Abstract
Only the latency-associated transcript (LAT) of the herpes simplex virus type 1 (HSV-1) genome is transcribed during latency, while the lytic genes are suppressed, possibly by LAT antisense mechanisms and/or chromatin modifications. In the present study, latently infected dorsal root ganglia were explanted to assess both relative levels of LAT and histone H3 (K9, K14) acetylation of the LAT locus and ICP0 promoter at early times postexplant. We observed that a decrease in both LAT enhancer histone H3 (K9, K14) acetylation and LAT RNA abundance occurs prior to an increase in acetylation, or transcriptional permissiveness, at the ICP0 promoter.
Herpes simplex virus type 1 (HSV-1) is characterized by its ability to establish latency as an episome in neurons (12). During this time, transcriptional activity is virtually nonexistent, with the exception of the latency-associated transcript (LAT), an 8.3- to 8.5-kb noncoding RNA that can be spliced to yield a 2.0-kb stable intron (5, 13, 16). One proposed function of the LAT is the suppression of nearby lytic phase transcripts ICP0, γ34.5, and ICP4 through antisense mechanisms, thereby promoting the establishment and maintenance of latency (3). Studies using LAT promoter and/or 5′ exon mutants demonstrate impaired establishment of latency and leaky expression of lytic phase transcripts (3). In addition, a recent study has demonstrated that the lytic gene regions of LAT mutants are associated with less of the repressive histone H3 K9 dimethyl, suggesting that the LAT plays a direct role in promoting a transcriptionally nonpermissive environment for lytic genes during latency (18). If the LAT is indeed responsible for suppressing lytic phase transcripts during latency, one might expect reactivation to directly regulate LAT levels.
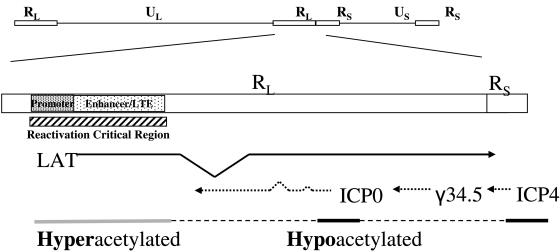
In addition to putative LAT-mediated suppression of lytic transcripts during latency, mounting evidence suggests that latent gene expression is also regulated at the chromatin level. The latent viral genome is known to associate with nucleosomes (4). Investigation of chromatin modification, in particular, the acetylation of histone H3 lysine residues 9 and 14 (K9 and K14, respectively), demonstrates that during latency, the lytic regions of the virus exist in a hypoacetylated, or transcriptionally nonpermissive, state, while the LAT promoter and 5′ exon/enhancer remain hyperacetylated, or transcriptionally permissive (9) (Fig. 1). However, LAT transcription is not a prerequisite, nor is it necessary to maintain the hyperacetylated, or structurally relaxed, chromatin state (8), suggesting that the enhancer within the LAT region is an important cis-acting DNA element.
FIG. 1.
Diagram of the HSV-1 genome. Regions of the long and short repeat (RL and RS, respectively) that have been analyzed for H3 acetylation are indicated by gray or black bars. The LAT promoter and enhancer components are encompassed by the reactivation critical region (rcr). These elements are maintained in a hyperacetylated state during latency, as indicated by the gray bar. The antisense immediate-early ICP0 and ICP4 promoters exist in a hypoacetylated state during latency, as indicated by the black bars. UL, unique long region; US, unique short region.
Reactivation of the latent viral genome has been linked to a reactivation critical region (rcr) that encompasses the LAT core promoter through the LAT 5′ exon/enhancer, since recombinants lacking this region display greatly reduced reactivation phenotypes (2, 6, 7, 10). However, this still does not address whether the regulatory elements in the rcr act at the RNA or DNA/chromatin level. An initial study using Northern blot analysis had detected a decrease in LAT at 24 and 36 h postexplant of latently infected murine trigeminal ganglia (14), suggesting that LAT expression may be incompatible with reactivation. Therefore, we sought to determine whether changes in LAT transcription and/or histone acetylation occur at early times during reactivation.
A model to study early molecular events of reactivation involves explant of latently infected murine dorsal root ganglia (DRG) into supplemented medium, a process that results in reactivation of latent virus (15). In the present study, explanted DRG were placed in medium and incubated for specific time intervals, followed by RNA isolation or chromatin cross-linking.
To determine the effect of explant on LAT levels, RNA was isolated from female murine DRG and reverse transcribed using random decamers, and the resulting cDNA was analyzed by real-time (TaqMan) PCR. Relative quantities of the LAT were normalized to either adenine phosphoribosyl transferase (APRT), a cytoplasmic cellular transcript, or to Xist, a nuclear noncoding RNA. As shown in Fig. 2, LAT abundance may transiently increase initially (1.5- to 2-fold), before decreasing between 2 and 3 h postexplant (hpe). Regardless of the cellular control used for normalization, the overall pattern of expression appears the same, suggesting that the decreases observed for the LAT following explant were not due to a general decrease in either total cellular or nuclear RNAs.
FIG. 2.
LAT RNA levels decrease at early times postexplant. RNA was isolated and pooled from DRG (4/mouse) explanted from three mice latently infected with 1 × 105 PFU/mouse of HSV-1 strain KOS as described previously (8). cDNA was analyzed in triplicate by real-time PCR using primers and a probe specific for the 5′ exon of the LAT (nucleotides 119326 to 119397) (8). Relative quantities of LAT RNA were normalized to the PCR of the cellular genes Xist (8) or APRT (forward, CTCAAGAAATCTAACCCCTGACTCA; reverse, GCGGGACAGGCTGAGA; probe, CCCCACACACACCTC). The results of two independent experiments are graphed as percent LAT 5′ exon RNA relative to the RNA level of the 0-hpe time point.
The observed effect of explant on LAT RNA levels suggests that early events in explant-induced reactivation may alter transcription of the LAT. Previously, we reported that the LAT region is maintained in a hyperacetylated state during latency, independently of LAT transcription; therefore, we sought to determine whether there was a change in histone H3 (K9, K14) acetylation following explant. Chromatin immunoprecipitation (ChIP) analysis of the LAT promoter following explant demonstrated a dramatic reduction in acetylation as early as 1 hpe (Fig. 3A). In order to be certain that this effect was not due to global changes in histone acetylation caused by explant-induced stress, subsequent experiments (Fig. 3B and C) were normalized to APRT, a constitutively expressed cellular gene. In the third experiment (Fig. 3C), the decrease in acetylation at the 1-hpe time point was not as dramatic, and in this experiment the acetylation actually increased between 1 and 2 hpe. Overall, the LAT promoter displayed a variable decrease in histone H3 acetylation occurring within the first hour of explant.
FIG. 3.
Explant causes a decrease in acetylation of the LAT enhancer, which precedes an increase in acetylation of the ICP0 promoter. ChIP analysis was performed as previously described (8) using DRG (6/mouse) explanted from mice latently infected with 1 × 105 PFU/mouse of HSV-1 strain KOS. Samples were analyzed in triplicate by real-time PCR. (A) Results from experiment 1 show the percentages of bound/input ratios relative to the 0-hpe time point for the postexplant times indicated. Two mice were used per time point. (B and C) Experiments 2 and 3, respectively, show the percentages of bound/input ratios normalized to APRT bound/input ratios and relative to the 0-hpe time point for the postexplant times indicated. Four mice were used per time point. B/I, bound/input.
Because it was previously reported that the LAT enhancer is capable of increasing LAT transcription (1) and because acetylation of the enhancer was shown to be independent of abundant LAT transcription (8), it seemed plausible that the observed decrease in LAT abundance might correlate with changes in the acetylation of histones associated with the LAT enhancer following explant-induced reactivation. Real-time PCR analyses of the LAT 5′ exon for the same three ChIP experiments that were analyzed for the LAT promoter displayed marked decreases of at least fivefold in acetylation occurring as early as 0.5 hpe (Table 1; Fig. 3A to C), with the decrease by 1 hpe being statistically significant among the three independent ChIP experiments (P > 0.02). It should be noted that in experiment 3, following a dramatic decrease in acetylation of the 5′ exon at 0.5 hpe, there is an increase in acetylation. This increase parallels the increase in acetylation observed at the LAT promoter in this same experiment and possibly reflects a more rapid return to the “latent” acetylation state in this set of ganglia. Overall, the dramatic and rapid decreases in the acetylation of histone H3 associated with the 5′ exon and LAT promoter suggest that a rapid change in transcriptional permissiveness precedes the decrease in LAT RNA abundance.
TABLE 1.
Calculation of changes in acetylation following explant-induced reactivation
| DNA target | Expt no. | Time (hpe) | B/Ia | B/I relative to APRT B/I | B/I relative to B/I at 0 hpe | % B/I relative to B/I at 0 hpe | Change (n-fold) relative to 0 hpe | Difference (n-fold) relative to 0 hpeb |
|---|---|---|---|---|---|---|---|---|
| LAT Promoter | 1 | 0 | 0.1430 | NDc | 1 | 100 | 1 | 0 |
| 1 | 0.0042 | ND | 0.0297 | 2.9724 | 33.6423 | −32.6423 | ||
| 2 | 0.0039 | ND | 0.0272 | 2.7223 | 36.7332 | −35.7332 | ||
| 3 | 0.0003 | ND | 0.0021 | 0.2079 | 480.9459 | −479.9459 | ||
| 4 | 0.0068 | ND | 0.0475 | 4.7537 | 21.0362 | −20.0362 | ||
| 2 | 0 | 2.4028 | 295.7442 | 1 | 100 | 1 | 0 | |
| 0.5 | 1.5483 | 60.5022 | 0.2046 | 20.4576 | 4.8882 | −3.8882 | ||
| 1 | 0.0248 | 1.4054 | 0.0048 | 0.4752 | 210.4340 | −209.4340 | ||
| 2 | 0.0096 | 0.5462 | 0.0018 | 0.1847 | 541.4208 | −540.4208 | ||
| 3 | 0.2627 | 12.7400 | 0.0431 | 4.3078 | 23.2138 | −22.2138 | ||
| 3 | 0 | 0.0098 | 1.7692 | 1 | 100 | 1 | 0 | |
| 0.5 | 0.0088 | 1.9255 | 1.0884 | 108.8363 | 0.9188 | 0.0812 | ||
| 1 | 0.0109 | 1.1141 | 0.6297 | 62.9720 | 1.5880 | −0.5880 | ||
| 2 | 0.0442 | 5.2463 | 2.9654 | 296.5368 | 0.3372 | 0.6628 | ||
| 3 | 0.0368 | 3.5309 | 1.9958 | 199.5778 | 0.5011 | 0.4989 | ||
| LAT 5′ exon | 1 | 0 | 0.1401 | ND | 1 | 100 | 1 | 0 |
| 1 | 0.0213 | ND | 0.1523 | 15.2301 | 6.5659 | −5.5659 | ||
| 2 | 0.0020 | ND | 0.0146 | 1.4622 | 68.3894 | −67.3894 | ||
| 3 | 0.0000 | ND | 0.0004 | 0.0350 | 2856.4697 | −2855.4697 | ||
| 4 | 0.0006 | ND | 0.0045 | 0.4541 | 220.2176 | −219.2176 | ||
| 2 | 0 | 3.2863 | 351.3441 | 1 | 100 | 1 | 0 | |
| 0.5 | 2.7672 | 17.0743 | 0.0486 | 4.8597 | 20.5774 | −19.5774 | ||
| 1 | 0.0951 | 4.2162 | 0.0120 | 1.2000 | 83.3318 | −82.3318 | ||
| 2 | 0.0086 | 0.5462 | 0.0016 | 0.1555 | 643.2079 | −642.2079 | ||
| 3 | 0.4751 | 15.0993 | 0.0430 | 4.2976 | 23.2690 | −22.2690 | ||
| 3 | 0 | 0.0885 | 14.9577 | 1 | 100 | 1 | 0 | |
| 0.5 | 0.0073 | 1.5404 | 0.1030 | 10.2985 | 9.7102 | −8.7102 | ||
| 1 | 0.0041 | 7.7986 | 0.5214 | 52.1381 | 1.9180 | −0.9180 | ||
| 2 | 0.0956 | 13.1157 | 0.8769 | 87.6856 | 1.1404 | −0.1404 | ||
| 3 | 0.1134 | 9.9748 | 0.6669 | 66.6869 | 1.4995 | −0.4995 | ||
| ICP0 promoter | 1 | 0 | 0.0029 | ND | 1 | 100 | 1 | 0 |
| 1 | 0.0008 | ND | 0.2580 | 25.7968 | 3.8765 | −2.8765 | ||
| 2 | 0.0006 | ND | 0.2194 | 21.9441 | 4.5570 | −3.5570 | ||
| 3 | 0.0006 | ND | 0.1995 | 19.9524 | 5.0119 | −4.0119 | ||
| 4 | 0.0100 | ND | 3.4450 | 344.5042 | 0.2903 | 0.7097 | ||
| 2 | 0 | 0.0145 | 1.7203 | 1 | 100 | 1 | 0 | |
| 0.5 | 0.0262 | 0.9733 | 0.5658 | 56.5787 | 1.7674 | −0.7674 | ||
| 1 | 0.0289 | 1.3528 | 0.7864 | 78.6380 | 1.2716 | −0.2716 | ||
| 2 | 0.0529 | 2.8914 | 1.6807 | 168.0709 | 0.5950 | 0.4050 | ||
| 3 | 0.0464 | 2.1909 | 1.2736 | 127.3556 | 0.7852 | 0.2148 | ||
| 3 | 0 | 0.0053 | 0.8496 | 1 | 100 | 1 | 0 | |
| 0.5 | 0.0020 | 0.3783 | 0.4453 | 44.5306 | 2.2456 | −1.2456 | ||
| 1 | 0.0060 | 0.6691 | 0.7875 | 78.7506 | 1.2698 | −0.2698 | ||
| 2 | 0.0074 | 0.9640 | 1.1346 | 113.4604 | 0.8814 | 0.1186 | ||
| 3 | 0.0299 | 2.6436 | 3.1114 | 311.1441 | 0.3214 | 0.6786 |
B/I, bound input ratio; average bound quantity divided by the average input quantity.
Difference = 1 − x, where x represents change (n-fold) relative to 0 hpe (value shown in preceding column).
ND, not determined.
To determine whether the observed changes in the LAT abundance are linked to a change in the transcriptional permissiveness of the ICP0 promoter, we also analyzed its acetylation status using the same ChIP experiments. As shown in Fig. 3A to C (far right column), there is a net increase in ICP0 promoter acetylation occurring as early as 2 hpe and increasing by as much as threefold by 3 hpe. Despite this increase in acetylated histone H3 associated with the ICP0 promoter, no significant increase in ICP0 transcription could be detected by 4 hpe (data not shown). Taken together, these data show a sequential process where changes in chromatin structure of the LAT enhancer and decreased transcription of the LAT allow for an increase in acetylation at the ICP0 promoter, perhaps facilitating productive reactivation in at least some neurons.
In order to extend these analyses and determine whether an increase in ICP0 transcription could be detected following longer incubations of the explants, we performed three independent ChIP and reverse transcription-PCR (RT-PCR) experiments at 8 and 12 hpe. We chose 12 hpe as our latest time point since it has been recently shown that infectious virus is first detected in the majority of explants at 14 hpe (11). ChIP analyses of the 8- and 12-hpe time points revealed that the LAT and ICP0 promoters and the LAT 5′ exon show comparable levels of acetylation at 8 and 12 h (Fig. 4A). In comparison to the levels of acetylation observed at 3 and 4 h, this represents a slight decrease in transcriptional permissiveness of the ICP0 promoter but is still higher than the baseline values for time zero (latent expression). RT-PCR analyses of these later time points clearly demonstrate that the levels of LAT RNA remain low through the 12-h time point (Fig. 4B). These results are consistent with, and extend, our observations at the earlier time points (Fig. 2). Nonetheless, we still failed to detect a significant increase in ICP0 transcription by 12 hpe. It should be noted that the earliest detection of a net increase in ICP0 transcripts in explanted ganglia was 96 h by Northern blotting (14) or 24 h by RT-PCR (17). Since it has been shown that the first round of reactivating virus produced by explant occurs by 14 h (11), it is likely that the inability to detect an increase in ICP0 RNA is because ICP0 transcription occurs in only the small subset of cells that ultimately go down the path to productive reactivation. It is well documented that reactivation occurs only in a small percentage of the total population of latently infected neurons, so detection of this small increase in ICP0 transcripts above the background of the entire latent population would be difficult, if not impossible. It is also likely that the ICP0 transcription detected at 24 h and later postexplant reflects secondary rounds of replication within the explanted ganglia, possibly in nonneuronal support cells (11).
FIG. 4.
LAT RNA levels remain low and the ICP0 promoter remains in a transcriptionally permissive state through 12 hpe. (A) Analysis of three independent ChIP experiments at 8 and 12 hpe. Three mice per time point per ChIP were precipitated with anti-H3 K9, 14 acetyl and analyzed by TaqMan PCR with primers and probes for the LAT promoter, 5′ exon, and ICP0 promoter. The ICP0 promoter remains hyperacetylated, though there is an apparent increase in the relative level of LAT 5′ exon acetylation by 12 hpe. (B) LAT and ICP0 RNA at 0, 8, and 12 hpe from three independent experiments of explanted DRG was analyzed by TaqMan real-time PCR. Reverse transcription using random decamer primers (Ambion) or a strand-specific primer for the ICP0 transcript (LAT I-1, GACACGGATTGGCTGGTGTAGTGGG; nucleotides 120797 to 120820) was performed using Omniscript reverse transcriptase (QIAGEN) according to the manufacturer's instructions. The cDNA was then analyzed with primers and probes for ICP0 (forward, GGCCGAGGGAGGTTTCC, nucleotides 121385 to 121401; reverse, CCGCTTCCGCCTCCTC, nucleotides 121438 to 121453; probe, CTCCCAGGGCACCGAC, nucleotides 121412 to 121427) or the LAT 5′ exon and then normalized relative to APRT and Xist cellular controls. A significant decrease in LAT RNA occurs between 0 and 8 hpe (P < 0.05) and remains at low levels through 12 hpe. No significant change in the amount of ICP0 RNA was detected between 0 and 12 hpe.
This study sought to determine the relationship between regulation of the LAT and the LAT region acetylation status at early reactivation times. The observations described here support the hypothesis that the LAT may act to suppress immediate-early genes and that the chromatin status of the LAT enhancer is linked to early reactivation events. Previous studies using the LAT promoter and/or 5′ exon mutants showed impaired establishment of latency and leaky expression of lytic phase transcripts during latency. Our finding that a significant and dramatic decrease in the LAT occurs at early times during explant-induced reactivation supports the notion that the LAT may be acting through antisense or other RNA-mediated mechanisms to suppress nearby lytic phase transcripts. Furthermore, the changes observed in the acetylation status of the LAT enhancer indicate that the LAT enhancer is both sensitive and responsive to reactivation signals. The increased level of acetylation at the ICP0 promoter following deacetylation of the LAT enhancer suggests that chromatin remodeling both at the LAT locus and at the ICP0 promoter may be directly linked during reactivation. It is therefore possible that the LAT enhancer functions to recruit a novel histone-modifying complex that helps establish and maintain the active expression of the LAT during latency. During reactivation, the complex may quickly respond to restructure the chromatin within the LAT region to facilitate nearby lytic phase gene expression. Work aimed at identifying such a complex is currently under way.
Acknowledgments
This work was supported by grant AI48633 from the National Institutes of Health and in part by the Investigators in Pathogenesis Award from the Burroughs Wellcome Fund (to D.C.B.). N.J.K. and N.V.G. received support from NIH training grant AI07110.
The authors thank P. McAnany for excellent technical assistance and J. Feller for helpful comments on the manuscript.
REFERENCES
- 1.Berthomme, H., J. Lokensgard, L. Yang, T. Margolis, and L. T. Feldman. 2000. Evidence for a bidirectional element located downstream from the herpes simplex virus type 1 latency-associated promoter that increases its activity during latency. J. Virol. 74:3613-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom, D. C., J. T. Hill, E. K. Wagner, L. F. Feldman, and J. G. Stevens. 1996. A 348-bp region in the latency-associated transcript facilitates herpes simplex virus type 1 reactivation. J. Virol. 70:2449-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, S. H., M. F. Kramer, P. A. Schaffer, and D. M. Coen. 1997. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 71:5878-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshmane, S. L., and N. W. Fraser. 1989. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J. Virol. 63:943-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell, M. J., A. T. Dobson, and L. T. Feldman. 1991. Herpes simplex virus latency-associated transcript is a stable intron. Proc. Natl. Acad. Sci. USA 88:790-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill, J. M., F. Sedarati, R. T. Javier, E. K. Wagner, and J. G. Stevens. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117-125. [DOI] [PubMed] [Google Scholar]
- 7.Jarman, R. G., J. M. Loutsch, G. B. Devi-Rao, M. E. Marquart, M. P. Banaszak, X. Zheng, J. M. Hill, E. K. Wagner, and D. C. Bloom. 2002. The region of the HSV-1 latency-associated transcript required for epinephrine-induced reactivation in the rabbit does not include the 2.0-kb intron. Virology 292:59-69. [DOI] [PubMed] [Google Scholar]
- 8.Kubat, N. J., A. L. Amelio, N. V. Giordani, and D. C. Bloom. 2004. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. J. Virol. 78:12508-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubat, N. J., R. K. Tran, P. McAnany, and D. C. Bloom. 2004. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. J. Virol. 78:1139-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leib, D. A., C. L. Bogard, V. M. Kosz, K. A. Hicks, D. M. Coen, D. M. Knipe, and P. A. Schaffer. 1989. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J. Virol. 63:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pesola, J. M., J. Zhu, D. M. Knipe, and D. M. Coen. 2005. Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production. J. Virol. 79:14516-14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock, D. L., and N. W. Fraser. 1985. Latent herpes simplex virus type 1 DNA contains two copies of the virion DNA joint region. J. Virol. 55:849-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rock, D. L., A. B. Nesburn, H. Ghaisi, J. Ong, T. L. Lewis, J. R. Lokensgard, and S. L. Wechsler. 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus. J. Virol. 61:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spivack, J. G., and N. W. Fraser. 1987. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J. Virol. 61:3841-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens, J. G., and M. L. Cook. 1971. Latent herpes simplex virus in spinal ganglia of mice. Science 173:843-845. [DOI] [PubMed] [Google Scholar]
- 16.Stevens, J. G., E. K. Wagner, R. G. B. Devi, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056-1059. [DOI] [PubMed] [Google Scholar]
- 17.Tal-Singer, R., T. M. Lasner, W. Podrzucki, A. Skokotas, J. J. Leary, S. L. Berger, and N. W. Fraser. 1997. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J. Virol. 71:5268-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, Q. Y., C. Zhou, K. E. Johnson, R. C. Colgrove, D. M. Coen, and D. M. Knipe. 2005. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc. Natl. Acad. Sci. USA 102:16055-16059. [DOI] [PMC free article] [PubMed] [Google Scholar]