HIV-1-specific CTL (31). However, there are problems associated with each of these approaches for eliciting CTL that will likely limit their ultimate effectiveness. Plasmid DNA has not proven nearly as immunogenic in humans as it has in laboratory animals (9, 33, 57). The immunogenicity of replication-defective adenovirus serotype 5 is limited in human populations by preexisting serotype-specific anti-adenovirus antibodies (37). Pox-vectored vaccines only elicit very short-lived immunity in humans (Andrew McMichael, personal communication), and production problems have slowed the development of alphavirus-based vaccine vectors (59). Better vector systems will therefore be needed to induce anti-HIV-1 cellular immunity and prime for broadly neutralizing antibody responses.
Mycobacteria have features that make them attractive as potential HIV-1 vaccine vectors. They can be readily engineered to stably express transgenes and can elicit long-lasting cellular and mucosal immune responses (28, 36). Most importantly, they have been used successfully as vaccines. The attenuated, nonpathogenic Mycobacterium bovis BCG is widely used as a vaccine for tuberculosis (TB) and leprosy (21, 27). Recombinant BCG (rBCG) vaccine constructs have shown immunogenicity and protection in murine models against various infectious agents, including Borrelia burgdorferi, Streptococcus pneumoniae, Bordetella pertussis, rodent malaria, leishmania, and measles virus (11, 15, 29, 34, 38, 50). In murine and monkey studies, we and others have shown that rBCG elicited antibody and cell-mediated responses against HIV-1 and simian immunodeficiency virus antigens (2, 20, 51, 65).
Mycobacterium smegmatis has a number of properties that may make it an effective vaccine vector. Some M. smegmatis strains are nonpathogenic and commensal in humans (5, 39, 45). Unlike other mycobacterial strains such as BCG that survive in host cells for months by inhibiting phagosome maturation, M. smegmatis is rapidly destroyed by phagolysosomal proteases in the phagosomes of infected cells (26, 32, 55, 56). Nevertheless, M. smegmatis can induce cytokine production by macrophages better than pathogenic mycobacterial species (8, 64) and can activate and induce the maturation of dendritic cells better than BCG by upregulation of major histocompatibility complex (MHC) class I and costimulatory molecules (10). M. smegmatis can also access the MHC class I pathway for presentation of mycobacterial antigens more efficiently than BCG (40). The present studies were initiated to assess the ability of recombinant M. smegmatis to elicit HIV-1 envelope-specific CD8+ T-cell responses.
MATERIALS AND METHODS
Generation of recombinant mycobacteria.
Mycobacterium smegmatis MC2155 was grown in Middlebrook 7H9 (Difco) supplemented with 10% albumin-dextrose saline and 0.05% Tween 80 (Fisher Scientific). Mycobacterium bovis BCG (Pasteur) was grown in 7H9 media supplemented with 10% oleic acid-albumin-dextrose-catalase (Difco) and 0.05% Tween 80. A human codon-optimized HIV-1 IIIB gp120 envelope gene (HXBc2) was cloned into the multicopy pJH222 and single-copy integrative pJH223 Escherichia coli/mycobacteria shuttle plasmids. A synthetic operon was constructed containing the viral envelope gene, which is regulated by the M. tuberculosis α antigen promoter and the M. tuberculosis 19-kDa signal sequence. For detection of the HIV-1 envelope protein, a hemagglutinin (HA) tag was fused to the C-terminal end of the envelope. Within the operon, a kanamycin resistance gene was cloned downstream of the viral gene. The multicopy and integrative plasmids with the HXBc2 envelope gene insert were transformed into the M. smegmatis MC2155 strain. Recombinant mycobacterial clones were selected for kanamycin resistance on 7H10 agar containing 20 μg/ml of kanamycin (Sigma). Single colonies were grown in 7H9 medium containing 20 μg/ml of kanamycin and grown by shaking for 2 to 3 days until an optical density at 600 nm (OD600) approximately equal to 1. Mycobacteria were then harvested and washed twice in ice-cold phosphate-buffered saline (PBS). Expression of the viral gp120 protein was assessed by Western blotting of mycobacterial lysates (1 μg of total protein) using an anti-HA monoclonal antibody (MAb) (clone 3F10) and a chemiluminescence detection kit according to the manufacturer's protocol (Roche Applied Science).
Mice and immunizations.
Eight- to 12-week-old female BALB/c mice were purchased from Taconic and Charles River laboratories. Mice were housed in a biosafety level 3 facility under specific-pathogen-free conditions at the Center for AIDS Research Animal Biohazard Containment Core Suite (Dana-Farber Cancer Institute). Research on mice was approved by the Dana-Farber Cancer Institute Animal Care and Use Committee. Recombinant M. smegmatis and BCG were grown in 7H9 medium until an OD600 approximately equal to 1. We estimated that bacterial growth to an OD value of 1 is equal to 5 × 108 CFU. For recombinant nonpathogenic Mycobacterium smegmatis MC2155 (rM. smegmatis) immunizations, approximately 106 or 108 CFU bacilli were injected via the intraperitoneal route in 200 μl of sterile PBS, 0.02% Tween. Approximately 106 CFU bacilli were injected subcutaneously for BCG immunization.
Tetramer staining and flow cytometric analysis.
H-2Dd tetrameric complexes folded with the P18 peptide (RGPGRAFVTI) (52), a sequence found in the V3 loop of HIV-1 HXBc2 envelope protein, were prepared as described previously (49). Mice were anesthetized with Isoflurane and bled retro-orbitally. Blood was collected in RPMI 1640 containing 40 U of heparin (American Pharmaceutical Partners) per ml. Peripheral blood mononuclear cells (PBMCs) were isolated using lympholyte-M (Cedarlane) and stained with the P18 tetramer conjugated with phycoerythrin (PE) and anti-CD8α MAb (Ly-2; Caltag) conjugated with allophycocyanin (APC) to detect P18-specific CD8+ T cells. The cells were washed in PBS containing 2% fetal bovine serum (FBS) and fixed with PBS containing 2% formaldehyde (Polysciences). CD8+ T cells were analyzed for tetramer staining using two-color flow cytometry on a FACS Array (BD Pharmingen). For phenotyping the P18-specific CD8+ T cells, splenocytes and PBMC were sampled 1 week after immunization of mice with recombinant mycobacteria and stained with anti-CD8α MAb (53-6.7; BD Pharmingen) conjugated with peridinin chlorophyll protein-Cy5.5, anti-CD62L MAb (MEL-14; BD Pharmingen) conjugated with APC, anti-CD44 MAb (IM-7; eBiosciences) conjugated with APC-Cy7, anti-CD127 MAb (A7R34; eBiosciences) conjugated with PE-Cy7, and the P18 tetramer conjugated with PE. Multicolor flow analysis was performed using the BD LSRII Cytometer (BD Biosciences) and the FlowJo software (Tree Star).
51Chromium release assay.
Splenocytes were harvested from mice 1 week after immunization with 107 CFU recombinant mycobacteria. The cells were resuspended in RPMI 1640 containing 10% FBS and cultured in a 24-well plate (8 × 106/well) with 10 ng of P18 epitope peptide per ml. Interleukin-2 (IL-2) (Sigma) was added to cultures on day 2 to a final concentration of 10 U/ml. On day 7, cells were harvested, washed once, and used as effectors in a 51Cr release assay with P815 target cells (American Type Culture Collection). P815 cells were cultured overnight in the presence of medium alone or with 100 ng of P18 peptide per ml. Cells (2 × 106) were labeled with 150 μCi of 51Cr for 1 h at 37°C, washed twice, and added to a 96-well round-bottom plate at 104/well in 100 μl of 10% RPMI medium. Titrations of effector cells were added to triplicate wells in 100 μl of medium. Lytic activity was assessed in a 4-h 51Cr release assay as previously described (46). Percent specific lysis was calculated as follows: 100 × (experimental − spontaneous release)/(maximum − spontaneous release).
IFN-γ ELISPOT assay.
An enzyme-linked immunospot (ELISPOT) assay was performed to measure gamma interferon (IFN-γ) production as previously described (46). Briefly, 96-well multiscreen HA plates (Millipore) were coated by overnight incubation (100 μl/well) at 4°C with rat anti-mouse IFN-γ MAb (clone R4-6A2; BD Pharmingen) at 10 μg/ml in PBS. Splenocytes were harvested from individual mice 1 week after immunization with 107 CFU recombinant mycobacteria. Effector cells were plated in triplicate at 2 × 105/well in a 100-μl final volume with medium alone, 4 μg of p18 epitope peptide per ml, or 4 μg of Env peptide pool per ml. The pool consisted of 47 overlapping 15-mer peptides spanning the HIV-1 IIIB gp120 protein (Centralized Facility for AIDS Reagents, Potters Bar, United Kingdom) and was used such that each peptide was present at a concentration of 4 μg/ml. After a 24-h incubation at 37°C, the plates were washed free of cells with PBS-0.05% Tween 20 and incubated overnight at 4°C with 100 μl of biotinylated rat anti-mouse IFN-γ MAb (clone XMG1.2; BD Pharmingen) per well at 5 μg/ml. Plates were washed four times, and 75 μl of streptavidin-alkaline phosphatase (Southern Biotechnology Associates) was added at a 1/500 dilution. After a 2-h incubation, plates were washed four times and developed with Nitro Blue Tetrazolium-5-bromo-4-chloro-3-indolylphosphate chromogen (Pierce). Plates were analyzed with an ELISPOT reader (Hitech Instruments).
Statistical analysis.
Data were expressed as means ± standard errors of the means (SEM). Statistical tests were performed using Student's t test. A P value of less than 0.05 was considered significant.
RESULTS
Generation of recombinant Mycobacterium smegmatis expressing HIV-1 gp120 envelope protein.
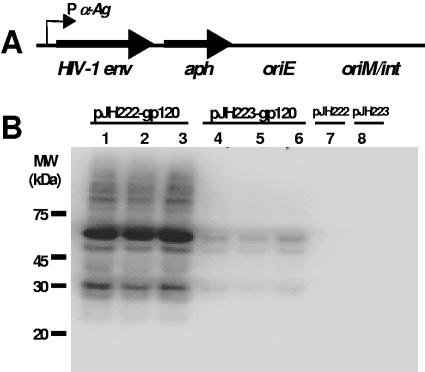
The pJH222 and pJH223 E. coli/mycobacteria shuttle plasmids were used to express a HIV-1 HXBc2 env gene codon-optimized for human cell expression. A human codon-optimized HIV-1 env was used, since the codons used by human cells are similar to those used by mycobacteria (3, 14, 44). pJH222 is a multicopy plasmid that replicates episomally in mycobacteria (47); pJH223 is an integration-proficient plasmid (30) that integrates as a single-copy DNA in the mycobacterial genome (Fig. 1A). Both plasmids contained the M. tuberculosis α antigen promoter to drive expression of HXBc2 env. The HIV-1 protein was fused to an HA tag at the C terminus. The N terminus of the HIV-1 envelope was fused to the 19-kDa signal sequence to facilitate expression of the viral protein in the mycobacterial membrane. Fusion with the 19-kDa signal sequence has been shown to increase immunogenicity of a heterologous protein (50).
FIG. 1.
Expression of HIV-1 HXBc2 gp120 envelope in nonpathogenic Mycobacterium smegmatis. (A) Codon-optimized HXBc2 gp120 env was cloned into the E. coli/mycobacteria shuttle plasmids pJH222 (multicopy) and pJH223 (integrative). The gp120 env in the plasmids is under the control of the M. tuberculosis α-Ag promoter (P α-Ag). A fusion protein was created in which the M. tuberculosis 19-kDa signal sequence was at the N terminus and an influenza hemagglutinin (HA) epitope tag was at the C terminus of the gp120 Env. Both plasmids contained the Tn903-derived aph gene conferring kanamycin resistance as a selectable marker and an E. coli origin of replication (oriE). The origin of replication (oriM) was inserted into the pJH222 plasmid, while the attP site and the int gene of mycobacteriophage L5 were included in pJH223. (B) Western blot analysis showed expression of the gp120 protein in recombinant M. smegmatis MC2155. The gp120 expression of three independent clones of mycobacteria transformed with either pJH222-gp120 (lanes 1 to 3) or pJH223-gp120 (lanes 4 to 6) was determined using an anti-HA MAb (clone 3F10). Mycobacteria transformed with either mock pJH222 or pJH223 containing an irrelevant gene (malaria msp1) were utilized as negative controls (lanes 7 and 8). MW, molecular mass.
Both env-containing plasmids were transformed into the efficient plasmid transformation mutant M. smegmatis MC2155 (48). Western blot analysis using an anti-HA MAb showed that recombinant M. smegmatis clones transformed with the multicopy plasmid had higher expression levels of the viral protein than those transformed with the integrating plasmid (Fig. 1B). Control rM. smegmatis constructs, which were transformed with either pJH222 or pJH223 containing the malaria msp1 gene, did not express the viral antigen. The predicted molecular weight of the envelope protein expressed by the rM. smegmatis constructs suggested an absence of the heavy glycosylation seen in the mature gp120 envelope of HIV-1 (63). These results show the successful expression of the full-length HIV-1 gp120 protein in the rapidly growing, nonpathogenic M. smegmatis.
rM. smegmatis immunization elicited functional HIV-1-specific CD8+ T cells.
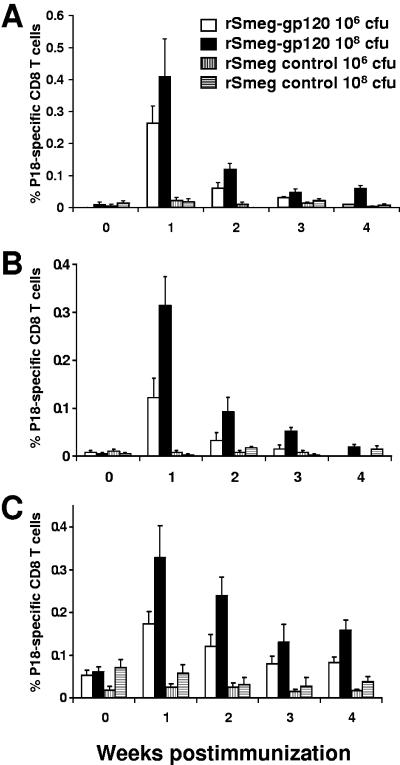
While BCG and Mycobacterium vaccae (19) have been assessed as potential vaccine vectors, other nonpathogenic species of mycobacteria have not been evaluated for this application. We therefore tested the rM. smegmatis constructs for their immunogenicity in mice. Immune responses were monitored in BALB/c mice using a tetramer assay, measuring CD8+ T-cell responses to the H-2Dd-restricted P18 epitope from the V3 loop of the HXBc2 envelope protein. Both the multicopy and the single-copy rM. smegmatis constructs expressing gp120 elicited peripheral blood HIV-specific CD8+ T-cell responses in mice immunized intraperitoneally with either 106 or 108 CFU bacilli (P < 0.05 versus control groups at weeks 1, 2, and 3 postimmunization) (Fig. 2A and B). Higher frequency responses were seen in mice immunized with 108 CFU than those immunized with 106 CFU bacilli. Interestingly, despite significantly lower viral antigen expression by rM. smegmatis containing the highly stable single-copy plasmid than rM. smegmatis containing the multicopy plasmid (Fig. 1B), the magnitudes of the immune responses elicited by both constructs were comparable. CD8+ T-cell responses peaked at week 1, rapidly declined thereafter, and were undetectable in the peripheral blood by week 4.
FIG. 2.
Recombinant M. smegmatis elicited HIV-1-specific CD8+ T-cell responses in mice. BALB/c mice were inoculated via the intraperitoneal route with approximately 106 CFU or 108 CFU gp120-expressing recombinant M. smegmatis (rSmeg-gp120) organisms transformed with either the integrative pJH223-gp120 plasmid (A) or multicopy pJH222-gp120 (B). As a negative control, mice were inoculated with the same dose of mycobacteria transformed with the control pJH222- and pJH223-msp1 plasmids (rSmeg control). (C) Mice were inoculated twice (10 weeks apart) with the same dose of either the rSmeg-gp120 (integrative) construct or the rSmeg control. The mean (± SEM) percent HIV-1 HXBc2 gp120 P18 tetramer-positive CD8 T cells from PBMC collected at the indicated time points is shown for each group of mice (n = 4 per group).
Mice were inoculated with rM. smegmatis expressing gp120 twice, at an interval of 10 weeks, to determine whether the T-cell responses could be boosted. P18-specific responses increased in magnitude and were seen 1 week after immunization (P < 0.05 versus control groups). However, these peak responses were not greater than those seen following a single inoculation (Fig. 2C). Responses in the boosted mice declined thereafter but remained detectable even 1 year following the initial immunization (data not shown). These results demonstrate that rM. smegmatis is capable of eliciting MHC class I-restricted CD8+ T cells specific for the HIV-1 envelope.
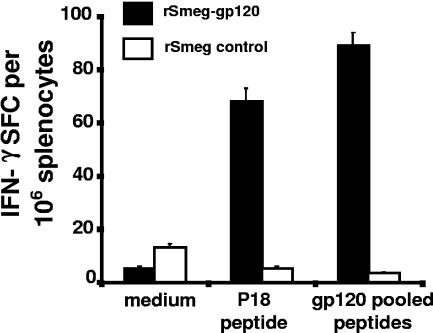
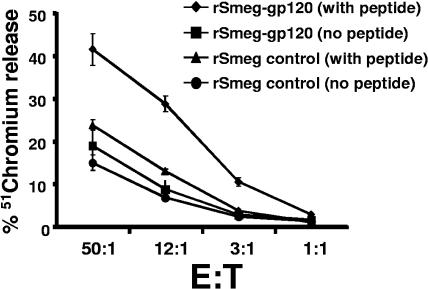
The functional capacity of the rM. smegmatis-induced HIV-1 envelope-specific CD8+ T cells was then determined. Splenocytes were harvested 1 week after immunization of mice with 107 CFU of the single-copy rM. smegmatis construct and evaluated in IFN-γ ELISPOT and 51Cr release assays. The splenocytes secreted IFN-γ after overnight stimulation with the dominant CD8+ T-cell P18 epitope peptide or a pool of overlapping peptides spanning the entire gp120 protein (P < 0.001 versus control groups) (Fig. 3). The cytotoxic activity of the rM. smegmatis-elicited CD8+ T cells was also assessed. Splenocytes were stimulated with the P18 peptide for 1 week in the presence of IL-2 and evaluated as effector cells in a 51Cr release assay. These effector cells were able to kill efficiently P815 target cells pulsed with the P18 peptide (P < 0.01 versus control groups at effector-to-target ratios 50:1, 12:1, and 3:1) (Fig. 4). Thus, the rM. smegmatis-elicited HIV-1-specific CD8+ T cells were capable of secreting IFN-γ and mediating cytolytic activity in response to HIV-1 envelope peptide stimulation.
FIG. 3.
rM. smegmatis-elicited HIV-1-specific CD8+ T cells secreted IFN-γ. Day 7 splenocytes from mice immunized with 107 CFU recombinant mycobacteria expressing gp120 (integrative) were exposed to no peptide, P18, or a gp120 peptide pool and evaluated in an ELISPOT assay. Splenocytes from mice immunized with rM. smegmatis expressing Msp1 were used as a control. The mean (± SEM) spot-forming cells (SFC) per 106 splenocytes for each group of mice (n = 4 per group) is shown.
FIG. 4.
Cytotoxic activity of HIV-1-specific CD8+ T cells elicited by rM. smegmatis immunization. HIV-1-specific CTL were expanded in vitro by stimulating splenocytes isolated from mice at day 14 after a single inoculation with 108 CFU rM. smegmatis expressing gp120 (integrative) with 10 ng/ml p18 peptide in the presence of rat IL-2 for 7 days. Cytotoxic activity of the effector cells for P815 target cells pulsed with or without p18 was assessed in a 51chromium release assay. Effector-to-target (E:T) ratios used in the study are indicated.
rM. smegmatis immunization elicited both effector and memory HIV-1 Env-specific CD8+ T cells.
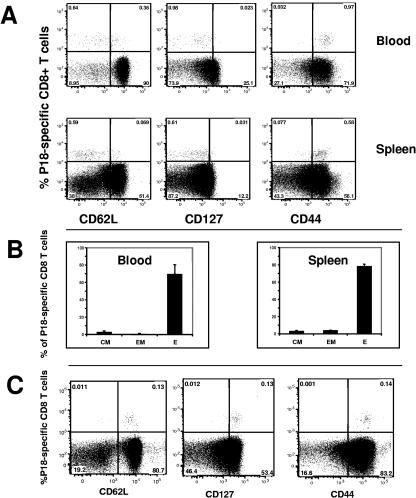
To further characterize the rM. smegmatis-induced CD8+ T cells, we evaluated these Env-specific T cells for their state of maturation and functional commitment by assessing their expression of CD62L, CD127, and CD44 using surface staining with monoclonal antibodies and flow cytometric analysis. Tetramer-positive CD8+ T cells were found in both the spleen and the peripheral blood 1 week after immunization with rM. smegmatis expressing gp120. In contrast, HIV-1-specific CD8+ T cells were not generated in mice immunized with the control mycobacteria construct. All the tetramer-positive CD8+ cells expressed CD44, indicating that they were activated (Fig. 5 A). Moreover, the majority of these CD8+ T cells were effector cells (tetramer positive, CD44hi, CD127−, and CD62Llo), and a small proportion was either effector memory (tetramer positive, CD44hi, CD127+, and CD62Llo) or central memory cells (tetramer positive, CD44hi, CD127+, and CD62Llo). This was seen both in the peripheral blood and spleen of the immunized mice (Fig. 5B). One year after immunization with rM. smegmatis, essentially all of the peripheral blood tetramer-positive CD8+ T cells were central memory cells (Fig. 5C). These data indicate that rM. smegmatis can generate effector, effector memory, and long-lived central memory HIV-specific CD8+ T cells.
FIG. 5.
Phenotype of HIV-1-specific CD8 T cells elicited by immunization with rM. smegmatis. Mice were immunized with 108 CFU rM. smegmatis expressing gp120 (integrative). (A) Flow cytometric analysis of week 1 PBMC and splenocytes from immunized mice revealed expression of CD44 on the surface of all rM. smegmatis-elicited tetramer-positive cells. CD62L and CD127 were expressed on a subset of the tetramer-positive cells. (B) The proportions of effector (P18-tetramer positive, CD127−, and CD62Llo), effector memory (P18-tetramer positive, CD127+, and CD62Llo), and central memory (P18-tetramer positive, CD127+, and CD62Lhi) cells in the blood and spleen of mice immunized with rM. smegmatis are shown. Effector, effector memory, and central memory cells are denoted E, EM, and CM, respectively. The mean (± SEM) percent E, EM, or CM for each group of mice (n = 4 per group) is shown. (C) Peripheral blood HIV-1-specific CD8+ T cells from mice 1 year after immunization with rM. smegmatis expressing gp120 were predominantly central memory cells. PBMC were pooled from 4 mice that were inoculated twice (10 weeks apart) with 108 CFU bacilli.
rM. smegmatis elicited HIV-1 Env-specific CD8+ T cells in BCG immune mice.
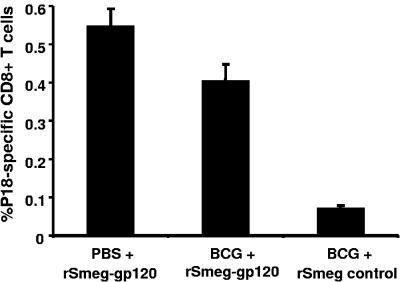
A large proportion of the human population has received BCG as a tuberculosis vaccine, and we were concerned that prior BCG exposure might substantially blunt the immunogenicity of an rM. smegmatis vaccine. We therefore evaluated whether anti-BCG immunity can affect the immunogenicity of rM. smegmatis constructs in mice. To induce anti-BCG immunity, mice were inoculated with 106 CFU wild-type BCG or PBS and 6 months later were inoculated with rM. smegmatis expressing gp120 (17). In fact, only a modest reduction in peak tetramer-positive CD8+ T-cell responses was observed in the BCG-preimmunized mice (Fig. 6). These results suggest that preexisting immunity to BCG may have only a marginal effect on the immunogenicity of rM. smegmatis.
FIG. 6.
Recombinant M. smegmatis-elicited HIV-1-specific CD8+ T-cell responses in mice preimmunized with BCG. Mice were immunized with wild-type BCG (Pasteur) or PBS and 6 months later with 108 CFU rM. smegmatis expressing gp120 (integrative) (indicated as BCG + rSmeg-gp120 and PBS + rSmeg-gp120, respectively). BCG-preimmunized mice that were subsequently inoculated with rSmeg control were used as a negative control (indicated as BCG + rSmeg control). Tetramer analysis was performed on the PBMC of mice 1 week after inoculation with the rM. smegmatis constructs. The mean (± SEM) percent of HIV-1 HXBc2 gp120 P18-tetramer-positive CD8+ T cells is shown for each group (n = 4 to 5 mice per group).
DISCUSSION
We have thus demonstrated that a novel vaccine vector, a recombinant nonpathogenic Mycobacterium smegmatis MC2155 (rM. smegmatis) expressing the entire HIV-1 HXBc2 gp120 envelope protein, was immunogenic in mice. The strain MC2155 is a mutant of M. smegmatis (ATCC 607) that is transformable with pAL5000 plasmids at 7 orders of magnitude higher frequency than the parental strain (48). The efficient plasmid transformation phenotype has caused MC2155 to be the surrogate host of choice for the analysis of genes from pathogenic mycobacteria (12, 13, 58) and has recently been sequenced by The Institute for Genomic Research (http://www.tigr.org). Moreover, this strain has been shown to be nonpathogenic following intravenous infections of SCID mice (5). We evaluated a variety of mycobacterial promoters and regulatory genes and found that the use of M. tuberculosis α antigen promoter and fusion of the transgene with the 19-kDa signal sequence optimized the immunogenicity of the vaccine construct. A number of factors probably contributed to this increased immunogenicity. This configuration clearly enhanced the expression of the HIV-1 gp120 envelope protein in mycobacteria (data not shown). Furthermore, fusion of the gp120 protein with the 19-kDa protein may have created a chimeric lipoprotein that is immunogenic, perhaps because of acylation of the signal sequence (66). Neyrolles et al. found that the acylated moiety was important for MHC class I antigen presentation of lipoproteins, perhaps because it facilitates lipoprotein interaction with Toll-like receptor 2 (TLR2) (18, 40).
It is interesting to speculate that the immunogenicity of the HIV-1 envelope 19-kDa fusion lipoprotein in the rapidly growing nonpathogenic rM. smegmatis mycobacteria may be associated with the inability of this recombinant vector to persist. Although lipoproteins are certainly immunogenic, persistent exposure to lipoproteins can lead to suppression of antigen presentation by macrophages (41, 53). Both M. bovis BCG and M. tuberculosis can persist in host cells and inhibit phagosome maturation (55, 56). Persisting M. tuberculosis and BCG may exert immunosuppressive effects through ligation of lipoproteins to TLRs localized to the phagosome (1, 42, 43, 53, 54). On the other hand, M. smegmatis does not inhibit phagosome maturation and is degraded rapidly by phagolysosomal proteases. An explanation for the robust immunogenicity of the rM. smegmatis constructs will likely come from parallel studies of the immunogenicity of the HIV envelope chimeric lipoprotein in rBCG and rM. smegmatis as well as an evaluation of the roles of persistence and lipoprotein-TLR interactions in the generation of CD8+ T-cell responses elicited by mycobacteria.
rM. smegmatis-elicited HIV-1-specific CD8+ T cells exhibited effector functions such as cytolysis and production of IFN-γ. The ability of recombinant, nonpathogenic rapidly growing mycobacteria to elicit antigen-specific CTL responses has never been reported previously. However, M. tuberculosis or recombinant M. bovis BCG have been shown to elicit antigen-specific CTL (2, 24). The ability of HIV-1 vaccine vectors to elicit strong CTL responses is likely to be critical for vaccine-induced immune containment of HIV-1 replication and prevention of AIDS (31).
Recombinant M. smegmatis was previously assessed as a vaccine in a mouse tumor model (10). Cheadle et al. showed that M. smegmatis was better than BCG at promoting DC maturation. However, recombinant M. smegmatis expressing a CTL epitope of the ovalbumin (OVA) antigen did not protect against challenge with a tumor expressing this epitope, whereas BCG expressing the same epitope protected mice against the OVA epitope-expressing tumor (10). This absence of anti-tumor activity elicited by recombinant M. smegmatis was associated with poor presentation of peptides by the nonpathogenic mycobacteria to an OVA-specific T-cell line in vitro (10). However, since the OVA-specific T-cell responses were not measured after immunization with the recombinant mycobacteria in this study, the inability of recombinant M. smegmatis to confer protection against a tumor challenge could not be attributed to inefficient induction of tumor antigen-specific CTL responses in vivo. In contrast to the findings of Cheadle et al., recombinant M. smegmatis was shown to access the MHC class I pathway better than BCG for presentation of peptide antigens (40). Furthermore, a recombinant M. smegmatis expressing TNF-α was shown to have anti-tumor properties in mice (67). The conflicting findings in these studies may be explained by the nature of the antigen expressed by mycobacteria. Neyrolles et al. expressed the influenza NP CTL epitope fused to the 19-kDa lipoprotein in mycobacteria (40). In contrast, Cheadle et al. expressed a secreted OVA CTL epitope in mycobacteria (10).
The kinetics of the rM. smegmatis-elicited T-cell responses differed from those of T-cell responses generated using other vaccine modalities. rM. smegmatis-elicited HIV-specific CD8+ T-cell responses were maximal 1 week after immunization. This peak T-cell response is earlier than responses elicited by plasmid DNA, adenoviral vectors, and vaccinia vectors, which generally are maximal 10 to 14 days postimmunization (6, 46). Interestingly, an early peak immune response has also been described in mice immunized with recombinant Listeria monocytogenes (23). rM. smegmatis also induced peak T-cell responses that were of lower magnitude than those induced by recombinant viral vectors but similar in magnitude to those elicited by plasmid DNA (46).
The maturation and differentiation status of the rM. smegmatis-elicited CD8+ T cells was defined using MAbs specific for CD44, CD62L, and CD127 (22). In both the peripheral blood and spleen of rM. smegmatis-immunized mice, the majority of the HIV-1-specific CTL generated were effector cells (CD44hi, CD127−, CD62Llo), and very few effector memory (CD44hi, CD127+, CD62Llo) and central memory (CD44hi, CD127+, CD62Lhi) CTL were seen at the time of peak immune responses. Interestingly, in mice receiving two immunizations with rM. smegmatis, we also found a small but stable population of HIV-1-specific central memory CD8+ T cells. These data therefore suggest that rM. smegmatis is capable of generating both HIV-1-specific effector and memory cells in vivo. The ability of the rM. smegmatis vector to generate central memory cells is particularly important, since these cells have been shown to expand in vivo and mediate protective immunity following a challenge with a pathogenic organism (62).
There is growing evidence that vector or pathogen persistence may have an adverse effect on the generation of T-cell memory. Persistent lymphocytic choriomeningitis virus and lentiviral infections result in the generation of T cells that have lost the ability to perform some important effector functions (4, 16, 35, 61, 68). Persistent mycobacterial infections by slow-growing M. tuberculosis and BCG may also adversely affect T-cell memory responses. On the other hand, vectors that do not persist can generate good T-cell memory (60). Therefore, we speculate that the rapidly growing M. smegmatis vector may be better at eliciting memory T-cell responses than persistent mycobacterial vectors because M. smegmatis is eliminated rapidly in the host.
Interestingly, we found that the multicopy and the single-copy vectors elicited comparable tetramer responses despite the fact that the multicopy rM. smegmatis construct expressed significantly more HIV-1 envelope protein. High levels of gp120 expression have been shown to be toxic to mycobacteria (51). Consistent with this finding, we observed that the in vitro growth of multicopy rM. smegmatis was slower than that of the single-copy vector (data not shown). Moreover, studies have shown that recombinant mycobacteria containing an integrated HIV transgene stably express that transgene and are highly immunogenic (36, 51). Thus, recombinant mycobacteria with integrated transgenes appear to be useful vaccine vectors.
A major limitation of the clinical utility of a number of vaccine vectors currently in development is the inhibition of vector immunogenicity by preexisting anti-vector immunity. For example, immunity to the HIV-1 vaccine vector adenovirus serotype 5 (rAd5) has been shown to blunt the immunogenicity of rAd5 vaccines (7, 37). Since BCG is administered to a large proportion of the human population as a TB vaccine, anti-mycobacterial immunity might diminish the immunogenicity of recombinant mycobacterial vectors such as rM. smegmatis. However, our data indicate that BCG immunity affects the immunogenicity of rM. smegmatis only modestly. Consistent with this observation, it was previously reported that mice immunized with wild-type BCG still developed T-cell and antibody responses to the HIV-1 Nef and β-galactosidase transgenes expressed in recombinant BCG (17). Preexisting immunologic memory responses to BCG could result in the rapid destruction of recombinant M. smegmatis, which might favor cross-priming of the heterologous HIV-1 gp120 antigen (25). Hence, recombinant mycobacterial vaccines may be useful in BCG-immunized individuals.
Acknowledgments
We are grateful to Barry Bloom, Michael Seaman, Dan Barouch, Joern Schmitz, Keith Reimann, Sampa Santra, Michael Newberg, Yue Sun, Shawn Sumida, and Adam Buzby for technical assistance and scientific discussions. Joseph Sodroski provided the codon-optimized HIV-1 HXBc2 DNA. The HIV-1 IIIB gp120 overlapping peptides were provided by the EU Program EVA/MRC Centralized Facility for AIDS Reagents, National Institute for Biological Standards and Control, United Kingdom.
This work was supported by National Institutes of Health grants AI52816 and AI067854.
REFERENCES
- 1.Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. [DOI] [PubMed] [Google Scholar]
- 2.Aldovini, A., and R. A. Young. 1991. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351:479-482. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, G. E., and P. M. Sharp. 1996. Codon usage in the Mycobacterium tuberculosis complex. Microbiology 142:915-925. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bange, F. C., F. M. Collins, and W. R. Jacobs, Jr. 1999. Survival of mice infected with Mycobacterium smegmatis containing large DNA fragments from Mycobacterium tuberculosis. Tuber. Lung Dis. 79:171-180. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 8.Beltan, E., L. Horgen, and N. Rastogi. 2000. Secretion of cytokines by human macrophages upon infection by pathogenic and non-pathogenic mycobacteria. Microb. Pathog. 28:313-318. [DOI] [PubMed] [Google Scholar]
- 9.Calarota, S., G. Bratt, S. Nordlund, J. Hinkula, A. C. Leandersson, E. Sandstrom, and B. Wahren. 1998. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351:1320-1325. [DOI] [PubMed] [Google Scholar]
- 10.Cheadle, E. J., D. O'Donnell, P. J. Selby, and A. M. Jackson. 2005. Closely related mycobacterial strains demonstrate contrasting levels of efficacy as antitumor vaccines and are processed for major histocompatibility complex class I presentation by multiple routes in dendritic cells. Infect. Immun. 73:784-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connell, N. D., E. Medina-Acosta, W. R. McMaster, B. R. Bloom, and D. G. Russell. 1993. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc. Natl. Acad. Sci. USA 90:11473-11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Converse, S. E., and J. S. Cox. 2005. A protein secretion pathway critical for Mycobacterium tuberculosis virulence is conserved and functional in Mycobacterium smegmatis. J. Bacteriol. 187:1238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Mendonca-Lima, L., M. Picardeau, C. Raynaud, J. Rauzier, Y. O. de la Salmoniere, L. Barker, F. Bigi, A. Cataldi, B. Gicquel, and J. M. Reyrat. 2001. Erp, an extracellular protein family specific to mycobacteria. Microbiology 147:2315-2320. [DOI] [PubMed] [Google Scholar]
- 14.de Miranda, A. B., F. Alvarez-Valin, K. Jabbari, W. M. Degrave, and G. Bernardi. 2000. Gene expression, amino acid conservation, and hydrophobicity are the main factors shaping codon preferences in Mycobacterium tuberculosis and Mycobacterium leprae. J. Mol. Evol. 50:45-55. [DOI] [PubMed] [Google Scholar]
- 15.Fennelly, G. J., J. L. Flynn, V. ter Meulen, U. G. Liebert, and B. R. Bloom. 1995. Recombinant bacille Calmette-Guerin priming against measles. J. Infect. Dis. 172:698-705. [DOI] [PubMed] [Google Scholar]
- 16.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477-486. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiu, M., M. R. Lagranderie, B. M. Gicquel, and C. D. Leclerc. 1994. Mycobacterium bovis BCG priming induces a strong potentiation of the antibody response induced by recombinant BCG expressing a foreign antigen. Infect. Immun. 62:4287-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grode, L., M. Kursar, J. Fensterle, S. H. Kaufmann, and J. Hess. 2002. Cell-mediated immunity induced by recombinant Mycobacterium bovis Bacille Calmette-Guerin strains against an intracellular bacterial pathogen: importance of antigen secretion or membrane-targeted antigen display as lipoprotein for vaccine efficacy. J. Immunol. 168:1869-1876. [DOI] [PubMed] [Google Scholar]
- 19.Hetzel, C., R. Janssen, S. J. Ely, N. M. Kristensen, K. Bunting, J. B. Cooper, J. R. Lamb, D. B. Young, and J. E. Thole. 1998. An epitope delivery system for use with recombinant mycobacteria. Infect. Immun. 66:3643-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda, M., K. Matsuo, T. Nakasone, Y. Okamoto, H. Yoshizaki, K. Kitamura, W. Sugiura, K. Watanabe, Y. Fukushima, S. Haga, Y. Katsura, H. Tasaka, K. Komuro, T. Yamada, T. Asano, A. Yamazaki, and S. Yamazaki. 1995. Protective immune responses induced by secretion of a chimeric soluble protein from a recombinant Mycobacterium bovis bacillus Calmette-Guerin vector candidate vaccine for human immunodeficiency virus type 1 in small animals. Proc. Natl. Acad. Sci. USA 92:10693-10697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huebner, R. E. 1996. BCG vaccination in the control of tuberculosis. Curr. Top. Microbiol. Immunol. 215:263-282. [DOI] [PubMed] [Google Scholar]
- 22.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 101:5610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamath, A. B., J. Woodworth, X. Xiong, C. Taylor, Y. Weng, and S. M. Behar. 2004. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J. Exp. Med. 200:1479-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufmann, S. H., and U. E. Schaible. 2005. Antigen presentation and recognition in bacterial infections. Curr. Opin. Immunol. 17:79-87. [DOI] [PubMed] [Google Scholar]
- 26.Kuehnel, M. P., R. Goethe, A. Habermann, E. Mueller, M. Rohde, G. Griffiths, and P. Valentin-Weigand. 2001. Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell Microbiol. 3:551-566. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni, H. R., and S. P. Zodpey. 1999. Differential protective effect of bacillus Calmette-Guerin vaccine against multibacillary and paucibacillary leprosy in Nagpur, India. Public Health 113:311-313. [DOI] [PubMed] [Google Scholar]
- 28.Lagranderie, M., A. M. Balazuc, B. Gicquel, and M. Gheorghiu. 1997. Oral immunization with recombinant Mycobacterium bovis BCG simian immunodeficiency virus nef induces local and systemic cytotoxic T-lymphocyte responses in mice. J. Virol. 71:2303-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langermann, S., S. R. Palaszynski, J. E. Burlein, S. Koenig, M. S. Hanson, D. E. Briles, and C. K. Stover. 1994. Protective humoral response against pneumococcal infection in mice elicited by recombinant bacille Calmette-Guerin vaccines expressing pneumococcal surface protein A. J. Exp. Med. 180:2277-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, M. H., L. Pascopella, W. R. Jacobs, Jr., and G. F. Hatfull. 1991. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guerin. Proc. Natl. Acad. Sci. USA 88:3111-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letvin, N. L. 2002. Strategies for an HIV vaccine. J. Clin. Investig. 110:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo, Y., X. Chen, A. Szilvasi, and M. A. O'Donnell. 2000. Co-expression of interleukin-2 and green fluorescent protein reporter in mycobacteria: in vivo application for monitoring antimycobacterial immunity. Mol. Immunol. 37:527-536. [DOI] [PubMed] [Google Scholar]
- 33.MacGregor, R. R., J. D. Boyer, K. E. Ugen, K. E. Lacy, S. J. Gluckman, M. L. Bagarazzi, M. A. Chattergoon, Y. Baine, T. J. Higgins, R. B. Ciccarelli, L. R. Coney, R. S. Ginsberg, and D. B. Weiner. 1998. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 178:92-100. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto, S., H. Yukitake, H. Kanbara, and T. Yamada. 1998. Recombinant Mycobacterium bovis bacillus Calmette-Guerin secreting merozoite surface protein 1 (MSP1) induces protection against rodent malaria parasite infection depending on MSP1-stimulated interferon gamma and parasite-specific antibodies. J. Exp. Med. 188:845-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKay, P. F., J. E. Schmitz, D. H. Barouch, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, D. A. Gorgone, and N. L. Letvin. 2002. Vaccine protection against functional CTL abnormalities in simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 168:332-337. [DOI] [PubMed] [Google Scholar]
- 36.Mederle, I., I. Bourguin, D. Ensergueix, E. Badell, J. Moniz-Peireira, B. Gicquel, and N. Winter. 2002. Plasmidic versus insertional cloning of heterologous genes in Mycobacterium bovis BCG: impact on in vivo antigen persistence and immune responses. Infect. Immun. 70:303-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molnar-Kimber, K. L., D. H. Sterman, M. Chang, E. H. Kang, M. ElBash, M. Lanuti, A. Elshami, K. Gelfand, J. M. Wilson, L. R. Kaiser, and S. M. Albelda. 1998. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum. Gene Ther. 9:2121-2133. [DOI] [PubMed] [Google Scholar]
- 38.Nascimento, I. P., W. O. Dias, R. P. Mazzantini, E. N. Miyaji, M. Gamberini, W. Quintilio, V. C. Gebara, D. F. Cardoso, P. L. Ho, I. Raw, N. Winter, B. Gicquel, R. Rappuoli, and L. C. Leite. 2000. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect. Immun. 68:4877-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newton, J. A., Jr., P. J. Weiss, W. A. Bowler, and E. C. Oldfield III. 1993. Soft-tissue infection due to Mycobacterium smegmatis: report of two cases. Clin. Infect. Dis. 16:531-533. [DOI] [PubMed] [Google Scholar]
- 40.Neyrolles, O., K. Gould, M. P. Gares, S. Brett, R. Janssen, P. O'Gaora, J. L. Herrmann, M. C. Prevost, E. Perret, J. E. Thole, and D. Young. 2001. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J. Immunol. 166:447-457. [DOI] [PubMed] [Google Scholar]
- 41.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom, and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 42.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pai, R. K., M. E. Pennini, A. A. Tobian, D. H. Canaday, W. H. Boom, and C. V. Harding. 2004. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect. Immun. 72:6603-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan, A., C. Dutta, and J. Das. 1998. Codon usage in highly expressed genes of Haemophillus influenzae and Mycobacterium tuberculosis: translational selection versus mutational bias. Gene 215:405-413. [DOI] [PubMed] [Google Scholar]
- 45.Pierre-Audigier, C., E. Jouanguy, S. Lamhamedi, F. Altare, J. Rauzier, V. Vincent, D. Canioni, J. F. Emile, A. Fischer, S. Blanche, J. L. Gaillard, and J. L. Casanova. 1997. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin. Infect. Dis. 24:982-984. [DOI] [PubMed] [Google Scholar]
- 46.Seaman, M. S., F. W. Peyerl, S. S. Jackson, M. A. Lifton, D. A. Gorgone, J. E. Schmitz, and N. L. Letvin. 2004. Subsets of memory cytotoxic T lymphocytes elicited by vaccination influence the efficiency of secondary expansion in vivo. J. Virol. 78:206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snapper, S. B., L. Lugosi, A. Jekkel, R. E. Melton, T. Kieser, B. R. Bloom, and W. R. Jacobs, Jr. 1988. Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc. Natl. Acad. Sci. USA 85:6987-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 49.Staats, H. F., C. P. Bradney, W. M. Gwinn, S. S. Jackson, G. D. Sempowski, H. X. Liao, N. L. Letvin, and B. F. Haynes. 2001. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J. Immunol. 167:5386-5394. [DOI] [PubMed] [Google Scholar]
- 50.Stover, C. K., G. P. Bansal, M. S. Hanson, J. E. Burlein, S. R. Palaszynski, J. F. Young, S. Koenig, D. B. Young, A. Sadziene, and A. G. Barbour. 1993. Protective immunity elicited by recombinant bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J Exp. Med. 178:197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi, H., Y. Nakagawa, C. D. Pendleton, R. A. Houghten, K. Yokomuro, R. N. Germain, and J. A. Berzofsky. 1992. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science 255:333-336. [DOI] [PubMed] [Google Scholar]
- 53.Tobian, A. A., N. S. Potter, L. Ramachandra, R. K. Pai, M. Convery, W. H. Boom, and C. V. Harding. 2003. Alternate class I MHC antigen processing is inhibited by Toll-like receptor signaling pathogen-associated molecular patterns: Mycobacterium tuberculosis 19-kDa lipoprotein, CpG DNA, and lipopolysaccharide. J. Immunol. 171:1413-1422. [DOI] [PubMed] [Google Scholar]
- 54.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 55.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 56.Via, L. E., R. A. Fratti, M. McFalone, E. Pagan-Ramos, D. Deretic, and V. Deretic. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111(Part 7):897-905. [DOI] [PubMed] [Google Scholar]
- 57.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 58.Wei, J., J. L. Dahl, J. W. Moulder, E. A. Roberts, P. O'Gaora, D. B. Young, and R. L. Friedman. 2000. Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages. J. Bacteriol. 182:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss, B. G., and S. Schlesinger. 1991. Recombination between Sindbis virus RNAs. J. Virol. 65:4017-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 63.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 64.Yadav, M., S. K. Roach, and J. S. Schorey. 2004. Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J. Immunol. 172:5588-5597. [DOI] [PubMed] [Google Scholar]
- 65.Yasutomi, Y., S. Koenig, S. S. Haun, C. K. Stover, R. K. Jackson, P. Conard, A. J. Conley, E. A. Emini, T. R. Fuerst, and N. L. Letvin. 1993. Immunization with recombinant BCG-SIV elicits SIV-specific cytotoxic T lymphocytes in rhesus monkeys. J. Immunol. 150:3101-3107. [PubMed] [Google Scholar]
- 66.Young, D. B., and T. R. Garbe. 1991. Lipoprotein antigens of Mycobacterium tuberculosis. Res. Microbiol. 142:55-65. [DOI] [PubMed] [Google Scholar]
- 67.Young, S. L., M. Murphy, X. W. Zhu, P. Harnden, M. A. O'Donnell, K. James, P. M. Patel, P. J. Selby, and A. M. Jackson. 2004. Cytokine-modified Mycobacterium smegmatis as a novel anticancer immunotherapy. Int. J. Cancer 112:653-660. [DOI] [PubMed] [Google Scholar]
- 68.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]